1. Introduction
Luminescent all-inorganic metal halides have emerged as versatile optoelectronic platforms due to their tunable electronic structures and crystallographic flexibility [
1,
2]. Lead halide perovskites represent a prominent subset, achieving near-unity photoluminescence (PL) quantum yields and broad spectral tunability [
3]. However, intrinsic lead toxicity and operational instability fundamentally constrain their device implementation. This has accelerated the development of lead-free alternatives, where ions with analogous electronic structures to Pb
2+ (e.g., Ge
2+, Sn
2+, Sb
3+ and Bi
3+) [
2,
4,
5,
6,
7,
8] show promise as B-site substitutes in ABX
3 frameworks.
Tin-based perovskites attract significant interest because of their distinctive self-trapped exciton (STE) emission and environmental benignity [
4,
9,
10,
11,
12,
13,
14,
15]. However, the facile oxidation of Sn(II) to Sn(IV) leads to irreversible phase transformation into vacancy-ordered A
2SnX
6 derivatives. Although thermodynamically stable, these phases typically exhibit severely quenched photoluminescence—a critical limitation attributed to dominant non-radiative recombination pathways.
Doping engineering has proved to be an effective strategy to overcome these limitations. Although Sb
3+/Bi
3+ doping has been shown to enable broadband emission [
16,
17], recent advances demonstrate that tetravalent dopants (e.g., Te
4+) are particularly promising for enhancing optoelectronic performance. For instance, Han et al. demonstrated that Te
4+-doped Cs
2SnCl
6 serves as an efficient system for CO
2 photoreduction, expanding the materials library for photocatalytic applications [
18]. Similarly, Tang et al. achieved intense yellow-green luminescence (580 nm) in Sn
4+/Te
4+ ion-exchanged materials via Jahn–Teller distortions, reaching a record PL quantum yield of 95.4% [
13]. Despite these advances, Te-doped (NH
4)
2SnCl
6 remains underexplored, with systematic studies on its optical properties still lacking.
In this paper, undoped (NH
4)
2SnCl
6 and Te-doped (NH
4)
2SnCl
6 were synthesized and we systematically investigated their structure and photoluminescence properties to reveal the correlated photoluminescence mechanism. The morphology and structure of the samples were characterized using techniques such as TEM, XPS and XRD. UV–Vis absorption spectroscopy and temperature-dependent photoluminescence spectra further confirmed strong electron–phonon coupling, which induces symmetry-breaking distortions that stabilize self-trapped excitons (STEs). DFT calculations revealed linear thermochromic behavior in Te-doped (NH
4)
2SnCl
6 over a broad temperature range, thereby bridging theoretical and experimental insights into the structure–property relationship. Notably, the pronounced temperature dependence of the emission peak position enables 0.5%Te:(NH
4)
2SnCl
6 to achieve highly accurate temperature-sensing across a wide range. Compared with conventional ratiometric thermometry based on emission intensity or intensity ratios, this peak-shift-based approach offers higher measurement accuracy and reduced susceptibility to environmental interference [
19,
20].
2. Materials and Methods
Chemicals: All reagents and solvents were used without further purification. Ammonium chloride (NH4Cl; 99.99%), stannic chloride hydrate (SnCl4∙5H2O; 99.995%) and tellurium tetrachloride (TeCl4; 99.9%) were purchased from Aladdin. Hydrochloric acid (HCl; 36.0~38.0% in water) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China.
Synthesis of (NH4)2SnCl6: (NH4)2SnCl6 polycrystalline powders from small-batch production were prepared by dissolving 1 mmol SnCl4∙5H2O in a 3.5 mL HCl solution using constant stirring with a magnetic stir bar under ambient conditions. Then, 2 mmol NH4Cl in 0.5 mL deionized water was immediately dropped into the above solution. Upon the addition of NH4Cl, a white polycrystalline precipitate was formed. The product was then extracted from the crude solution via centrifugation (4500 rpm; 3 min, TG16-WS, Beijing North Tech Sichuang Scientific Instrument Co., Ltd., Beijing, China), washed with a HCl solution twice and dried under pressure overnight. The Te-doped (NH4)2SnCl6 samples were prepared via precipitation reactions following the same procedure with the addition of a nominal amount of TeCl4 in the HCl solution.
Characterization: The samples were characterized by transmission electron microscopy (TEM). TEM images were obtained using a JEM-2200FS (JEOL, Tokyo, Japan) machine equipped with an emission gun operated at 200 kV.
Optical Measurements: The optical absorption spectra measurements were carried out between 250 and 1000 nm using a deuterium–halogen light source and recorded with an optical fiber spectrometer (QE65000, Ocean Optics, Orlando, FL, USA) and a data collection time of 10 s. The PL experiments were carried out using a 355 nm excitation laser with 10 mW power.
Structural characterization: The structural characterization of the synthesized samples was performed using an X-ray diffractometer (XRD, D8 Advance Bruker, Berlin, Germany) with Cu Kα radiation (λ = 1.5406 Å), scanning over a range of 10–60° (2θ). The Raman spectra were recorded using a spectrometer equipped with liquid-nitrogen-cooled CCD (iHR 550, Symphony II, Horiba Jobin Yvon, Irvine, CA, USA). A 671 nm single-mode DPSS laser was utilized to excite the sample and the output power was 10 mW. The resolution of the system was 1 cm−1. All the high-pressure experiments were performed at room temperature.
3. Results and Discussion
The (NH
4)
2SnCl
6 and Te:(NH
4)
2SnCl
6 samples were synthesized according to the report in [
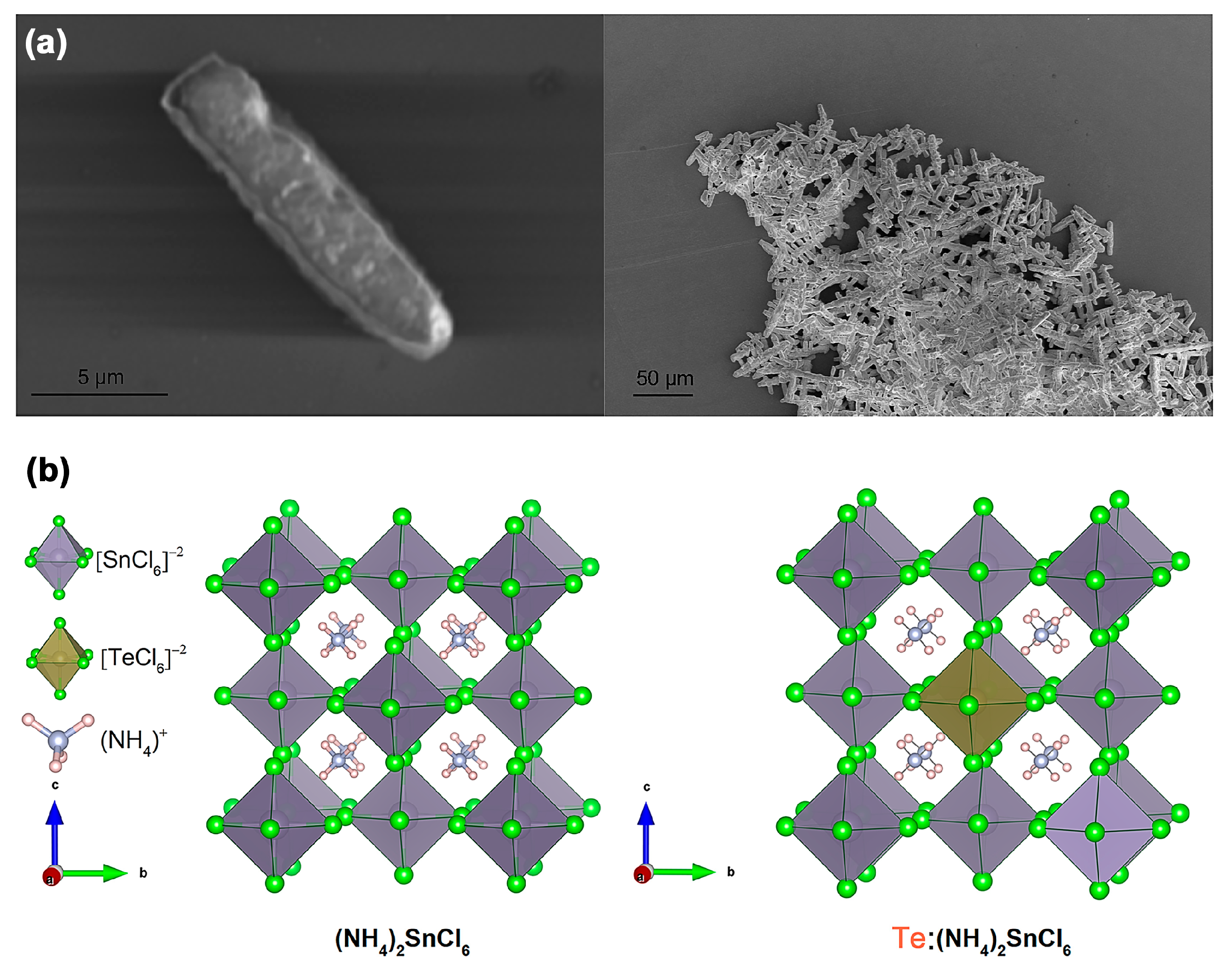
9]. The details were provided in the experimental section. The transmission electron microscopy (TEM) images reveal that the polycrystalline powders synthesized at room temperature consist of rod-shaped particles with a uniform morphology, having lengths of approximately 15 μm (
Figure 1a). Such morphological uniformity plays a critical role in influencing device performance and operational stability. Enhanced structural homogeneity helps to reduce defects, thereby optimizing the functional response of the device (such as response sensitivity and operational stability). As depicted in
Figure 1b, lead-free vacancy-ordered (NH
4)
2SnCl
6 perovskite crystallizes in the cubic phase (left panel). Notably, Te is the same periodic element as Sn and they have similar coordination characteristics with halogen, suppressing the doping. This results in a lattice defect and strain as far as possible. After doping the Te
4+ ions, the [SnCl
6]
2− octahedron in (NH
4)
2SnCl
6 can be partly substituted by the formed [TeCl
6]
2− octahedron (right panel). The [Sn(Te)Cl
6]
2− octahedra are isolated from each other, similar to the typical structure of Cs
2SnX
6 (X = Cl, Br and I).
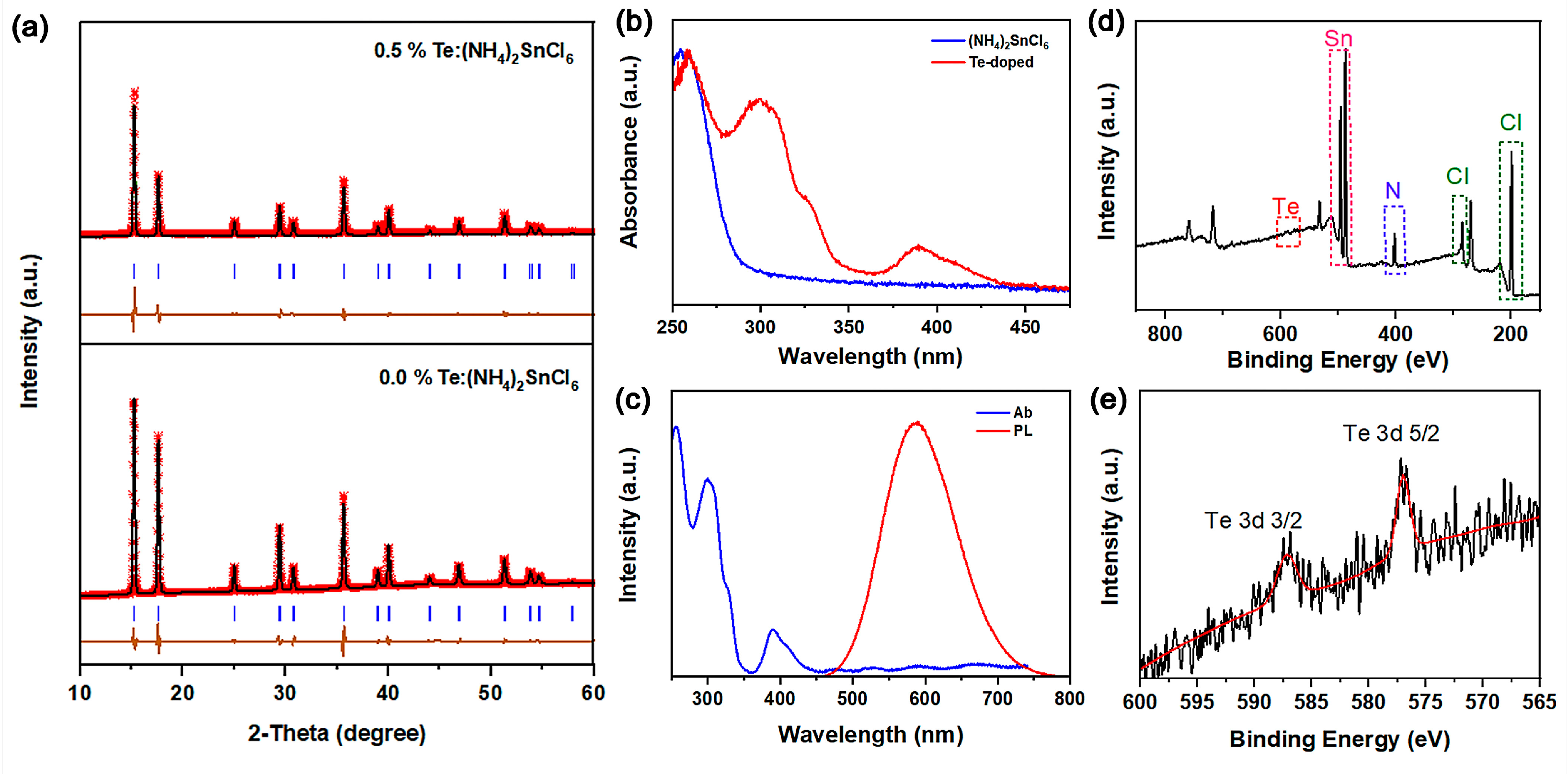
To confirm the successful incorporation of Te without inducing structural phase transitions, the XRD patterns were recorded. The XRD of the as-synthesized undoped (NH
4)
2SnCl
6 sample corresponded well with the standard pattern of (NH
4)
2SnCl
6. No impurity diffraction peaks were observed, indicating that the as-synthesized (NH
4)
2SnCl
6 was pure. No impurity phase was detected in 0.5% Te-doped (NH
4)
2SnCl
6, indicating the high purity of 0.5%Te:(NH
4)
2SnCl
6. The refinement profiles of the (NH
4)
2SnCl
6:xTe (x = 0 and 0.5%) samples showed well-refined simulated patterns (
Figure 2a). Furthermore, as is well-established, the ion radius of Te
4+ (VI, 0.97 Å) is bigger than that of Sn
4+ (VI, 0.69 Å). With Te doping, the XRD peaks gradually shifted to a smaller diffraction angle, verifying that the partial Sn
4+ was successfully replaced by the bigger Te
4+ in the (NH
4)
2SnCl
6 lattice. Detailed Rietveld refinements are provided in
Table S1. Furthermore, the stable oxidation states of Sn
4+ and Te
4+ ensure excellent ambient and thermal stability. As shown in
Figure 2b, the absorption spectrum of pure (NH
4)
2SnCl
6 shows a bandgap of 4.26 eV and the strongest band edge absorption, located at 291 nm, is slightly smaller than the reported value of (NH
4)
2SnCl
6 (310 nm). The absorption tail in the long-wavelength region could be attributed to defect or surface states, similar to those observed in Cs
2SnCl
6 NCs. As Te
4+ is incorporated, the absorption spectrum shows two distinct absorption humps at 300–360 nm and 370–500 nm. As shown in
Figure S1, the bandgap of 0.5%Te: (NH
4)
2SnCl
6 significantly reduced to 2.66 eV compared with (NH
4)
2SnCl
6 (4.26 eV). The newly emerging absorption region between 350 and 500 nm could be assigned to localized Te 6s
2 → 6s
1p
1 transitions in [TeCl
6]
2− octahedra. The long-wavelength absorption feature centered at ~390 nm can be assigned to the
1S
0 →
3P
1 transition of Te
4+, which is a doublet caused by the dynamic Jahn–Teller effect. Correspondingly, the peak of the short-wavelength band at ~330 nm is assigned to the
1S
0 →
1P
1 transition.
At room temperature, Sn(IV)-based compounds are more stable in air than tin(II)-based counterparts. However, the absence of ns
2 active lone pairs cannot effectively promote the formation of Jahn–Teller-like STEs; thus, the pure Sn(IV)-based halides of the (NH
4)
2SnCl
6 white powder showed no emission under a 355 nm UV lamp. Upon the exchange of Sn/Te ions, the introduction of Te
4+ ions with active 5s
2 lone pairs effectively promotes the formation of Jahn–Teller distortions in the lattice structure [
13]. The Jahn–Teller distortions formed during the expansion process are conducive to the improvement in light emission performance. Hence, after doping the Te
4+ ions, the formed 0.5%Te: (NH
4)
2SnCl
6 perovskite emits a strong yellow light under a 355 nm UV lamp. As shown in
Figure 2c, the photoluminescence (PL) spectra show a bright broadband orange emission peak at 585 nm with a full width at half maximum (FWHM) at about 116 nm and a large Stokes shift. The broadband PL spectrum profile and large Stokes shift are due to the intense lattice distortion and robust Jahn–Teller effect. Hence, a significant improvement in photoluminescence was observed from the Te
4+ substitution. Moreover, the exact element content was determined by XPS. In
Figure 2d,e, the peaks located at 597.78 and 587.48 eV belong to Te
4+ 3d
3/2 and 3d
5/2, respectively. These XPS profiles indicate that Te prefers to replace the Sn site, forming the TeCl
6 octahedron in 0.5%Te: (NH
4)
2SnCl
6 perovskite variants.
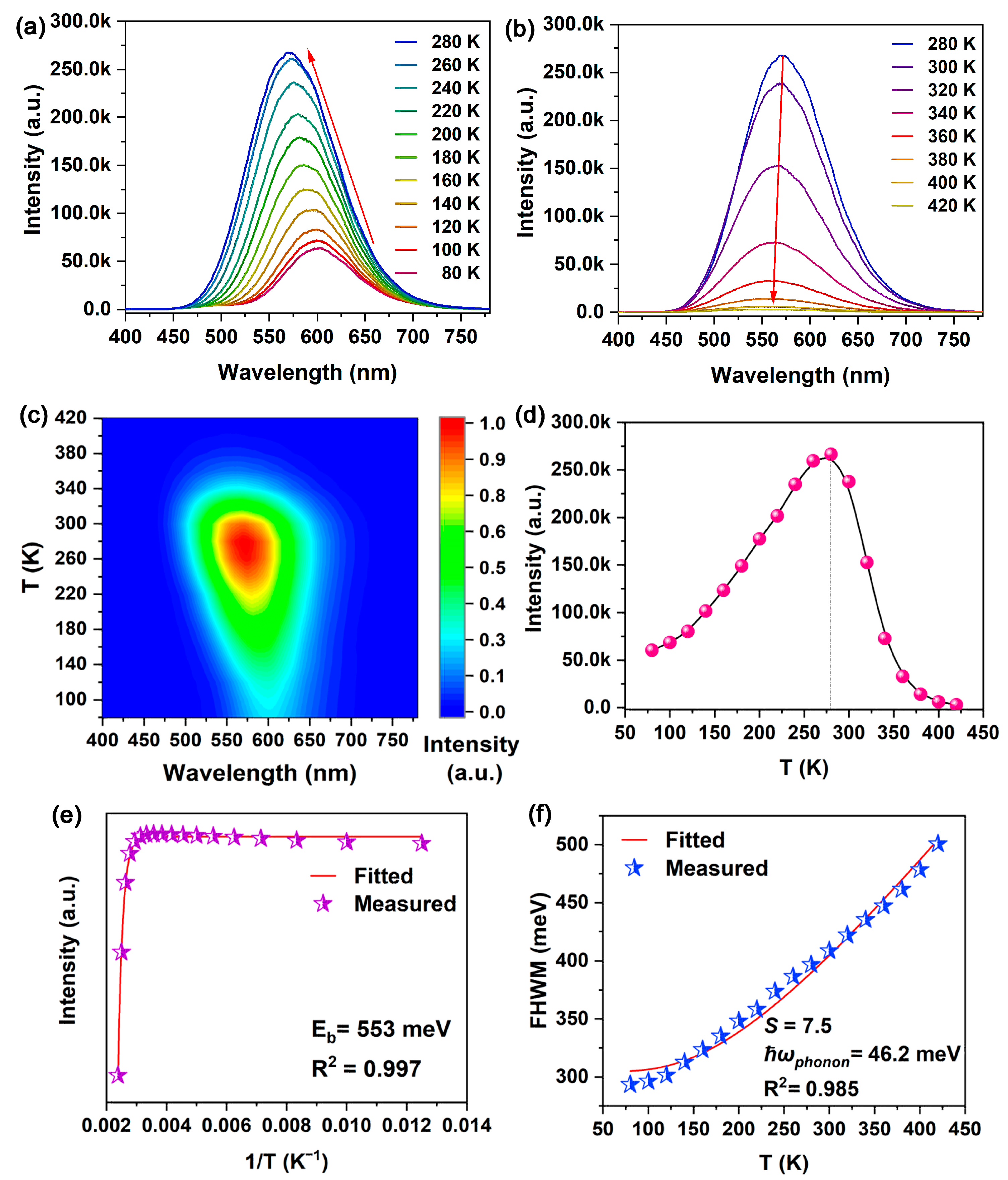
To further clarify the emission mechanism, the PL emission spectra at different temperatures were measured from 80 K to 420 K (
Figure 3a,b). A 2D projection of the emission spectra with contour lines demonstrated the evolution of luminescence with temperature (
Figure 3c). The emission intensity first increased as the temperature increased from 80 K to 280 K, but then decreased with temperatures from 280 K to 420 K (
Figure 3d). Such behavior suggests that PL could originate from thermally activated delayed fluorescence, which involves reverse intersystem crossing (RISC) from long-lived triplet-excited states back to short-lived singlet-excited states [
21] via the thermally activated non-radiative recombination. A comparison with the previous commonly observed PL shifts with temperature in ABX
3 and A
2BB’X
6 perovskites (X = Cl, Br and I) [
22,
23,
24] revealed that this abnormal PL blue shift monotonically corresponds with increasing temperatures and is ascribed to important thermal lattice expansion [
22]. As shown in
Figure 3e, the variation in PL intensity with temperature can be expressed as follows:
where
I0 and
I are the emission intensities at 0 K and measurement temperature, respectively.
Ea is the thermal activation energy, which is considered to evaluate the exciton binding energy [
25].
kB is the Boltzmann constant. The
Ea of 0.5%Te:(NH
4)
2SnCl
6 was calculated to be 553 meV, which is much higher than Te:Cs
2SnCl
6 (270 meV) and Rb
2TeCl
6 (117 meV) [
10,
11]. The
Ea of 0.5%Te:(NH
4)
2SnCl
6 is much higher than the thermal energy at room temperature and indicates the formation of stable STEs with highly localized behavior. The electron–phonon coupling degree can be effectively reflected by the variation in FWHM with temperature (
Figure 3f). The temperature-dependent FWHM can be fitted as follows:
where
S is the electron–phonon coupling parameter, ħ
ω is the phonon frequency and
kB is the Boltzmann constant. The
S of 7.5 and ħ
ω of 46.2 meV were obtained by fitting. The ħ
ω value is consistent with the Raman spectra (
Figure S2). Such a value of
S indicates robust electron–phonon coupling, implying prominent lattice distortion and the resulting Jahn–Teller effect in 0.5%Te: (NH
4)
2SnCl
6.
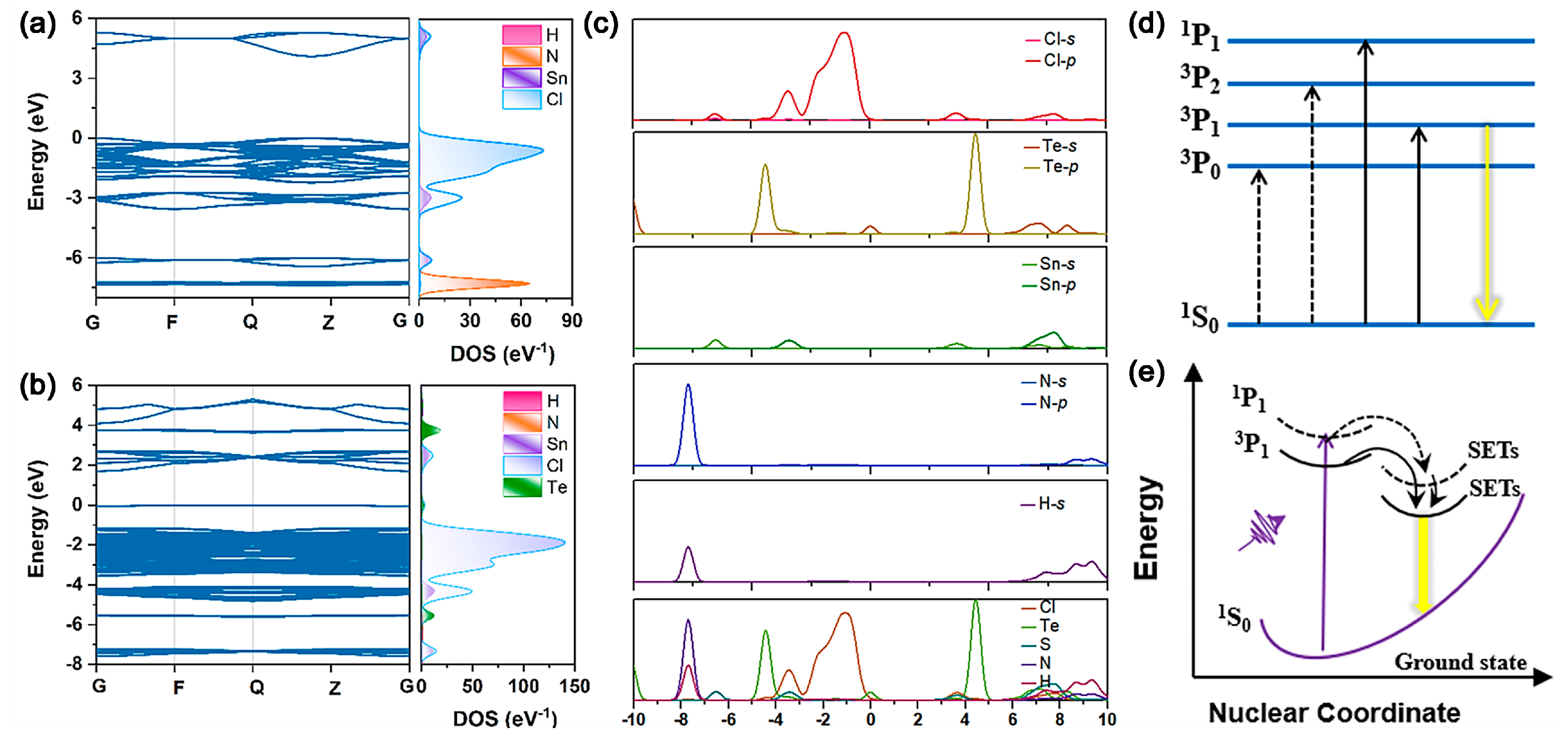
Generally, the DFT calculations provide an insight into the optical properties and energy band structure of undoped (NH
4)
2SnCl
6 and Te-doped(NH
4)
2SnCl
6 (
Figure 4a,b). In the undoped compound, the VBM mainly consists of Cl-
p orbitals, while the conduction band minimum (CBM) is dominated by Cl-
p and Sn-
s orbitals. The NH
4+ ions do not contribute near the band edges. Upon Te doping, the electronic density of states reveals a new VBM above the original valence band. The bandgap is abruptly reduced to 1.78 eV, which is mainly attributed to the newly formed VBM, consisting of states of the Cl 3
p and filled pseudo-closed Te 5
s orbitals. It is well-established that the STE emission of Te-doped perovskites is likely a result of the interplay between the 5s
2 lone pair of Te
4+ [
11]. As an ion whose outer electron configuration is s
2, the energy bands of Te
4+ are generally composed of ground state
1S
0, singlet-excited state
1P
1 and triplet-excited state
3P
n (
n = 0, 1 and 2) [
26]. In combination with the above results, the luminescence mechanism diagram of 0.5%Te:(NH
4)
2SnCl
6 is illustrated in
Figure 4d,e. Due to the soft lattice structure of 0.5%Te:(NH
4)
2SnCl
6, the excited carriers are readily localized to form bound excitons, which then relax to the trap state. Electrons are emitted through energy transfer to the Te
4+ triplet STEs, followed by non-radiation relaxation, resulting in STE emission. For the Te
4+ of s
2 electron configuration,
1S
0 →
3P
1 and
1S
0 →
1P
1 are parity-allowed due to spin-orbit coupling. The electrons are transferred to the singlet state with high energy, then undergo the intersystem crossing (ISC) process from singlet STEs to triplet STEs. Finally, the compound from triplet STEs to the ground state results in a wide emission band with a large Stokes shift [
24]. At low temperatures (80–280 K), the
3P
1 state is partially frozen because the thermal energy is insufficient to overcome its energy barrier. As the temperature rises from 80 K to 280 K, this state becomes thermally activated, enhancing the reverse intersystem crossing (RISC) and leading to an increase in the photoluminescence (PL) intensity. However, a further increase in temperature beyond 280 K enhances non-radiative recombination pathways, which subsequently quenches the PL emission. Therefore, the emission intensity of 0.5%Te:(NH
4)
2SnCl
6 reaches a maximum at 280 K. The emission blue shift monotonically corresponding with increasing temperature is ascribed to important thermal lattice expansion and enhanced electron–phonon coupling. The electron–phonon coupling coefficient is basically scaled to the temperature because of atom motion in the lattice. There is also a positive correlation between the electron–phonon coupling coefficient and temperature in perovskites. Previous studies prove that higher temperatures lead to a larger electron–phonon coupling constant [
27], resulting in a blue shift of the emission. However, the influence of the electron–phonon coupling constant on emission intensity is non-linear: a strong coupling constant contributes to enhanced emission intensity, whereas an excessively large coupling constant promotes non-radiative recombination, leading to diminished emission intensity. With an increase in temperature from 280 K to 420 K, the excessively large electron–phonon coupling accounts for the crossover between the GS and the STEs, promoting phonon-assisted non-radiative recombination and thereby reducing the emission intensity of 0.5%T Te:(NH
4)
2SnCl
6.
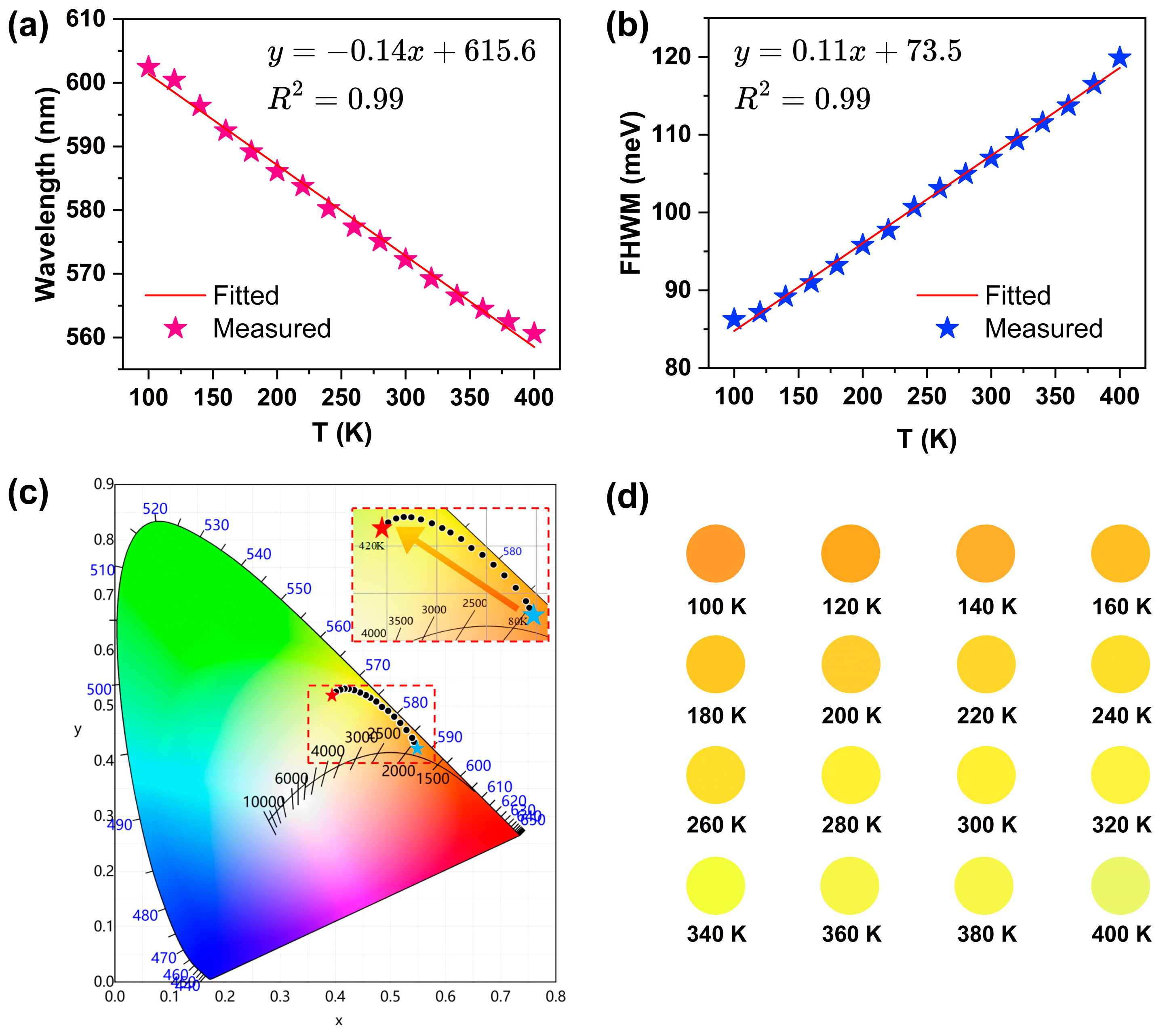
Additionally, temperature-dependent luminescence data were processed to generate plots depicting the evolution of the peak position and full width at half maximum (FWHM) as functions of temperature (
Figure 5a,b). The emission peak exhibits a linear blue shift with increasing temperature according to λ = −0.14 T +615.6 nm (R
2 = 0.99), where T denotes temperature in Kelvin. This temperature sensitivity corresponds with an absolute temperature sensitivity. Therefore, to quantify observed changes, the absolute temperature sensitivity can be determined as
, with Δλ representing the spectral shift per temperature increment of ΔT. The absolute sensitivity (S
A) is 0.14 nm/K and relative sensitivity (Sr) is 0.23% K
−1 at 100 K. Concurrently, the FWHM of the Te-doped emission broadens linearly as FWHM = 0.11T +73.5 meV (R
2 = 0.99). Based on the research findings, 0.5%Te:(NH
4)
2SnCl
6 demonstrates significant potential as a promising candidate for non-contact dual-parameter thermometry across a wide temperature range of 100–400 K. The chromaticity coordinates of 0.5%Te:(NH
4)
2SnCl
6 can effectively be controlled by temperature, ranging from orange-yellow to light yellow, which can be observed from the emission color changes throughout the temperature-increase process (
Figure 5c). Moreover, the CIE coordinates are (0.5439, 0.4303) at 80 K and (0.3943, 0.5195) at 420 K. The CIE(x,y), CCT(K) and CRI of 0.5%Te:(NH
4)
2SnCl
6 at different temperature are presented in
Table S2. In addition, the typical thermochromism behavior, linear variation in the emission wavelength and FWHM of the emission band with temperature make it a promising candidate for temperature sensors (
Figure 5d). The linear thermochromic behavior of the material originates from temperature-dependent changes in the crystal structure. DFT calculations were performed to simulate the crystal structures at various temperatures and the lattice parameters were plotted as a function of temperature (
Figure S4). The results indicate a nearly linear expansion of the lattice parameters with an increasing temperature. This structural expansion is identified as the primary mechanism responsible for the observed linear thermochromism. Temperature variations can alter the crystallinity and interfacial stress of a material, thereby affecting its performance. Given that perovskite materials possess a soft lattice structure, reversible structural and optical changes are achievable within a certain temperature range. The XRD patterns of (NH
4)
2SnCl
6 and 0.5%Te:(NH
4)
2SnCl
6 were observed before and after storing them under ambient conditions for 20 months. All patterns show a pure-phase crystal structure, indicating no structural evolution and, therefore, good stability of the materials under ambient conditions (
Figure S3). Furthermore, the sample can withstand the extreme condition of immersion in water without any pretreatment and maintain a bright luminescence. This stability significantly broadens its application in the field of temperature-sensing. These results suggest that the luminescence behavior of 0.5%Te:(NH
4)
2SnCl
6 can be promoted by modulating the temperature, which makes it a potential candidate for modern highly efficient and visual (colorimetric) optical temperature sensors.