1. Introduction
Mavrud has been cultivated in Bulgaria since ancient Thracian times (fourth century BCE). Traditional methods were rustic but effective in extracting the grape’s bold character. Typical Mavrud grapes are dark-skinned. This variety is indigenous to Bulgaria, specifically in the Thracian Valley region, and is often protected under Bulgarian PDO (Protected Designation of Origin) classifications. It is known for producing deeply colored, full-bodied red wines with a rich, complex flavor. Clay/loam, marl, and alluvial soils contribute to Mavrud’s structured profile. These wines typically exhibit notes of dark fruits, such as blackberries and plums, along with earthy and spicy undertones. Mavrud wines are naturally high in tannins and acidity, contributing to their aging potential. This was confirmed by Gerginova and Simova [
1], where samples of Mavrud wine were tested using nuclear magnetic resonance (NMR), and some of the wine’s contents were detected and quantified.
Historically, Mavrud has played a significant role in Bulgarian winemaking and continues to be a cherished variety representing a unique expression of the region’s terroir. While it is primarily cultivated in Bulgaria, it can also be found in other countries.
Looking at current consumption levels, wine, especially of the red variety, is among the top three alcoholic drinks in the world according to the “Global status report on alcohol and health and treatment of substance use disorders” provided by the World Health Organization in 2024. It is well established that moderate consumption of red wine is associated with various health benefits, primarily due to its rich content of polyphenols [
2]. The consumption of red wine is associated with a decrease in cardiovascular diseases. It has been reported that moderate red wine consumption reduces the risk of cardiovascular diseases and mortality [
3]. This is attributed to the antioxidant, anti-inflammatory, and vasodilatory properties of polyphenols, particularly resveratrol [
4].
However, a demand for lower-alcohol wines is observed today. This is driven by health and economic considerations, as well as evolving consumer preferences [
5]. Indeed, red wine carries some benefits for human health. Still, the consumption of alcoholic beverages is reduced due to various restrictions, which are connected to the negative influence of the alcohol content on the human body. Various reports indicate that the removal of ethanol from red wine does not decrease its health-benefiting effects [
6].
Another major factor to consider is the impact of global warming on the alcohol content in wine [
7]. Global warming has a significant influence on the wine industry, primarily by increasing the sugar content in grapes, leading to higher alcohol levels in wine. This phenomenon is driven by the earlier ripening of the grapes due to rising temperatures, resulting in musts with elevated sugar concentrations [
8]. Simultaneously, the increase in light radiation exposure raises the content of flavonols and anthocyanins, according to Gerginova and Simova [
1].
Due to the warmer and sunnier climate, the wine becomes higher in alcohol content, as well as invaluable bioactive substances. After a particular volumetric concentration of ethanol is reached, this becomes a significant constraint for the producer. As an example, according to the Law on Wine and Spirits in Bulgaria, in a wine, the alcohol strength should not fall below 12 vol.% and not exceed 24 vol.% [
9]. Since the Bulgarian Laws are harmonized with the European Commission (EC) regulation [
10], and as follows from the document, the wine producers are allowed to apply the processes of lowering the alcohol content in the wine. Still, it should not be more than two percentage points of ethanol. The resulting concentration must be at least 8.5 vol.%. If the process continues further, below the limit, the produced beverage cannot be named “wine”. Using heat-based processes, a wine with very low alcohol content (<0.5 vol.%) can be obtained. Since heat is used in this manner, most essential bioactive substances (such as volatile compounds and volatile polyphenolics) are destroyed during the removal of the alcohol. Membrane-based processes are a promising approach to reducing the alcohol content of Mavrud wine [
11].
Membrane separation processes have shown promise for dealcoholizing wines while retaining key aroma and flavor compounds [
12]. Membrane processes offer several advantages over traditional distillation methods, as they operate at lower temperatures and can preserve the original wine composition and quality [
13]. Key parameters of the mentioned processes are the permeate flux and rejection coefficient, influenced by the membrane pore size, transmembrane pressure, and fluid flow rate (in cross-flow filtration). They define operational efficiency and the quality of the resulting dealcoholized wine.
Previous works have contributed to the present results. The work of Dencheva-Zarkova et al. [
14] aimed to investigate the flux and separation efficiency in the ethanol separation from water/ethanol systems. The commercially available flat-sheet membrane NADIR NP030P, with a molecular weight cut-off (MWCO) of 500 Da, was used for the experiment. In the research, water/alcohol mixtures and red wine (Mavrud) were tested using nanofiltration. Another factor for the stable process operation was understanding the nanomembrane fouling while filtering the Mavrud wine [
15].
It is important to note that Mavrud wine, as a part of red wines, contains bioactive components [
1,
16], which are beneficial for human health [
17]. Other researchers also described the aroma profile and composition of
Cabernet Sauvignon red wine, which is a well-founded reference point for this study [
18]. Another beneficial reference point for this study is understanding how to mitigate the volatile compounds during the process; proper answers have been found in the research of Montevecchi et al. [
19].
Catarino and Mendes tested four NF membranes (including NF99 HF and NF99) and one RO membrane in diafiltration mode for removing ethanol from a 12 vol.% red wine and observed promising organoleptic properties. The pervaporated aroma compounds were added to the reduced alcohol wine to increase the flavor sensations [
13].
The studies of Kumar focus on the behavior of NF and RO membranes used for dealcoholizing both model ethanol/water mixtures and real wine. For the production of low-alcohol white wine, the study found the nanofiltration membranes HL and NF99 to be most effective while retaining the rest of the wine compounds. The authors reported good preservation of total acidity and sugars after diafiltration as well as a potential for obtaining favorable sensory characteristics of the final product. After filtration with NF-DK and RO-SG membranes, a specific decrease in the physicochemical properties in the reduced alcohol wine was observed, such as total and volatile acidity, as well as dry extract [
20].
Monitoring of key components of the wine took place during the process, including some carboxylic acids, sugars, and alcohols: acetic acid, succinic acid, lactic acid, malic acid, tartaric acid, and citric acid; glucose and fructose; and ethanol and glycerol. This research forms a balanced conclusion on the separation efficiency based on the compound’s concentration. Additionally, the concentration of these compounds is crucial for understanding how the organoleptic qualities have changed during the nanofiltration process. This study applied a high-performance liquid chromatography system with a refractive index detector to quantify and identify the named compounds.
2. Mavrud Wine Dealcoholization and Composition
2.1. Sample Collection and Mass Balance
The object of this research—Mavrud dry red wine, vintage 2020—was provided by the Bulgarian wine cellar, Harmanli, Bulgaria. The wine’s composition was introduced in the work of Tsibranska et al. [
21]. Samples were collected before (for the initial concentration) and during the filtration process to obtain information about the changes in the composition.
The gathered samples were analyzed by high-performance liquid chromatography (HPLC); as a result, two groups of components were monitored—carboxylic acids and sugars, and ethanol as a separate compound. The results of the analyses revealed the concentrations of the wine (feed solution—CF), and the final average concentration in the retentate (CR) and the permeate (CP).
The calculations follow the formulas given below, where
m(
Fi) is the mass of the feed’s stream components (initial wine);
m(
Ri) is the mass of the retentate’s stream components;
m(
Pi) is the mass of the permeate’s stream components.
Equation (1) defines the mass of the
i component (in g) in the
F stream (initial mass), Equation (2) defines the mass of the component in
P stream, and Equation (3) gives the
R stream component’s mass. An exception to the mass balance is the ethanol, presented in terms of volume (in mL), from each stream.
Equation (4) presents how the EtOH volume was defined, where i indicates the stream (F, R, or P), and the C(EtOHi, vol.%) is delivered by the HPLC analysis.
Then the mass balance equation will have the form presented by Equation (5):
where
i is the component (in g) in the feed stream.
The EtOH volume balance is calculated by Equation (7):
The calculated mass for each component m(calc.i) was obtained after the process according to the HPLC analysis, where m(Ri) + m(Pi) = m(calc.i) is the calculated mass.
Deviation (
Dev,%) from the initial mass (or volume, for EtOH) of each component was calculated by Equations (8) and (9):
where
m(
calc.i) is the sum of each component from the
R and
P streams.
2.2. Membrane Equipment
The experiments utilized a laboratory constant-pressure cross-flow filtration system (MaxiMem, Prozesstechnik GmbH, Basel, Switzerland) with a rectangular flat-sheet membrane of 215 cm
2 active area.
Figure 1 illustrates the operational diagram of the equipment. The filtration system operated with three different commercially available flat-sheet membranes: NP030P (from MANN + HUMMEL), NF99HF, and RO99 (both from Alfa Laval). The applied transmembrane pressure varied between 10 and 50 bars for the NF99HF membrane and 30–50 bars for the NP030P and RO99 membranes. Based on previously published results, the optimal cross-flow rate was set to 1.2 L/min. The permeate flux (L m
2 h
−1) mean value and development over time were calculated based on measurements at every 10 mL permeated volume. Before the experiments, the membrane is pre-washed to restore its initial flux. Throughout the experiments, distilled water from the “GFL Type 2004” water is still used. After the nanofiltration or reverse osmosis process, the hydraulically reversible fouling is removed from the membrane, backwashing with distilled water. In case the normalized permeate flow drops by about 10–15%, chemical cleaning is applied with 0.01 to 0.05% aqueous sodium hydroxide solutions (from “Valerus—Valeri Rusinov ET”) under the following conditions: pressure 5 bar, temperature 30–35 °C, circulation time 10–30 min, followed by a 10–30 min soaking period and then a final 30 min’ recirculation before flushing. For high fouling intensities, the solution may need to be circulated through the membrane for up to 3 h.
The average permeate (
c(
Pi)) and retentate (
c(
Ri)) concentrations for the respective component
i are used to calculate the rejection coefficient (
Ri), as per Equation (9).
Due to the significant temperature influence on the alcohol evaporation from the Mavrud wine, it is essential to control the temperature. For the present experimental investigation, a temperature regime is achieved by the thermostatic cooling bath LAUDA Alpha RA 8. The cooling is important for the used filtration system because, when the pump is running at a high flow rate and pressure and no cooling is applied, the medium heats up rapidly. For the effective maintenance of the filtration system, the temperature of the cooling medium should be at least 15 °C lower than the desired operating temperature of the unit.
2.3. Analytical Methods
This study aimed to define how the ethanol content and other components in the wine changed during the research. The HPLC analytical technique was chosen for its ability to determine changes in the concentration of different components simultaneously with the ethanol. The HPLC analysis helped to define the following compounds: citric acid, tartaric acid, succinic acid, lactic acid, acetic acid, glucose, fructose, glycerol, and ethanol. Before the analysis, standard mass concentration water solutions were prepared.
The ethanol solution was prepared in volumetric concentration (vol.%). The following standard concentrations were prepared: 20. 10. 5. 0.5 vol.% (±0.1%). The ethanol used was 99%, produced by VWR. The prepared solutions were immediately subjected to analysis and also triple-injected. The parameters of the analysis are described below. For the ethanol content, the standard deviation was determined by the equation of the calibration curve. The defined standard deviation (SD) for the EtOH was 0.05553. Then, based on Equations (10) and (11), the defined limit of detection (LoD) and limit of quantification (LoQ) were defined. The values are presented in
Table 1.
The other compounds were separated into groups—carboxylic acids and sugars (where the glycerol was included). The solution for each group contained 1 g/L (±0.0001 g) of each component. Then the solutions were diluted to 0.1 g/L and 0.01 g/L. Each standard solution was tripled. All of the chemicals were 99%, obtained from Sigma-Aldrich; only the lactic acid was 98%, obtained from Thermo-Fischer Scientific.
The following HPLC equipment was used: Agilent 1260 Infinity II, equipped with an autosampler and a refractive index detector (RID). All samples were injected with a 5 μL injection volume and were kept at a constant temperature of 15 °C in the autosampler. An Agilent HiPlex H analytical column was used (300 × 7.7 mm, 8 μm particle size). It has a matrix of monodisperse sulfonated styrene/divinylbenzene resin in hydrogen ionic form. The eluent was a 5 mM H
2SO
4 water solution with 5% acetonitrile (AcN), pumped at flow 0.6. The method’s runtime was set at 27 min, due to the EtOH elution time. The temperature of the column was set to 70 °C. The temperature was set according to proposed applications based on the studies of Schneider et al. [
22] and Ding et al. [
23] provided by the manufacturer of the analytical column. The RI detector cell temperature was set to 35 °C. After each sample run, a blank injection was performed with 20 vol.%. AcN to clear any strongly retained compounds. Characteristic chromatograms of the analyzed compounds are given on
Figure 2. The Agilent’s OpenLab CDS v.2.4 software performed the peak integration and other calculations. The concentration of the components is defined as an average over the individual injections for each sample.
The collected samples from the filtration were duplicated in the HPLC analysis.
2.4. Filtration Configurations and Operation Parameters
Figure 3 presents the applied filtration configuration modes. The concentration mode (
Figure 3a) allows increasing the content of high molecular compounds in the initial wine, while “small” molecules (like water and ethanol) can pass into the permeate stream. Diafiltration experiments (
Figure 3b) aim to reduce the ethanol content. In constant volume diafiltration, water is added in equal amounts to the permeated volume so that the retentate volume remains constant. The two-step filtration (
Figure 3c) aimed at almost complete retention of high-molecular-weight bioactive substances in the first stage (NF) and better separation of ethanol in the second stage (RO), the second membrane having higher ethanol rejection.
Table 2 summarizes the scope of the filtration experiments and the range of variation of the investigated parameters; the effect of the latter on the rejection of ethanol and bioactive substances was reported by the authors in their previous papers.
In the present study, the rejection coefficients of the wine components, such as organic acids and sugars, were investigated to illustrate the effect of pressure and the applied operation mode.
2.5. Statistical Analysis of the Results
Statistical analysis of the results for the received rejection coefficients was performed by defining the sample standard deviation by the following formula:
where
S is the sample standard deviation (SSD),
xavg is the sample average, and
n is the number of values in the sample.
3. Results and Discussion
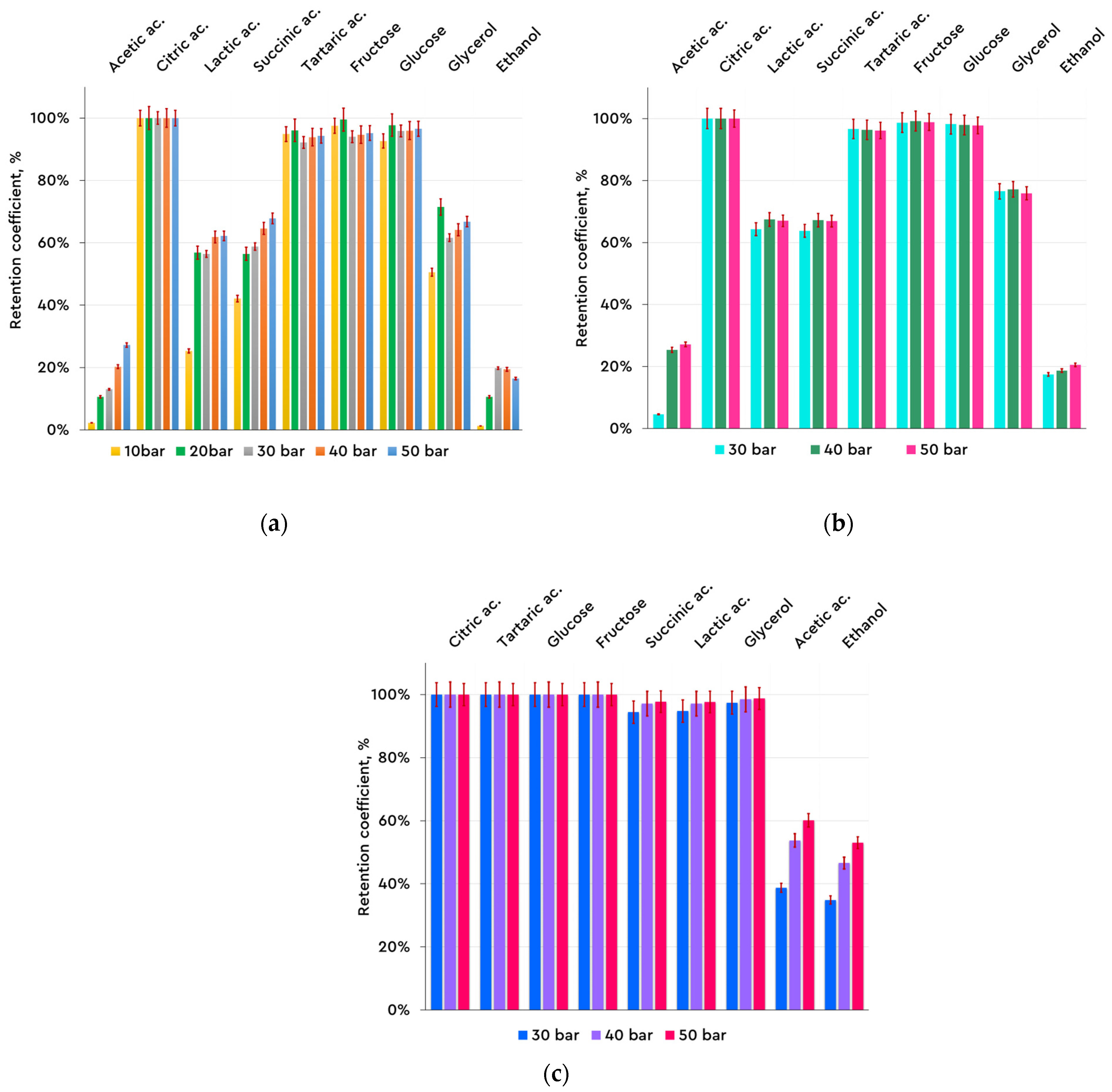
Figure 4, which is presented below, reveals the results concerning the rejection of different groups of compounds (alcohols, sugars, and organic acids) found in the Mavrud wine after filtration with each membrane type.
3.1. Mavrud Wine Composition in Relation to the Mode of Operation—Concentration Mode
For the NF membranes, the highest rejection values (over 90%) were observed for citric acid, tartaric acid, glucose, and fructose, with approximately 60% of the remaining low-molecular-weight compounds rejected. For the acetic acid and ethanol nanofiltration, lower rejection was observed. The RO membrane showed higher rejection rates, exceeding 80% for all compounds, except for ethanol and acetic acid.
As is clear from
Table 3, the range of the TMP on the NF99HF membrane starts at 10 bars, which is allowed by the membrane morphology. The NP030P and RO99 membranes were also tested at 10 and 20 bars of TMP, but no permeate flux was observed; therefore, results for these membranes were lacking. Nevertheless,
Figure 4a presents the rejection results of the NF99HF membrane starting with 10 bars of TMP.
Regarding the effect of transmembrane pressure, most of the results show a slight increase with increasing pressure. The formation of a compression and/or fouling layer on the membrane surface could explain the latter. In numerous cases, this effect is small enough to consider the retention as practically constant (e.g., the NF99HF membrane); however, there are also some exceptions to the observed trend, where this influence is limited to approximately 40 bar. A reduction in retention is usually related to the effect of concentration polarization, which increases the driving force for solute transfer through the membrane; however, there are also other reasons, such as experimental imperfections, for this effect.
In
Table 4, the mass balance deviation between the components before and after the nanofiltration of the Mavrud wine is presented. The presented table below is divided into three sections, which represent the deviation in the mass balance for each of the applied TMP and different membranes used in concentration mode of operation.
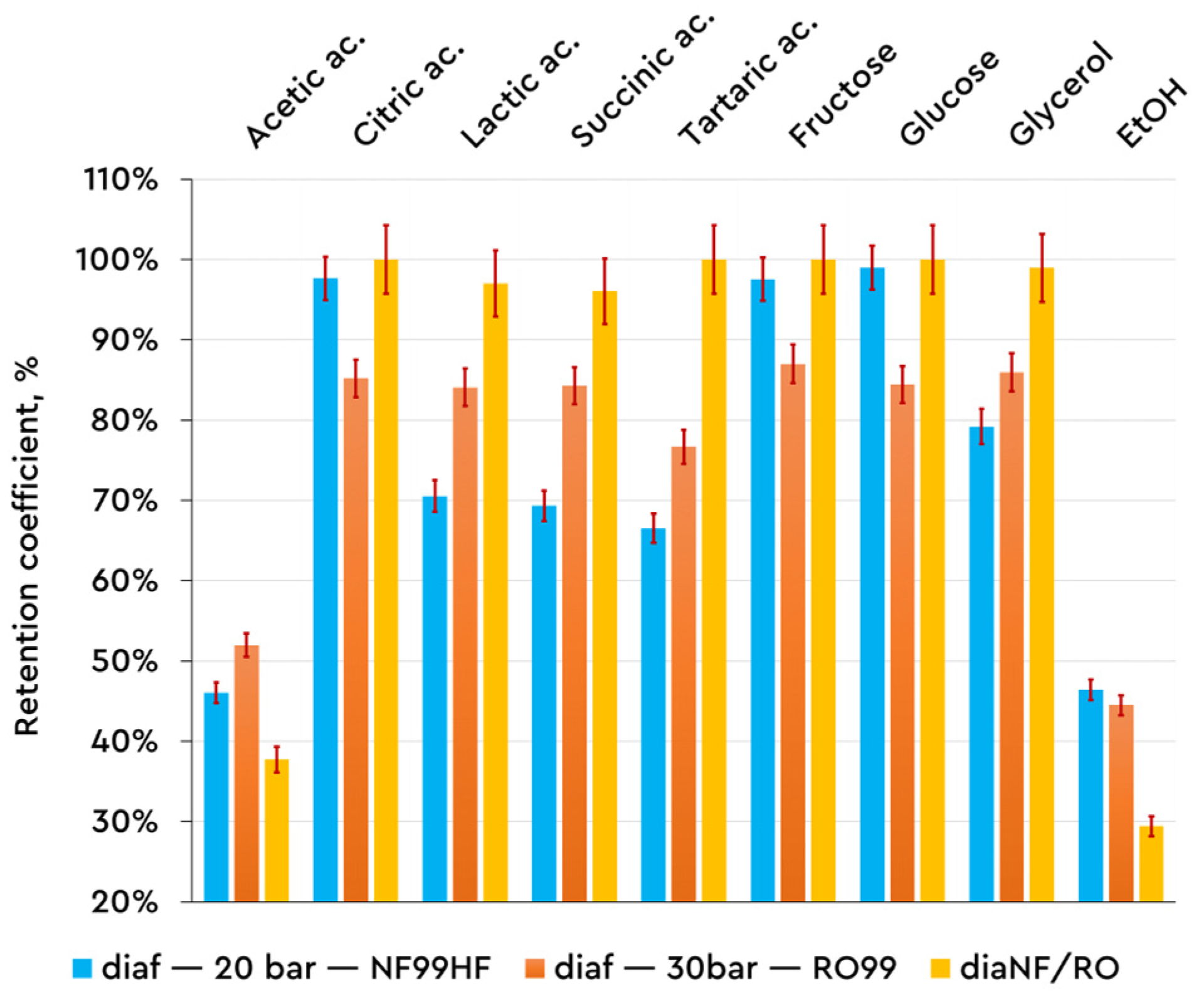
3.2. Mavrud Wine Composition in Relation to the Mode of Operation—Diafiltration and Sequential Mode
Previously conducted studies on Mavrud wine show that the rejection values for ethanol and high-molecular-weight bioactive compounds in the diafiltration runs are preserved as compared to the concentration mode. These studies were presented and described in the
Introduction section. Based on previously gathered information and conclusions (the papers of Tsibranska et al. [
21] and Dencheva-Zarkova et al. [
14]), it can be noted that the present study confirms the previously published results, specifically the observed rejection coefficients for the organic acids and sugars present in the examined Mavrud wine.
Figure 5 presents the obtained results from the described experimental work above.
High rejection rates (Rej > 80%) are obtained, through the mass balance, for carboxylic acids and sugars, except for ethanol and acetic acid. Kumar et al. published results in this direction that reported strong preservation of total acidity and sugars during the diafiltration of white wine concerning the NF99 membrane [
24]. The previously published works by Tsibranska et al. [
21] and Dencheva-Zarkova et al. [
14] describe a positive effect on the permeate flux. The last is associated with the different concentration conditions as compared to the concentration mode of filtration. From the perspective of high permeate flux and appropriate retention, the NF99HF membrane is most successful for wine dealcoholization in diafiltration mode.
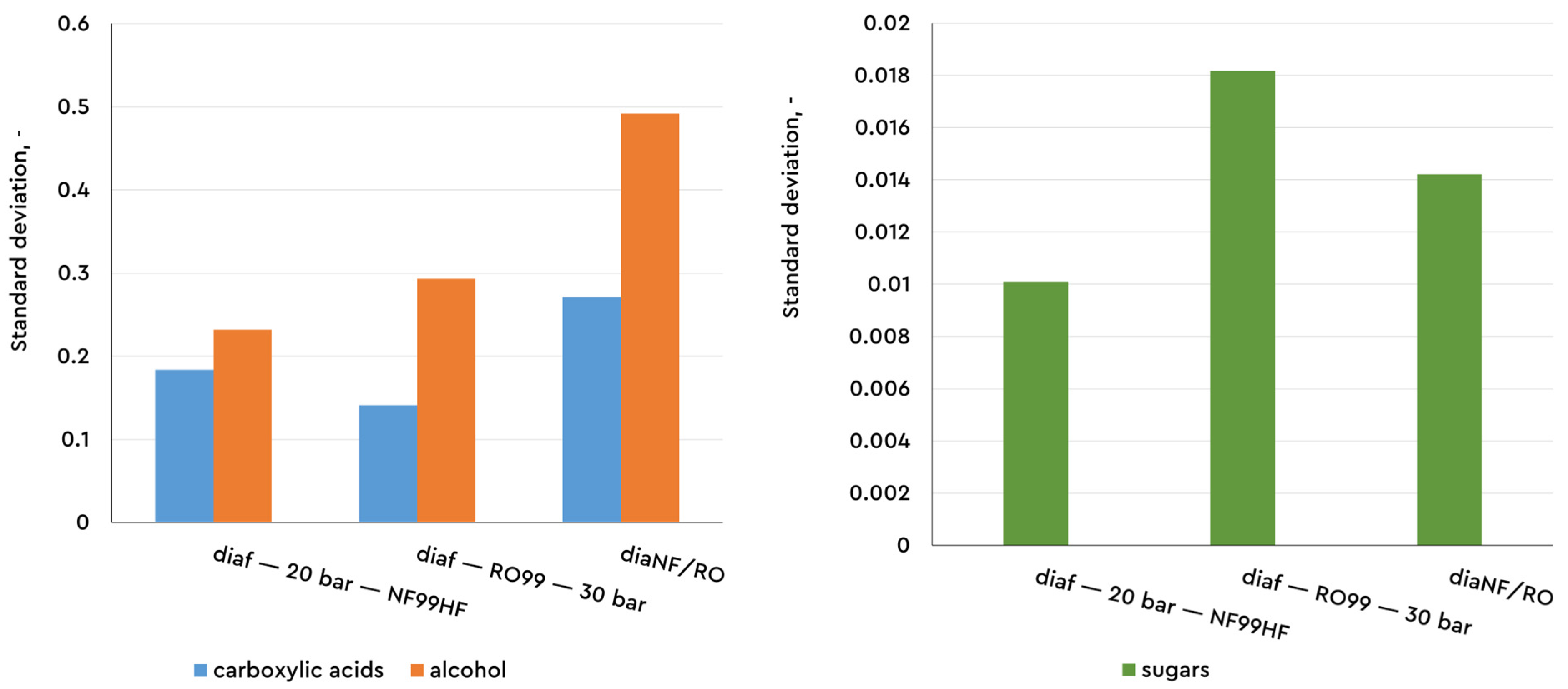
The comparison of the individual membranes shows increasing rejection coefficients in the direction of NF and RO (in single-stage constant volume diafiltration) and sequential NF/RO under the same operating conditions. In the latter case, the first stage (NF99HF at 20 bar) operates in diafiltration mode, and the second stage (RO99 at 30 bar) processes the permeate flow after nanofiltration to separate the ethanol. In the second filtration stage, the composition and concentrations of the incoming flow differ significantly from those of the wine (represented by the assessment of the standard deviation for the rejection coefficients, graphically represented in
Figure 6 and
Figure 7). Good retention (Rej > 80%) of lactic, succinic, and tartaric acids, as well as of fructose and glucose, both of which have Rej > 90%, is observed. The acetic and citric acids have lower retention by the membrane, Rej < 50%. Calculated as a whole in the two-stage filtration scheme (final products are retentate in the first stage and permeate from the second stage), the observed rejection coefficients are in the range of 90–100% for all the compounds except for acetic acid and ethanol.
In a recent study published by Tsibranska et al. [
21], experiments were conducted on NF-based diafiltration runs with two nanofiltration membranes (NF99HF, NP030P) and one reverse osmosis membrane (RO99) to assess separation ability towards ethanol, polyphenols, and anthocyanins in Mavrud red wine. The membrane NF99HF has proven to be the most effective for moderate wine dealcoholizing, high retention of bioactive compounds, and preservation of antioxidant activity. The use of sequential NF99HF(dia)/RO99 filtration has successfully improved the recovery of the separated ethanol.
The Nadir NP030P membrane was used before for diafiltration [
25]. The experimental investigation was aimed at concentrating larger volumes of valuable substances extracted from plant material. The results revealed provide information on the correlation between pressure and rejection change for glucose and phenolics. These conclusions were necessary for the experiments conducted and the research presented here.
The present study investigates the behavior of organic substances and volatile compounds during partial dealcoholization of Mavrud red wine by membrane filtration. Presented and discussed are the observed rejection coefficients for lowering the alcohol content in the wine.
4. Conclusions
The present study compares the rejection behavior of several groups of substances contained in Mavrud red wine, subject to membrane separation with two nanofiltration membranes and one reverse osmosis membrane. The performed experiments were in concentration mode, single-stage constant-volume diafiltration, and sequential NF/RO membrane separation.
The two NF membranes (NP030P and NF99HF) show high rejection rates for part of the organic acids and sugars contained in the wine (Rej over 90% for citric acid, tartaric acid, glucose, and fructose), the retention of the remaining wine components (lactic acid, succinic acid, glycerol) being about 60%. The NF99HF membrane retains the highest percentages of glucose, fructose, citric acid, and tartaric acid.
The RO99 membrane exhibits higher rejection rates, over 80% for all compounds. The observed exception is in the rejection of ethanol and acetic acid. Since ethanol rejection is important for dealcoholization purposes, our results show the lowest rejection values for the NF99HF membrane, followed by the NP030P and RO99 membranes in that order. The latter means that the main difference between the rejection coefficients of ethanol and the other compounds observed in the wine with the NF99HF membrane is a favorable condition for conducting diafiltration aimed at ethanol separation.
On the other hand, the significantly higher retention of the RO99 membrane for all tested components, including ethanol, makes it a suitable membrane for subsequent treatment of the diafiltration effluent. Mainly because this membrane exhibits a low permeate flux and a more pronounced tendency to foul when applied directly for wine filtration, treating the permeate effluent from the diafiltration step, where both composition and concentration conditions are different, can help mitigate these disadvantages. The two-step NF99HF(dia)-RO99 filtration is based on the results obtained in concentration and diafiltration modes with the studied membranes, selecting conditions that minimize fouling and yield good, generally comparable permeate fluxes in both stages. Such a filtration configuration allows for the observation of the highest rejection coefficients for the organic acids and sugars contained in the wine, as compared to the final permeate. It is worth emphasizing that the obtained results could be helpful for the production of Mavrud wine with controlled alcohol content while preserving its unique content profile.
Within the studied ranges of experimental conditions in concentration and diafiltration mode, no significant differences in the rejection coefficients were observed. Besides preserving the rejection values in the two filtration modes, the transmembrane pressure affects the rejection coefficients. In most cases of wine filtration, the pressure increase leads to a corresponding increase in the rejection coefficients, as well as increased fouling, as previously shown. To minimize the impact of this effect, the change in permeate flow over time is monitored. The conclusions from the monitoring led to the selection of lower pressure values to minimize membrane fouling.
The results obtained by this experimental study regarding the separation ability of the studied membranes for organic acids, sugars, and ethanol are encouraging. The last is promising for future commercial applications of nanofiltration, reverse osmosis, and their sequential operation. The last will allow the winemaking sector to produce Mavrud wine with lower alcohol content while preserving the wine’s unique profile.