Synthesis, Phase Formation, and Raman Spectroscopy of Ni and Zn(Mg) Codoped Bismuth Stibate Pyrochlore
Abstract
1. Introduction
2. Experimental Part
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Giampaoli, G.; Siritanon, T.; Day, B.; Li, J.; Subramanian, M.A. Temperature in-dependent low loss dielectrics based on quaternary pyrochlore oxides. Prog. Solid. State Chem. 2018, 50, 16–23. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid. State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- McCauley, R.A. Structural Characteristics of Pyrochlore Formation. J. Appl. Phys. 1980, 51, 290–294. [Google Scholar] [CrossRef]
- Vanderah, T.A.; Lufaso, M.W.; Adler, A.U.; Levin, I.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Subsolidus phase equilibria and properties in the system Bi2O3:Mn2O3±x:Nb2O5. J. Solid State Chem. 2006, 179, 3467–3477. [Google Scholar] [CrossRef]
- Vanderah, T.A.; Siegrist, T.; Lufaso, M.W.; Yeager, M.C.; Roth, R.S.; Nino, J.C.; Yates, S. Phase Formation and Properties in the System Bi2O3:2CoO1+x:Nb2O5. Eur. J. Inorg. Chem. 2006, 2006, 4908–4914. [Google Scholar] [CrossRef]
- Lufaso, M.W.; Vanderah, T.A.; Pazos, I.M.; Levin, I.; Roth, R.S.; Nino, J.C.; Provenzano, V.; Schenck, P.K. Phase formation, crystal chemistry, and properties in the system Bi2O3–Fe2O3–Nb2O5. J. Solid State Chem. 2006, 179, 3900–3910. [Google Scholar] [CrossRef]
- Du, H.; Wang, H.; Yao, X. Observations on structural evolution and dielectric properties of oxygen-deficient pyrochlores. Ceram. Int. 2004, 30, 1383–1387. [Google Scholar] [CrossRef]
- Huiling, D.; Xi, Y. Structural trends and dielectric properties of Bi-based pyrochlores. J. Mater. Sci. Mater. Electron. 2004, 15, 613–616. [Google Scholar] [CrossRef]
- Cann, D.P.; Randall, C.A.; Shrout, T.R. Investigation of the dielectric properties of bismuth pyrochlores. Solid State Commun. 1996, 100, 529–534. [Google Scholar] [CrossRef]
- Khaw, C.C.; Tan, K.B.; Lee, C.K. High temperature dielectric properties of cubic bismuth zinc tantalate. Ceram. Int. 2009, 35, 1473–1480. [Google Scholar] [CrossRef]
- Murugesan, S.; Huda, M.N.; Yan, Y.; Al-Jassim, M.M.; Subramanian, V. Band-Engineered Bismuth Titanate Pyrochlores for Visible Light Photocatalysis. J. Phys. Chem. C 2010, 114, 10598–10605. [Google Scholar] [CrossRef]
- Valant, M.; Babu, G.S.; Vrcon, M.; Kolodiazhnyi, T.; Axelsson, A.-K. Pyrochlore range from Bi2O3–Fe2O3–TeO3 system for LTCC and photocatalysis and the crystal structure of new Bi3(Fe0.56Te0.44)3O11. J. Am. Ceram. Soc. 2011, 95, 644–650. [Google Scholar] [CrossRef]
- Lomakin, M.S.; Proskurina, O.V.; Sergeev, A.A.; Buryanenko, I.V.; Semenov, V.G.; Voznesenskiy, S.S.; Gusarov, V.V. Crystal structure and optical properties of the Bi–Fe–W–O pyrochlore phase synthesized via a hydrothermal method. J. Alloys Compd. 2022, 889, 161598. [Google Scholar] [CrossRef]
- Pandey, J.; Shrivastava, V.; Nagarajan, R. Metastable Bi2Zr2O7 with Pyrochlore-like Structure: Stabilization, Oxygen Ion Conductivity, and Catalytic Properties. Inorg. Chem. 2018, 57, 13667–13678. [Google Scholar] [CrossRef]
- Lomakin, M.S.; Proskurina, O.V.; Danilovich, D.P.; Panchuk, V.V.; Semenov, V.G.; Gusarov, V.V. Hydrothermal synthesis, phase formation and crystal chemistry of the pyrochlore/Bi2WO6 and pyrochlore/α–Fe2O3 composites in the Bi2O3–Fe2O3–WO3 system. J. Solid State Chem. 2020, 282, 121064. [Google Scholar] [CrossRef]
- Perenlei, G.; Talbot, P.C.; Martens, W.N. Sol-gel synthesis and characterization of cubic bismuth zinc niobium oxide nanopowders. J. Nanomater. 2014, 2014, 695973. [Google Scholar] [CrossRef]
- Kahoul, A.; Nkeng, P.; Hammouche, A.; Naamoune, F.; Poillerat, G. A Sol–Gel Route for the Synthesis of Bi2Ru2O7 Pyrochlore Oxide for Oxygen Reaction in Alkaline Medium. J. Solid State Chem. 2001, 161, 379–384. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Badanina, K.A.; Korolev, R.I.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Belyy, V.A.; Makeev, B.A. Sol–gel derived Bi2NiNb2O9 pyrochlore: Synthesis, characterization and dielectric properties. Ceram. Int. 2024, 50, 50397–50409. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Gajtko, O.M.; Rudnev, P.O.; Ellert, O.G.; Ivanov, V.K. Synthesis of Bi–Fe–Sb–O pyrochlore nanoparticles with visible-light photocatalytic activity. Eur. J. Inorg. Chem. 2016, 2016, 2193–2199. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Plukchi, K.R.; Golodukhina, S.V.; Liberman, E.Y.; Ellert, O.G. Hydrothermal synthesis of highly dispersed Bi–Ni–Sb–O pyrochlore for catalytic oxidation of carbon monoxide. Mendeleev Commun. 2023, 33, 608–610. [Google Scholar] [CrossRef]
- Jusoh, F.A.; Tan, K.B.; Zainal, Z.; Chen, S.K.; Khaw, C.C.; Lee, O.J.; Balachandran, R.; Murthy, H.C.A. Novel pyrochlore-structured bismuth iron antimonates: Structural, impedance and electrochemical studies. Results Phys. 2021, 27, 104542. [Google Scholar] [CrossRef]
- Miles, G.C.; West, A.R. Pyrochlore Phases in the System ZnO-Bi2O3-Sb2O5: I. Stoichiometries and Phase Equilibria. J. Am. Ceram. Soc. 2006, 89, 1042–1046. [Google Scholar] [CrossRef]
- Whitaker, M.J.; Marco, J.F.; Berry, F.J.; Raith, C.; Blackburn, E.; Greaves, C. Structural and magnetic characterisation of the pyrochlores Bi2−xFex(FeSb)O7, (x = 0.1, 0.2, 0.3), Nd1.8Fe0.2(FeSb)O7 and Pr2(FeSb)O7. J. Solid State Chem. 2013, 198, 316–322. [Google Scholar] [CrossRef]
- Rahman, R.A.U.; Ruth, D.E.J.; Ramaswamy, M. Emerging scenario on displacive cubic bismuth pyrochlores (Bi,M)MNO7-δ (M = transition metal, N = Nb, Ta, Sb) in context of their fascinating structural, dielectric and magnetic properties. Ceram. Int. 2020, 46, 14346–14360. [Google Scholar] [CrossRef]
- Muraviov, V.A.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Korolev, R.I.; Zhuk, N.A. Synthesis of Bi2NiTa2O9 with a pyrochlore-type structure. Glass Ceram. 2022, 95, 40–46. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Korolev, R.I. Effect of magnesium and zinc on phase formation of pyrochlore-type Bi2Mg(Zn)1-xMxTa2O9.5-Δ (M-Cr, Fe) ceramics. Ceram. Int. 2023, 49, 5496–5509. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Kovalenko, S.Y.; Korolev, R.I.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Sivkov, D.V.; Nekipelov, S.V.; Sivkov, V.N.; Yermolina, M.V. Features of Phase Formation of Pyrochlore-type Ceramics Bi2Mg(Zn)1-xNixTa2O9. ACS Omega 2023, 8, 11351–11363. [Google Scholar] [CrossRef]
- Nino, J.C.; Lanagan, M.T.; Randall, C.A. Phase formation and reactions in the Bi2O3–ZnO–Nb2O5–Ag pyrochlore system. J. Mater. Res. 2001, 16, 1460–1464. [Google Scholar] [CrossRef]
- Zanetti, S.M.; da Silva, S.A.; Thim, G.P. A chemical route for the synthesis of cubic bismuth zinc niobate pyrochlore nanopowders. J. Solid State Chem. 2004, 177, 4546–4551. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Makeev, B.A.; Korolev, R.I.; Sivkov, D.N.; Shpynova, A.D. A study of phase evolution of Fe-doped bismuth tantalate pyrochlore. Ceram. Int. 2022, 48, 31965–31969. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Nekipelov, S.V.; Sivkov, D.V.; Badanina, K.A. Features of the Phase Formation of Cr/Mn/Fe/Co/Ni/Cu Codoped Bismuth Niobate Pyrochlore. Crystals 2023, 13, 1202. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Badanina, K.A.; Korolev, R.I.; Makeev, B.A.; Krzhizhanovskaya, M.G.; Kharton, V.V. Phase Formation of Co and Cr Co-Doped Bismuth Niobate with Pyrochlore Structure. Inorganics 2023, 11, 288. [Google Scholar] [CrossRef]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Koroleva, A.V.; Nekipelov, S.V.; Kharton, V.V.; Sekushin, N.A. Thermal Expansion, XPS Spectra, and Structural and Electrical Properties of a New Bi2NiTa2O9 Pyrochlore. Inorg. Chem. 2021, 60, 4924–4934. [Google Scholar] [CrossRef] [PubMed]
- Zhuk, N.A.; Krzhizhanovskaya, M.G.; Sekushin, N.A.; Kharton, V.V.; Koroleva, A.V.; Nekipelov, S.V.; Sivkov, D.V.; Sivkov, V.N.; Makeev, B.A.; Lebedev, A.M.; et al. Novel Ni-Doped Bismuth–Magnesium Tantalate Pyrochlores: Structural and Electrical Properties, Thermal Expansion, X-ray Photoelectron Spectroscopy, and Near-Edge X-ray Absorption Fine Structure Spectra. ACS Omega 2021, 6, 23262–23273. [Google Scholar] [CrossRef]
- Akselrud, L.G.; Grin, Y.N.; Zavalii, P.Y.; Pecharsky, V.K.; Fundamenskii, V.S. CSD-universal program package for single crystal or powder structure data treatment. In Proceedings of the Collected Abstracts of XII European Crystallographic Meeting, Moscow, Russia, 22–29 August 1989. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Levin, E.M.; Roth, R.S. Polymorphism of bismuth sesquixide. II. Effect of oxide additions on the polymorphism of Bi2O3. J. Res. Natl. Bur. Stand. A Phys. Chem. 1964, 68A, 189–206. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Pang, L.-X.; Zhou, D.; Wang, H.; Wu, Y.; Guo, J.; Chen, Y.-H. Phase evolution and microwave dielectric properties of Bi3SbO7 ceramic. J. Phys. Chem. Solids 2011, 72, 882–885. [Google Scholar] [CrossRef]
- Dutta, D.P. Composites of Sb2O4 and Biomass-Derived Mesoporous Disordered Carbon as Versatile Anodes for Sodium-Ion Batteries. ChemistrySelect 2020, 5, 1846–1857. [Google Scholar] [CrossRef]
- Egorysheva, A.; Ellert, O.; Zubavichus, Y.; Gajtko, O.; Efimov, N.; Svetogorov, R.; Murzin, V. New complex bismuth oxides in the Bi2O3–NiO–Sb2O5 system and their properties. J. Solid State Chem. 2015, 225, 97–104. [Google Scholar] [CrossRef]
- Egorysheva, A.V.; Ellert, O.G.; Gajtko, O.M.; Efimov, N.N.; Svetogorov, R.D.; Zubavichus, Y.V.; Grigorieva, A.V. The Bi2O3–Fe2O3–Sb2O5 system phase diagram refinement, Bi3FeSb2O11 structure peculiarities and magnetic properties. J. Solid State Chem. 2015, 225, 278–284. [Google Scholar] [CrossRef]
- Sleight, A.W.; Bouchard, R.J. A new cubic KSbO3 derivative structure with interpenetrating networks. Crystal structure of Bi3GaSb2O11. Inorg. Chem. 1973, 12, 2314–2316. [Google Scholar] [CrossRef]
- Golodukhina, S.V.; Razvorotneva, L.S.; Plukchi, K.R.; Popova, E.F.; Egorysheva, A.V. Successful and not so successful examples of using the citrate method for the synthesis of La and Bi antimonates. Abstr. All-Russ. Conf. VIII Russ. Rare Earth Day Nizhny Novgorod NNSU IMC RAS 2024, 258, 149–150. Available online: https://iomc.ras.ru/wp-content/uploads/2024/02/rdrz2024_abstracts.pdf (accessed on 26 June 2025).
- Ramesha, K.; Prakash, A.S.; Sathiya, M.; Madras, G.; Shukla, A.K. Synthesis of new (Bi, La)3MSb2O11 phases (M = Cr, Mn, Fe) with KSbO3-type structure and their magnetic and photocatalytic properties. Bull. Mater. Sci. 2011, 34, 271–277. [Google Scholar] [CrossRef]
- Evans, I.R.; Shanwen, T.; John, T.; Irvine, S.; Judith Howard, A.K. Synthesis, Crystal Structure, and Oxide Ion Conductivity in Bi4.6Ca1.1VO10.5. Chem. Mater. 2002, 14, 3700–3704. [Google Scholar] [CrossRef]
- Badanina, K.A.; Nekipelov, S.V.; Lebedev, A.M.; Zhuk, N.A.; Beznosikov, D.S. Conversion of Cr(III) and Co(III) During the Synthesis of Co/Cr Codoped Bismuth Niobate Pyrochlore According to NEXAFS Data. J. Sib. Fed. Univ. Math. Phys. 2024, 17, 559–569. [Google Scholar]
- Brown, S.; Gupta, H.C.; Alonso, J.A.; Martinez-Lope, M.J. Vibrational spectra and force field calculation of A2Mn2O7(A=Y, Dy, Er, Yb) pyrochlores. J. Raman Spectrosc. 2003, 34, 240–243. [Google Scholar] [CrossRef]
- Fischer, M.; Malcherek, T.; Bismayer, U.; Blaha, P.; Schwarz, K. Structure and stability of Cd2Nb2O7 and Cd2Ta2O7 explored by ab initio calculations. Phys. Rev. B 2008, 78, 014108. [Google Scholar] [CrossRef]
- Patterson, C.H. First-principles calculation of the structure and dielectric properties of Bi2Ti2O7. Phys. Rev. B 2010, 82, 155103. [Google Scholar] [CrossRef]
- Krayzman, V.; Levin, I.; Woicik, J.C. Local structure of displacively disordered pyrochlore dielectrics. Chem. Mater. 2007, 19, 932–936. [Google Scholar] [CrossRef]
- Jana, Y.M.; Nand, S.; Gupta, H.C. Lattice dynamics and force constant calculation for the Raman and infra-red wave numbers of cubic bismuth-based pyrochlore compounds. J. Mol. Struct. 2018, 1154, 463–468. [Google Scholar] [CrossRef]
- Sudheendran, K.; Raju, K.C.J.; Singh, M.K.; Katiyar, R.S. Microwave dielectric and Raman scattering studies on bismuth zinc niobate thin films. J. Appl. Phys. 2008, 104, 104104. [Google Scholar] [CrossRef]
- Gupta, H.C.; Brown, S.; Rani, N.; Gohel, V.B. Lattice dynamic investigation of the zone center wavenumbers of the cubic A2Ti2O7 pyrochlores. J. Raman Spectrosc. 2001, 32, 41–44. [Google Scholar] [CrossRef]
- Arenas, D.J.; Gasparov, L.V.; Qiu, W.; Nino, J.C.; Patterson, C.H.; Tanner, D.B. Raman study of phonon modes in bismuth pyrochlores. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 214302. [Google Scholar] [CrossRef]
- Chen, M.; Tanner, D.B.; Nino, J.C. Infrared study of the phonon modes in bismuth pyrochlores. Phys. Rev. B 2005, 72, 54303. [Google Scholar] [CrossRef]
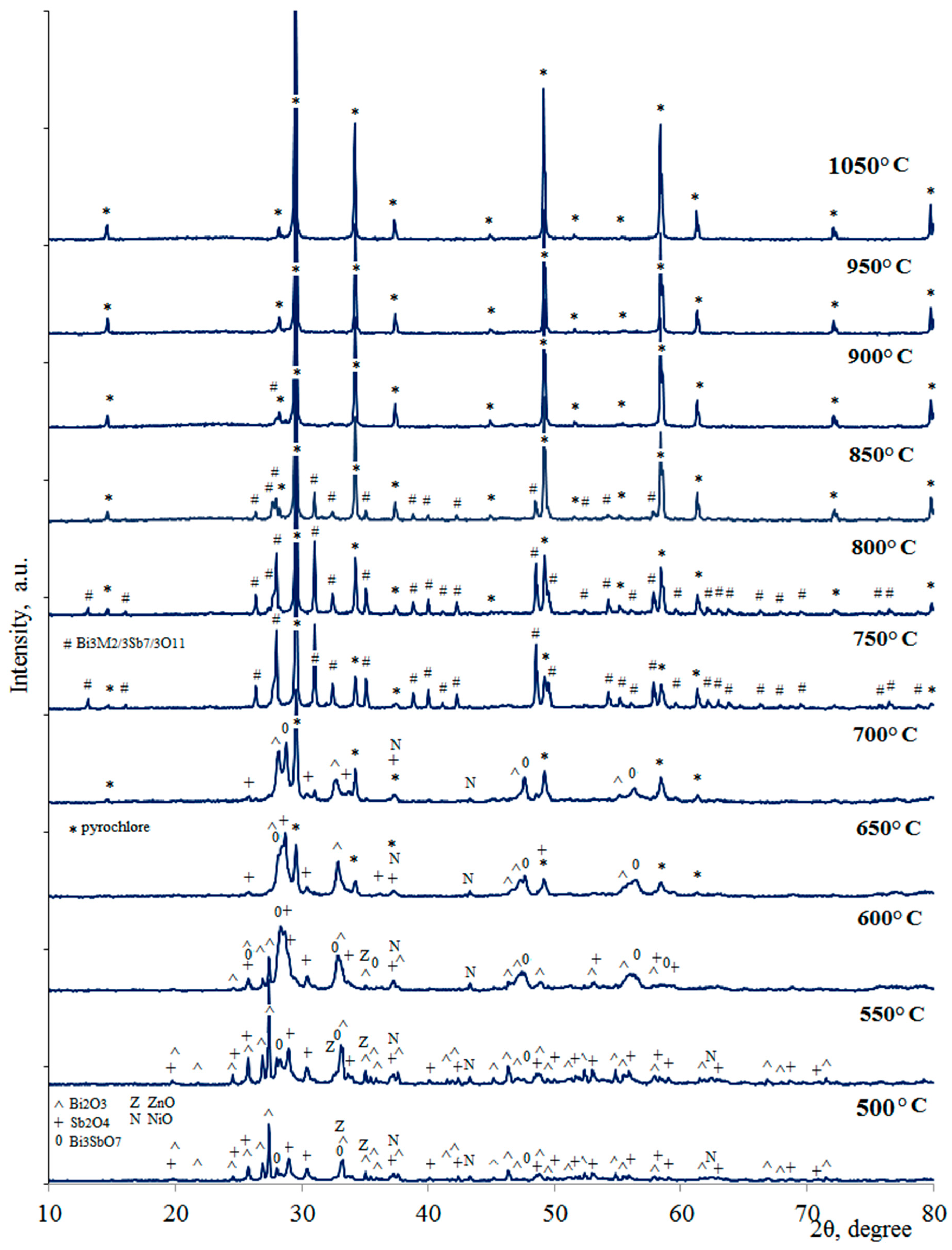

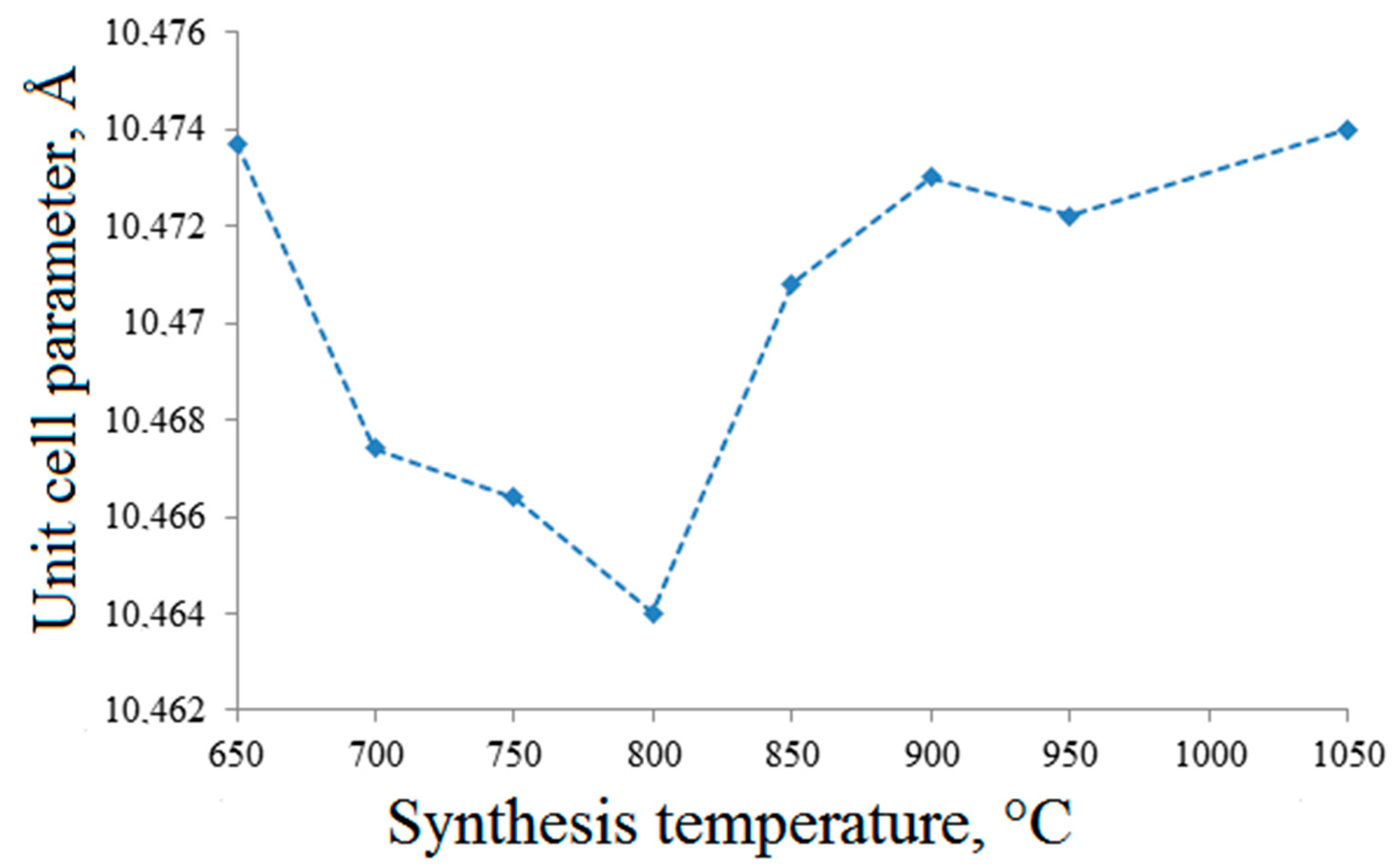
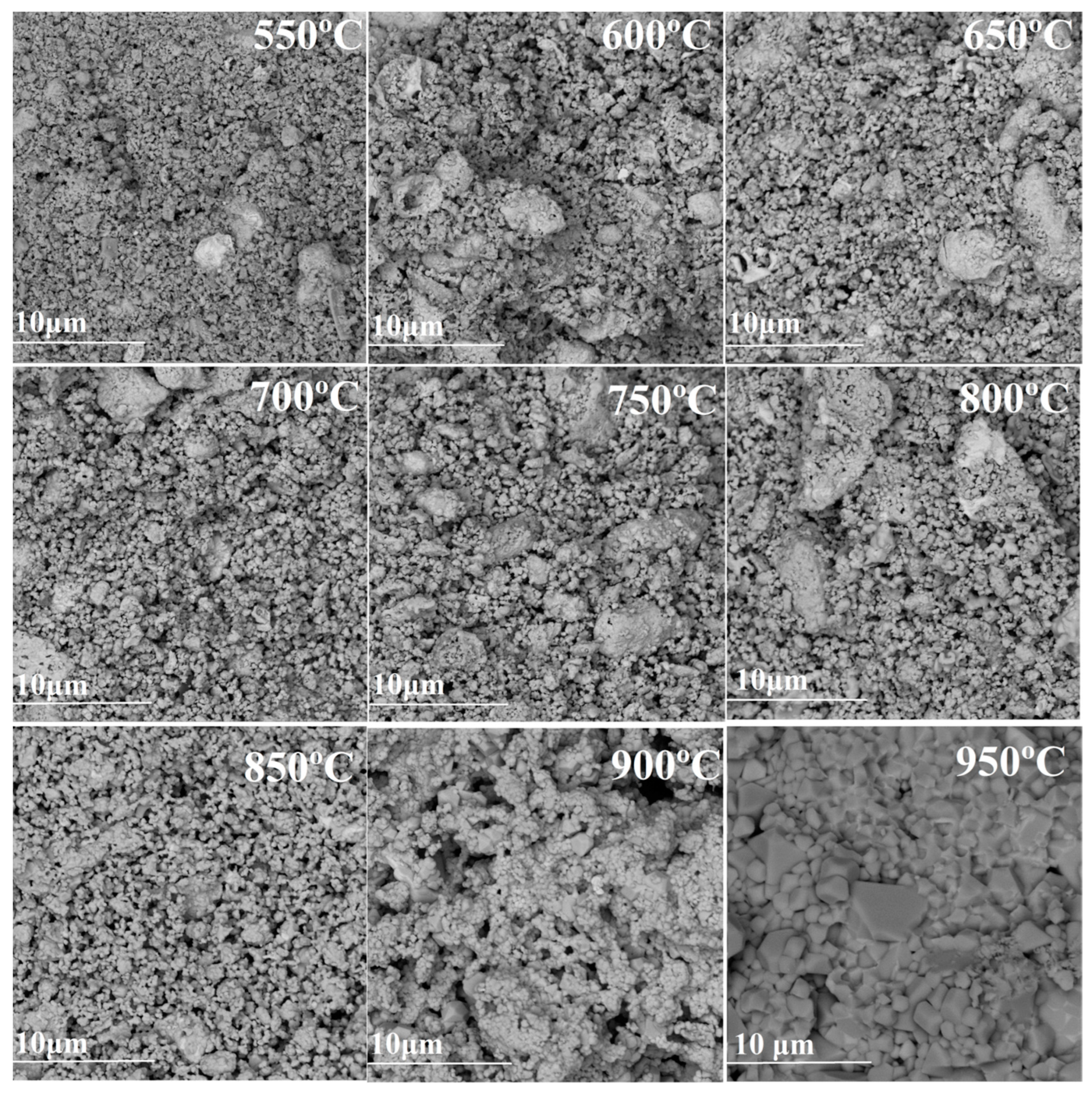
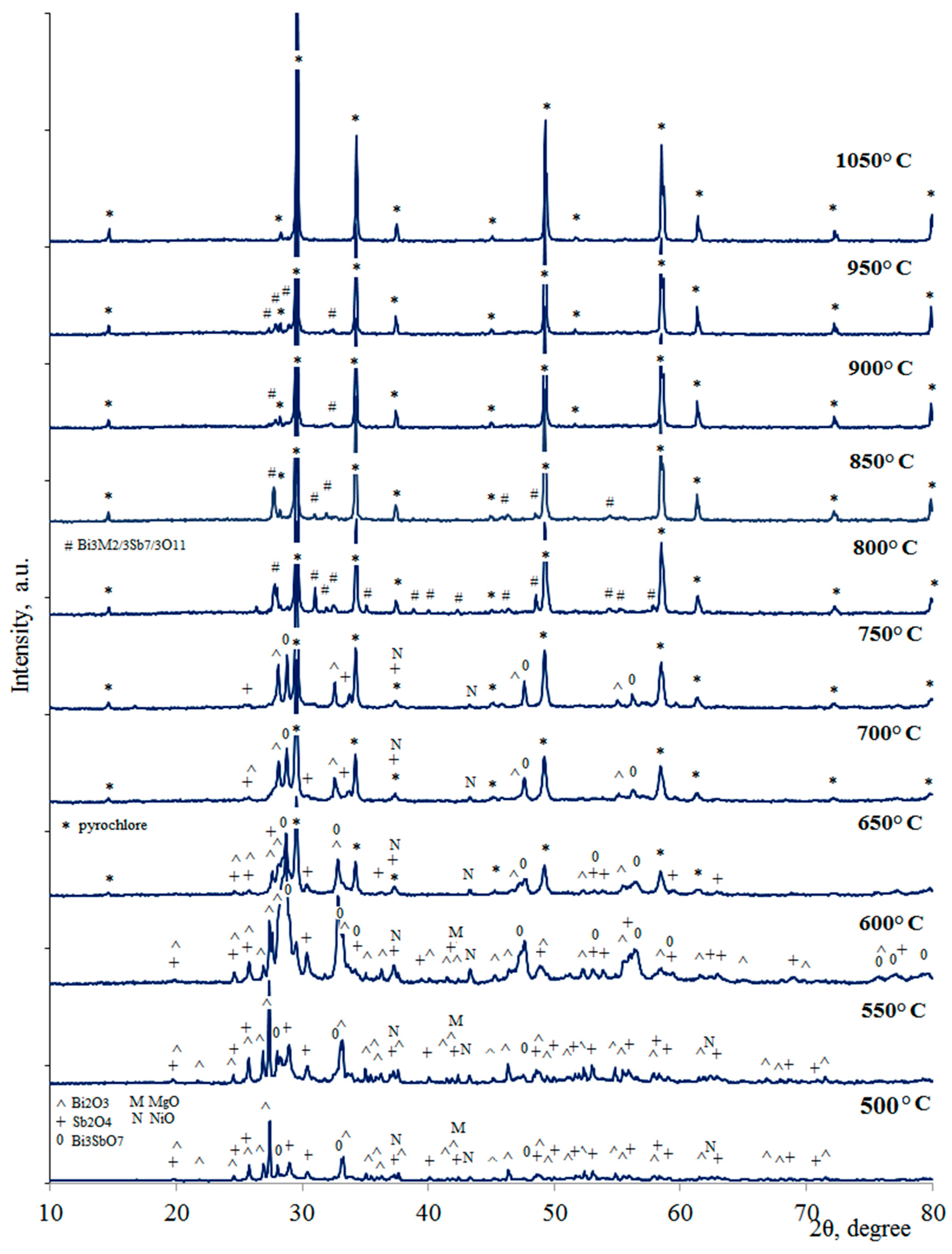



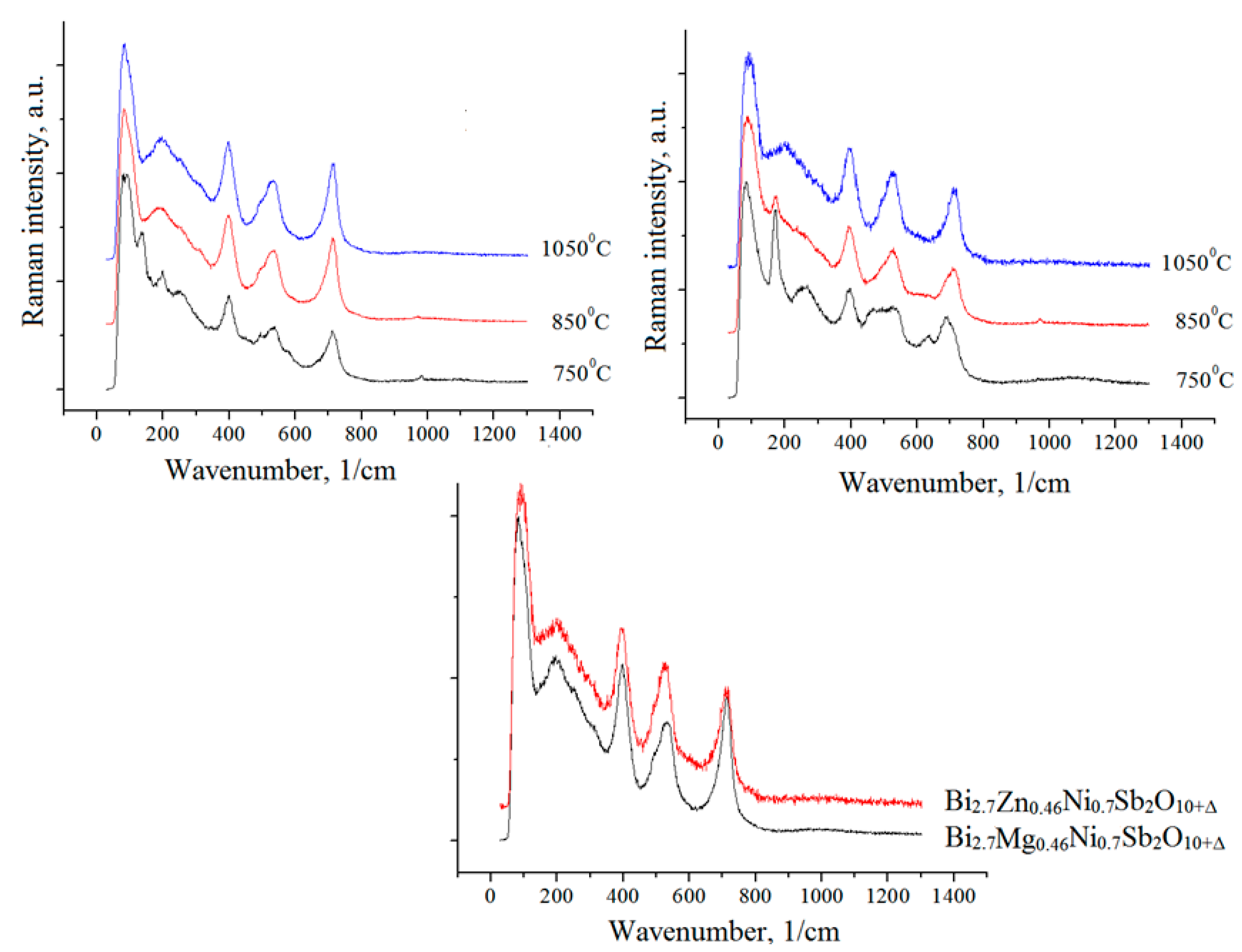
| Synthesis Temperature, °C | Phase Composition of the Sample |
|---|---|
| 1050 | pyrochlore |
| 950 | pyrochlore |
| 900 | Bi3M2/3Sb7/3O11 (traces), pyrochlore |
| 850 | Bi3M2/3Sb7/3O11, pyrochlore |
| 800 | Bi3M2/3Sb7/3O11, pyrochlore, NiO (traces) |
| 750 | Bi3M2/3Sb7/3O11, pyrochlore, NiO, ZnO (traces) |
| 700 | Bi2O3, Sb2O4, Bi3SbO7 (traces), pyrochlore, NiO, ZnO |
| 650 | Bi2O3, Sb2O4, Bi3SbO7, pyrochlore, NiO, ZnO |
| 600 | Bi2O3, Sb2O4, Bi3SbO7, NiO, ZnO |
| 550 | Bi2O3, Sb2O4, Bi3SbO7, NiO, ZnO |
| 500 | Bi2O3, Sb2O4, Bi3SbO7, NiO, ZnO |
| Synthesis Temperature, °C | Synthesis Temperature, °C |
|---|---|
| 1050 | pyrochlore |
| 950 | Bi3Mg2/3Sb7/3O11, pyrochlore |
| 900 | Bi3Mg2/3Sb7/3O11, pyrochlore |
| 850 | Bi3Mg2/3Sb7/3O11, pyrochlore |
| 800 | Bi3Mg2/3Sb7/3O11, pyrochlore |
| 750 | Bi2O3, Sb2O4, Bi3SbO7, pyrochlore, MgO |
| 700 | Bi2O3, Sb2O4, Bi3SbO7, pyrochlore, NiO, MgO |
| 650 | Bi2O3, Sb2O4, Bi3SbO7, pyrochlore, NiO, MgO |
| 600 | Bi2O3, Sb2O4, Bi3SbO7, NiO, MgO |
| 550 | Bi2O3, Sb2O4, Bi3SbO7, NiO, MgO |
| 500 | Bi2O3, Sb2O4, Bi3SbO7, NiO, MgO |
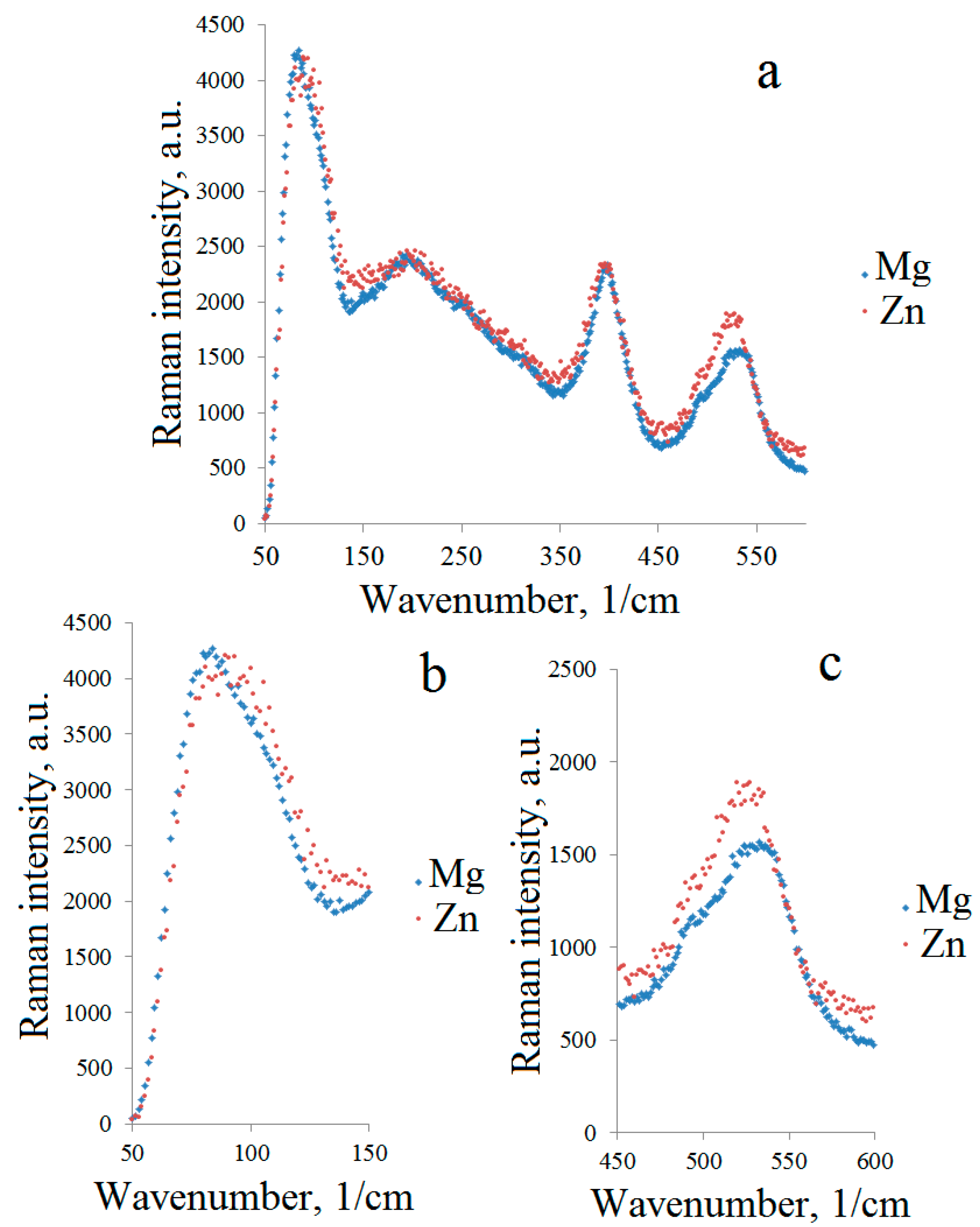
| Frequency (sm−1) | Symmetry | Identification | |
|---|---|---|---|
| Ni/Mg | Ni/Zn | ||
| Pyrochlore | |||
| 80 | 93 | F1u | Angular oscillations O-A-O, O-A-O′, O′-A-O′, chemical bond vibrations A-BO6 |
| 195 | 203 | Eg + F2g | Chemical bond vibrations A-O |
| 400 | 397 | F2g | Chemical bond vibrations B-O |
| 493 | 493 | A1g | Bond O-Sb-O in the octahedron SbO6 |
| 539 | 527 | A1g | Bond O-M-O in the octahedron MO6 (M-Ni, Mg, Zn) |
| 710 | 714 | F2g | Chemical bond vibrations B-O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuk, N.A.; Nekipelov, S.V.; Petrova, O.V.; Makeev, B.A.; Isaenko, S.I.; Krzhizhanovskaya, M.G.; Parshukova, K.N.; Korolev, R.I.; Simpeleva, R.A. Synthesis, Phase Formation, and Raman Spectroscopy of Ni and Zn(Mg) Codoped Bismuth Stibate Pyrochlore. Chemistry 2025, 7, 110. https://doi.org/10.3390/chemistry7040110
Zhuk NA, Nekipelov SV, Petrova OV, Makeev BA, Isaenko SI, Krzhizhanovskaya MG, Parshukova KN, Korolev RI, Simpeleva RA. Synthesis, Phase Formation, and Raman Spectroscopy of Ni and Zn(Mg) Codoped Bismuth Stibate Pyrochlore. Chemistry. 2025; 7(4):110. https://doi.org/10.3390/chemistry7040110
Chicago/Turabian StyleZhuk, Nadezhda A., Sergey V. Nekipelov, Olga V. Petrova, Boris A. Makeev, Sergey I. Isaenko, Maria G. Krzhizhanovskaya, Kristina N. Parshukova, Roman I. Korolev, and Ruslana A. Simpeleva. 2025. "Synthesis, Phase Formation, and Raman Spectroscopy of Ni and Zn(Mg) Codoped Bismuth Stibate Pyrochlore" Chemistry 7, no. 4: 110. https://doi.org/10.3390/chemistry7040110
APA StyleZhuk, N. A., Nekipelov, S. V., Petrova, O. V., Makeev, B. A., Isaenko, S. I., Krzhizhanovskaya, M. G., Parshukova, K. N., Korolev, R. I., & Simpeleva, R. A. (2025). Synthesis, Phase Formation, and Raman Spectroscopy of Ni and Zn(Mg) Codoped Bismuth Stibate Pyrochlore. Chemistry, 7(4), 110. https://doi.org/10.3390/chemistry7040110






