Abstract
The influence of sodium 4-vinylbenzenesulfonate on the electrooxidation of phenols and naphthols was studied in dimethylformamide (DMF). The usually observed deactivation of phenol in non-aqueous environments was suppressed upon addition of sodium 4-vinylbenzenesulfonate, and signals of all studied compounds were highlighted due to the diminished background current. As in other cases, the latter is attributable to the solvent. The 4-vinylbenzenesulfonate salt underwent electroinitiated polymerization close to the electrode surface excluding all other compounds from it within the timescale of the measurements. The polymeric products were partially removed by dissolution; therefore, improved signal reproducibilities could be reached. The investigation was extended to pulsed voltammetric techniques widely used in analytical chemistry (differential pulse, normal pulse, square wave voltammetry). Among these techniques, differential pulse voltammetry showed the best performance. Therefore, during its use, the analytical usefulness of adding the unsaturated additive in optimal concentration was estimated in the case of some phenols and naphthols. Successful calibration for picric acid was attained in DMF.
1. Introduction
Nowadays, there is a high interest in electrode modifications with organic layers. Many analytical applications are based on the advantageous properties of the electrochemically formed conducting or insulating layers, which themselves have size-exclusion or accumulation capabilities, thereby improving selectivity and/or sensitivity [1,2]. These properties come from their porosities, and the latter property makes possible these types of utilization. Electropolymerized natural phenolics and chemical aromatic alcohols serve as a platform for the immobilization of nanomaterials [3,4,5] and enzymes [6]. Molecularly imprinted polymers can also be created by electropolymerization, which also has significant practical interest in food, environmental and clinical analyses [7,8].
The suppression of electropolymerization by adding additional reagents is a topic of fundamental research. Coexisting aniline with N-(3-sulfopropyl) aniline in acidic solution decreased the rate of polyaniline formation, as anionogenic interlayers played the role of anion doping of the oxidized polymer chain, thereby contributing to diminished conductivity [9]. The application of a magnetic field impedes the growth of polypyrrole film in definite directions, resulting in a round-shaped and compact deposit [10]. In acetonitrile solvent, Rehan found that the presence of methyl naphthyl ether suppresses the growth of layer formed from 1-naphthol [11].
The presence of a carbon–carbon double bond in a molecule can lead to complications in the anodic polymerization process. For example, when 2-allylphenol was electrochemically polymerized [12], multiple modes of coupling took place, primarily characteristic for phenols and olefins. Eugenol, a natural phenolic compound, also has an allyl group, and its anodic oxidation has been studied in detail [13,14,15,16,17,18]. This electrochemical process begins, as usual for phenolic compounds, with the formation of the corresponding phenolic radical. The methoxy group found in the ortho position can be transformed to the oxo group leading to the introduction of quinone moieties into the polymer chain. The double bond found in the allylic group also becomes saturated, resulting in the insertion of alkyl chains between the aromatic and quinone rings.
There is a recent study in which phenol was investigated in dimethyl sulfoxide in the presence of 4-vinylbenzenesulfonate ions [19]. The results highlighted that the unsaturated additive suppresses the formation of poly (phenyleneoxide) deposits, thereby improving the determination of unreacted phenol. At higher potentials, dimethyl sulfoxide oxidizes easily. Its oxidation was also suppressed temporarily due to the presence of copolymer on the electrode for a short time. Thus, visible anodic peaks could be obtained. That study highlighted that the suppression of electropolymerization might offer possibilities for analytical purposes mainly for phenolic compounds. Therefore, the aim of this work was to conduct further research in dimethylformamide and to test pulsed voltammetric methods in this context. Similarly to dimethyl sulfoxide, DMF dissolves sodium 4-vinylbenzenesulfonate well; therefore, its performance in studying polymerizable compounds through electrooxidation will be evaluated in combination with electroanalytical methods.
2. Materials and Methods
The solid materials were analytical reagent grade (Reanal (Budapest, Hungary), TCI Chemicals (Tokyo, Japan) and AlfaAesar (Kandel, Germany)), while the solvent DMF was chromatographic grade (Hipersolv (Fontenay-sous-Bois, France)). The platinum working electrode (a 1 mm disc-shaped electrode sealed in a polyetheretherketone insulating sheath and a product of eDAQ) was connected to a Pt wire counter electrode and a silver wire reference electrode in a three-electrode cell during measurements. This cell was connected to a potentiostat (Dropsens, Oviedo, Spain). When it was necessary, the surface of the working electrode was cleaned by polishing with an aqueous suspension of alumina slurry with a 1 μm average particle diameter. This step was followed by thorough washing with deionized water and drying by rinsing with dry acetone. The supporting electrolyte was tetrabutylammonium perchlorate (TBAP).
3. Results
3.1. Assessment of the Influence of Sodium 4-Vinylbenzenesulfonate on the Voltammograms of Phenol
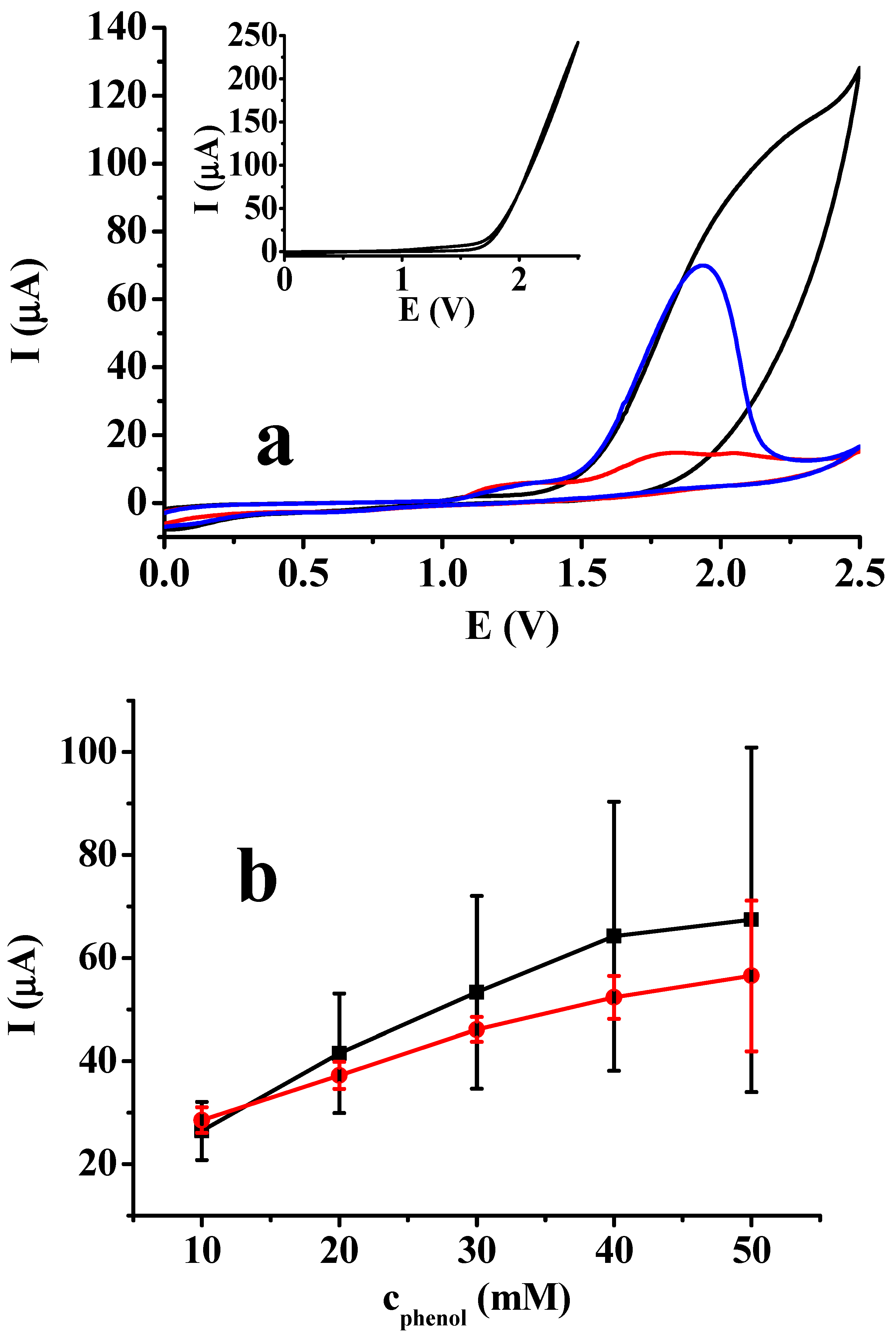
In order to shed light on how the addition of sodium 4-vinylbenzenesulfonate affects the voltammetric behavior of phenol cyclic, voltammograms were recorded between 0 and 2.5 V with a 0.1 V/s scan rate. The corresponding curves are shown in Figure 1a to illustrate the effect of phenol and the additive. The voltammogram of phenol in DMF alone does not contain a well-defined peak, as the solvent itself has a very high contribution to the current enhancement seen in the curve. Compared with the curve recorded in DMF containing only 50 mM TBAP, the surface blocking by poly (phenyleneoxide) is very significant, as seen from the diminished currents close to 2.5 V. Therefore, this also indicates that the high porosity of the forming polymer layer enhances the diffusion of solvent molecules through it, as it was also shown in an earlier study on dimethyl sulfoxide [19].

Figure 1.
Cyclic voltammetric curves of 50 mM phenol in DMF in different conditions. Part (a): black curve: 50 mM phenol alone; red curve: 50 mM sodium 4-vinylbenzenesulfonate alone; blue curve: 50 mM phenol in the presence of 50 mM sodium 4-vinylbenzenesulfonate); part (b) dependence of average voltammetric peak heights of phenol in DMF at different concentrations: alone (black curve) and in presence of 50 mM sodium 4-vinylbenzenesulfonate (red curve) (scan rate 0.1 V/s, with 50 mM TBAP as the supporting electrolyte). The inset graph in part (a) reveals the voltammetric curve in DMF containing only 50 mM TBAP.
When the above experiment was repeated for sodium 4-vinylbenzenesulfonate, the corresponding curve had remarkable characteristics. Although there is also a small anodic peak at around 1.9 V attributable to it, its height suggests that a few molecules are oxidized, triggering an electroinitiated polymerization. This result is very similar to that obtained in dimethyl sulfoxide [19]. The most remarkable finding is the measuring of drastically diminished currents at potentials at which solvent oxidation is very significant. This is due to the polymer formation close to the electrode surface, excluding solvent molecules from it. When both phenol and sodium 4-vinylbenzenesulfonate were present in DMF, a well-defined oxidation peak appeared, attributable mainly to phenol oxidation. However, its height was half of the maximum current when only phenol was present, due to the stronger adherence of the phenol and additive copolymer. The exclusion of solvent is visible, as indicated by the significant drop in current near 2.5 V. As a matter of fact, phenol is the real electroactive compound, and the anodically generated phenoxyl radicals slow down the coverage of the platinum electrode. Under these conditions, the products contain numerous phenoxyl groups.
The phenol concentration dependence and current reproducibility were also investigated (Figure 1b) at a constant concentration of 50 mM sodium 4-vinylbenzenesulfonate and in the absence of this additive. Five data points were averaged for each concentration with a renewed electrode before measurements in each solution. The electrooxidation of phenol without the additive showed a concentration-dependent trend, but the current signals were highly scattered. These large scatterings are due to increasingly significant electrode deactivation. In a previous study [20], we found a rapid process, therefore the peak current values showed significant differences. However, the error bars do not reflect that the currents of repeated scans for each concentration had a strong decreasing tendency. This trend became increasingly evident with increasing concentrations, verifying the continuous coverage of the electrode with electropolymerized phenol. The presence of sodium 4-vinylbenzenesulfonate resulted in significantly reduced scatterings, and the currents did not show a decreasing tendency (this is not evident from the figure). This indicates that the product copolymers dissolve more readily in DMF compared to poly (phenyleneoxide) especially by agitating the solution through movement of the electrode between the parallel scans.
3.2. Effect of Sodium 4-Vinylbenzenesulfonate Concentration
In the previous section, the voltammograms of phenol showed that the additive has a remarkable effect on the shape of cyclic voltammetric curves. Its concentration was varied by keeping the phenol concentration (50 mM) constant. Figure 2 shows the obtained curves. The heights of the peaks do not show a remarkable dependence, but it is clearly evident that the enhanced amount has a negative effect. The depths of the current drops following the peaks are almost the same, which seems very promising from an analytical viewpoint. Therefore, a smaller quantity of additive is enough to obtain visible anodic peaks.

Figure 2.
Cyclic voltammetric curves of 50 mM phenol at different concentrations of sodium 4-vinylbenzenesulfonate (scan rate 0.1 V/s, with 50 mM TBAP as the supporting electrolyte).
3.3. Effect of Water Content
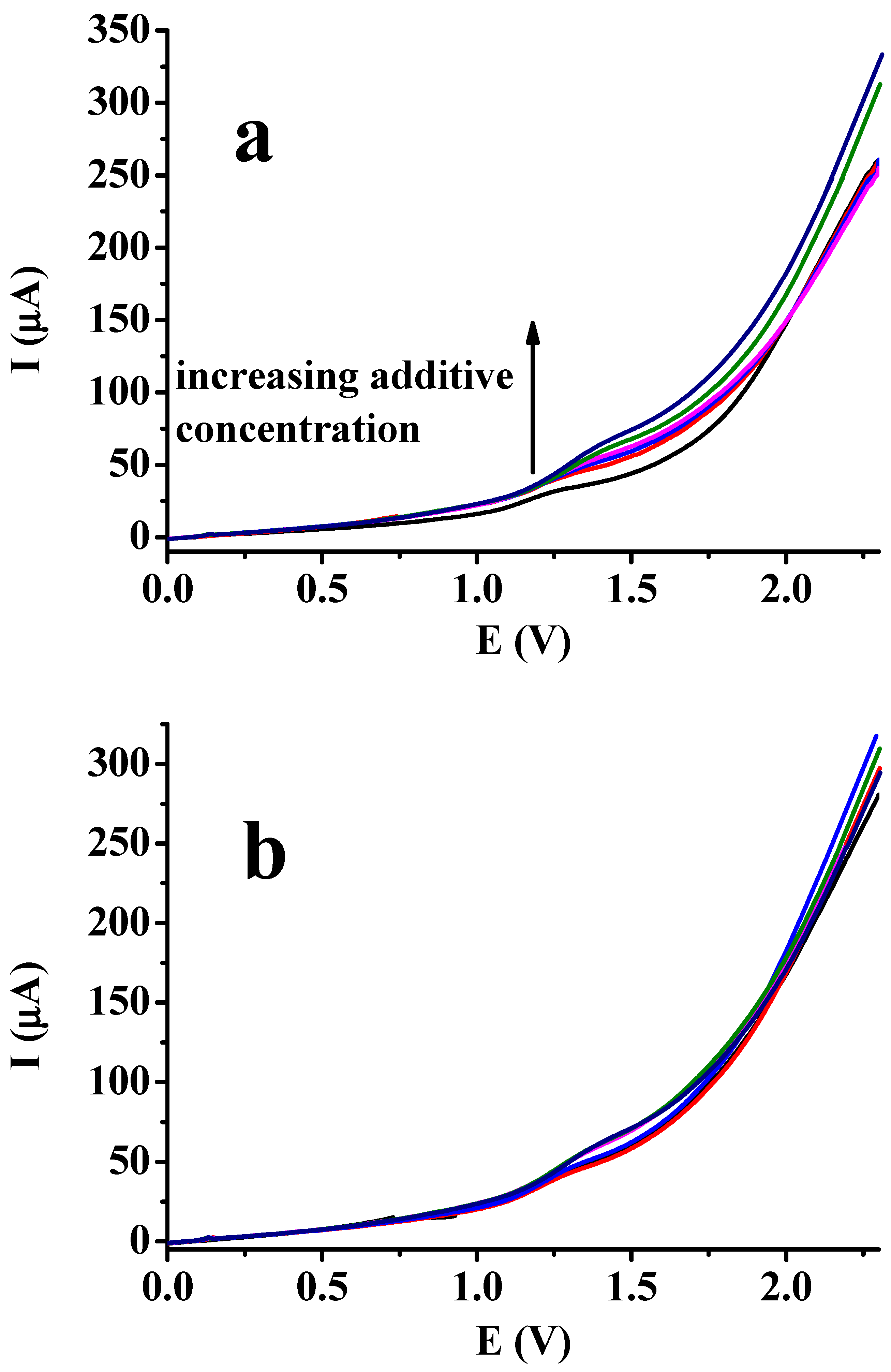
As aqueous media are by far the most interesting in the quantification of different compounds, the water content was varied in the experiment. The corresponding curves in Figure 3 reveal the significant shifting of peak potentials in the cathodic direction with increasing water content, as expected. The permittivity of solvent mixtures will be higher at higher water content. The really important finding in this experiment is the drastic continuous drop of peak heights as the volume ratio of water increased. This can be explained by the fact that water usually dissolves electrochemically formed polymers less readily than many organic solvents, and numerous studies support this fact [20,21,22,23]. In summary, cyclic voltammetric curves show that water also has a negative effect on the magnitude of the signal in the presence of sodium 4-vinylbenzenesulfonate, despite the fact that the polymer of this vinyl compound dissolves readily in water due to the sulfonate groups. Segments of polyphenol parts trigger the settling down of polymers on the electrode.

Figure 3.
Cyclic voltammetric curves of 50 mM phenol by different v/v% of water mixed with DMF (scan rate: 0.1 V/s; supporting electrolyte: 50 mM TBAP; additive 50 mM).
3.4. Assessment of Pulsed Voltammetric Methods
Pulsed voltammetric methods play a significant role in electroanalytical chemistry, mainly in the detection of molecules at low concentrations. These techniques have not been investigated in detail by reactions where electropolymer formation occurs, so they were also studied. After optimizing the parameters, their performance will be revealed. One of these techniques is differential pulse voltammetry (DPV), where the potential is changed from an initial value to the end value incrementally, similarly to linear sweep voltammetry. During this scan, potential pulses are superimposed at a definite rate, and the differences in current, registered at the end of the pulse and at the actual base potential, are plotted as a function of the actual base potential. During the experiments, compounds that are prone to film formation were collected during their electrooxidation.
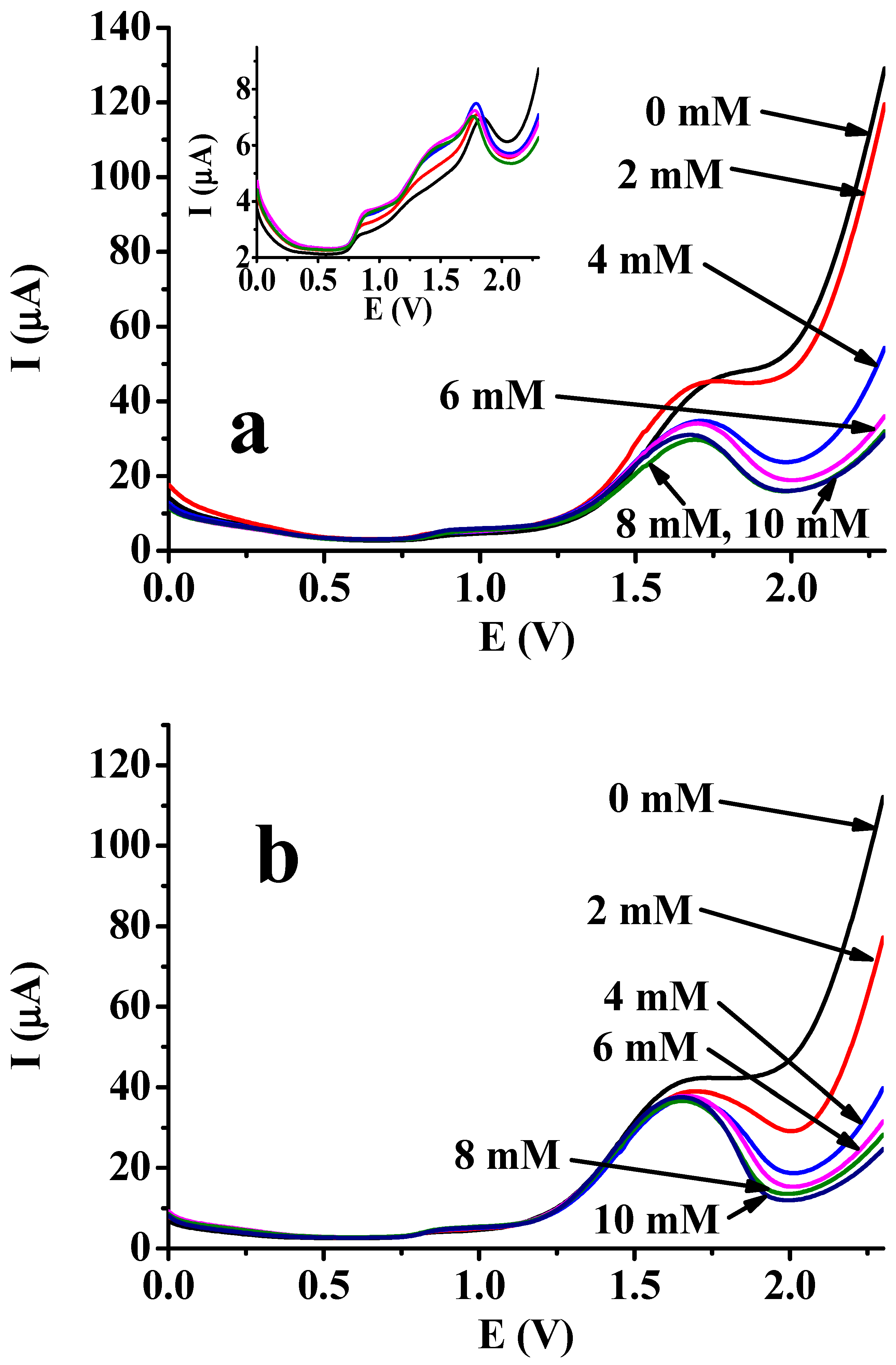
At the first stage of this study, two different phenolic compounds were selected, and the main difference is between them is their fouling ability in non-aqueous solvents. Earlier studies verified that 4-chlorophenol is generally not susceptible to fouling in the mmol/L concentration range in the commonly used non-aqueous solvents [24]. Bare phenol is much more susceptible to it. As Figure 4 illustrates, the DPV curves have very similar characteristics to the cyclic voltammograms shown before. At the highest potentials, the currents dropped after the appearance of peaks at around 1.8 V. The reason is the same as in cyclic voltammetry, which is not promising, as this technique involves a continuous increase in anodic potential, leading to the electroinitiated polymerization of the unsaturated additive. The observed peaks consist of two contributions, namely the electrooxidation of the phenolic compound and 4-vinylbenzenesulfonate ions.


Figure 4.
Differential pulse voltammetric curves of 10 mM 4-chlorophenol (a) and 10 mM phenol (b) in DMF at varied concentrations of sodium 4-vinylbenzenesulfonate (supporting electrolyte: 20 mM TBAP; Epulse = 0.25 V; tpulse = 5 ms; Estep = 0.004 V; scan rate: 0.04 V/s). The inset graph in part a shows the curves recorded in the same conditions as in the main graph without 4-chlorophenol for sodium 4-vinylbenzenesulfonate in DMF at the same concentrations (the colors of the curves represent the same concentrations as in the main figure).
The inset graph in Figure 4a displays the curves recorded in the presence of only the additive in different concentrations (from 2 to 10 mM). The heights of the anodic peaks show high uniformity at around 1.9 V, indicating a lack of concentration independence due to the electrochemically initiated polymerization and the settling of the polymer on the platinum surface. In contrast, peaks at around 1 V show some concentration dependence, probably as a result of the electrosorption of sulfonate anions.
The main portion of the figures illustrates in fact the effect of addition of sodium 4-vinylbenzenesulfonate salt in different quantities while the solutions contained the phenols in uniform concentrations (10 mM). In the case of both phenols, the anodic peak heights are almost uniform, and the large differences can be observed at the end of the curves (2.3 V). The increasing concentration of the additive resulted in continuous and increasingly deeper drops in the current. This verifies that the analyte aromatic alcohol has an inhibiting effect on the polymerization of sodium 4-vinylbenzenesulfonate, as it can terminate the growth of polymers. Thus, they will be more rapidly removed by dissolution in the solution bulk. On the other hand, the 6 mM concentration for this substance is a limiting value, as at higher concentrations, the current drops did not show any trend, being comparable to the curve at 6 mM concentration.
The other most commonly used pulsed voltammetric technique is square wave voltammetry (SWV), so it was also investigated with 4-chlorophenol and phenol by using the same solutions as before, measured by DPV. This technique applies not only to forward pulses but also to immediately following backward pulses. The current differences between the ends of the forward and backward pulses will be plotted as a function of incrementally changed base potential. That is why this method is very sensitive to reversible electroactive compounds. As shown in the inset graph in Figure 5a, the curves indicate that the currents of sodium 4-vinylbenzenesulfonate do not show significant differences, mainly due to the continuous scanning of the base potential in SWV as well. This verifies that electroinitiation leads to irreversible polymerization irrespective of the used potential program. Similarly to DPV, the curves exhibit continuously increasing current drops near 2.3 V with increasing additions of the sulfonate salt.

Figure 5.
Square wave voltammetric curves of 10 mM 4-chlorophenol (a) and 10 mM phenol (b) in DMF with varying concentrations of sodium 4-vinylbenzenesulfonate (supporting electrolyte: 20 mM TBAP; Epulse = 0.25 V; tpulse = 5 ms; Estep = 0.005 V; frequency: 10 Hz).
There is a less frequently applied voltammetric method which involves increasing potential pulses, and between these pulses, the electrode potential is kept at the base potential. This is called normal pulse voltammetry (NPV). Usually, the operation of this detection method is based on the application of incrementally increasing potential pulses from the base potential to the end value. After all pulses, the potential is returned to the base value by ensuring that nothing happens in the vicinity of the electrode surface. The NPV curves in Figure 6 show that this technique is ineffective in this context due to the almost uniform nature of curves, irrespective of concentration. However, the currents did not drop at higher anodic potentials, indicating a lack of electrode blockage. As a matter of fact, the few polymers formed during the anodic pulses could dissolve in the time intervals inserted between the pulses, so their 100 ms width seemed to be enough.

Figure 6.
Normal pulse voltammetric curves of 10 mM 4-chlorophenol (a) and 10 mM phenol (b) in DMF with varying concentrations of sodium 4-vinylbenzenesulfonate (supporting electrolyte: 20 mM TBAP; tint = 100 ms; tpulse = 5 ms; Estep = 0.005 V; Ebase = 0 V). The arrow in part (a) indicates the increase in additive concentration; the concentrations of the additive were 0 (black), 2 (red), 4 (blue), 6 (magenta), 8 (green), 10 mM (navy).
3.5. Voltammetric Studies with Sodium Benzenesulfonate
In the previous sections, notable declines in current were observed by adding sodium vinylbenzenesulfonate to the solutions. The possible explanation for it was the electroinitiated polymerization of the additive, but other interactions cannot be excluded, including electrosorption. To better understand what happens close to the electrode surface, a similar compound was studied without a carbon–carbon unsaturated bond and without a functional group being able to oxidize in the potential range where phenols and naphthols usually undergo anodic oxidation. This was sodium benzenesulfonate, and its differential pulse voltammetric curves are displayed in Figure 7 in the concentration range 2–10 mM in DMF, in presence of 20 mM TBAP. The curves clearly show the lack of anodic peaks and current drops at high potentials. This finding supports the earlier results as complementary evidence showing that by dissolving sodium vinylbenzenesulfonate, electroinitiated polymerization really occurs at potentials higher than approximately 1.3 V. The anodic curves observed by using the DPV technique appear to be related to the solvent.

Figure 7.
Differential pulse voltammetric curves of sodium benzenesulfonate at different concentrations (supporting electrolyte: 0.02 M TBAP).
3.6. Study of Other Phenols and Naphthols in DMF in Presence of Sodium 4-Vinylbenzenesulfonate
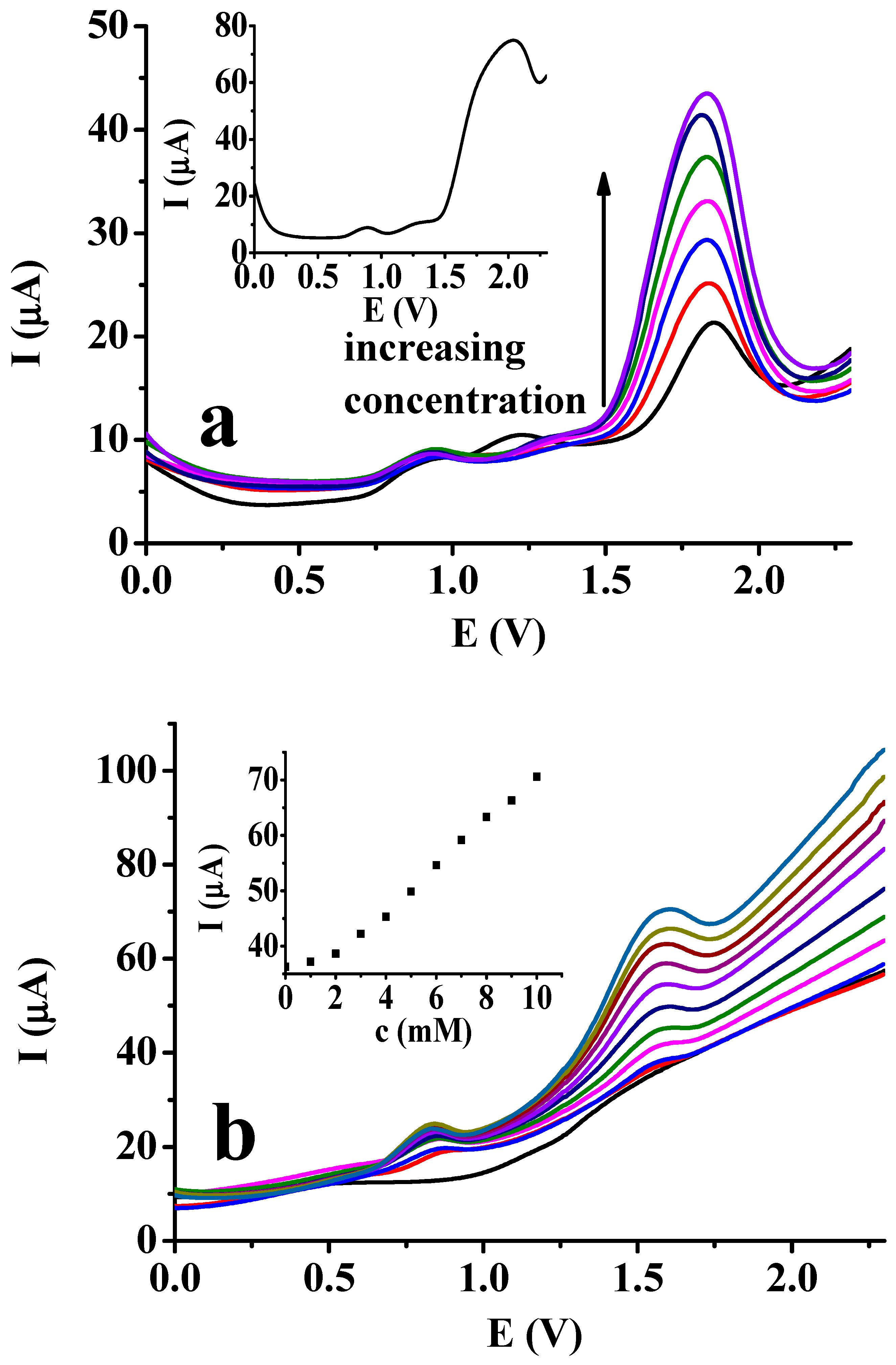
Previous studies of phenol showed that the solvent DMF can be favorably utilized in presence of sodium 4-vinylbenzenesulfonate. Therefore, more organic compounds which are susceptible to electrochemical polymerization were also tested by using the DPV technique. Taking into account the obtained curves, DPV seemed to be the most appropriate for further investigation.
The experiments were repeated for other aromatic alcohols in DMF by changing the concentration of sodium 4-vinylbenzenesulfonate between 0 and 10 mM and by setting the analyte concentration to 10 mM. The corresponding current values at 2.3 V can be seen in Figure 8, and similar magnitudes of current drops can be observed. It can be observed that in the case of the majority of aromatic alcohols, the current drops are very significant until an additive concentration of 4 mM can be reached. The highest change was observed with picric acid; moreover, the peak current was by far the highest both in the absence of the additive and in general. Among the studied aromatic alcohols, picric acid has a minimum affinity to fouling due to the occupation of para and the two ortho positions substituted with nitro groups. When it is oxidized, the corresponding phenoxyl radical forms, which terminates any kind of forming polymer in close proximity to the platinum surface. The results also suggest that the copolymers will be easily removed by dissolving in the solution, as current drops are not significant until sodium 4-vinylbenzenesulfonate is not dissolved at a concentration higher than 4 mM. Resorcinol is the exception, as the largest drop occurred around 2 mM additive concentration, probably because the electropolymer of resorcinol also has poor solubility.

Figure 8.
DPV currents at 2.3 V for different aromatic alcohols (c = 10 mM for each phenolic compounds) as a function of sodium 4-vinylbenzenesulfonate concentration (supporting electrolyte: 20 mM TBAP).
Sodium 4-vinylbenzenesulfonate has a carbon atom in the neighborhood of the aromatic ring in the vinyl group, so it can be oxidized. This was demonstrated in the case of a similar compound, phenylacetylene electropolymerization. The authors found that a radical forms in the acetylene group, and it promotes polymerization, and another electrogenerated radical terminates the chain growth [25]. Summarizing the voltammetric results, it can be concluded that the ethylene group can be oxidized in molecules of sodium 4-vinylbenzenesulfonate, producing a radical. When this additive was studied on its own, very small peaks appeared, compared to the case in presence of phenol. This suggests that only some molecules oxidize, and fast polymerization leads to the propagation of film, which then covers the electrode. When another electropolymerizable compound coexists in the solution, its radical can also initiate the polymerization of the unsaturated additive, and, depending on its concentration, the degree of polymerization varies. A higher concentration of the phenolic compound results in lower molecular weight polymers due to the frequent termination of additive polymerization, as Scheme 1 shows. Therefore, phenols play a predominant role in the initiation and termination of polymerization, and as a consequence, the lower degree of polymerization leads to a product that is significantly more soluble than the homopolymer of the additive, which exhibits strong adherence.

Scheme 1.
Electroinitiated polymerization of sodium 4-vinylbenzenesulfonate: alone and the main pathway in the presence of a phenolic compound.
3.7. Estimation of Effect of Sodium 4-Vinylbenzenesulfonate on Analytical Signal of Picric Acid
Picric acid has a large oxidation potential due to the presence of three electron-withdrawing nitro groups, and the significant interference of the background current of DMF should be taken into account. As it is shown in the inset graph of Figure 9a, only a small anodic peak appeared in the 10 mM concentration solution due to solvent interference in the absence of the additive. The main part displays the DPV curves for concentrations in the linear range in presence of 6 mM 4-vinylbenzenesulfonate, and the significant difference can be seen in the shape of the curves, as they resemble to the ones typically observed in DPV. Above the linear range, the results were unuseful, as there was no proportionality to the concentration and a saturation-like dependence was observed.

Figure 9.
DPV curves of picric acid in DMF covering the linear range (a) and in 50–50 v/v% DMF-water mixtures (b) in the presence of 6 mM sodium 4-vinylbenzenesulfonate (background electrolyte: 20 mM TBAP; Epulse = 0.25 V; tpulse = 5 ms; Estep = 0.004 V; scan rate: 0.04 V/s). The inset graph in part (a) shows a curve measured in the absence of the additive for 10 mM picric acid, and the inset in part (b) reveals the corresponding calibration curve.
By directly reading peak current values from the curves and fitting a linear function to the first seven points, a calibration equation can be obtained with a detection limit of 147 μM, based on the 3σ criterion: I(μA) = 21.554 + 7.685c (mM) (R2 = 0.996). The dynamic range was between 0 and 3 mM for picric acid, and the concentrations changed with 0.5 mM increments. As seen from the graph, the dependence is approximately linear at these points. To calculate the detection limit, the scatter of five peak current values was used, measured in the DMF solution containing only the supporting electrolyte and the unsaturated additive.
The above experiments were repeated in a 50–50 v/v% solvent mixture of DMF and water (Figure 9b). Significantly smaller peaks appeared due to the excellent solvation of the formed polymeric product, similarly to pure water. The calibration curve in the inset graph shows that the dynamic range begins only at around 2 mM picric acid concentration. The increasing background currents indicate this, obviously leading to decreased analytical signals. Therefore, extending the method to aqueous samples by mixing them with DMF is not suggested.
In summary, the results generally show that both voltammetric peak heights and the depths of current drops at higher potentials can be used as an analytical signal. In fact, the latter is a measure for the quantity of solvent molecules able to reach the electrode to undergo electrooxidation. These depths also depend on the concentration of the analyte, similarly to peak heights. The results with picric acid show that addition of sodium 4-vinylbenzenesulfonate is very promising for concentration measurement in the potential range where solvent also has high background current. The analytical signal of the analyte can be extracted from this highly interfering contribution. Specifically, in the case of picric acid, this electrochemical method is promising and highlights the importance of solvent–solute interactions.
4. Conclusions
The investigations in dimethylformamide showed that the addition of sodium 4-vinylbenzenesulfonate leads to promising results in the analysis of aromatic alcohols in media where interference with solvent electrooxidation is large by suppressing the background current of the solvent. Our findings inspire the development of new electroanalytical methods in non-aqueous environments. They can serve as an illustration of the effect of an additive, also observable with frequently used pulsed voltammetric techniques, and the strategy used here will gain utility for the determination of more important compounds in certain solvents. Concentration optimization of sodium 4-vinylbenzenesulfonate or other olefins can help in the determination of compounds. The modified shape of the curves, due to the unsaturated additive, offers new possibilities for signal processing, as it was suggested by the examples of phenol and 4-clorophenol, and they highlighted that in cases when oxidative radical formation occurs, we observe the same characteristics.
Author Contributions
Conceptualization, L.K.; methodology, L.K.; investigation, L.K.; writing—original draft preparation, L.K. and A.K.; writing—review and editing, A.K.; supervision, L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hungarian National Research Development and Innovation Office (NKFI), grant number NKFI-137793, Chinese-Hungarian Intergovernmental S&T Cooperation Programme (Project no.: CH-10-6/2024 and 2024-1.2.5-TÉT-2024-00006), and by the New National Excellence Program of the Ministry for In-novation and Technology Project no. TKP2021-EGA-17. The financial support offered by the Uni Pécs under the project 015_2024_PTE_RK/31 is highly appreciated.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| DMF | Dimethylformamide |
| TBAP | Tetrabutylammonium perchlorate |
| DPV | Differential pulse voltammetry |
| SWV | Square wave voltammetry |
| NPV | Normal pulse voltammetry |
References
- Zhang, Y.; Zhuang, H. Poly(acridine orange) film modified electrode for the determination 1-naphthol in the presence of 2-naphthol. Electrochim. Acta 2009, 54, 7364–7369. [Google Scholar] [CrossRef]
- Guerrieri, A.; Lattanzio, V.; Palmisano, F.; Zambonin, P.G. Electrosynthesized poly(pyrrole)/poly(2-naphthol) bilayer membrane as an effective anti-interference layer for simultaneous determination of acethylcholine and choline by a dual electrode amperometric biosensor. Biosens. Bioelectron. 2006, 21, 1710–1718. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Zhao, L.; Shen, S.; Yuan, M.; Liu, W.; Tu, Q.; Yu, R.; Wang, J. Au nanoparticles/poly(caffeic acid) composite modified glassy carbon electrode for voltammetric determination of acetaminophen. Talanta 2016, 159, 356–364. [Google Scholar] [CrossRef]
- Jin, G.P.; Peng, X.; Chen, Q.Z. Preparation of novel arrays silver nanoparticles modified polyrutin coat-paraffin-impregnated graphite electrode for tyrosine and tryptophan’s oxidation. Electroanal 2008, 20, 907–915. [Google Scholar] [CrossRef]
- Jin, G.P.; Chen, L.L.; Hang, G.P.; Yang, S.Z.; Wu, X.J. Stripping chronopotentiometric analysis of cysteine on nano-silver coat polyquercetin-MWCNT modified platinum electrode. J. Solid State Electrochem. 2009, 14, 1163–1169. [Google Scholar] [CrossRef]
- Ciriello, R.; Cataldi, T.R.I.; Centonze, D.; Guerrieri, A. Permselective behavior of an electrosynthesized, nonconducting thin film of poly(2-naphthol) and its application to enzyme immobilization. Electroanal 2000, 12, 825–830. [Google Scholar] [CrossRef]
- Kiss, L.; David, V.; David, I.G.; Lazar, P.; Mihailciuc, C.; Stamatin, I.; Ciobanu, A.; Stefanescu, C.D.; Nagy, L.; Nagy, G.; et al. Electropolymerized molecular imprinting on glassy carbon electrode for voltammetric detection of dopamine in biological samples. Talanta 2016, 160, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Shojaei, S.; Nasirizadeh, N.; Entezam, M.; Koosha, M.; Azimzadeh, M. An electrochemical nanosensor based on molecularly imprinted polymer (MIP) for detection of gallic acid in fruit juices. Food Anal. Methods 2016, 9, 2721–2731. [Google Scholar] [CrossRef]
- Malinauskas, A.; Holze, R. Suppression of the “first cycle effect” in self-doped polyaniline. Electrochim. Acta 1998, 43, 515–520. [Google Scholar] [CrossRef]
- Mogi, I.; Kamiko, M. Suppression of growth instability in electropolymerization of pyrrole. Bull. Chem. Soc. Jpn. 1996, 69, 1889–1892. [Google Scholar] [CrossRef]
- Rehan, H.H. Electrosynthesis and characterization of new conducting copolymer films from 1-naphthol and methyl naphthyl ether. Polym. Int. 2000, 49, 645–653. [Google Scholar] [CrossRef]
- Cihaner, A.; Önal, A.M. Electroinitiated polymerization of 2-allylphenol. Polym. Bull. 2000, 45, 45–52. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G. Polyeugenol-modified platinum electrode for selective detection of dopamine in the presence of ascorbic acid. Anal. Chem. 1999, 71, 1055–1061. [Google Scholar] [CrossRef]
- Toniolo, R.; Dossi, N.; Pizzariello, A.; Susmel, S.; Bontempelli, G. Simultaneous detection of ascorbic acid and hydrogen peroxide by flow-injection analysis with a thin layer dual-electrode detector. Electroanal 2011, 23, 628–636. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G. Preparation and general properties of chemically modified electrodes based on electrosynthesized thin polymeric films derived from eugenol. Electroanal 2001, 13, 860–867. [Google Scholar] [CrossRef]
- Okumura, L.L.; Stradiotto, N.R.; Rees, N.V.; Compton, R.G. Modifying glassy carbon (GC) electrodes to confer selectivity for the voltammetric detection of L-cysteine in the presence of DL-homocysteine and glutathione. Electroanal 2008, 20, 916–918. [Google Scholar] [CrossRef]
- Paul, D.W.; Prajaprati, I.; Reed, M.L. Electropolymerized eugenol: Evaluation as a protective film for oxygen sensing. Sens. Actuators B Chem. 2013, 183, 129–135. [Google Scholar] [CrossRef]
- El Qouatli, S.; Ngono, R.T.; Najih, R.; Chtaini, A. Eugenol modified titanium electrode for the analysis of carbocysteine. Zastita Materijala 2011, 52, 242–246. [Google Scholar]
- Kiss, L.; Kunsági-Máté, S.; Szabó, P. Studies of phenol electrooxidation performed on platinum electrode in dimethyl sulphoxide medium. Determination of unreacted phenol by the effect of 4-vinylbenzenesulfonate on the electrooxidation process. Electroanal 2023, 35, e202200268. [Google Scholar] [CrossRef]
- Kiss, L.; Bősz, D.; Kovács, F.; Li, H.; Nagy, G.; Kunsági-Máté, S. Investigation of phenol electrooxidation in aprotic non-aqueous solvents by using cyclic and normal pulse voltammetry. Polym. Bull. 2019, 76, 5849–5864. [Google Scholar] [CrossRef]
- Fino, D.; Jara, C.C.; Saracco, G.; Specchia, V.; Spinelli, P.J. Deactivation and regeneration of Pt anodes for the electro-oxidation of phenol. J. Appl. Electrochem. 2005, 35, 405–411. [Google Scholar] [CrossRef]
- Jeffrey, A.R.; Paul, E.W.; Dennis, H.E. Electrochemical oxidation of 2,4,6-tri-tert-butylphenol. J. Electroanal. Chem. 1975, 53, 311–327. [Google Scholar]
- Dulal, S.M.S.I.; Won, M.; Shim, Y. Carbon fiber supported platinum nanoparticles for electrooxidation of methanol and phenol. J. Alloys Compd. 2010, 494, 463–467. [Google Scholar] [CrossRef]
- Kiss, L.; Kunsági-Máté, S. Scan rate and concentration dependence of the voltammograms of substituted phenols on electrodes with different size, diffusion coefficients of phenols in different solvents. Can. J. Chem. 2023, 101, 297–305. [Google Scholar] [CrossRef]
- García-Canadas, J.; Rodríguez, J.G.; Lafuente, A.; Marcos, M.L.; Velasco, J.G. Electropolymerization of phenylacetylene in acetonitrile. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 2407–2416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).