Metal-Free Synthesis of Benzimidazolinones via Oxidative Cyclization Under Hypervalent Iodine Catalysis
Abstract
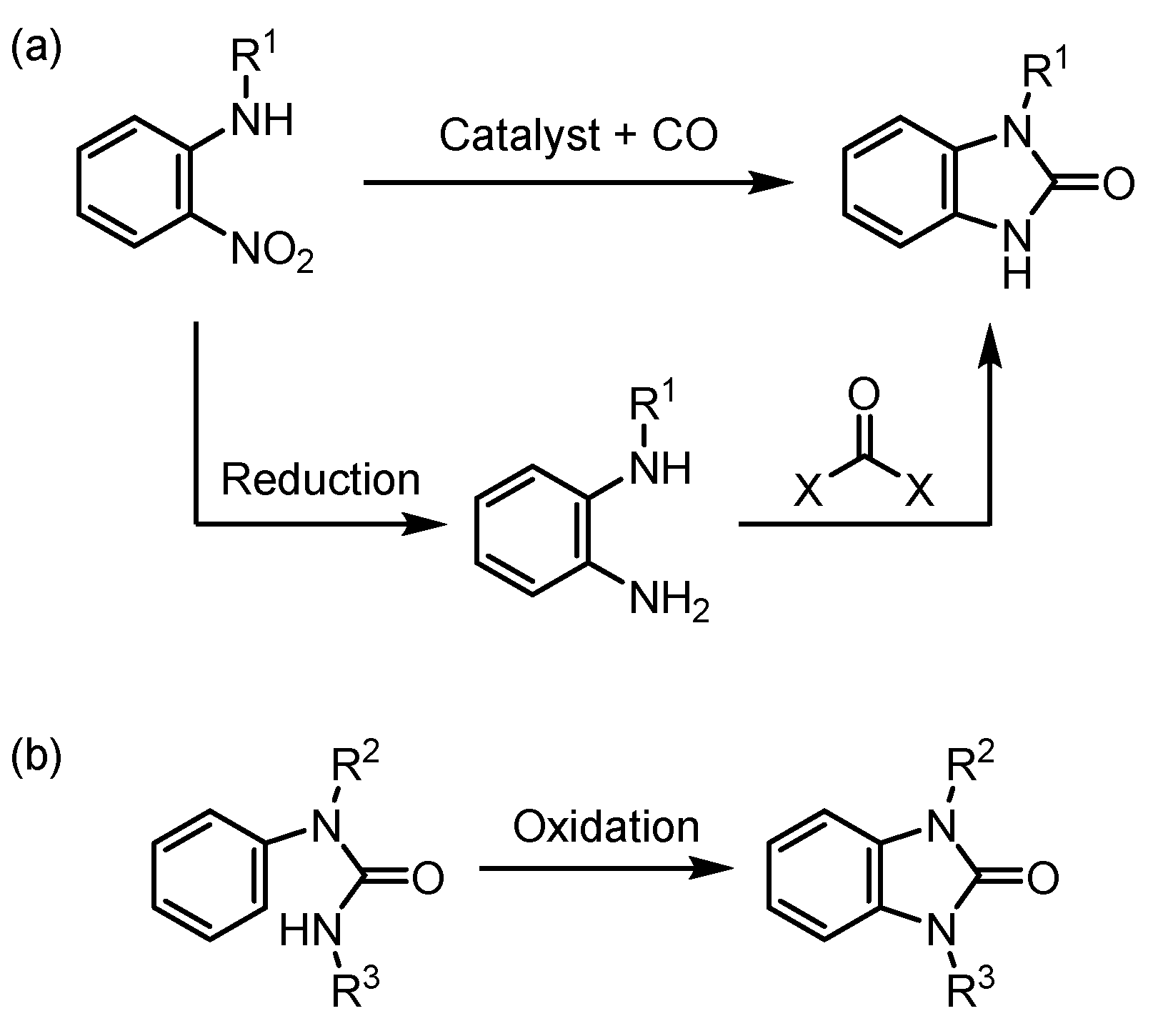
1. Introduction
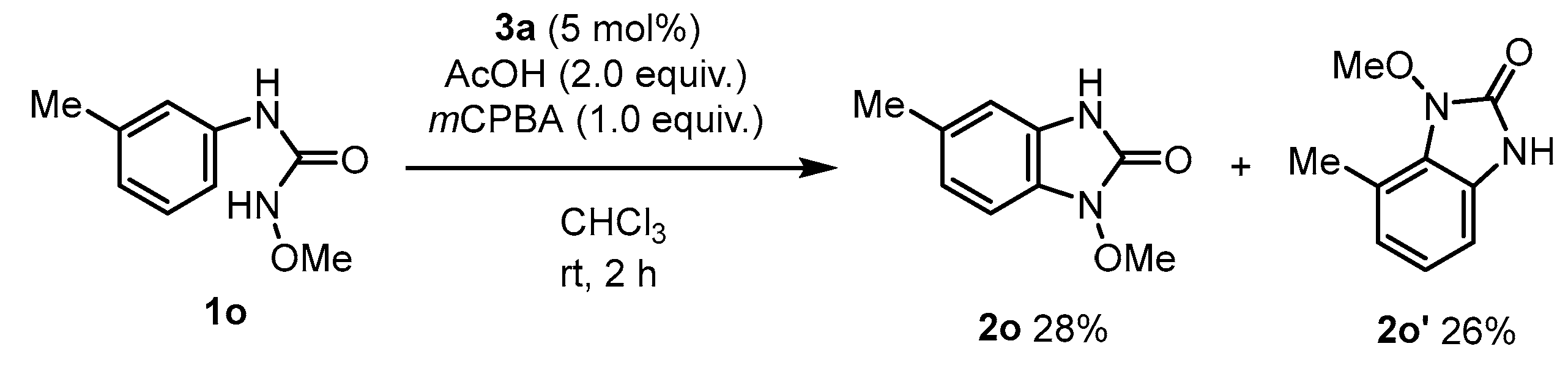
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Urea Derivatives
General Procedure for Synthesis of Urea Substrate 1
3.3. Hypervalent Iodine Catalysis
3.3.1. General Procedure for Cyclization of Aryl Urea-1 Using Oxygen-Bridged Hypervalent Iodine Catalyst
3.3.2. 1-Methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2a
3.3.3. 6-Bromo-1-methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2b
3.3.4. 6-Chloro-1-methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2c
3.3.5. 6-Fluoro-1-methoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2d
3.3.6. 1-Methoxy-6-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2e
3.3.7. 1,6-Dimethoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2f
3.3.8. 1-Methoxy-6-(trifluoromethyl)-1,3-dihydro-2H-benzo[d]imidazol-2-one 2g
3.3.9. 1-Methoxy-4-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2h
3.3.10. 1-Methoxy-4-phenyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2i
3.3.11. 1-Methoxy-5,7-dimethyl-1,3-dihydro-2H-benzo[d]imidazol-2-one 2j
3.3.12. 3-Methoxy-1,3-dihydro-2H-naphtho[1,2-d]imidazol-2-one 2k
3.3.13. 1-(Benzyloxy)-1,3-dihydro-2H-benzo[d]imidazol-2-one 2l
3.3.14. 1-Ethoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2m
3.3.15. 1-Butoxy-1,3-dihydro-2H-benzo[d]imidazol-2-one 2n
3.3.16. 1-Methoxy-5-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one (2o) and 1-Methoxy-7-methyl-1,3-dihydro-2H-benzo[d]imidazol-2-one (2o’) (Mixture)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Devi, N.; Kumar, S.; Pandey, S.K.; Singh, V. 1(3)-Formyl-β-carbolines: Potential Aldo-X Precursors for the Synthesis of β-Carboline-Based Molecular Architectures. Asian J. Org. Chem. 2018, 7, 6–36. [Google Scholar]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [PubMed]
- Shearer, J.; Castro, J.L.; Lawson, A.D.G.; MacCoss, M.; Taylor, R.D. Rings in Clinical Trials and Drugs: Present and Future. J. Med. Chem. 2022, 65, 8699–8712. [Google Scholar] [PubMed]
- Zakharychev, V.V.; Martsynkevich, A.M. Development of novel pyridine-based agrochemicals: A review. Adv. Agrochem. 2025, 4, 30–48. [Google Scholar]
- Bellotti, P.; Koy, M.; Hopkinson, M.N.; Glorius, F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021, 5, 711–725. [Google Scholar]
- Awouters, F.; Niemegeers, C.J.E.; Van den Berk, J.; Van Nueten, J.M.; Lenaerts, F.M.; Borgers, M.; Schellekens, K.H.L.; Broeckaert, A.; De Cree, J.; Janssen, P.A.J. Oxatomide, a new orally active drug which inhibits both the release and the effects of allergic mediators. Experientia 1977, 33, 1657–1659. [Google Scholar]
- Kasanami, Y.; Ishikawa, C.; Kino, T.; Chonan, M.; Toyooka, N.; Takashima, Y.; Iba, Y.; Sekiguchi, F.; Tsubota, M.; Ohkubo, T.; et al. Discovery of pimozide derivatives as novel T-type calcium channel inhibitors with little binding affinity to dopamine D2 receptors for treatment of somatic and visceral pain. Eur. J. Med. Chem. 2022, 243, 114716. [Google Scholar]
- Perry, C.M. Azilsartan Medoxomil A Review of its Use in Hypertension. Clin. Drug Investig. 2012, 32, 621–639. [Google Scholar]
- Saïdani, N.; Botté, C.Y.; Deligny, M.; Bonneaud, A.-L.; Reader, J.; Lasselin, R.; Merer, G.; Niepceron, A.; Brossier, F.; Cintrat, J.-C.; et al. Discovery of Compounds Blocking the Proliferation of Toxoplasma gondii and Plasmodium falciparum in a Chemical Space Based on Piperidinyl-Benzimidazolone Analogs. Antimicrob. Agents Chemother. 2014, 58, 2586–2597. [Google Scholar]
- Wu, Q.; Chen, J.; Liu, Z.; Xu, Y. CO Activation Using Nitrogen-Doped Carbon Nanotubes for Reductive Carbonylation of Nitroaromatics to Benzimidazolinone and Phenyl Urea. ACS Appl. Mater. Interfaces 2020, 12, 48700–48711. [Google Scholar]
- Wang, X.; Ling, G.; Xue, Y.; Lu, S. Selenium-Catalyzed Reductive Carbonylation of 2-Nitrophenols to 2-Benzoxazolones. Eur. J. Org. Chem. 2005, 2005, 1675–1679. [Google Scholar]
- Qi, X.; Zhou, R.; Peng, J.-B.; Ying, J.; Wu, X.-F. Selenium-Catalyzed Carbonylative Synthesis of 2-Benzimidazolones from 2-Nitroanilines with TFBen as the CO Source. Eur. J. Org. Chem. 2019, 2019, 5161–5164. [Google Scholar]
- Tsuji, Y.; Takeuchi, R.; Watanabe, Y. Platinum complex catalyzed synthesis of urea derivatives from nitroarenes and amines under carbon monoxide. J. Organomet. Chem. 1985, 290, 249. [Google Scholar]
- English, J.P.; Clapp, R.C.; Cole, Q.P.; Halverstadt, I.F.; Lampen, J.O.; Roblin, R.O., Jr. Studies in Chemotherapy. IX. Ureylenebenzene and Cyclohexane Derivatives as Biotin Antagonist. J. Am. Chem. Soc. 1945, 67, 295–302. [Google Scholar]
- Purandare, A.V.; Gao, A.; Poss, M.A. Solid-phase synthesis of ‘diverse’ heterocycles. Tetrahedron Lett. 2002, 43, 3903–3906. [Google Scholar]
- Acharya, A.N.; Ostresh, J.M.; Houghten, R.A. Solid-phase parallel synthesis of substituted dihydroimidazolyl dihydrobenzimidazol-2-ones. Tetrahedron 2002, 58, 2095–2100. [Google Scholar]
- Nale, D.B.; Bhanage, B.M. Copper-catalyzed efficient synthesis of a 2-benzimidazolone scaffold from 2-nitroaniline and dimethyl carbonate via a hydrosilylation reaction. Green Chem. 2015, 17, 2480. [Google Scholar]
- Cooley, J.H.; Jacobs, P.T. Oxidative Ring Closure of 1-Benzyloxy-3-arylureas to 1-Benzyloxybenzimidazolones. J. Org. Chem. 1975, 40, 552–557. [Google Scholar]
- Dohi, T.; Maruyama, A.; Minamitsuji, Y.; Takenaga, N.; Kita, Y. First hypervalent iodine(III)-catalyzed C–N bond forming reaction: Catalytic spirocyclization of amides to N-fused spirolactams. Chem. Commun. 2007, 12, 1224–1226. [Google Scholar]
- Dohi, T.; Takenaga, N.; Fukushima, K.; Uchiyama, T.; Kato, D.; Shiro, M.; Fujioka, H.; Kita, Y. Designer μ-oxo-bridged hypervalent iodine(III) organocatalysts for greener oxidations. Chem. Commun. 2010, 46, 7697–7699. [Google Scholar]
- Dohi, T.; Sasa, H.; Dochi, M.; Yasui, C.; Kita, Y. Oxidative Coupling of N-Methoxyamides and Related Compounds toward Aromatic Hydrocarbons by Designer μ-Oxo Hypervalent Iodine Catalyst. Synthesis 2019, 51, 1185–1195. [Google Scholar] [CrossRef]
- Sasa, H.; Mori, K.; Kikushima, K.; Kita, Y.; Dohi, T. μ-Oxo-Hypervalent-Iodine-Catalyzed Oxidative C-H Amination for Synthesis of Benzolactam Derivatives. Chem. Pharm. Bull. 2022, 70, 106–110. [Google Scholar] [CrossRef]
- Sasa, H.; Hamatani, S.; Hirashima, M.; Takenaga, N.; Hanasaki, T.; Dohi, T. Efficient Metal-Free Oxidative C–H Amination for Accessing Dibenzoxazepinones via μ-Oxo Hypervalent Iodine Catalysis. Chemistry 2023, 5, 2155–2165. [Google Scholar] [CrossRef]
- Miyamoto, N.; Kikushima, K.; Sasa, H.; Katagiri, T.; Takenaga, N.; Kita, Y.; Dohi, T. Transition-metal-free dibenzoxazepinone synthesis by hypervalent iodine-mediated chemoselective arylocyclizations of N-functionalized salicylamides. Chem. Commun. 2025, 61, 1882–1885. [Google Scholar] [CrossRef]
- Zheng, H.; Sang, Y.; Houk, K.N.; Xue, X.-S.; Cheng, J.-P. Mechanism and Origins of Enantioselectivities in Spirobiindane-Based Hypervalent Iodine(III)-Induced Asymmetric Dearomatizing Spirolactonizations. J. Am. Chem. Soc. 2019, 141, 16046–16056. [Google Scholar] [CrossRef]
- Wang, Q.; An, J.; Alper, H.; Xiao, W.-J.; Beauchemin, A.M. Catalytic substitution/cyclization sequences of O-substituted Isocyanates: Synthesis of 1-alkoxybenzimidazolones and 1-alkoxy-3,4-dihydroquinazolin-2(1H)-ones. Chem. Commun. 2017, 53, 13055–13058. [Google Scholar] [CrossRef]





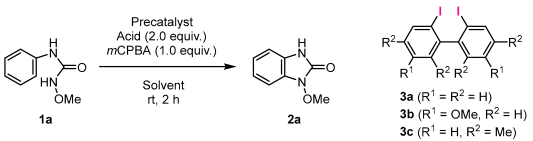 | ||||
|---|---|---|---|---|
| Entry | Precatalyst | Acid | Solvent | Isolated Yield of 2a |
| 1 | 3a (5 mol%) | CF3COOH | HFIP | Complex mixture |
| 2 | 3a (5 mol%) | CF3COOH | CH2Cl2 | Complex mixture |
| 3 | 3a (5 mol%) | CF3COOH | CHCl3 | Complex mixture |
| 4 | 3a (5 mol%) | AcOH | HFIP | Complex mixture |
| 5 | 3a (5 mol%) | AcOH | CH2Cl2 | 51% |
| 6 | 3a (5 mol%) | AcOH | CHCl3 | 67% |
| 7 b | 3a (5 mol%) | AcOH | CHCl3 | 42% |
| 8 | 3b (5 mol%) | AcOH | CHCl3 | 72% |
| 9 | 3c (5 mol%) | AcOH | CHCl3 | 72% |
| 10 | PhI (10 mol%) | AcOH | CHCl3 | 55% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirashima, M.; Hamatani, S.; Sasa, H.; Takenaga, N.; Hanasaki, T.; Dohi, T. Metal-Free Synthesis of Benzimidazolinones via Oxidative Cyclization Under Hypervalent Iodine Catalysis. Chemistry 2025, 7, 50. https://doi.org/10.3390/chemistry7020050
Hirashima M, Hamatani S, Sasa H, Takenaga N, Hanasaki T, Dohi T. Metal-Free Synthesis of Benzimidazolinones via Oxidative Cyclization Under Hypervalent Iodine Catalysis. Chemistry. 2025; 7(2):50. https://doi.org/10.3390/chemistry7020050
Chicago/Turabian StyleHirashima, Mayu, Syotaro Hamatani, Hirotaka Sasa, Naoko Takenaga, Tomonori Hanasaki, and Toshifumi Dohi. 2025. "Metal-Free Synthesis of Benzimidazolinones via Oxidative Cyclization Under Hypervalent Iodine Catalysis" Chemistry 7, no. 2: 50. https://doi.org/10.3390/chemistry7020050
APA StyleHirashima, M., Hamatani, S., Sasa, H., Takenaga, N., Hanasaki, T., & Dohi, T. (2025). Metal-Free Synthesis of Benzimidazolinones via Oxidative Cyclization Under Hypervalent Iodine Catalysis. Chemistry, 7(2), 50. https://doi.org/10.3390/chemistry7020050







