2,4,6-Trichlorophenyl-Substituted [3]Triangulene with Enhanced Stability
Abstract
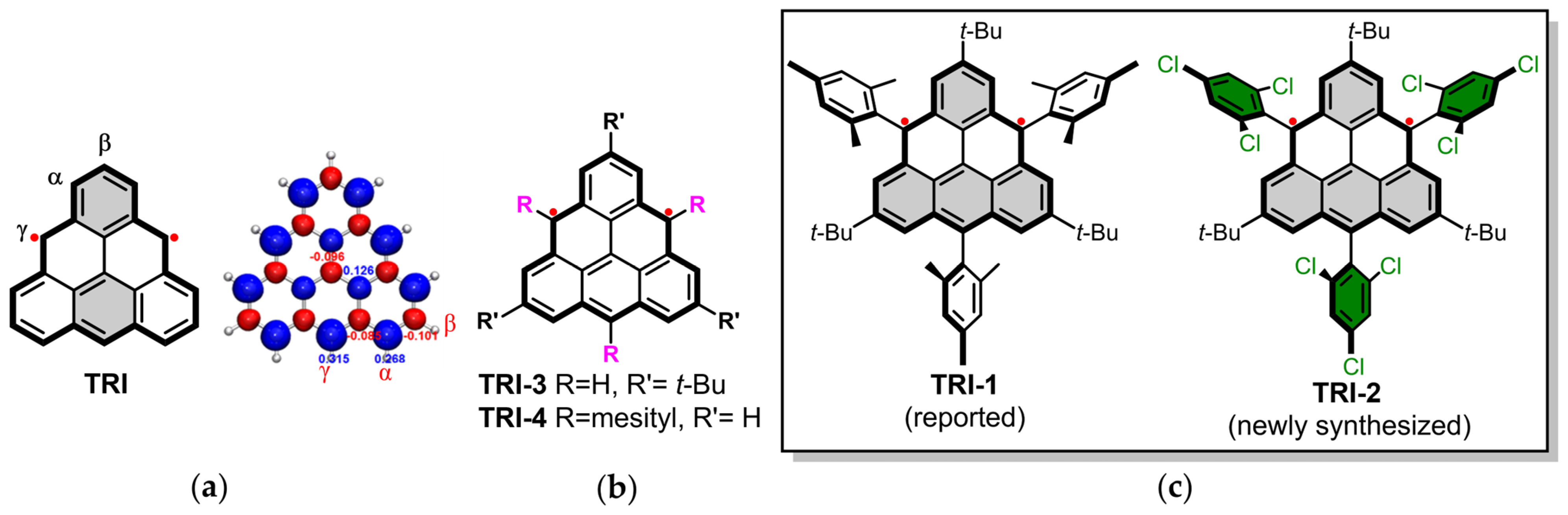
1. Introduction
2. Materials and Methods
3. Results
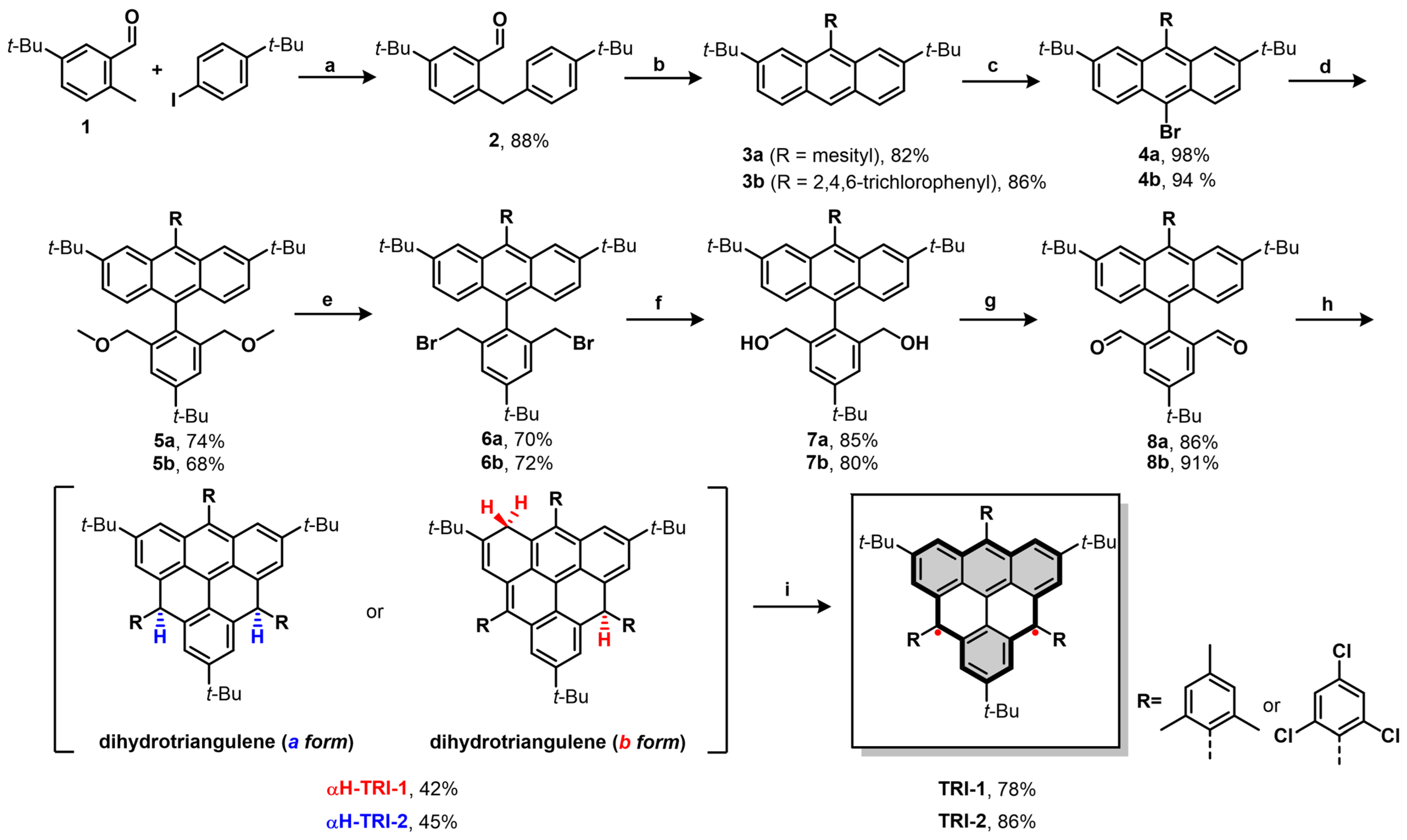
3.1. Design and Synthesis
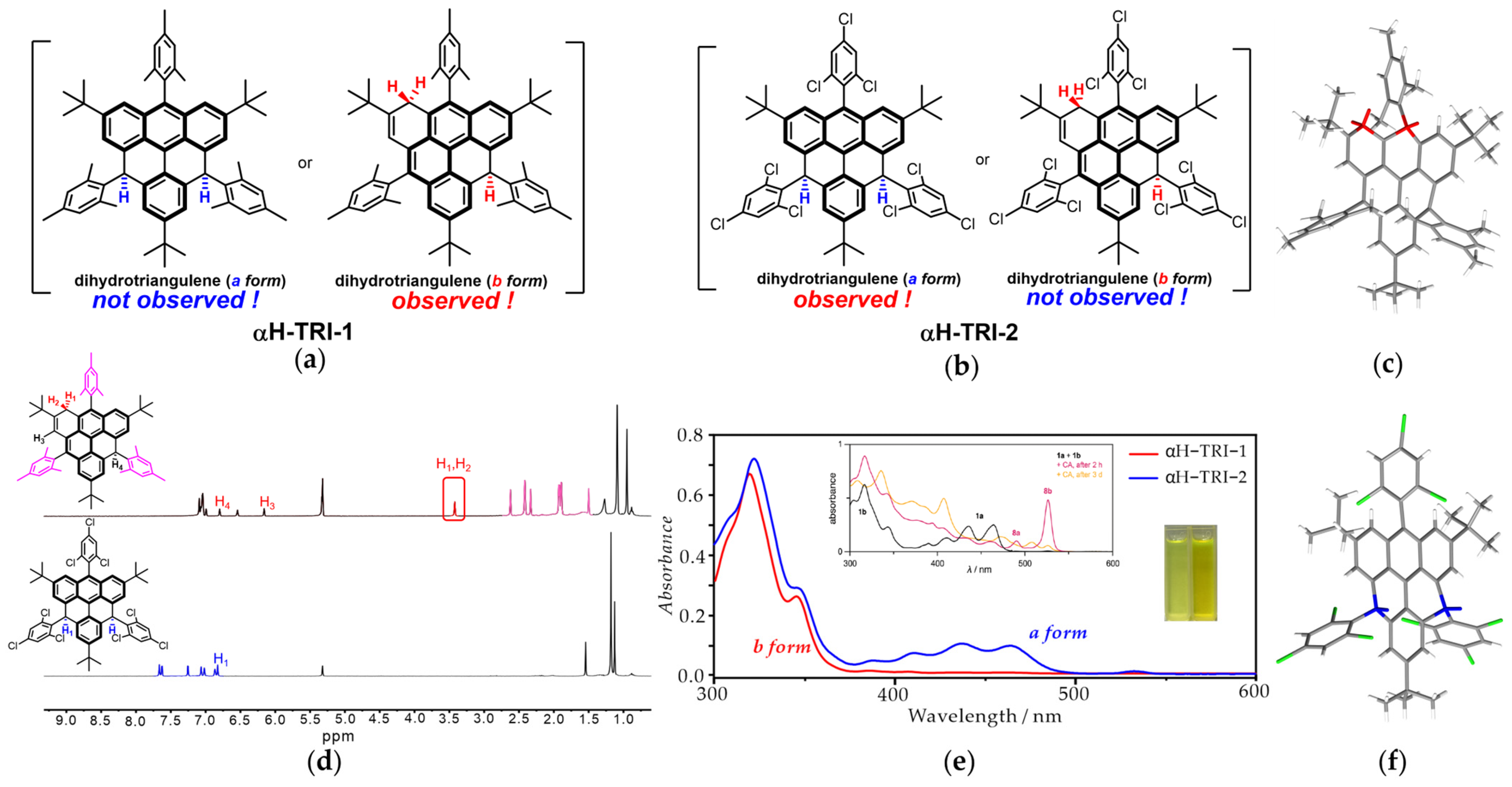
3.2. Structural Characterization of Two Dihydro-Triangulene Isomers
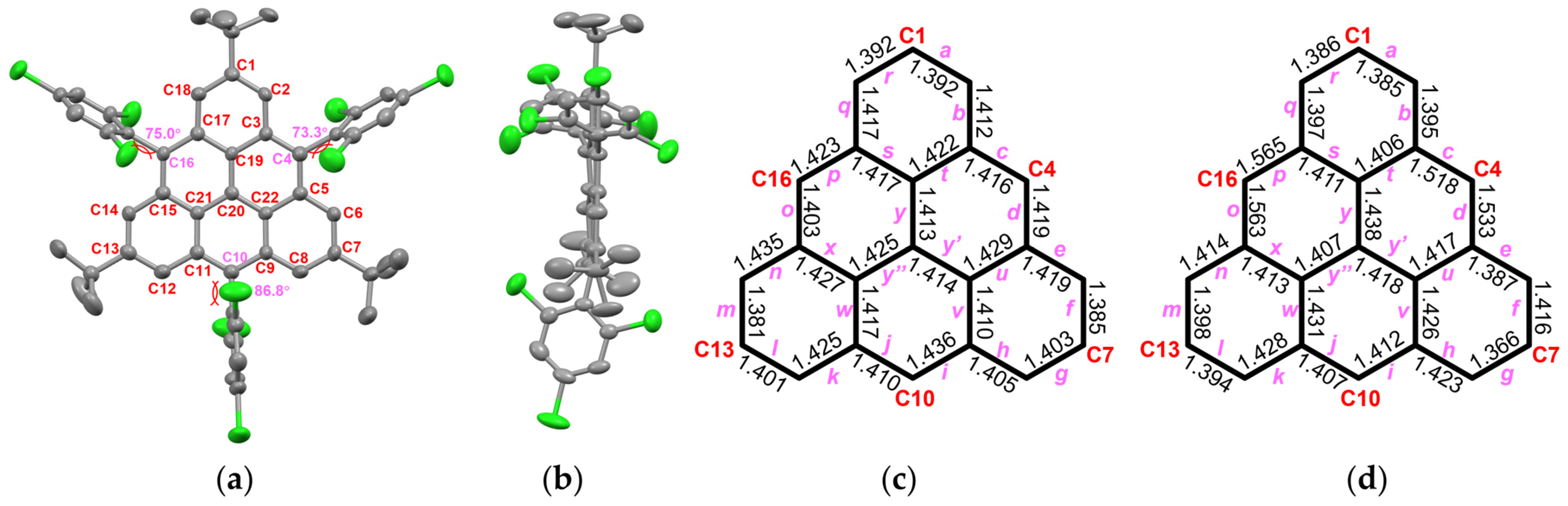
3.3. Optical and Structural Characterization of TRI-1 and TRI-2
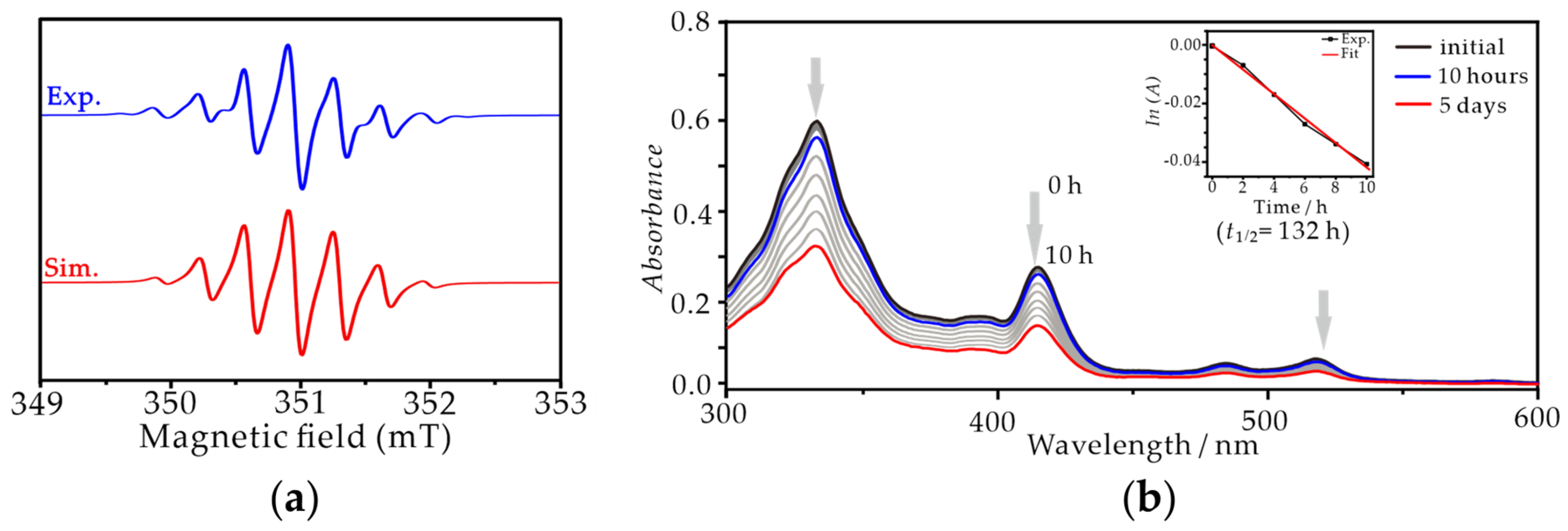
3.4. EPR and Stability Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morita, Y.; Suzuki, S.; Sato, K.; Takui, T. Synthetic Organic Spin Chemistry for Structurally Well-Defined Open-Shell Graphene Fragments. Nat. Chem. 2011, 3, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Wu, J. Open-Shell Graphene Fragments. Chem 2021, 7, 358–386. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X. Recent Advances in Open-Shell Graphene Fragments. Angew. Chem. Int. Ed. 2020, 59, 23386–23401. [Google Scholar] [CrossRef]
- Morita, Y.; Nishida, S. Phenalenyls, Cyclopentadienyls, and Other Carbon-Centered Radicals. In Stable Radicals; Hicks, R.G., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2010; Volume 3, pp. 81–145. [Google Scholar]
- Han, W.; Kawakami, R.K.; Gmitra, M. Graphene Spintronics. Nat. Nanotechnol. 2014, 9, 794–807. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S. Delocalized Magnetism in Low-Dimensional Graphene System. About Acta Phys. Sin. 2022, 71, 188101. [Google Scholar] [CrossRef]
- Yazyev, O.V. Emergence of Magnetism in Graphene Materials and Nanostructures. Rep. Prog. Phys. 2010, 73, 056501. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fukui, K.; Enoki, T.; Kusakabe, K.; Kaburagi, Y. Observation of Zigzag and Armchair Edges of Graphite Using Scanning Tunneling Microscopy and Spectroscopy. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 193406. [Google Scholar] [CrossRef]
- Philpott, M.R.; Cimpoesu, F.; Kawazoe, Y. Geometry, Bonding and Magnetism in Planar Triangulene Graphene Molecules with D3h Symmetry: Zigzag Cm**2+4m+1H3m+3 (m = 2,⋯, 15). Chem. Phys. 2008, 354, 1–15. [Google Scholar] [CrossRef]
- Bearpark, M.J.; Robb, M.A.; Bernardi, F.; Olivucci, M. Molecular Mechanics Valence Bond Methods for Large Active Spaces. Application to Conjugated Polycyclic Hydrocarbons. Chem. Phys. Lett. 1994, 217, 513–519. [Google Scholar] [CrossRef]
- Das, A.; Müller, T.; Plasser, F.; Lischka, H. Polyradical Character of Triangular Non-Kekulé Structures, Zethrenes, p-Quinodimethane-Linked Bisphenalenyl, and the Clar Goblet in Comparison: An Extended Multireference Study. J. Phys. Chem. A 2016, 120, 1625–1636. [Google Scholar] [CrossRef]
- Pavliček, N.; Mistry, A.; Majzik, Z.; Moll, N.; Meyer, G.; Fox, D.J.; Gross, L. Synthesis and Characterization of Triangulene. Nat. Nanotechnol. 2017, 12, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Narayan, S.; Dabhi, S.D.; Jha, P.K. Tailoring the Electronic and Magnetic Properties of Peculiar Triplet Ground State Polybenzoid “Triangulene”. ChemistrySelect 2018, 3, 2390–2397. [Google Scholar] [CrossRef]
- Lang, J.; Brabec, J.; Saitow, M.; Pittner, J.; Neese, F.; Demel, O. Perturbative Triples Correction to Domain-Based Local Pair Natural Orbital Variants of Mukherjee’s State Specific Coupled Cluster Method. Phys. Chem. Chem. Phys. 2019, 21, 5022–5038. [Google Scholar] [CrossRef]
- Melle-Franco, M. When 1+1 is Odd. Nat. Nanotechnol. 2017, 12, 292–293. [Google Scholar] [CrossRef]
- Ghising, P.; Biswas, C.; Lee, Y.-H. Graphene Spin Valves for Spin Logic Devices. Adv. Mater. 2023, 35, 2209137. [Google Scholar] [CrossRef]
- Song, S.; Su, J.; Telychko, M.; Li, J.; Li, G.; Li, Y.; Su, C.; Wu, J.; Lu, J. On-Surface Synthesis of Graphene Nanostructures with π-Magnetism. Chem. Soc. Rev. 2021, 50, 3238–3262. [Google Scholar] [CrossRef]
- Turco, E.; Mishra, S.; Melidonie, J.; Eimre, K.; Obermann, S.; Pignedoli, C.A.; Fasel, R.; Feng, X.; Ruffieux, P. On-Surface Synthesis and Characterization of Super-nonazethrene. J. Phys. Chem. Lett. 2021, 12, 8314–8319. [Google Scholar] [CrossRef]
- Sil, S.; Santha Bhaskaran, A.; Chakraborty, S.; Singh, B.; Kuniyil, R.; Mandal, S.K. Reduced-Phenalenyl-Based Molecule as a Super Electron Donor for Radical-Mediated C–N Coupling Catalysis at Room Temperature. J. Am. Chem. Soc. 2022, 144, 22611–22621. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, L.; Shi, X. Chalcogen Effect of Atom Substitution on the Properties of Tris(2,4,6-trichlorophenyl)methyl(TTM) Radical. Chem. Res. Chin. Univ. 2023, 39, 197–201. [Google Scholar] [CrossRef]
- Clar, E.; Stewart, D.G. Aromatic Hydrocarbons. LXV. Triangulene Derivatives. J. Am. Chem. Soc. 1953, 75, 2667–2672. [Google Scholar] [CrossRef]
- Clar, E.; Stewart, D.G. Aromatic Hydrocarbons. LXVIII. Triangulene Derivatives. J. Am. Chem. Soc. 1954, 76, 3504–3507. [Google Scholar] [CrossRef]
- Valenta, L.; Juríček, M. The Taming of Clar’s Hydrocarbon. Chem. Commun. 2022, 58, 10896–10906. [Google Scholar] [CrossRef] [PubMed]
- Hara, O.; Tanaka, K.; Yamamoto, K.; Nakazawa, T.; Murata, I. The Chemistry of Phenalenium Systems. XXV the Triangulenyl Dianion. Tetrahedron Lett. 1977, 18, 2435–2436. [Google Scholar] [CrossRef]
- Holt, C.J.; Wentworth, K.J.; Johnson, R.P. A Short and Efficient Synthesis of the [3]Triangulene Ring System. Angew. Chem. Int. Ed. 2019, 58, 15793–15796. [Google Scholar] [CrossRef]
- Ribar, P.; Sǒolomek, T.; Jurícek, M. Gram-Scale Synthesis and Supramolecular Complex of Precursors of Clar’s Hydrocarbon Triangulene. Org. Lett. 2019, 21, 7124–7128. [Google Scholar] [CrossRef]
- Allinson, G.; Bushby, R.J.; Paillaud, J.L.; Oduwole, D.; Sales, K. ESR Spectrum of a Stable Triplet π Biradical: Trioxytriangulene. J. Am. Chem. Soc. 1993, 115, 2062–2064. [Google Scholar] [CrossRef]
- Allinson, G.; Bushby, R.J.; Paillaud, J.-L.; Thornton-Pett, M. Synthesis of a Derivative of Triangulene; the First Non-Kekulé Polynuclear Aromatic. J. Chem. Soc. Perkin Trans. 1995, 1, 385–390. [Google Scholar] [CrossRef]
- Allinson, G.; Bushby, R.J.; Jesudason, M.V.; Paillaud, J.-L.; Taylor, N. The Synthesis of Singlet Ground State Derivatives of Non-Kekulé Polynuclear Aromatics. J. Chem. Soc. Perkin Trans. 1997, 2, 147–156. [Google Scholar] [CrossRef]
- Su, J.; Telychko, M.; Song, S.; Lu, J. Triangulenes: From Precursor Design to On-Surface Synthesis and Characterization. Angew. Chem. Int. Ed. 2020, 59, 7658–7668. [Google Scholar] [CrossRef]
- Liu, P.; Wu, M.X.; Yu, M.; Kang, H.; Huang, B.; Yang, H.-B.; Zhao, X.-L.; Shi, X. Synthesis of Polycyclic Aromatic Compounds by Electrocyclization–Dehydrogenation of Diradicaloids. Org. Lett. 2024, 26, 7914–7919. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.; Wu, M.X.; Kang, H.; Zhao, X.L.; Xu, L.; Liu, L.; Li, X.; Fang, J.; Fang, Z.; et al. Double [5]carbohelicene: Facile synthesis, chiroptical properties, isomerization study, and lasing application. Sci. China Chem. 2025, 68, 233–240. [Google Scholar] [CrossRef]
- Shen, T.; Dijkstra, D.; Farrando-Pérez, A.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Zou, Y.; Hou, X.; Wei, H.; Li, Z.; et al. Fused Triangulene Dimers: Facile Synthesis by Intramolecular Radical-Radical Coupling and Application for Near-Infrared Lasers. Angew. Chem. Int. Ed. 2023, 62, e202304197. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Boto, R.A.; García-Martínez, N.; Sancho-García, J.C.; Melle-Franco, M.; Fernández-Rossier, J. Exchange Rules for Diradical π-Conjugated Hydrocarbons. Nano Lett. 2019, 19, 5991–5997. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Xue, Z.; Li, C.; Liu, Y.; Xiang, L.; Ke, Y.; Yan, K.; Wang, S.; Ping, Y. On-Surface Synthesis of Triangulene Trimers via Dehydration Reaction. Nat. Commun. 2022, 13, 1705. [Google Scholar] [CrossRef]
- Inoue, J.; Fukui, K.; Kubo, T.; Nakazawa, S.; Sato, K.; Shiomi, D.; Morita, Y.; Yamamoto, K.; Takui, T.; Nakasuji, K. The First Detection of a Clar’s Hydrocarbon, 2,6,10-Tri-tert-butyltriangulene: A Ground-State Triplet of Non-Kekulé Polynuclear Benzenoid Hydrocarbon. J. Am. Chem. Soc. 2001, 123, 12702–12703. [Google Scholar] [CrossRef]
- Valenta, L.; Mayländer, M.; Kappeler, P.; Blacque, O.; Sǒlomek, T.; Richert, S.; Juríček, M. Tri-mesityltriangulene: A Persistent Derivative of Clar’s Hydrocarbon. Chem. Commun. 2022, 58, 3019–3022. [Google Scholar] [CrossRef]
- Arikawa, S.; Shimizu, A.; Shiomi, D.; Sato, K.; Shintani, R. Synthesis and Isolation of a Kinetically Stabilized Crystalline Triangulene. J. Am. Chem. Soc. 2021, 143, 19599–19605. [Google Scholar] [CrossRef]
- Zhang, F.; Hong, K.; Li, T.; Park, H.; Yu, J. Functionalization of C(sp3)–H Bonds Using a Transient Directing Group. Science 2016, 351, 252–256. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, X.; Gopalakrishna, T.Y.; Phan, H.; Wu, J. Graphene-like Molecules with Four Zigzag Edges. Angew. Chem. Int. Ed. 2018, 57, 6541–6545. [Google Scholar] [CrossRef]
- Turco, E.; Bernhardt, A.; Krane, N.; Valenta, L.; Fasel, R.; Juríček, M.; Ruffieux, P. Observation of the magnetic ground state ofthe two smallest triangular nanographenes. JACS Au 2023, 3, 1358–1364. [Google Scholar] [CrossRef]
- Yu, H.; Heine, T. Magnetic Coupling Control in Triangulene Dimers. J. Am. Chem. Soc. 2023, 145, 19303–19311. [Google Scholar] [CrossRef] [PubMed]
- Mallory, F.B.; Butler, K.E.; Bérubé, A.; Luzik, E.D.; Mallory, C.W.; Brondyke, E.J.; Hiremath, R.; Ngo, P.; Carroll, P.J. Phenacenes: A family of graphite ribbons. Part 3: Iterative strategies for the synthesis of large phenacenes. Tetrahedron 2001, 57, 3715–3724. [Google Scholar] [CrossRef]
- Umemoto, T.; Singh, R.P.; Xu, Y.; Saito, N. Discovery of 4-tert-Butyl-2,6-dimethylphenylsulfur Trifluoride as a Deoxofluorinating Agent with High Thermal Stability as Well as Unusual Resistance to Aqueous Hydrolysis, and Its Diverse Fluorination Capabilities Including Deoxofluoro-Arylsulfinylation with High Stereoselectivity. J. Am. Chem. Soc. 2010, 132, 18199–18205. [Google Scholar] [PubMed]
- Tian, Y.; Uchida, K.; Kurata, H.; Hirao, Y.; Nishiuchi, T.; Kubo, T. Design and Synthesis of New Stable Fluorenyl-Based Radicals. J. Am. Chem. Soc. 2014, 136, 12784–12793. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, P.; Zhao, X.; Shi, X. 2,4,6-Trichlorophenyl-Substituted [3]Triangulene with Enhanced Stability. Chemistry 2025, 7, 39. https://doi.org/10.3390/chemistry7020039
Yang Y, Liu P, Zhao X, Shi X. 2,4,6-Trichlorophenyl-Substituted [3]Triangulene with Enhanced Stability. Chemistry. 2025; 7(2):39. https://doi.org/10.3390/chemistry7020039
Chicago/Turabian StyleYang, Yiming, Peipei Liu, Xiaoli Zhao, and Xueliang Shi. 2025. "2,4,6-Trichlorophenyl-Substituted [3]Triangulene with Enhanced Stability" Chemistry 7, no. 2: 39. https://doi.org/10.3390/chemistry7020039
APA StyleYang, Y., Liu, P., Zhao, X., & Shi, X. (2025). 2,4,6-Trichlorophenyl-Substituted [3]Triangulene with Enhanced Stability. Chemistry, 7(2), 39. https://doi.org/10.3390/chemistry7020039






