Addition of a Perfluoroalkyl Acetyl Group to the C-Vertex of a Carborane Anion to Enhance Its Solubility in Fluorinated Ether Solvents
Abstract
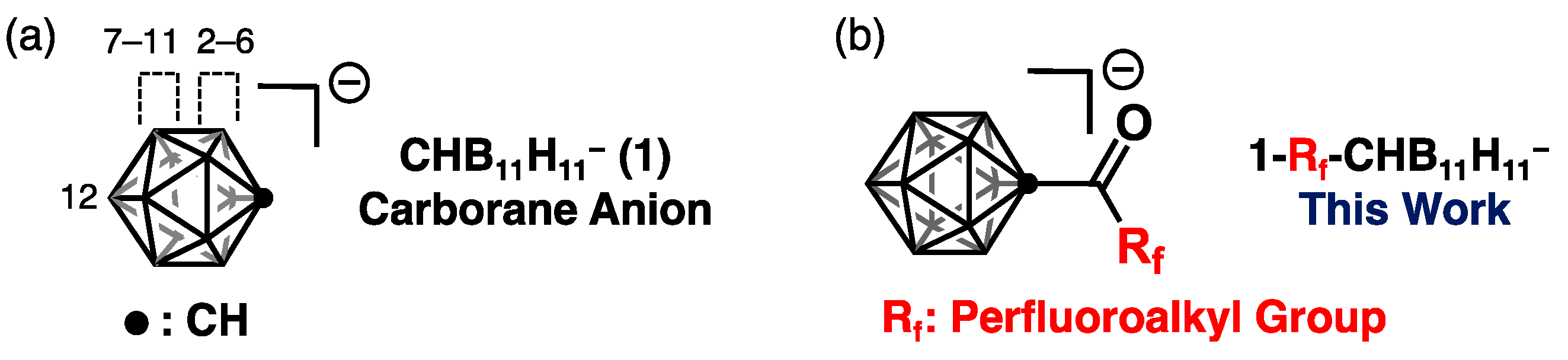
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Materials
2.3. Experimental Procedures
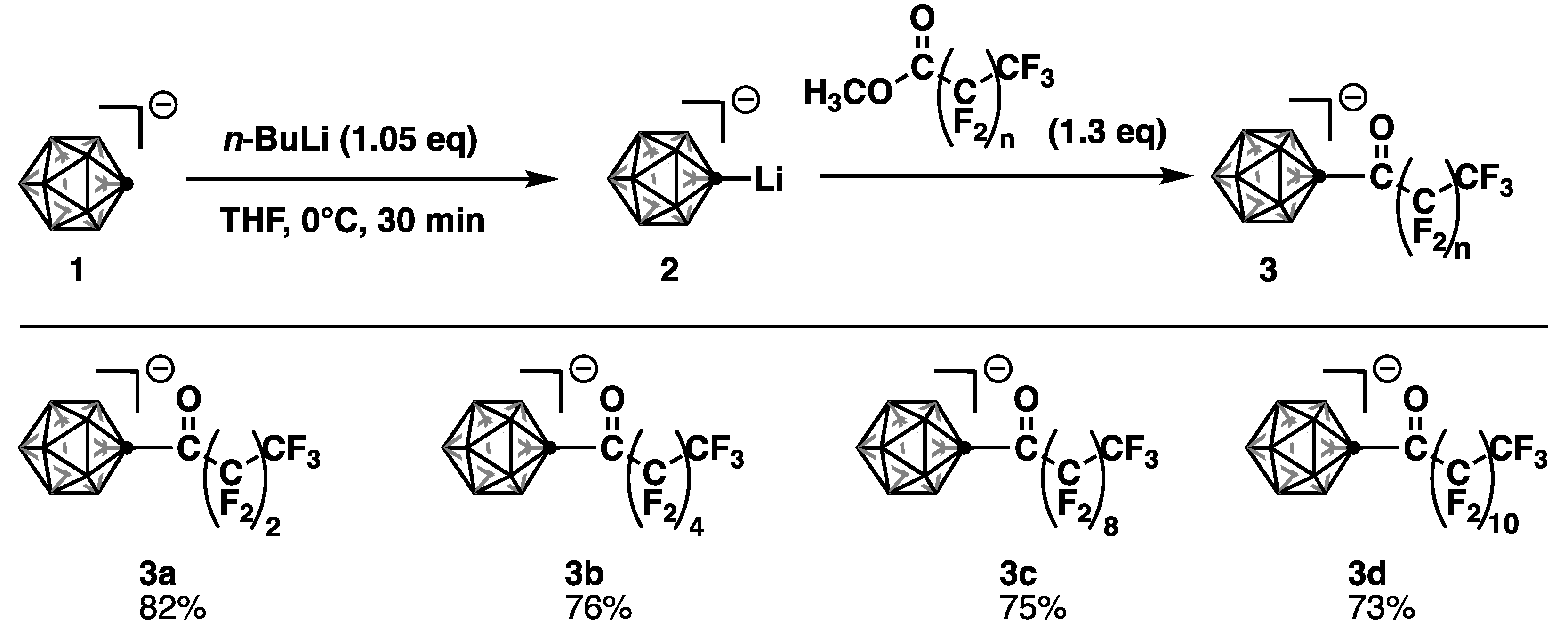
2.3.1. General Procedure for the Perfluoroalkyl Acylation of C-Vertex of Carborane Anion
Compound: [Cs+][1-(CO(CF2)2CF3)–CB11H11‒] (Cs·3a)
Compound: [Cs+][1-(CO(CF2)4CF3)–CB11H11‒] (Cs·3b)
Compound: [Cs+][1-(CO(CF2)8CF3)–CB11H11‒] (Cs·3c)
Compound: [Cs+][1-(CO(CF2)10CF3)–CB11H11‒] (Cs·3d)
Compound: [Cs+][1-(CO(CH2)4CH3)–CB11H11‒] (Cs·3e)
2.3.2. Synthesis of Tri-n-octylammonium Salt of [1-(CO(CF2)2CF3)–CB11H11‒] (3b)
Compound: [Ag+][1-(CO(CF2)4CF3)–CB11H11‒] (Ag·3b)
Compound: [HN(octyl)3+][1-(CO(CF2)4CF3)–CB11H11‒] (HN(octyl)3·3b)
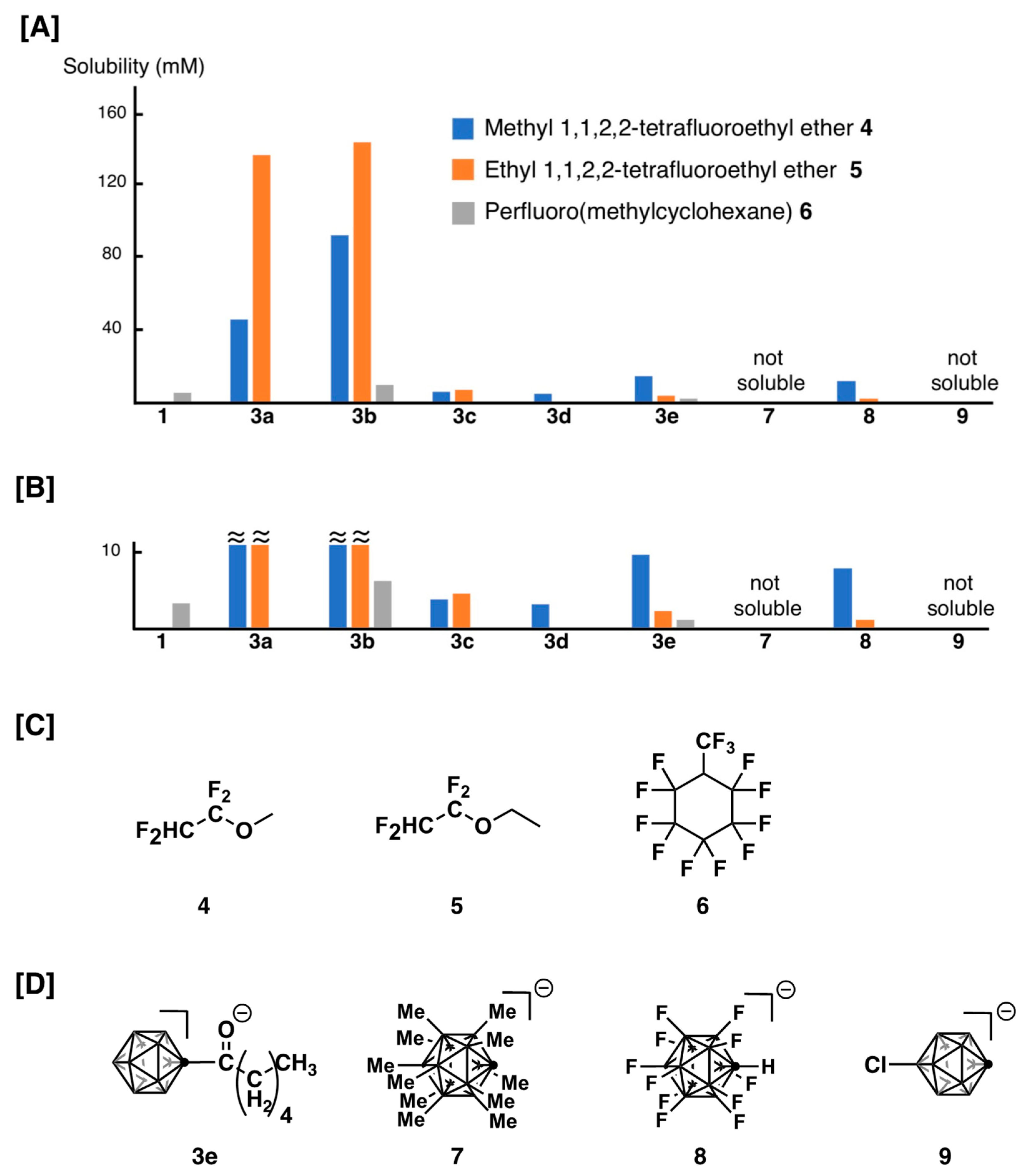
2.3.3. Solubility of Cesium Salts in Fluorinated Solvents
3. Results
3.1. Synthesis
3.2. Solubility of Cesium Salts of 3 in Fluorinated Solvents
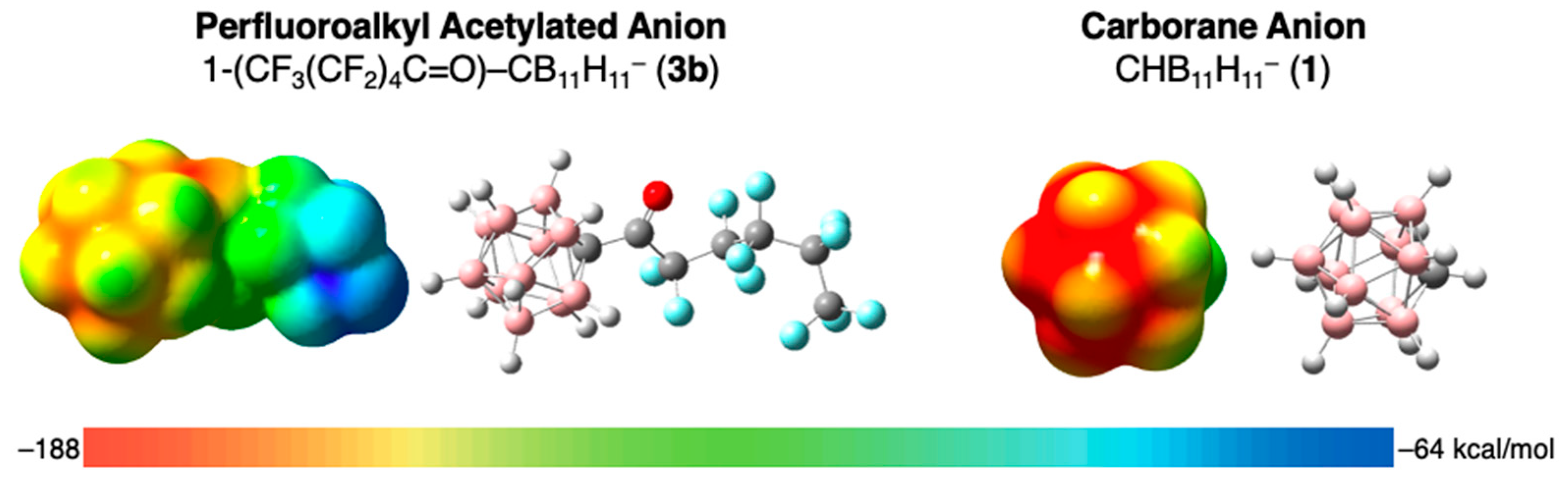
3.3. Coordinating Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Douvris, C.; Michl, J. Update 1 of: Chemistry of the Carba-closo-dodecaborate (−) Anion, CB11H12‒. Chem. Rev. 2013, 113, PR179–PR233. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.P.; Tomich, A.W.; Guo, J.; Lavallo, V. Teaching an Old Dog New Tricks: New Directions in Fundamental and Applied closo-Carborane Anion Chemistry. Chem. Commun. 2019, 55, 1684–1701. [Google Scholar] [CrossRef]
- Fisher, S.P.; Tomich, A.W.; Lovera, S.O.; Kleinsasser, J.F.; Guo, J.; Asay, M.J.; Nelson, H.M.; Lavallo, V. Nonclassical Applications of closo-Carborane Anions: From Main Group Chemistry and Catalysis to Energy Storage. Chem. Rev. 2019, 119, 8262–8290. [Google Scholar] [CrossRef]
- Kanazawa, J.; Kitazawa, Y.; Uchiyama, M. Recent Progress in the Synthesis of the Monocarba-closo-dodecaborate (−) Anions. Chem. Eur. J. 2019, 25, 9123–9132. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.; Duttwyler, S.; Lin, F.; Zhang, Y. Chemistry of Three-Dimensional Icosahedral Boron Clusters Anions: Closo-Dodecaborate (2-) [B12H12]2‒ and Carba-closo-Dodecaborate (-) [CB11H12]-. Coord. Chem. Rev. 2024, 516, 215974. [Google Scholar] [CrossRef]
- Jelinek, T.; Baldwin, P.; Scheidt, W.R.; Reed, C.A. New Weakly Coordinating Anions. 2. Derivatization of the Carborane Anion CB11H12−. Inorg. Chem. 1993, 32, 1982–1990. [Google Scholar] [CrossRef]
- Reed, C.A. Carboranes: A New Class of Weakly Coordinating Anions for Strong Electrophiles, Oxidants, and Superacids. Acc. Chem. Res. 1998, 31, 133–139. [Google Scholar] [CrossRef]
- Krossing, I.; Raabe, I. Noncoordinating Anions—Fact or Fiction? A Survey of Likely Candidates. Angew. Chem. Int. Ed. 2004, 43, 2066–2090. [Google Scholar] [CrossRef]
- Engesser, T.A.; Lichtenthaler, M.R.; Schleep, M.; Krossing, I. Reactive P-Block Cations Stabilized by Weakly Coordinating Anions. Chem. Soc. Rev. 2016, 45, 789–899. [Google Scholar] [CrossRef]
- Riddlestone, I.M.; Kraft, A.; Schaefer, J.; Krossing, I. Taming the Cationic Beast: Novel Developments in the Synthesis and Application of Weakly Coordinating Anions. Angew. Chem. Int. Ed. 2018, 57, 13982–14024. [Google Scholar] [CrossRef]
- Carter, T.J.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Zhang, R.; Shirai, S.; Kampf, J.W. Boron Clusters as Highly Stable Magnesium-Battery Electrolytes. Angew. Chem. Int. Ed. 2014, 53, 3173–3177. [Google Scholar] [CrossRef] [PubMed]
- McArthur, S.G.; Geng, L.; Guo, J.; Lavallo, V. Cation Reduction and Comproportionation as Novel Strategies to Produce High Voltage, Halide Free, Carborane Based Electrolytes for Rechargeable Mg Batteries. Inorg. Chem. Front. 2015, 2, 1101–1104. [Google Scholar] [CrossRef]
- Tutusaus, O.; Mohtadi, R.; Arthur, T.S.; Mizuno, F.; Nelson, E.G.; Sevryugina, Y.V. An Efficient Halogen-Free Electrolyte for Use in Rechargeable Magnesium Batteries. Angew. Chem. Int. Ed. 2015, 54, 7900–7904. [Google Scholar] [CrossRef] [PubMed]
- McArthur, S.G.; Jay, R.; Geng, L.; Guo, J.; Lavallo, V. Below the 12-Vertex: 10-Vertex Carborane Anions as Non-Corrosive, Halide Free, Electrolytes for Rechargeable Mg Batteries. Chem. Commun. 2017, 53, 4453–4456. [Google Scholar] [CrossRef]
- Hahn, N.T.; Seguin, T.J.; Lau, K.-C.; Liao, C.; Ingram, B.J.; Persson, K.A.; Zavadil, K.R. Enhanced Stability of the Carba-closo-Dodecaborate Anion for High-Voltage Battery Electrolytes through Rational Design. J. Am. Chem. Soc. 2018, 140, 11076–11084. [Google Scholar] [CrossRef]
- Jay, R.; Tomich, A.W.; Zhang, J.; Zhao, Y.; De Gorostiza, A.; Lavallo, V.; Guo, J. Comparative Study of Mg(CB11H12)2 and Mg(TFSI)2 at the Magnesium/Electrolyte Interface. ACS Appl. Mater. Interfaces 2019, 11, 11414–11420. [Google Scholar] [CrossRef]
- Watanabe, M.; Kanazawa, J.; Hamamura, T.; Shimokawa, T.; Miyamoto, K.; Hibino, M.; Nakura, K.; Inatomi, Y.; Kitazawa, Y.; Uchiyama, M. Boron-Vertex Modification of Carba-closo-Dodecaborate for High-Performance Magnesium-Ion Battery Electrolyte. Mater. Adv. 2021, 2, 937–941. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Shinohara, T.; Zhao, K.; Züttel, A.; Orimo, S. Monocarborane Cluster as a Stable Fluorine-Free Calcium Battery Electrolyte. Sci. Rep. 2021, 11, 7563. [Google Scholar] [CrossRef]
- Tomich, A.; Park, J.; Son, S.-B.; Kamphaus, E.; Lyu, X.; Dogan, F.; Carta, V.; Gim, J.; Li, T.; Cheng, L.; et al. A Carboranyl Electrolyte Enabling Highly Reversible Sodium Metal Anodes via a “Fluorine-Free” SEI. Angew. Chem. Int. Ed. 2022, 61, e202208158. [Google Scholar] [CrossRef]
- Tomich, A.W.; Chen, J.; Carta, V.; Guo, J.; Lavallo, V. Electrolyte Engineering with Carboranes for Next-Generation Mg Batteries. ACS Cent. Sci. 2024, 10, 264–271. [Google Scholar] [CrossRef]
- Fan, X.; Chen, L.; Borodin, O.; Ji, X.; Chen, J.; Hou, S.; Deng, T.; Zheng, J.; Yang, C.; Liou, S.-C.; et al. Non-Flammable Electrolyte Enables Li-Metal Batteries with Aggressive Cathode Chemistries. Nat. Nanotechnol. 2018, 13, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Jia, H.; Xu, W.; Zhang, J.-G. Review—Localized High-Concentration Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2021, 168, 010522. [Google Scholar] [CrossRef]
- Pecyna, J.; Rončević, I.; Michl, J. Insertion of Carbenes into Deprotonated Nido-Undecaborane, B11H13(2-). Molecules 2019, 24, 3779. [Google Scholar] [CrossRef] [PubMed]
- King, B.T.; Körbe, S.; Schreiber, P.J.; Clayton, J.; Němcová, A.; Havlas, Z.; Vyakaranam, K.; Fete, M.G.; Zharov, I.; Ceremuga, J.; et al. The Sixteen CB11HnMe12-n‒ Anions with Fivefold Substitution Symmetry: Anodic Oxidation and Electronic Structure. J. Am. Chem. Soc. 2007, 129, 12960–12980. [Google Scholar] [CrossRef]
- Ivanov, S.V.; Rockwell, J.J.; Polyakov, O.G.; Gaudinski, C.M.; Anderson, O.P.; Solntsev, K.A.; Strauss, S.H. Highly Fluorinated Weakly Coordinating Monocarborane Anions. 1-H-CB11F11‒, 1-CH3-CB11F11‒, and the Structure of [N(n-Bu)4]2[CuCl(CB11F11)]. J. Am. Chem. Soc. 1998, 120, 4224–4225. [Google Scholar] [CrossRef]
- Kitazawa, Y.; Watanabe, M.; Masumoto, Y.; Otsuka, M.; Miyamoto, K.; Muranaka, A.; Hashizume, D.; Takita, R.; Uchiyama, M. “Dumbbell”- and “Clackers”-Shaped Dimeric Derivatives of Monocarba-closo-Dodecaborate. Angew. Chem. Int. Ed. 2018, 130, 1517–1520. [Google Scholar] [CrossRef]
- Dontha, R.; Zhu, T.-C.; Shen, Y.; Wörle, M.; Hong, X.; Duttwyler, S. A 3D Analogue of Phenyllithium: Solution-Phase, Solid-State, and Computational Study of the Lithiacarborane [Li−CB11H11]−. Angew. Chem. Int. Ed. 2019, 58, 19007–19013. [Google Scholar] [CrossRef]
- Stoyanov, E.S.; Kim, K.-C.; Reed, C.A. An Infrared νNH Scale for Weakly Basic Anions. Implications for Single-Molecule Acidity and Superacidity. J. Am. Chem. Soc. 2006, 128, 8500–8508. [Google Scholar] [CrossRef]

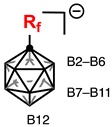 | |||
|---|---|---|---|
| Compound/Position | B12 | B7–B11 | B2–B6 |
| 1 | –6.9 | –13.3 | –16.2 |
| 3a | –3.1 | –12.3 | –13.9 |
| 3b | –3.1 | –12.3 | –13.7 |
| 3c | –2.9 | –12.3 | –13.7 |
| 3d | –3.1 | –12.3 | –14.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwashita, S.; Kimura, M.; Kitazawa, Y. Addition of a Perfluoroalkyl Acetyl Group to the C-Vertex of a Carborane Anion to Enhance Its Solubility in Fluorinated Ether Solvents. Chemistry 2024, 6, 1449-1457. https://doi.org/10.3390/chemistry6060087
Iwashita S, Kimura M, Kitazawa Y. Addition of a Perfluoroalkyl Acetyl Group to the C-Vertex of a Carborane Anion to Enhance Its Solubility in Fluorinated Ether Solvents. Chemistry. 2024; 6(6):1449-1457. https://doi.org/10.3390/chemistry6060087
Chicago/Turabian StyleIwashita, Sota, Mutsumi Kimura, and Yu Kitazawa. 2024. "Addition of a Perfluoroalkyl Acetyl Group to the C-Vertex of a Carborane Anion to Enhance Its Solubility in Fluorinated Ether Solvents" Chemistry 6, no. 6: 1449-1457. https://doi.org/10.3390/chemistry6060087
APA StyleIwashita, S., Kimura, M., & Kitazawa, Y. (2024). Addition of a Perfluoroalkyl Acetyl Group to the C-Vertex of a Carborane Anion to Enhance Its Solubility in Fluorinated Ether Solvents. Chemistry, 6(6), 1449-1457. https://doi.org/10.3390/chemistry6060087






