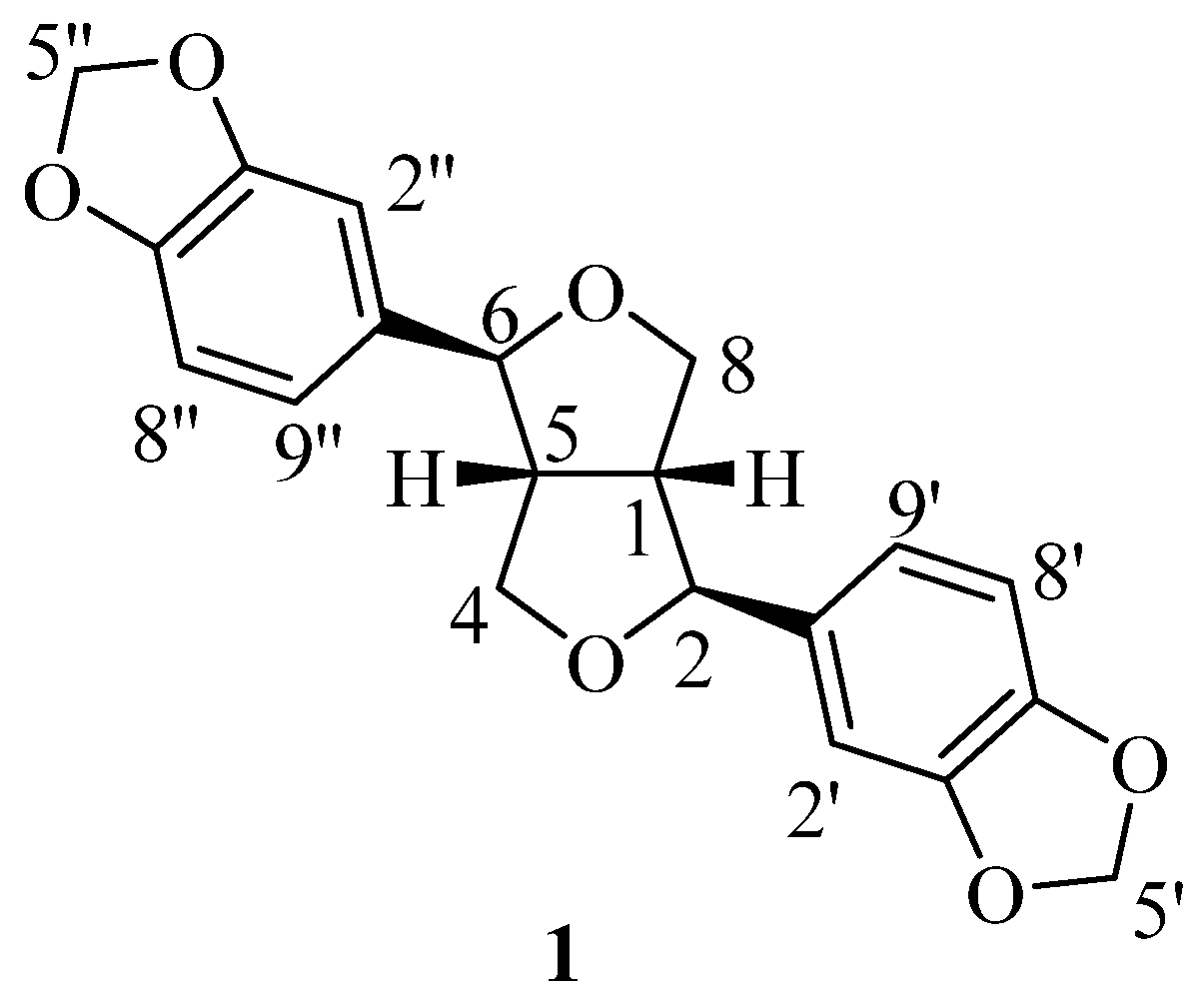
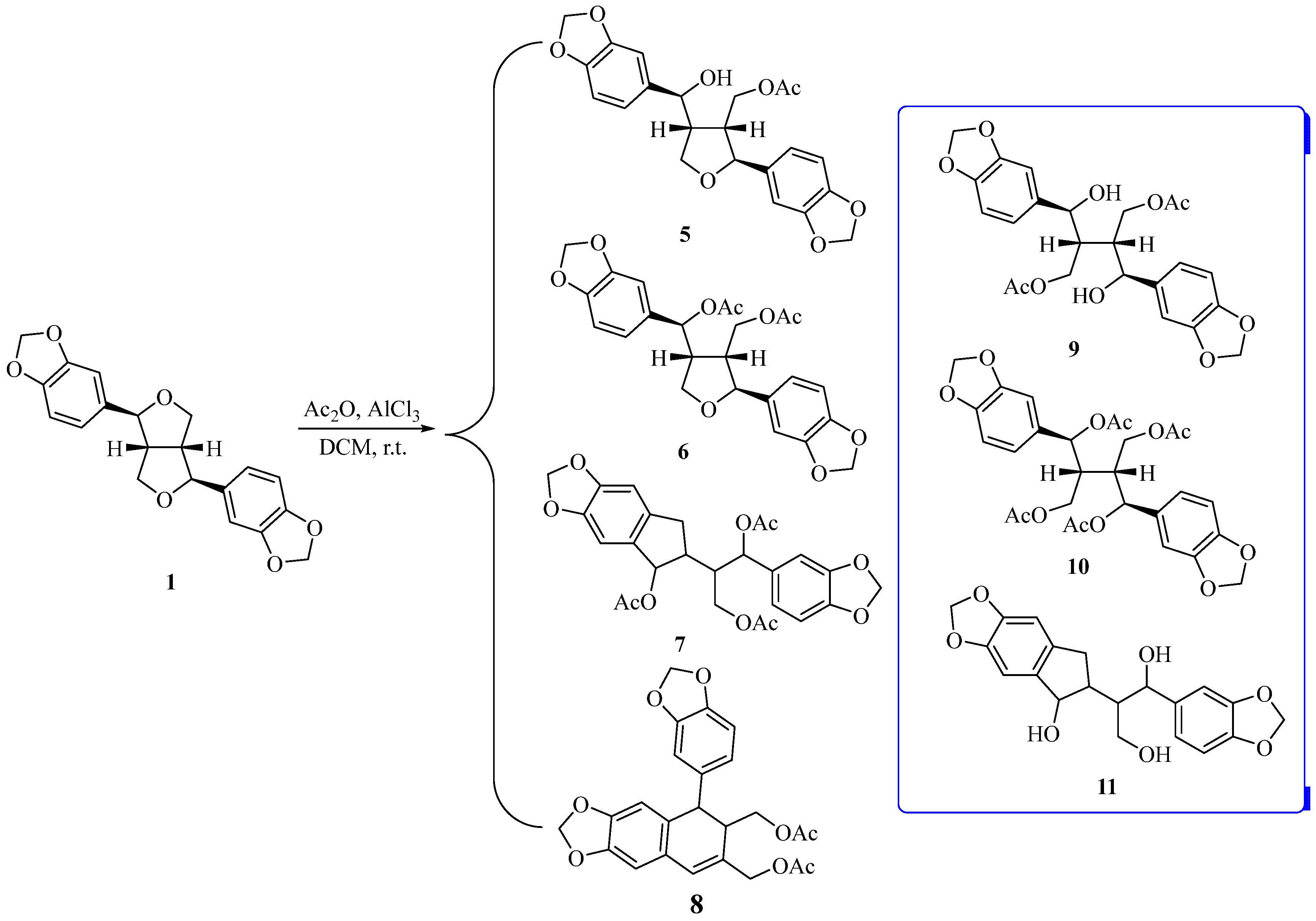
3.2. Experimental Section
(1) Cleavage of the ethereal bond.
To a solution of 1 (1.1 g, 3.2 mmol) in CH2Cl2 (20 mL), acetic anhydride (5 mL) was added. The reaction mixture was cooled down to 0 °C, followed by the addition of anhydrous AlCl3 (1.2 g, 9.0 mmol) in an ice bath. Then, hydrochloric acid (20 mL, 2 mol/L) was added to quench the reaction, and the mixture was extracted with EtOAc (2 × 50 mL). The combined organic layers were washed with brine (30 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuo and the residue was purified via flash column chromatography (petroleum ether/EtOAc = 2:1) to obtain the products 5–8.
Compound 5: colorless solid, yield: 11.0%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.84–6.70 (m, 6H), 5.94 (d, J = 4.60 Hz, 4H), 4.83 (d, J = 5.76 Hz, 1H), 4.80 (d, J = 5.04 Hz, 1H), 4.33 (dd, J = 11.12, 6.36 Hz, 1H), 4.23–4.12 (m, 2H), 4.12–4.03 (m, 1H), 2.86–2.75 (m, 1H), 2.39–2.28 (m, 1H), 2.00 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 171.0, 148.2, 147.9, 147.4, 147.1, 137.2, 136.5, 119.7, 119.3, 108.5, 108.2, 106.6, 106.3, 101.3, 101.1, 83.7, 72.6, 69.8, 62.8, 48.7, 47.0, 21.0.
Compound 6: yellow transparent oil, yield: 17.0%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.85 (dd, J = 7.96, 1.84 Hz, 1H), 6.83–6.73 (m, 5H), 5.95 (s, 2H), 5.94 (d, J = 1.40 Hz, 2H), 5.84 (d, J = 9.04 Hz, 1H), 4.85 (d, J = 4.28 Hz, 1H), 4.26 (t, J = 7.68 Hz, 1H), 4.18–4.04 (m, 2H), 3.94 (t, J = 9.00 Hz, 1H), 3.09–2.96 (m, 1H), 2.29–2.20 (m, 1H), 2.03 (s, 3H), 2.02 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.9, 169.9, 148.1, 148.0, 147.9, 147.1, 136.4, 132.6, 121.4, 118.9, 108.5, 108.2, 107.5, 106.1, 101.4, 101.2, 83.8, 74.2, 70.7, 62.5, 47.9, 45.1, 21.3, 21.0.
Compound 8: colorless transparent oil, yield: 35.0%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.68–6.62 (m, 2H), 6.58 (s, 1H), 6.51–6.44 (m, 3H), 5.91 (dd, J = 5.32, 1.16 Hz, 2H), 5.86 (dd, J = 5.60, 1.20 Hz, 2H), 4.61 (d, J = 13.04 Hz, 1H), 4.52 (d, J = 12.88 Hz, 1H), 4.18 (dd, J = 10.92, 5.04 Hz, 1H), 4.00 (s, 1H), 3.84 (dd, J = 10.88, 9.00 Hz, 1H), 2.77–2.69 (m, 1H), 2.06 (s, 3H), 1.93 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 171.0, 170.7, 147.6, 147.5, 146.8, 146.1, 137.3, 129.6, 129.0, 128.0, 126.4, 120.6, 110.2, 108.1, 108.1, 107.4, 101.2, 100.9, 66.5, 64.4, 45.2, 43.2, 21.0, 20.8.
(2) Synthesis of compound 11.
Compound 7 (249.1 mg, 0.5 mmol) was dissolved in CH3OH (15 mL) and H2O (5 mL), followed by the addition of K2CO3 (276.4 mg, 2.0 mmol). The mixture was stirred at room temperature overnight. The solvent was then removed under reduced pressure, and the residue was purified directly using silica gel column chromatography (petroleum ether/EtOAc = 1:1), yielding 149.6 mg of the product 11.
Compound 11: white solid, yield: 80.4%. 1H NMR (400 MHz, DMSO-d6, J in Hz) δ (ppm): 7.01 (s, 1H), 6.83 (d, J = 7.80 Hz, 1H), 6.63–6.55 (m, 2H), 6.09 (s, 1H), 5.97 (s, 2H), 5.89 (d, J = 11.68 Hz, 2H), 5.21 (d, J = 7.24 Hz, 1H), 4.57 (t, J = 8.52 Hz, 1H), 4.53–4.41 (m, 2H), 3.96 (d, J = 9.80 Hz, 1H), 3.86–3.77 (m, 1H), 3.71–3.62 (m, 1H), 3.53–3.44 (m, 1H), 3.20–3.11 (m, 1H), 1.90–1.79 (m, 1H), 1.65–1.53 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 147.3, 145.6, 145.5, 145.4, 139.9, 135.2, 132.7, 122.2, 109.0, 108.4, 107.9, 106.2, 100.8, 100.5, 67.4, 59.4, 58.7, 45.9, 44.8, 43.4. HRMS m/z 395.1110 [M + Na]+ (calcd for C20H20O7Na, 395.1101).
(3) General procedure for the synthesis of compounds 18a–18f.
To a stirred solution of compound 5 (324.7 mg, 0.8 mmol) in CH2Cl2 (25 mL), arylamine (2.4 mmol) and PPh3 (411.3 mg, 1.6 mmol) were added successively. The reaction mixture was cooled down to 0 °C and DIAD (320 μL, 1.6 mmol) was added with a syringe. At the end of the reaction, the mixture was diluted with 20 mL of H2O, then extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine (40 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuo and the residue was then purified using flash column chromatography to obtain the products 18.
Compound 18a: colorless transparent oil, yield: 90.4%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.11 (t, J = 7.48 Hz, 2H), 6.83–6.72 (m, 6H), 6.67 (t, J = 7.20 Hz, 1H), 6.53 (d, J = 7.84 Hz, 2H), 5.95 (s, 2H), 5.92 (dd, J = 6.20, 1.32 Hz, 2H), 4.91 (d, J = 7.12 Hz, 1H), 4.61 (d, J = 4.52 Hz, 1H), 4.42–4.24 (m, 2H), 4.19–4.09 (m, 2H), 2.84–2.74 (m, 1H), 2.61–2.53 (m, 1H), 2.00 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.9, 148.3, 148.1, 147.2, 146.9, 146.3, 137.1, 136.2, 129.4, 120.0, 119.1, 117.8, 113.7, 113.5, 108.6, 108.3, 107.0, 106.1, 101.2, 83.4, 70.4, 62.4, 55.8, 49.8, 48.4, 21.0.
Compound 18b: colorless transparent oil, yield: 82.9%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.18 (d, J = 8.68 Hz, 2H), 6.82–6.73 (m, 6H), 6.40 (d, J = 8.68 Hz, 2H), 5.95 (s, 2H), 5.93 (d, J = 4.60 Hz, 2H), 4.88 (d, J = 7.28 Hz, 1H), 4.55 (d, J = 4.40 Hz, 1H), 4.40–4.24 (m, 2H), 4.17–4.06 (m, 2H), 2.81–2.71 (m, 1H), 2.64–2.53 (m, 1H), 2.00 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.8, 148.3, 148.1, 147.2, 147.0, 145.4, 136.9, 135.6, 132.2, 132.1, 119.9, 119.1, 115.1, 109.4, 108.7, 108.3, 106.9, 106.0, 101.3, 101.2, 83.3, 70.2, 62.3, 55.8, 49.8, 48.3, 20.9.
Compound 18c: colorless transparent oil, yield: 68.9%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.84–6.72 (m, 8H), 6.45 (s, 2H), 5.95 (s, 2H), 5.93 (dd, J = 4.28, 1.12 Hz, 2H), 4.90 (d, J = 7.00 Hz, 1H), 4.51 (d, J = 4.56 Hz, 1H), 4.40–4.23 (m, 2H), 4.14 (d, J = 4.20 Hz, 2H), 2.84–2.70 (m, 1H), 2.60–2.52 (m, 1H), 2.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.9, 148.3, 148.1, 147.2, 147.0, 137.0, 120.0, 119.1, 116.0, 115.8, 114.4, 108.63, 108.3, 106.9, 106.1, 101.3, 101.2, 83.4, 70.4, 62.4, 56.5, 49.7, 48.3, 21.0.
Compound 18d: colorless transparent oil, yield: 81.6%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.05 (d, J = 8.76 Hz, 2H), 6.82–6.71 (m, 6H), 6.43 (d, J = 8.76 Hz, 2H), 5.96 (s, 2H), 5.93 (dd, J = 5.24, 1.16 Hz, 2H), 4.88 (d, J = 7.32 Hz, 1H), 4.85 (br s, 1H), 4.55 (d, J = 4.20 Hz, 1H), 4.40–4.24 (m, 2H), 4.19–4.06 (m, 2H), 2.80–2.72 (m, 1H), 2.65–2.55 (m, 1H), 2.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.8, 148.4, 148.1, 147.2, 147.0, 144.9, 136.9, 135.7, 129.3, 122.4, 119.9, 119.1, 114.6, 108.7, 108.4, 106.9, 106.0, 101.3, 101.2, 83.4, 70.3, 62.3, 55.9, 49.8, 48.4, 21.0.
Compound 18e: colorless transparent oil, yield: 91.7%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.92 (d, J = 8.20 Hz, 2H), 6.82–6.71 (m, 6H), 6.46 (d, J = 6.12 Hz, 2H), 5.95 (s, 2H), 5.92 (dd, J = 6.00, 1.12 Hz, 2H), 4.91 (d, J = 6.88 Hz, 1H), 4.56 (d, J = 4.44 Hz, 1H), 4.41–4.22 (m, 2H), 4.14 (d, J = 4.08 Hz, 2H), 2.88–2.73 (m, 1H), 3.60–2.48 (m, 1H), 2.20 (s, 3H), 2.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.8, 148.2, 148.1, 147.2, 146.8, 137.1, 129.9, 120.0, 119.1, 113.7, 108.6, 108.3, 107.0, 106.1, 101.2, 83.5, 70.5, 62.5, 49.7, 48.2, 29.8, 21.0, 20.5.
Compound 18f: colorless transparent oil, yield: 82.3%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.81–6.68 (m, 8H), 6.56–6.42 (m, 2H), 5.95 (s, 2H), 5.92 (d, J = 3.12 Hz, 2H), 4.92 (d, J = 6.52 Hz, 1H), 4.49 (brs, 1H), 4.38–4.22 (m, 2H), 4.20–4.08 (m, 2H), 3.70 (s, 3H), 2.90–2.70 (m, 1H), 2.58–2.42 (m, 1H), 2.01 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 170.9, 148.2, 148.1, 147.1, 137.1, 119.0, 115.0, 108.6, 108.3, 106.1, 101.2, 101.2, 83.5, 70.7, 62.5, 55.8, 49.6, 21.0.
(4) General procedure for the synthesis of compounds 19a–19f.
To a solution of compound 18 (0.7 mmol) in methanol (30 mL), K2CO3 (107.8 mg, 0.8 mmol), and H2O (5 mL) were added. The reaction mixture was then stirred at room temperature. At the end of the reaction, CH2Cl2 (100 mL) was added, and then the organic layers were washed with H2O (30 mL) and brine (40 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuo and the residue was purified using flash column chromatography to obtain the product 19.
Compound 19a: white solid, yield: 70.8%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.11 (t, J = 8.56 Hz, 2H), 6.84–6.70 (m, 6H), 6.67 (t, J = 7.52 Hz, 1H), 6.55 (d, J = 7.48 Hz, 2H), 5.95 (s, 2H), 5.91 (dd, J = 6.40, 1.44 Hz, 2H), 4.90 (d, J = 7.20 Hz, 1H), 4.75 (d, J = 4.28 Hz, 1H), 4.20–4.03 (m, 2H), 4.00–3.80 (m, 2H), 2.85–2.75 (m, 1H), 2.51–2.40 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.1, 146.7, 146.4, 137.6, 136.5, 129.4, 119.9, 118.9, 117.8, 113.7, 108.5, 108.3, 107.1, 106.0, 101.7, 83.1, 70.2, 60.8, 55.7, 53.0, 48.4.
Compound 19b: white solid, yield: 60.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.18 (d, J = 8.68 Hz, 2H), 6.82–6.71 (m, 6H), 6.41 (d, J = 8.72 Hz, 2H), 5.95 (s, 2H), 5.93 (d, J = 4.80 Hz, 2H), 4.85 (d, J = 7.28 Hz, 1H), 4.69 (d, J = 3.92 Hz, 1H), 4.16 (dd, J = 9.52, 3.00 Hz, 1H), 4.06 (dd, J = 9.40, 6.16 Hz, 1H), 3.93–3.84 (m, 2H), 2.84–2.74 (m, 1H), 2.53–2.46 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.3, 148.1, 147.1, 146.9, 137.4, 132.1, 119.9, 118.9, 115.3, 108.6, 108.4, 107.0, 106.0, 101.3, 101.2, 83.1, 70.2, 60.9, 53.0, 48.4, 31.7.
Compound 19c: white solid, yield: 80.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.85–6.71 (m, 8H), 6.51–6.44 (m, 2H), 5.95 (s, 2H), 5.92 (dd, J = 4.64, 1.44 Hz, 2H), 4.91 (d, J = 7.08 Hz, 1H), 4.66 (d, J = 4.36 Hz, 1H), 4.16 (dd, J = 9.52, 4.04 Hz, 1H), 4.09 (dd, J = 9.44, 6.32 Hz, 1H), 3.96–3.84 (m, 2H), 2.85–2.75 (m, 1H), 2.51–2.40 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 155.0, 148.3, 148.1, 147.1, 146.8, 142.5, 137.5, 136.1, 120.0, 118.9, 116.0, 115.7, 114.8, 108.6, 108.3, 107.1, 106.0, 101.2, 101.2, 83.1, 70.2, 60.8, 56.7, 52.9, 48.4.
Compound 19d: white solid, yield: 86.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.09–7.01 (m, 2H), 6.83–6.70 (m, 6H), 6.47 (d, J = 8.80 Hz, 2H), 5.95 (s, 2H), 5.93 (dd, J = 5.36, 1.40 Hz, 2H), 4.86 (d, J = 7.20 Hz, 1H), 4.69 (d, J = 4.04 Hz, 1H), 4.16 (dd, J = 9.56, 3.56 Hz, 1H), 4.07 (dd, J = 9.52, 6.20 Hz, 1H), 3.93–3.83 (m, 2H), 2.85–2.75 (m, 1H), 2.54–2.43 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.3, 148.1, 147.1, 146.9, 145.0, 137.4, 136.0, 129.2, 122.5, 119.9, 118.9, 114.8, 108.6, 108.3, 107.0, 106.0, 101.3, 101.2, 83.1, 70.2, 60.9, 56.0, 53.0, 48.4.
Compound 19e: white solid, yield: 94.5%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.93 (d, J = 8.20 Hz, 2H), 6.84–6.70 (m, 6H), 6.48 (d, J = 8.32 Hz, 2H), 5.98–5.85 (m, 4H), 4.93 (t, J = 7.16 Hz, 1H), 4.72 (t, J = 3.68 Hz, 1H), 4.15 (dd, J = 9.40, 3.72 Hz, 1H), 4.08 (dd, J = 9.32, 6.32 Hz, 1H), 3.95–3.80 (m, 2H), 2.87–2.74 (m, 1H), 2.50–2.38 (m, 1H), 2.20 (t, J = 11.72 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.0, 146.7, 144.0, 137.6, 136.7, 129.9, 127.1, 119.9, 118.9, 113.9, 108.5, 108.3, 107.1, 106.0, 101.1, 83.1, 70.2, 60.7, 56.0, 53.0, 48.5, 20.5.
Compound 19f: white solid, yield: 73.7%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.83–6.69 (m, 8H), 6.51 (d, J = 8.88 Hz, 2H), 5.94 (s, 2H), 5.91 (dd, J = 5.12, 1.40 Hz, 2H), 4.95 (d, J = 7.00 Hz, 1H), 4.65 (d, J = 4.52 Hz, 1H), 4.17–4.04 (m, 2H), 3.93–3.84 (m, 2H), 3.70 (s, 3H), 2.81–2.73 (m, 1H), 2.45–2.37 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 152.4, 148.2, 148.0, 147.0, 146.7, 140.3, 137.6, 136.6, 119.9, 118.9, 115.3, 115.0, 108.5, 108.3, 107.1, 106.0, 101.1, 83.0, 70.2, 60.6, 56.8, 55.8, 52.9, 48.5.
(5) General procedure for the synthesis of N-aryl-azasesamins 20.
To a stirred solution of compound 19 (0.4 mmol) in CH2Cl2 (15 mL), PPh3 (204.9 mg, 0.8 mmol) was added. The reaction mixture was cooled down to 0 °C and then DIAD (155 μL, 0.8 mmol) was added. At the end of the reaction, the mixture was diluted with H2O (20 mL) and extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine (40 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuo to obtain residue, which was then purified using flash column chromatography to obtain product 20.
Compound 20a: white solid, yield: 90.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.15 (t, J = 7.72 Hz, 2H), 6.88–6.71 (m, 7H), 6.60 (d, J = 8.24 Hz, 2H), 5.95 (s, 2H), 5.94 (d, J = 3.28 Hz, 2H), 4.75 (d, J = 5.84 Hz, 1H), 4.70 (d, J = 8.28 Hz, 1H), 3.91 (dd, J = 9.72, 3.48 Hz, 1H), 3.82 (t, J = 8.52 Hz, 1H), 3.63–3.53 (m, 2H), 3.46–3.36 (m, 1H), 2.93–2.85 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.3, 148.1, 147.2, 146.6, 136.4, 134.1, 128.9, 120.0, 119.3, 118.0, 115.5, 108.5, 108.3, 107.4, 106.4, 101.2, 101.1, 86.8, 70.4, 65.4, 55.1, 51.3, 50.2. HRMS m/z 430.1648 [M + H]+ (calcd for C26H24NO5, 430.1649).
Compound 20b: white solid, yield: 92.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.21 (d, J = 8.96 Hz, 2H), 6.87–6.66 (m, 6H), 6.45 (d, J = 9.00 Hz, 2H), 5.95 (s, 2H), 5.95 (d, J = 1.80 Hz, 2H), 4.73 (d, J = 5.76 Hz, 1H), 4.67 (d, J = 8.28 Hz, 1H), 3.90–3.78 (m, 2H), 3.60–3.51 (m, 2H), 3.47–3.36 (m, 1H), 2.96–2.86 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.2, 147.1, 146.8, 136.2, 133.4, 131.6, 119.9, 119.3, 117.0, 110.3, 108.6, 108.3, 107.3, 106.4, 101.2, 86.7, 70.2, 65.5, 55.0, 51.3, 50.2. HRMS m/z 508.0778 [M + H]+ (calcd for C26H23BrNO5, 508.0754).
Compound 20c: white solid, yield: 91.3%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.88–6.73 (m, 8H), 6.58 (dd, J = 8.96, 4.40 Hz, 2H), 5.95 (s, 2H), 5.94 (d, J = 6.56 Hz, 2H), 4.73 (d, J = 6.12 Hz, 1H), 4.60 (d, J = 8.16 Hz, 1H), 3.88 (dd, J = 9.76, 3.12 Hz, 1H), 3.80 (t, J = 8.44 Hz, 1H), 3.56 (t, J = 6.92 Hz, 1H), 3.50–3.35 (m, 2H), 2.92–2.82 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 155.3, 148.1, 147.2, 146.7, 144.7, 136.1, 133.4, 120.1, 119.4, 117.1, 117.1, 115.5, 115.3, 108.6, 108.3, 107.5, 106.4, 101.2, 87.0, 70.3, 66.0, 56.2, 51.1, 50.3. HRMS m/z 448.1555 [M + H]+ (calcd for C26H23FNO5, 448.1555).
Compound 20d: white solid, yield: 90.3%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.08 (d, J = 8.96 Hz, 2H), 6.88–6.68 (m, 6H), 6.53 (d, J = 8.96 Hz, 2H), 5.95 (s, 2H), 5.95 (d, J = 1.72 Hz, 2H), 4.75 (d, J = 5.80 Hz, 1H), 4.67 (d, J = 8.28 Hz, 1H), 3.88 (dd, J = 9.88, 3.68 Hz, 1H), 3.82 (dd, J = 9.32, 7.80 Hz, 1H), 3.61–3.51 (m, 2H), 3.47–3.36 (m, 1H), 2.96–2.86 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.2, 146.8, 146.5, 136.1, 133.3, 128.8, 123.3, 120.0, 119.3, 116.7, 108.6, 108.3, 107.3, 106.4, 101.2, 101.2, 86.7, 70.2, 65.7, 55.3, 51.2, 50.2. HRMS m/z 464.1261 [M + H]+ (calcd for C26H23ClNO5, 464.1259).
Compound 20e: white solid, yield: 86.8%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.95 (d, J = 8.36 Hz, 2H), 6.88–6.73 (m, 6H), 6.54 (d, J = 8.44 Hz, 2H), 5.95 (s, 2H), 5.93 (d, J = 3.36 Hz, 2H), 4.72 (d, J = 6.08 Hz, 1H), 4.62 (d, J = 8.20 Hz, 1H), 3.90 (dd, J = 9.80, 3.20 Hz, 1H), 3.79 (t, J = 9.16 Hz, 1H), 3.55 (dd, J = 9.20, 6.84 Hz, 1H), 3.48 (dd, J = 9.72, 8.04 Hz, 1H), 3.44–3.33 (m, 1H), 2.91–2.80 (m, 1H), 2.21 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.1, 148.0, 147.2, 146.6, 146.2, 136.3, 134.1, 129.4, 120.0, 119.4, 116.1, 108.5, 108.3, 107.5, 106.5, 101.2, 101.1, 87.0, 70.4, 65.6, 55.7, 51.2, 50.2, 20.5. HRMS m/z 444.1806 [M + H]+ (calcd for C27H26NO5, 444.1805).
Compound 20f: white solid, yield: 80.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 6.91–6.70 (m, 10H), 5.95 (s, 2H), 5.93 (d, J = 1.56 Hz, 2H), 4.78 (d, J = 3.36 Hz, 1H), 4.57 (d, J = 7.96 Hz, 1H), 3.91 (d, J = 8.84 Hz, 1H), 3.79 (t, J = 8.68 Hz, 1H), 3.72 (s, 3H), 3.66–3.49 (m, 1H), 3.39 (t, J = 9.32 Hz, 2H), 2.89–2.80 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.1, 148.0, 147.2, 146.6, 136.2, 120.2, 119.5, 117.8, 114.4, 108.5, 108.3, 107.7, 106.5, 101.2, 101.1, 87.2, 70.5, 65.9, 55.7, 53.6, 51.1, 50.3. HRMS m/z 460.1761 [M + H]+ (calcd for C27H26NO6, 460.1755).
(6) General procedure for the synthesis of N-aryl-azasesamins 21.
Compound 20 (0.2 mmol), arylboric acid (0.2 mmol), K2CO3 (49.7 mg, 0.4 mmol), Pd (PPh3)4 (20.8 mg, 0.02 mmol), and toluene (20 mL) were added to a 50 mL round-bottom flask. The reaction was heated to 80 °C under nitrogen and reacted overnight. At the end of the reaction, toluene was removed by use of a rotary evaporator, and the concentrate was diluted with H2O (20 mL) and extracted with CH2Cl2 (3 × 20 mL). The combined organic layers were washed with brine (20 mL) and dried over anhydrous Na2SO4. The solvent was removed under vacuo to obtain residue, which was then purified using flash column chromatography to obtain the product 21.
Compound 21a: white solid, yield: 75.0%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.39 (d, J = 8.72 Hz, 2H), 7.34–7.27 (m, 2H), 7.25 (t, J = 1.40Hz, 1H), 6.89–6.71 (m, 6H), 6.62 (d, J = 8.72 Hz, 2H), 5.96 (s, 2H), 5.95 (d, J = 3.08 Hz, 2H), 4.76 (t, J = 6.20 Hz, 2H), 3.94 (dd, J = 9.92, 3.72 Hz, 1H), 3.83 (dd, J = 9.28, 7.80 Hz, 1H), 3.67–3.55 (m, 2H), 3.48–3.38 (m, 1H), 2.97–2.87 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.1, 148.1, 147.4, 147.2, 146.7, 142.5, 136.3, 134.0, 127.0, 126.2, 125.9, 120.0, 119.3, 118.1, 115.7, 108.6, 108.3, 107.4, 106.4, 101.2, 86.7, 70.3, 65.6, 55.0, 51.3, 50.2. HRMS m/z 512.1533 [M + H]+ (calcd for C30H26NO5S, 512.1526).
Compound 21b: white solid, yield: 85.3%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.50 (dd, J = 8.44, 1.32 Hz, 2H), 7.42 (d, J = 8.72 Hz, 2H), 7.37 (t, J = 7.48 Hz, 2H), 7.28–7.22 (m, 1H), 6.89–6.73 (m, 8H), 5.96 (s, 2H), 5.95 (t, J = 2.16 Hz, 2H), 4.85 (d, J = 5.84 Hz, 1H), 4.78 (d, J = 8.28 Hz, 1H), 3.99 (dd, J = 10.08, 3.64 Hz, 1H), 3.84 (dd, J = 9.36, 7.84 Hz, 1H), 3.71–3.61 (m, 2H), 3.50–3.41 (m, 1H), 2.99–2.90 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.2, 146.9, 141.0, 136.2, 128.8, 127.6, 126.6, 126.5, 120.2, 119.4, 116.2, 108.6, 108.3, 107.5, 106.4, 101.2, 86.6, 70.2, 66.3, 55.5, 51.2, 50.2. HRMS m/z 506.1957 [M + H]+ (calcd for C32H28NO5, 506.1962).
Compound 21c: white solid, yield: 81.3%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.44–7.31 (m, 6H), 6.88–6.76 (m, 6H), 6.66 (d, J = 8.72 Hz, 2H), 5.96 (s, 2H), 5.95 (d, J = 2.32 Hz, 2H), 4.78 (t, J = 6.12 Hz, 2H), 3.95 (dd, J = 10.00, 3.84 Hz, 1H), 3.84 (dd, J = 9.32, 7.80 Hz, 1H), 3.70–3.56 (m, 2H), 3.49–3.39 (m, 1H), 2.98–2.89 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.6, 147.2, 146.8, 139.6, 136.2, 133.7, 132.2, 129.4, 128.9, 128.9, 127.7, 127.4, 120.0, 119.3, 115.7, 108.7, 108.6, 108.3, 107.3, 106.4, 101.2, 86.6, 70.3, 65.6, 55.0, 51.3, 50.2. HRMS m/z 540.1581 [M + H]+ (calcd for C32H27ClNO5, 540.1572).
Compound 21d: white solid, yield: 88.7%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.40 (d, J = 6.00 Hz, 2H), 7.38 (d, J = 6.48 Hz, 2H), 7.18 (d, J = 8.00 Hz, 2H), 6.88–6.76 (m, 6H), 6.66 (d, J = 8.12 Hz, 2H), 5.96 (s, 2H), 5.95 (d, J = 2.44 Hz, 2H), 4.76 (dd, J = 9.92, 5.84 Hz, 2H), 3.95 (dd, J = 9.96, 3.64 Hz, 1H), 3.84 (dd, J = 9.32, 7.84 Hz, 1H), 3.67–3.56 (m, 2H), 3.48–3.39 (m, 1H), 2.97–2.87 (m, 1H), 2.36 (s, 3H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.1, 148.1, 147.4, 147.2, 146.7, 138.3, 136.3, 136.0, 134.0, 130.8, 129.5, 127.3, 126.4, 126.3, 120.0, 119.3, 115.7, 108.6, 108.3, 107.4, 106.4, 101.2, 101.2, 86.7, 70.3, 65.6, 55.0, 51.4, 50.2, 21.2. HRMS m/z 520.2126 [M + H]+ (calcd for C33H30NO5, 520.2118).
Compound 21e: white solid, yield: 71.9%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.44 (dd, J = 8.76, 5.40 Hz, 2H), 7.34 (d, J = 8.72 Hz, 2H), 7.05 (t, J = 8.72 Hz, 2H), 6.89–6.72 (m, 6H), 6.65 (d, J = 8.72 Hz, 2H), 5.96 (s, 2H), 5.95 (d, J = 2.56 Hz, 2H), 4.76 (t, J = 5.56 Hz, 2H), 3.95 (dd, J = 9.92, 3.80 Hz, 1H), 3.84 (dd, J = 9.32, 7.80 Hz, 1H), 3.68–3.55 (m, 2H), 3.48–3.40 (m, 1H), 2.96–2.90 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 160.8, 148.2, 148.1, 147.5, 147.2, 146.8, 137.3, 136.3, 133.9, 129.8, 128.0, 127.9, 127.4, 120.0, 119.3, 115.7, 115.7, 115.5, 108.6, 108.3, 107.4, 106.4, 101.2, 86.7, 70.3, 65.5, 55.0, 51.4, 50.2. HRMS m/z 524.1863 [M + H]+ (calcd for C32H27FNO5, 524.1868).
Compound 21f: white solid, yield: 66.5%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.32 (d, J = 8.52 Hz, 2H), 7.29–7.24 (m, 1H), 7.21–7.09 (m, 2H), 6.88–6.73 (m, 6H), 6.66 (d, J = 8.60 Hz, 2H), 5.95 (s, 2H), 5.94 (d, J = 2.92 Hz, 2H), 4.78 (dd, J = 7.88, 5.92 Hz, 2H), 3.95 (dd, J = 9.96, 3.80 Hz, 1H), 3.83 (t, J = 8.92 Hz, 1H), 3.70–3.57 (m, 2H), 3.50–3.38 (m, 1H), 3.00–2.89 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.6, 147.2, 146.8, 138.3, 136.2, 133.5, 128.8, 127.4, 122.2, 122.1, 122.1, 120.0, 119.3, 117.5, 117.4, 115.8, 115.2, 115.0, 108.6, 108.3, 107.3, 106.4, 101.2, 86.6, 70.2, 65.7, 55.0, 51.3, 50.2. HRMS m/z 542.1777 [M + H]+ (calcd for C32H26F2NO5, 542.1774).
Compound 21g: white solid, yield: 74.5%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.43 (d, J = 8.68 Hz, 2H), 7.36 (d, J = 8.68 Hz, 2H), 6.94–6.77 (m, 8H), 6.67 (d, J = 8.68 Hz, 2H), 5.96 (s, 2H), 5.95 (d, J = 2.60 Hz, 2H), 4.76 (dd, J = 14.20, 5.84 Hz, 2H), 3.95 (dd, J = 9.92, 3.56 Hz, 1H), 3.88–3.83 (m, 1H), 3.82 (s, 3H), 3.65–3.56 (m, 2H), 3.49–3.37 (m, 1H), 2.97–2.86 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 158.5, 148.1, 148.1, 147.2, 147.2, 146.7, 136.3, 134.0, 133.9, 130.6, 127.5, 127.5, 127.4, 127.1, 120.0, 119.3, 115.8, 114.2, 108.6, 108.3, 107.4, 106.4, 101.2, 101.2, 86.8, 70.3, 65.6, 55.4, 55.1, 51.3, 50.2. HRMS m/z 536.2068 [M + H]+ (calcd for C33H30NO6, 536.2068).
Compound 21h: white solid, yield: 63.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 8.15 (d, J = 5.36 Hz, 1H), 7.47 (d, J = 8.76 Hz, 2H), 7.30 (d, J = 5.36 Hz, 1H), 7.02 (s, 1H), 6.87–6.70 (m, 6H), 6.65 (d, J = 8.80 Hz, 2H), 5.96 (s, 2H), 5.95 (d, J = 1.20 Hz, 2H), 4.84 (d, J = 8.36 Hz, 1H), 4.80 (d, J = 5.44 Hz, 1H), 3.94 (dd, J = 10.00, 4.24 Hz, 1H), 3.86 (dd, J = 9.32, 7.80 Hz, 1H), 3.75 (t, J = 8.36 Hz, 1H), 3.62 (dd, J = 9.40, 6.48 Hz, 1H), 3.51–3.43 (m, 1H), 3.03–2.94 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 166.0, 149.1, 148.3, 148.1, 147.8, 147.6, 147.3, 146.9, 136.1, 133.4, 127.6, 125.5, 123.6, 119.9, 119.2, 118.4, 118.4, 115.3, 108.7, 108.3, 107.2, 106.3, 105.3, 101.3, 101.2, 86.4, 70.1, 65.4, 54.4, 51.4, 50.1. HRMS m/z 525.1814 [M + H]+ (calcd for C31H26FN2O5, 525.1820).
Compound 21i: white solid, yield: 60.6%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 8.30 (d, J = 2.20 Hz, 1H), 7.71 (dd, J = 8.56, 2.20 Hz, 1H), 7.32 (d, J = 8.60 Hz, 2H), 6.88–6.75 (m, 7H), 6.66 (d, J = 8.60 Hz, 2H), 5.96 (s, 2H), 5.95–5.89 (m, 2H), 4.77 (t, J = 5.96 Hz, 2H), 3.96 (s, 3H), 3.93 (d, J = 3.60 Hz, 1H), 3.84 (t, J = 8.24 Hz, 1H), 3.69–3.55 (m, 2H), 3.48–3.40 (m, 1H), 2.99–2.90 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 162.9, 148.2, 148.1, 147.6, 147.2, 146.7, 144.0, 137.3, 136.2, 133.8, 130.2, 127.4, 127.1, 124.2, 123.6, 119.9, 119.3, 119.2, 115.8, 110.8, 108.6, 108.3, 107.3, 106.4, 101.2, 86.7, 70.3, 65.5, 54.9, 53.8, 51.3, 50.2. HRMS m/z 537.2020 [M + H]+ (calcd for C32H29N2O6, 537.2020).
Compound 21j: green solid, yield: 91.5%. 1H NMR (400 MHz, DMSO-d6, J in Hz) δ (ppm): 8.86 (dd, J = 3.72, 1.36 Hz, 1H), 8.37 (dd, J = 8.24, 1.28 Hz, 1H), 7.88 (d, J = 7.32 Hz, 1H), 7.67 (d, J = 6.24 Hz, 1H), 7.60 (t, J = 7.68 Hz, 1H), 7.52 (dd, J = 8.24, 4.12 Hz, 1H), 7.46 (d, J = 8.56 Hz, 2H), 6.99–6.79 (m, 6H), 6.65 (d, J = 8.56 Hz, 2H), 6.01 (s, 2H), 5.99–5.94 (m, 2H), 4.83 (d, J = 8.52 Hz, 1H), 4.74 (d, J = 6.16 Hz, 1H), 3.99 (dd, J = 10.04, 3.08 Hz, 1H), 3.77 (t, J = 7.56 Hz, 1H), 3.60 (t, J = 8.28 Hz, 1H), 3.53–3.45 (m, 1H), 3.43–3.39 (m, 1H), 2.94–2.87 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δ (ppm): 149.9, 147.5, 147.4, 147.0, 146.5, 146.0, 145.3, 139.9, 136.9, 136.4, 134.6, 131.0, 129.3, 128.5, 127.8, 126.8, 126.5, 121.2, 120.0, 119.1, 114.1, 108.3, 108.0, 107.3, 106.3, 100.9, 85.5, 69.4, 64.5, 53.8, 51.2, 49.4. HRMS m/z 557.2077 [M + H]+ (calcd for C35H29N2O5, 557.2071).
Compound 21k: white solid, yield: 75.1%. 1H NMR (400 MHz, CDCl3, J in Hz) δ (ppm): 7.50 (d, J = 1.92 Hz, 1H), 7.19 (d, J = 8.72 Hz, 2H), 6.88–6.72 (m, 6H), 6.63 (d, J = 8.72 Hz, 2H), 6.22 (d, J = 1.80 Hz, 1H), 5.96 (s, 2H), 5.95–5.90 (m, 2H), 4.79 (t, J = 4.44 Hz, 2H), 3.94 (dd, J = 9.88, 3.96 Hz, 1H), 3.88 (s, 3H), 3.85 (dd, J = 9.40, 7.80 Hz, 1H), 3.70 (dd, J = 9.80, 8.24 Hz, 1H), 3.60 (dd, J = 9.40, 6.44 Hz, 1H), 3.50–3.42 (m, 1H), 3.00–2.93 (m, 1H). 13C NMR (100 MHz, CDCl3) δ (ppm): 148.2, 148.1, 147.2, 146.8, 138.0, 136.2, 133.6, 129.7, 129.4, 124.2, 123.6, 119.9, 119.2, 116.0, 115.0, 113.2, 108.7, 108.3, 107.2, 106.3, 105.5, 101.2, 86.5, 70.2, 65.5, 54.6, 51.4, 50.1, 29.8. HRMS m/z 510.2030 [M + H]+ (calcd for C30H28N3O5, 510.2023).
(7) Crystal structure determination.
A crystal with optimum size was placed on a Bruker D8 Venture single crystal diffractometer. Under the optimized conditions of graphite monochromatic GaKα ray (λ = 1.34139 Å) and 213.96 K, the diffraction points were measured in a fixed range of θ. A direct method was used to solve the structure, and the hydrogen and nonhydrogen atoms were hydrogenated by use of isotropic and anisotropic thermal parameters, respectively. The crystal structure was corrected and analyzed by SHELX 2018 (Sheldrick, 2018) and SHELXL 2018 (Sheldrick, 2015). The relevant crystallographic and structural correction data are shown in
Table S1.
(8) Bioactivity evaluation.
Antifungal activity evaluation: The broth microdilution method based on the Clinical and Laboratory Standards Institute (CLSI) was used to evaluate the antifungal activity of N-aryl-azasesamins. The compounds were dissolved in DMSO at a stock concentration of 4 mg/mL. Exponentially growing cultures (OD600 = 0.03–0.06) of each strain were prepared from overnight cultures. Cultures were diluted in broth (1:10) and added to a 96-well plate (195 μL/well). C. neoformans was used directly without dilutions. Fluconazole was used as positive control for C. albicans and C. neoformans. Plates were read at 600 nm after 48 h incubation. Inhibition was calculated by subtracting the absorbance of the blank wells, dividing by the average value for the DMSO only wells, and multiplying by 100.
Antitumor activity evaluation: HepG2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin in an incubator set to 5% CO2, 37 °C, and 100% humidity conditions. The medium was normally exchanged every 2–3 days, and cells were passaged every 6–7 days. During the exponential growth phase, cells were passaged 3 to 4 times before being used for experiments.
For the assays, cells were detached using 0.25% trypsin, and the resulting suspension was seeded into 96-well culture plates at a density of approximately 5000 cells/mL/well. Cells were treated with increasing concentrations of N-aryl-azasesamins solution and cultured for 24 h. About 10 μL of freshly prepared MTT (5 mg/mL) solution was added to each well and incubated for 4 h. Following incubation, the supernatant was removed, and 150 μL of DMSO was added to dissolve the formazan crystals. Optical density (OD) values were measured at 570 nm with a reference wavelength of 450 nm using a microplate reader. The IC50 values of each compound, representing the concentration required to achieve 50% inhibition of cell growth, were calculated.
(9) Data Analysis.
Data were averaged and statistically analyzed using SPSS 17.0 (IBM, Armonk, NY, USA) with one-way ANOVA (Tukey test) and Student’s T-tests to compare mean differences at a significance level of p < 0.05. GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) was used for IC50 calculation. There were three biological and three technical replicates performed for each sample.