Understanding the Enhanced Separation Mechanism of C2H4/C2H6 at Low Pressure by HKUST−1
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Synthesis
2.2. Gas Sorption Measurements
2.3. Computational Simulation
3. Results and Discussion
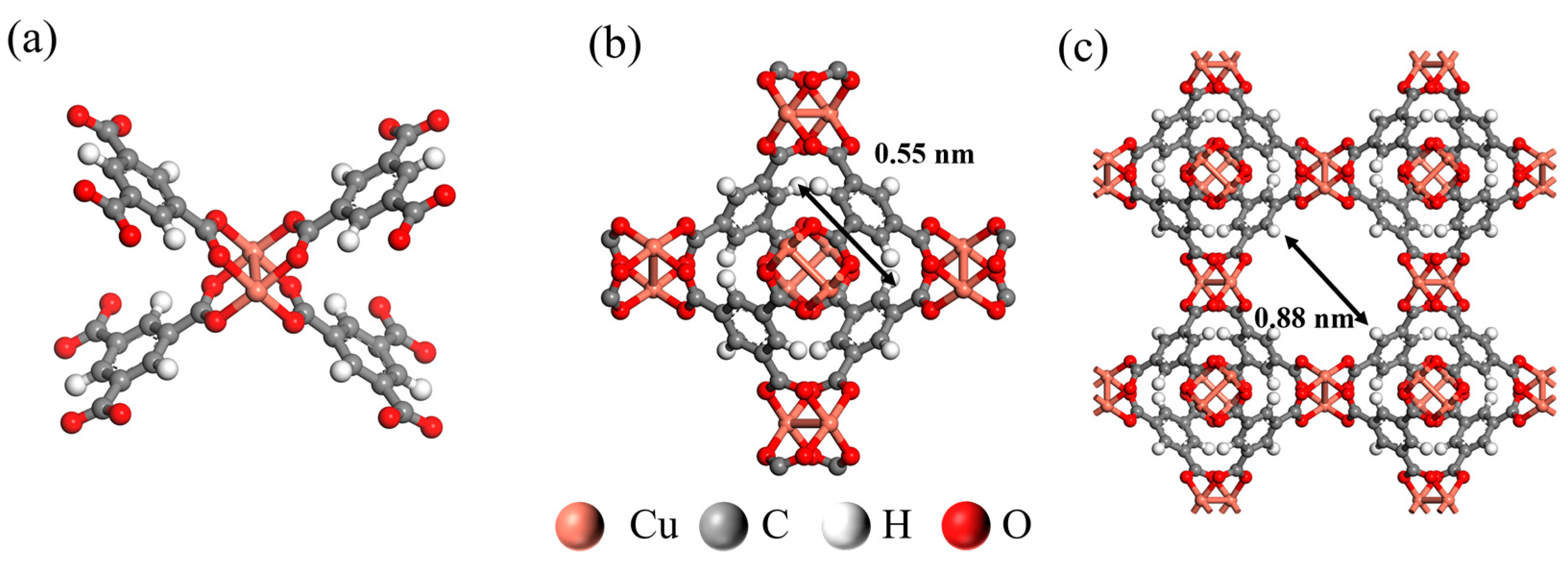
3.1. Structural Analysis
3.2. Adsorption Study of C2H6 and C2H4
3.3. Gas Separation Properties
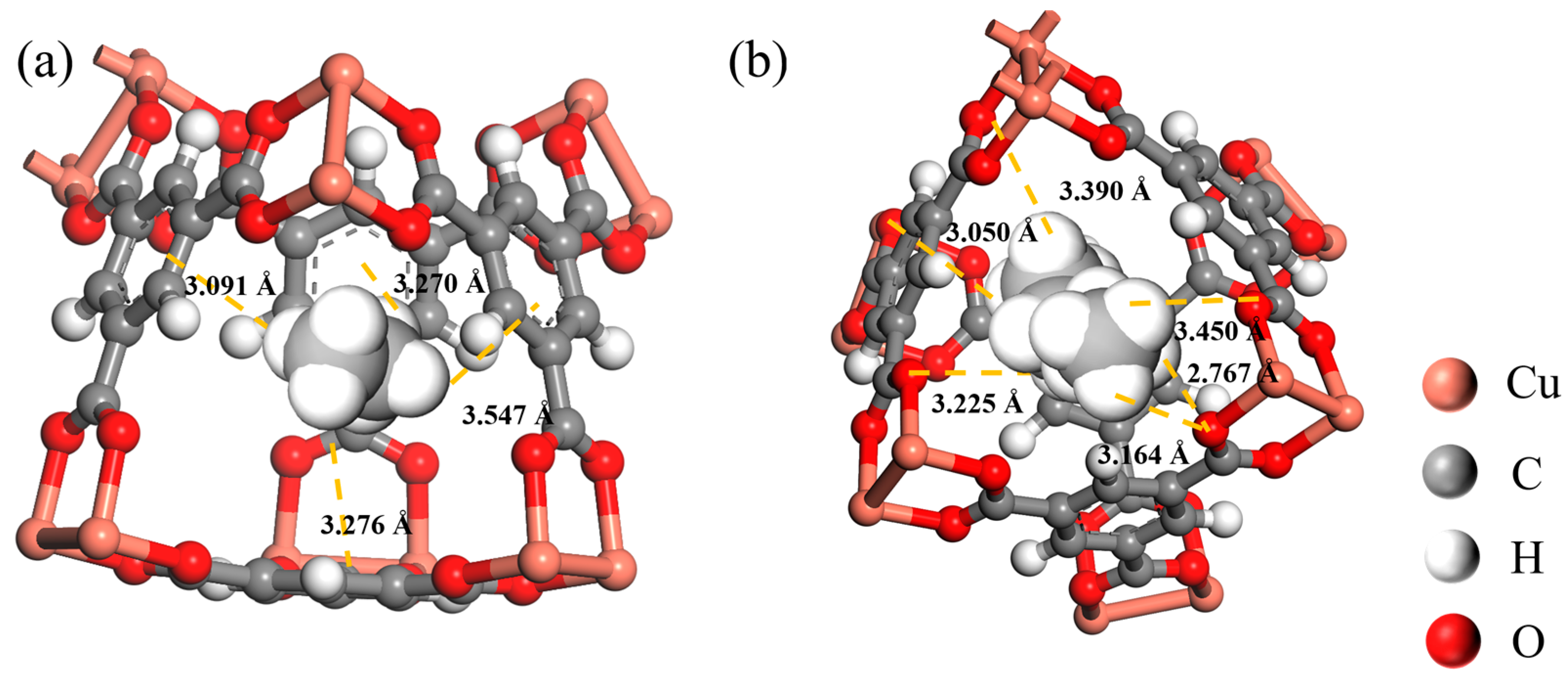
3.4. Molecular Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sensharma, D.; Chen, K.J.; Zaworotko, M.J. Crystal engineering of porous coordination networks to enable separation of C2 hydrocarbons. Chem. Commun. 2020, 56, 10419–10441. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Yang, L.; Qian, S.; Wang, X.; Cui, X.; Chen, B.; Xing, H. Energy-efficient separation alternatives: Metal-organic frameworks and membranes for hydrocarbon separation. Chem. Soc. Rev. 2020, 49, 5359–5406. [Google Scholar] [CrossRef]
- Gao, M.Y.; Song, B.Q.; Sensharma, D.; Zaworotko, M.J. Crystal engineering of porous coordination networks for C3 hydrocarbon separation. SmartMat 2020, 2, 38–55. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, W.; Wu, J.; Miao, Y.; Xiang, C.; Chen, C.; Ge, B.; Gan, Z.; Yang, F.; Zhang, M.; et al. Recent advances in direct air capture by adsorption. Chem. Soc. Rev. 2022, 51, 6574–6651. [Google Scholar] [CrossRef]
- Wang, Y.S.; Zhang, X.J.; Ba, Y.Q.; Li, T.Y.; Hao, G.P.; Lu, A.H. Recent Advances in Carbon-Based Adsorbents for Adsorptive Separation of Light Hydrocarbons. Research 2022, 2022, 9780864. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Y.; Yue, B.; Wang, C.; Qin, B.; Chai, Y.; Wu, G.; Li, J.; Han, X.; da-Silva, I.; et al. Regulating Extra-Framework Cations in Faujasite Zeolites for Capture of Trace Carbon Dioxide. Chem. Eur. J. 2022, 28, e202201659. [Google Scholar] [CrossRef]
- Ye, Y.; Ma, Z.; Lin, R.-B.; Krishna, R.; Zhou, W.; Lin, Q.; Zhang, Z.; Xiang, S.; Chen, B. Pore Space Partition within a Metal–Organic Framework for Highly Efficient C2H2/CO2 Separation. J. Am. Chem. Soc. 2019, 141, 4130–4136. [Google Scholar] [CrossRef]
- Gao, J.; Qian, X.; Lin, R.B.; Krishna, R.; Wu, H.; Zhou, W.; Chen, B. Mixed Metal–Organic Framework with Multiple Binding Sites for Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2020, 59, 4396–4400. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Wang, X.; Zhang, H.; He, L.; Huang, J.L.; Wei, W.J.; Yuan, Z.; Xiong, Z.L.; Chen, H.D.; Xiang, S.C.; et al. A Microporous Hydrogen Bonded Organic Framework for Highly Selective Separation of Carbon Dioxide over Acetylene. Angew. Chem. Int. Ed. 2023, 62, e202311419. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Krungleviciute, V.; Eryazici, I.; Hupp, J.T.; Farha, O.K.; Yildirim, T. Methane Storage in Metal–Organic Frameworks: Current Records, Surprise Findings, and Challenges. J. Am. Chem. Soc. 2013, 135, 11887–11894. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, M.; Otake, K.I.; Song, B.Q.; van Wyk, L.M.; Yang, Q.Y.; Kumar, N.; Feldmann, W.K.; Pham, T.; Suepaul, S.; Space, B.; et al. Benchmark Acetylene BindingAffinity and Separation through Induced Fit in aFlexible Hybrid Ultramicroporous Material. Angew.Chem. Int. Ed. 2021, 60, 20383–20390. [Google Scholar] [CrossRef]
- Gutiérrez-Sevillano, J.J.; Vicent-Luna, J.M.; Dubbeldam, D.; Calero, S. Molecular Mechanisms for Adsorption in Cu-BTC Metal Organic Framework. J. Phys. Chem. C 2013, 117, 11357–11366. [Google Scholar] [CrossRef]
- Wang, Q.M.; Shen, D.M.; Bülow, M.; Lau, M.L.; Deng, S.G.; Fitch, F.R.; Lemcoff, N.O.; Semanscin, J. Metallo-organic molecular sieve for gas separation and purification. Microporous Mesoporous Mater. 2002, 55, 217–230. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Bhatia, S.K. Electrostatically Mediated Specific Adsorption of Small Molecules in Metallo-Organic Frameworks. J. Phys. Chem. B 2006, 110, 24834–24836. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, X.; Chen, M.; Mishima, A.; Li, L.; Hori, A.; Wu, X.; Ding, L.; Kusaka, S.; Matsuda, R. Design of a MOF based on octa-nuclear zinc clusters realizing both thermal stability and structural flexibility. Chem. Commun. 2022, 58, 1139–1142. [Google Scholar] [CrossRef]
- Foo, M.L.; Matsuda, R.; Hijikata, Y.; Krishna, R.; Sato, H.; Horike, S.; Hori, A.; Duan, J.G.; Sato, Y.; Kubota, Y.; et al. An Adsorbate Discriminatory Gate Effect in a Flexible Porous Coordination Polymer for Selective Adsorption of CO2 over C2H2. J. Am. Chem. Soc. 2016, 138, 3022–3030. [Google Scholar] [CrossRef]
- Wang, W.; Wang, G.D.; Zhang, B.; Li, X.Y.; Hou, L.; Yang, Q.Y.; Liu, B. Discriminatory Gate-Opening Effect in a Flexible Metal–Organic Framework for Inverse CO2/C2H2 Separation. Small 2023, 19, 2302975. [Google Scholar] [CrossRef]
- Al-Janabi, N.; Hill, P.; Torrente-Murciano, L.; Garforth, A.; Gorgojo, P.; Siperstein, F.; Fan, X. Mapping the Cu-BTC metal–organic framework (HKUST−1) stability envelope in the presence of water vapour for CO2 adsorption from flue gases. Chem. Eng. J. 2015, 281, 669–677. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Yang, Y.; Li, X.; Li, J.; Li, Z. Competitive adsorption and selectivity of benzene and water vapor on the microporous metal organic frameworks (HKUST−1). Chem. Eng. J. 2015, 259, 79–89. [Google Scholar] [CrossRef]
- Ismail, R.; Azman, N.H.N.; Mohanadas, D.; Mustafa, M.N.; Mohd Abdah, M.A.A.; Raman, V.; Abdullah, J.; Sulaiman, Y. Facile ultrasonication synthesis of MXene/HKUST-1 composite as positive electrode for supercapattery. J. Energy Storage 2024, 94, 112461. [Google Scholar] [CrossRef]
- Ursueguía, D.; Daniel, C.; Collomb, C.; Cardenas, C.; Farrusseng, D.; Díaz, E.; Ordóñez, S. Evaluation of HKUST−1 as Volatile Organic Compound Adsorbents for Respiratory Filters. Langmuir 2022, 38, 14465–14474. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Shang, M.G.; Zhang, M.N.; Yu, S.M.; Yuan, Z.P.; Yi, X.B.; Filatov, S.; Zhang, J. Konjac glucomannan/cellulose nanofibers composite aerogel supported HKUST−1 for CO2 adsorption. Carbohydr. Polym. 2022, 293, 119720. [Google Scholar] [CrossRef]
- Pan, J.Y.; Bai, X.T.; Li, Y.Y.; Yang, B.H.; Yang, P.Y.; Yu, F.; Ma, J. HKUST−1 derived carbon adsorbents for tetracycline removal with excellent adsorption performance. Environ. Res. 2022, 205, 112425. [Google Scholar] [CrossRef]
- Zhao, X.B.; Xiao, B.; Fletcher, A.J.; Thomas, K.M. Hydrogen adsorption on functionalized nanoporous activated carbons. J. Phys. Chem. B 2005, 109, 8880–8888. [Google Scholar] [CrossRef]
- Cole, J.H.; Everett, D.H.; Marshall, C.T.; Paniego, A.R.; Powl, J.C.; Rodriguez-Reinoso, F. Thermodynamics of the high temperature adsorption of some permanent gases by porous carbons. J. Chem. Soc. Faraday Trans. 1 1974, 70, 2154–2169. [Google Scholar] [CrossRef]
- Zhao, X.B.; Villar-Rodil, S.; Fletcher, A.J.; Thomas, K.M. Kinetic isotope effect for H2 and D2 quantum molecular sieving in adsorption/desorption on porous carbon materials. J. Phys. Chem. B 2006, 110, 9947–9955. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Fu, Q.; Kong, X.; Yuan, X.; Yang, L.; Yan, L.; Zhao, X. Understanding the Enhanced Separation Mechanism of C2H4/C2H6 at Low Pressure by HKUST−1. Chemistry 2024, 6, 1326-1335. https://doi.org/10.3390/chemistry6060077
Xie W, Fu Q, Kong X, Yuan X, Yang L, Yan L, Zhao X. Understanding the Enhanced Separation Mechanism of C2H4/C2H6 at Low Pressure by HKUST−1. Chemistry. 2024; 6(6):1326-1335. https://doi.org/10.3390/chemistry6060077
Chicago/Turabian StyleXie, Wenpeng, Qiuju Fu, Xiangjun Kong, Xiangsen Yuan, Lingzhi Yang, Liting Yan, and Xuebo Zhao. 2024. "Understanding the Enhanced Separation Mechanism of C2H4/C2H6 at Low Pressure by HKUST−1" Chemistry 6, no. 6: 1326-1335. https://doi.org/10.3390/chemistry6060077
APA StyleXie, W., Fu, Q., Kong, X., Yuan, X., Yang, L., Yan, L., & Zhao, X. (2024). Understanding the Enhanced Separation Mechanism of C2H4/C2H6 at Low Pressure by HKUST−1. Chemistry, 6(6), 1326-1335. https://doi.org/10.3390/chemistry6060077






