Abstract
Small bifunctional molecules are attractive for use as models in different areas of knowledge. How can their functional groups interact in solids? This is important to know for the prediction of the physical and chemical properties of the materials based on them. In this study, two new hydrogen-bonded organic frameworks (HOFs) based on sterically demanding molecular compounds, bis(1-hydroxy-2-methylpropane-2-aminium) sulfate (1) and 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate (2), were synthesized and fully characterized by means of FTIR and NMR spectroscopies, as well as by X-ray powder diffraction and thermogravimetric analyses. Their molecular and crystal structures were established through single-crystal X-ray diffraction analysis. It was shown that both compounds have a layered structure due to the formation of a 2D hydrogen-bonding network, the layers being linked by systematically arranged Van der Waals contacts between the methyl groups of organic cations. To unveil some dependencies between the chemical nature of bifunctional molecules and their solid structure, Hirschfeld surface (HS) analysis was carried out for HOFs 1, 2, and their known congeners 1-hydroxy-2-methylpropan-2-aminium hemicarbonate (3) and 1-hydroxy-2-methylpropan-2-aminium (1-hydroxy-2-methylpropan-2-yl) carbamate (4). HS was performed to quantify and visualize the close intermolecular atomic contacts in the crystal structures. It is clearly seen that H–H contacts make the highest contributions to the amino alcohol based compounds 1, 3 and 4, with a maximal value of 65.2% for compound 3 having CO32− as a counterion. A slightly lower contribution of H–H contacts (64.4%) was found for compound 4, in which the anionic part is represented by 1-hydroxy-2-methylpropan-2-yl carbamate. The significant contribution of the H–H contacts in the bifunctional moieties is due to the presence of a quaternary carbon atom with a short three-carbon chain.
1. Introduction
Bifunctional small organic molecules are very attractive for use as models of different active sites in biological systems since they are capable of coordinating vital d-metal cations such as Co(II), Cu(II), Fe(II/III) and Zn(II) [1,2,3]. Bifunctional molecules can also be useful as starting compounds for the synthesis of heterocyclic organic ligands. For example, amino alcohols have been used to obtain sterically hindered cyclic amines, such as five-membered oxazolidines [4] and six-membered oxazinanes [5], which give stable nitroxyl radicals after oxidation [6,7]. However, sterically crowded amino alcohols themselves, and their possible precursors amino ketones, are poorly studied, as are their salts and coordination compounds. This is particularly relevant when studying the supramolecular organization of such objects, as they could be applied as materials based on ionic hydrogen-bonded organic frameworks (HOFs) [8,9,10]. Since the presence of two functional groups in a molecule provides a well-developed network of hydrogen bonds, making crystals of bifunctional molecules containers for carbon dioxide or other molecular moieties having donor electron pairs is possible [11,12,13,14,15].
Hydrogen-bonded organic frameworks (HOFs), a class of solids built on weak non-covalent intermolecular interactions (hydrogen bonding and π–π stacking), have rapidly evolved in the recent past into multifunctional materials for proton conductivity [16,17,18], separation and purification [19,20,21,22], gas storage [23,24], sensing [25,26,27], enzyme encapsulation [28,29,30], antibacterial activity [31,32] and other applications [33,34,35,36,37]. By exploiting hydrogen bond features, it is possible to design non-porous molecular crystals involving hydrogen bonds that exhibit pore-opening responses to certain guest molecules. This phenomenon can greatly enhance their usefulness in molecule recognition and separation.
2-amino-2-methyl-1-propanol (Scheme 1) is a hygroscopic substance, which is capable of easily absorbing carbon dioxide from air. Recently, it was shown that this amino alcohol can be used for CO2 uptake [11,12]. In this process, two main products are formed, 1-hydroxy-2-methylpropan-2-aminium hemicarbonate (3) and 1-hydroxy-2-methylpropan-2-aminium (1-hydroxy-2-methylpropan-2-yl)carbamate (4). For compound 3, both studied polymorphs are layered (2D) ionic HOFs, whereas a crystal of 4 is a neutral HOF in which carbamate and amino alcohol molecules alternate in a 1:1 ratio. A free base, 2-amino-2-methyl-propan-1-ol, well known as a starting material for the synthesis of DOXYL nitroxyl radicals [38], can be recovered from CO2 absorption products by treating them with strong acid followed by neutralization. Another bifunctional molecule, 4-amino-4-methylpentan-2-one (Scheme 1), was also obtained previously in the form of its hydroxalate hydrate [39], but the crystal and molecular structures of the latter have not yet been established.

Scheme 1.
Bifunctional molecules amino alcohol and amino ketone, as well as their derivatives.
This paper concerns the preparation of two new ionic HOFs based on salts of sterically crowded bifunctional cations, bis(1-hydroxy-2-methylpropan-2-aminium) sulfate (1) and the not yet structurally described diacetoneamine hydroxalate hydrate (2). A modified recipe was developed for the synthesis of the latter. In addition to the structural and thermal characterization of the compounds, special attention has been paid to the comparative study of supramolecular interactions in HOFs of 1–4 by means of the analysis of the Hirschfield surface, which, along with IR spectra, are considered as the fingerprints for organic molecular compounds. Furthermore, we have performed a meticulous search of the CCDC database to detect and classify HOFs that include cations 1-hydroxy-2-methylpropane-2-aminium and 2-methyl-4-oxopentan-2-aminium.
2. Experimental Section
2.1. Materials and Methods
2.1.1. Materials
Solvents, concentrated sulfuric and solid oxalic acids of the reagent grade (Vecton, Saint Petersburg, Russia), and 2-amino-2-methyl-1-propanol (99%) (Sigma-Aldrich, Saint Louis, MO, USA) were used as received. The compounds were synthesized under aerobic conditions.
2.1.2. Elemental Analysis
The contents of C, H, N and S were determined by standard methods with a Euro-Vector 3000 analyzer (Eurovector, Redavalle, Italy).
2.1.3. Fourier Transform Infrared (FTIR) Spectroscopy
FTIR spectra were recorded in KBr pellets with a Perkin–Elmer System 2000 FTIR spectrometer (Perkin Elmer, Waltham, MA, USA) in the 4000–400 cm−1 range.
2.1.4. Nuclear Magnetic Resonance Spectroscopy
The 1H NMR (500.13 MHz), 13C {1H} NMR (125.76 MHz) and HSQS spectra were recorded at 298 K in D2O as a solvent on a Bruker Avance III 500 spectrometer (Bruker Biospin, Karlsruhe, Baden-Württemberg, Germany).
2.1.5. Investigation of the Compounds Thermal Stability
Thermal analyses were carried out on a TG 209 F1 Iris thermo-balance (NETZSCH, Selb, Germany). The measurements were conducted in a helium flow using the heating rate of 10 °C min−1; the gas flow rate was 60 mL min−1 in Al crucibles for the samples with the mass of ~5 mg. The melting points were measured on a heating table BOETSIUS RN MK 05 with an observation microscope (Franz Küstner Nachfolger KG, HMK, Dresden, Germany).
2.1.6. Powder X-ray Diffraction (PXRD)
The XRD analyses of the polycrystalline powders were performed on a Shimadzu XRD-7000 diffractometer (CuKα radiation, Ni filter, linear One Sight detector, 0.0143° 2θ step, 2 s per step).
2.1.7. Intermolecular Interactions Analysis
The analysis of Hirschfeld surface was performed using the CrystalExplorer program (version 17.5) [40,41].
2.1.8. Crystal Structure Visualization
The compound structures were visualized and their drawings were made with the help of the Diamond 3.0 (Crystal Impact GbR: Bonn, Germany) and OLEX2-1.5 [42] software.
2.2. Single-Crystal X-ray Crystallography Data Collection and Refinement
Diffraction data for a single crystal of 1 were obtained at 150 K on an automated Bruker D8 Venture diffractometer (Karlsruhe, Germany) equipped with a CMOS PHOTON III detector and IμS 3.0 source (MoKα, graphite monochromator, φ- and ω-scans with a step of 0.5°). Absorption corrections were applied using SADABS [43]. Diffraction data for single crystals 2 and 3 were obtained at 291 K on an automated Agilent Xcalibur diffractometer equipped with an area CCD AtlasS2 detector (MoKα, graphite monochromator, and ω-scans with a step of 0.5°). The integration, absorption correction, and determination of unit cell parameters were performed using the CrysAlisPro program package [44]. The structures were solved by the dual space algorithm (SHELXT [45]) and refined by the full-matrix least squares technique (SHELXL [46]) in the anisotropic approximation (except hydrogen atoms). For compound 1, some low-angle reflections were ignored during the refinement due to their truncation by the beam stop. The positions of hydrogen atoms for organic cations were calculated geometrically and refined in the riding model, while the rotation of CH3, NH3 and OH groups was allowed. The positions of hydrogen atoms of a water molecule were found from the difference Fourier map, and the O–H and H…H distances were restrained during the refinement. The crystallographic data and details of the structure refinements are summarized in Table 1. CCDC 2265962, 2265963 and 2220607 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/ accessed on 30 July 2024.

Table 1.
Crystal data and structure refinement for 1 and 2.
2.3. Synthesis
2.3.1. Bis(1-hydroxy-2-methylpropan-2-aminium) Sulfate (1)
The crystalline colorless product of compound 1 was obtained by the slow evaporation of an ethanol solution of amino alcohol and sulfuric acid in a 1:1 ratio. An additional product can be obtained by saturating the mother liquor with diethyl ether. The average yield for a series of three syntheses was 94%. Anal. calcd. (%) for C8H24N2O6S: C, 34.77; H, 8.75; N, 10.14; S, 11.6. Found: C, 34.8; H, 3.7; N, 10.0; S, 11.3; IR see in SM.
2.3.2. 2-Methyl-4-oxopentan-2-aminium Hydrogen Ethanedioate Hydrate (2)
The title compound was synthesized according to the known procedure [39], which was modified. A mixture of mesityl oxide (58 g) [47] and 80 mL of concentrated (27%) aqueous solution of NH3 was agitated during three days at room temperature. Then, the yellowish reaction mixture was bubbled with air for three days to remove the nonreacted ammonia and, upon agitation, was gradually added to a cold ethanol (300 mL) solution of oxalic acid (75 g). The clear precipitate of 2 was filtered from orange brownish mother liquor, and washed a few times with cold ethanol (4 × 50 mL) and once with 70 mL of Et2O. After air-drying, slightly rose-colored polycrystalline powder of the desired product was obtained. Yield: 105 g (80%). 1H NMR spectrum (500 MHz, D2O), δ, ppm: 1.31 (6H, C(CH3)2); 2.16 (3H, C(O)CH3); 2.91 (2H, CH2); 4.54 (exchange peak from OHn and NHn species); 13C {1H} NMR spectrum (126 MHz, D2O), δ, ppm: 212.41 (>C=O), 165.61 (oxalate), 52.42(>C=), 49.47 (CH2), 30.53 (C(O)CH3), 25.01 (C(CH3)2). Anal. calcd. (%) for C8H17NO6 (Mw = 223.22): C, 43.04; H, 7.68; N, 6.27. Found: C, 42.8; H, 7.7; N, 6.3.For IR spectrum see in Supplementary Materials.
The colorless single crystals of 2 for SCXRD analysis (mp. 125–127 °C) were grown during the slow evaporation of the combined alcohol washes from the reaction beaker and filter crystals.
3. Results and Discussion
3.1. Synthetic Aspects
Formerly, 2-amino-2-methyl-1-propanol was used to synthesize DOXYL-type nitroxyl radicals [6,38,48], and their tripodal derivative [49], which we used for the preparation of the spin-exchanged coupled LnIII complexes [50,51]. For this purpose, the first step toward a paramagnetic tripodal ligand is the condensation of an amino alcohol with bis-(2-pyridyl) ketone in the presence of sulfuric acid as a catalyst [49]. We have observed that when a large excess of the amino alcohol was used, a white precipitate was formed during the prolonged reaction in aerobic conditions. This powder, formed upon crystallization from an aqueous solution, gave crystals identical to the earlier-studied polyform of 1-hydroxy-2-methylpropan-2-aminium hemicarbonate [11], the latter having evolved into gaseous carbon dioxide when sulfuric acid solution was added either to the initial white powder or to the crystals as well. Thus, through the sulfate production step, followed by neutralization and extraction, 2-amino-2-methyl-1-propanol can be recycled or purified. This is particularly relevant because, being hygroscopic, amino alcohol actively absorbs moisture and carbon dioxide from air during prolonged storage, and,2 in any case, needs to be purified before use. On the other hand, the crystalline bis(1-hydroxy-2-methylpropane-2-aminium) sulfate (1), which was obtained by the method indicated in the experimental part, is not hygroscopic, and does not change its composition for a long time.
When reacting with concentrated aqueous ammonia, mesityl oxide [47] attaches a molecule of NH3 at the double bond to form 4-amino-4-methyl-pentan-2-one (also named diacetoneamine, top of the Scheme 2), which is too volatile to be treated by the classical distillation method. For this reason, it is more convenient to isolate and purify diacetoneamine as its acid oxalate (2) (see Scheme 2, bottom). Pure amino ketone could be reduced to its corresponding amino alcohol (4-amino-4-methyl-pentan-2-ol) immediately prior to subsequent use as a reagent for nitroxyl radical synthesis [5,6,7,52,53]. Compound 2 was obtained in a satisfactory yield (see experimental section). The single crystals (colorless prisms) were formed during compound crystallization from an ethanol solution (See photo of the crystals in Figure S1, Supplementary Materials (SM)).

Scheme 2.
Reaction of addition of ammonia to mesityl oxide (top); titration of the reaction mixture with an ethanol solution of oxalic acid (bottom).
3.2. FTIR Spectra
In general, the main organization of the band-rich IR spectrum of 1 (Figure S2, SM) is similar to that of the spectrum of bis(1-hydroxy-2-methylpropan-2-aminium) selenate [54], with the only difference that the sulfate bands are significantly shifted compared to the positions of selenate bands (Figure S3, SM). The FTIR spectrum of compound 2 evidences the presence of a loaded hydrogen bonding network, and is almost perfectly matched with the spectrum of diacetonamine hydrogen oxalate from the Wiley spectral data base; see Figure S4, SM.
3.3. Thermal Analysis Results
Thermogravimetric analyses were carried out for both crystalline materials of compounds 1 and 2. As can be seen on Figures S5 and S6 (SM), despite the close chemical nature of the cations constituting compounds 1 and 2, the thermal behaviors of these substances are very different. Alcohol derivative 1 is much more robust when heating in an inert atmosphere compared to amino ketone species 2. This is primarily due to the absence of water molecules in the crystals of substance 1. The latter starts to lose weight only above 200 °C, whereas the polycrystalline sample of compound 2, after a slight loss of natural moisture in the temperature range 31–96 °C, begins to decompose. The further thermal degradation process is very different for the sulfate salt of amino alcohol and for the hydroxalate salt of amino ketone. When the temperature reaches 248 °C (there are peaks on the DTG and c-DTA curves), substance 1 loses about 65% of its weight, which corresponds to the complete removal of the amino alcohol. Consequently, upon further heating, the sulfuric acid transformations occur.
The process of the thermal decomposition of compound 2 is much richer in transformations compared to the thermolysis process of substance 1. This is mostly because oxalic acid decomposes into carbon dioxide and formic acid when heated. In addition, the sublimation or decomposition processes of an organic bifunctional molecule can proceed in parallel. In the temperature range 96–137 °C, according to DTG and DTA curves, two processes take place; one is the loss of solvated water, which is superimposed on the second, the melting process. In this case, the total mass loss is about 9%, which agrees well with the calculated value of 8.06%. Nevertheless, the presence of minor peaks on the DTG and c-DTA curves for 2 is possibly an indication of the presence of small amounts of impurities. These impurities may be related to the ammonium salts of oxalic acid and their hydrates, due to the incomplete removal of ammonia from the final reaction mixture. A detailed investigation of these processes is beyond the scope of this study.
Since the thermal analysis for compound 2 was performed on a freshly settled fine crystalline sample, we decided to accomplish NMR spectroscopy analyses for the same batch of 2 in order to identify possible impurities.
3.4. NMR Spectrocopy Investigations
The results of the 13C and 1H NMR spectroscopic studies of the D2O solution of 2 are shown in Figures S7 and S8 (SM), respectively. Signals attribution was performed using the HSQS NMR spectrum, Figure S9. It should be mentioned that due to the lack of an internal standard in the solutions used for the spectroscopic study, the chemical shift values are slightly moved in one direction, but this does not prevent their assignment to specific atoms. Nevertheless, none of the spectra revealed the presence of a carbon-containing impurity other than the main substance, organic bifunctional cation or oxalate anion. This indicates that the outer species is, most likely, an NH4+ containing salt of oxalic acid (intermediate or acidic), as the broad peak at ~4.7 ppm can include a number of impurities (Figure S8 (SM)). As an additional option, X-ray powder diffraction analysis was carried out on both compounds. The PXRD study results are placed immediately after the next section on crystal structure analysis.
3.5. Crystal Structures
Single crystals of compounds 1 and 2 were grown by the slow evaporation of the corresponding ethanolic solutions at room temperature. Both compounds crystallize in monoclinic space group P21/n (Table 1).
3.5.1. Crystal Structure of the Compound 1
The crystals of compound 1 are colorless prisms. There are two 1-hydroxy-2-methylpropan-2-aminium cations for one SO42− dianion in the asymmetric unit in the absence of any solvent molecules (Figure 1a).

Figure 1.
Asymmetric unit of the compounds: (a) bis(1-hydroxy-2-methylpropane-2-ammonium) sulfate (1); (b) 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate (2).
In the structure, all hydrogen atoms of the protonated amino groups of the bifunctional moiety are hydrogen-bonded; in addition, all four oxygen atoms of the sulfate anion are also included in the hydrogen bond (HB) network, forming 2D ionic HOF (Figure 2). All types of HBs in compound 1 are visualized in Figure 3, and their geometric parameters are presented in Table S1, SM. Three oxygen atoms of the SO42− anion form two HBs each, while the fourth forms only one. The alcohol group, OH, of the first cation is involved in the two HBs, while the OH group of the second cation participates in only one HB.

Figure 2.
Formation of a layer in the crystal of compound 1 due to hydrogen bonding (view along b axis).

Figure 3.
Eight types of hydrogen bonds in compound 1. The distances of the hydrogen bonds are given between the atoms O and N or O and O in Å.
The supramolecular layers are situated on (202) planes. Similar layers, outlined by the (CH3)2–C–CH2– alkane groups, were also found in the closely related HOFs formed in the crystal structures of 2(Me2(NH3)CH2OH)XO4, where X = SeO42− [54] or MoO42− [55], respectively, as well as in 1-hydroxy-2-methylpropan-2-aminium hemicarbonates [11,56] (Figure S10, SM). However, in carbamate (4) [11] (Figure S11, SM), which is “formally obtained by dehydration of compound 3”, such a systematic arrangement of the Van der Waals contacts between methyl groups is absent because the hydrogen bonding in compound 4 has a three-dimensional character, classifying it as 3D ionic HOF. Despite the commercial availability of the amino alcohol precursor, we were able to find only a little more than a dozen records in the CCDC database listing the 1-hydroxy-2-methylpropan-2-aminium cation. With one exception [57], all solids are ionic HOFs. Most of them belong to 2D HOFs, six including an inorganic anion [54,55,56,58,59,60] and only three comprising an organic one [13,61,62]. In addition, a series of five solids representing 3D HOFs were detected by analyzing the CCDC search results. Two of them are the halide salts of HOCH2(C)Me2NH3+X− (X = Cl, Br) [63,64], and one is a pentaborate-containing species [65]. Another comprises a bifunctional terpenoid derivative as an anion [66]. The last representative of this series is a rare example of a three-component HOF that includes a neutral Cu(II) bischelate of deprotonated form of 2-amino-2-methyl-1-propanol, in addition to its singly protonated cation and a formate anion [67].
3.5.2. Crystal Structure of Compound 2
The asymmetric part of compound 2, comprising only one bifunctional cation, 2-methyl-4-oxopentan-2-aminium, one hydrogen oxalate anion and one molecule of water, is shown in Figure 1b. The main dissimilarity between the compounds 2 and 1 is the presence of an aqua molecule in the former. In addition, the hydrooxalate anion differs very much from the sulfate anion in shape and chemical nature. Some of the donor atoms of the molecular units in compound 2 do not participate in the formation of the HB network; for example, an oxygen of the ketone group and one from the four oxygen atoms belonging to the hydrooxalate anion. As a result, one of the hydrogen atoms of an ammonium group is outside of the HB system. The geometry parameters of the HBs for compound 2 are accumulated in Table S2, SM. Similar to compound 1, the layers here are formed in a crystal of 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate (see Figure 4 and Figure S12, SM). The supramolecular layers are located on (101) planes. The formation of the 2D HOF in 2 is mainly due to the hydrogen oxalate anions and water molecules. In addition, two of the three H atoms of the {–NH3+} group of the bifunctional moiety participate in the 2D supramolecular structure of 2. Furthermore, the layers, formed in this way, are “cross-linked” due to the Van der Waals contacts between the alkane’s hydrogens of the 2-methyl-4-oxopentan-2-aminium cations (Figure 4). In the literature, the aminoketone is reported to form rare 1D ionic HOFs upon crystallization in the presence of 3,5-dinitrobenzoic [68] or salicylic acid [69], while its interaction with a solution of 2-sulfamoyl-benzoic acid leads to 2D ionic HOF [70].

Figure 4.
The 2D network organization and the interlayer Van der Waals interactions, which are realized through contacts between the methyl groups of 2-methyl-4-oxopentan-2-aminium moieties in the crystal of compound 2 (view along b axis).
The only known compound where the cation 2-methyl-4-oxopentan-2-aminium is coordinated to the metal ion is the oxalate complex of copper(II), [Cu(Oxalate)2(MeC(O)CH2(C)Me2NH3+)2] [71]. In its crystal structure, there are layers formed by HBs between oxygen atoms of bidentate coordinated oxalate and hydrogen atoms of NH3+ groups. The layers, in turn, are connected in a 3D network by the coordination bonds of the copper–ketone group of 2.66 Å. Another 2D anionic HOF is a combination of the 2-methyl-4-oxopentan-2-aminium cation and anion complex of beryllium biphthalate [72]. The only structurally characterized compound of 4-amino-4-methyl-pentan-2-ol, a bifunctional molecule, derived from the corresponding ketone, is 4-hydroxy-2,4-dimethylpentane-2-amminium iodide [53], which belongs to the class of 2D HOFs.
It should be emphasized that unlike compound 2, containing one water molecule, solids 1 and 3 lack the molecules of H2O, while the latter were present in sufficient amounts in the crystallization medium. The analysis of the Cambridge structural database revealed only one other HOF with H2O in its composition [58].
In order to estimate the intermolecular interactions in the crystals, we have performed the calculation of the Hirschfeld surface (HS) for the crystals of 1, 2, and their related compounds 3 and 4. The results of these calculations are discussed in Section 3.7.
3.6. Powder X-ray Diffraction Investigations
The experimental powder X-ray diffraction (PXRD) patterns and the theoretically calculated ones, obtained from the single-crystal data for compounds 1 and 2, are presented in Figures S13 and S14 (SM), respectively. Considering that the experimental PXRD pattern for the sulfate of amino propanol, 1, was recorded at room temperature while the simulated one was calculated from the single-crystal X-ray data (SCXRD) collected at 150 K, there is a satisfactory coincidence between the diffractograms. This confirms the phase purity of compound 1. However, the same conclusion cannot be drawn for 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate, 2. The diffractograms for the latter are depicted in Figure S14, SM. The experimental pattern for the bulk sample of 2, obtained directly from the synthesis, is colored in black. Another, colored in red, is a batch of compound 2, recrystallized from ethanol. In the black plot some additional reflections, marked with a blue asterisk, are missing in the green plot, calculated from the SCXRD data. After testing several ammonia compounds of oxalic acid as impurities, it was concluded that the extra peaks in the black plot belong to ammonium hydrogen oxalate hemihydrate, (NH4)2C2O4(H2O) (5) [73]. In the figure, the diffractogram of the latter is colored blue. Hence, it is possible to remove an impurity of compound 5 present in a raw sample by a simple recrystallization of the synthesis product from ethanol. In addition to C, H and N analysis, the phase purity of the recrystallized product was confirmed by PXRD data (the red pattern in Figure S14 (SM)).
3.7. Hirschfeld Surface Analysis
3.7.1. Short Theoretical Introduction in the Hirschfeld Surface Analysis
Historically, the concept of the Hirschfeld surface arose as a result of an attempt to determine the space occupied by a molecule in a crystal in order to localize the electron density of a whole solid into molecular fragments [74,75]. HS is used to determine the spatial arrangement of contacts created by different segments of a molecule. HS was named after F.L. Hirschfeld, who had proposed a scheme for separating the electron density in a crystal, and a weight function for each atom in a molecule [76], i.e., such surfaces are obtained by converting the weight function describing an atom in a molecule to the weight function of a molecule in a crystal. The resulting isosurface, with a specified weight function w(r) = 0.5, envelops the molecule and, by subdividing the electron density of the molecular fragments, delineates the space occupied by a molecule in a crystal [77]. Other molecular surfaces (Van der Waals surface, outer surface of the electron density) are determined only by the molecule itself [78], while the Hirschfeld surface is determined by the molecule and the proximity of its nearest neighbors, thus encoding information about intermolecular interactions [75].
3.7.2. Hirschfeld Surface Analysis for the Solids of 1 and 2 and Their Two Related Compounds
HS analysis [79] was carried out to quantify and visualize the close intermolecular atomic contacts in the crystal structures for all compounds. The directions and strengths of intermolecular interactions within the molecular crystal are mapped onto the Hirschfeld surface using the descriptor dnorm. The normalized contact distance (dnorm) is based on the two contact distances between nearest atoms present inside (di) and outside (de) the surface, respectively, and is expressed as dnorm= ((di − rivdw)/rivdw)⁄((de − revdw)/revdw), where rivdw and revdw are the Van der Waals radii of the appropriate atoms internal and external to the surface, respectively [75]. The crystal structure packing of the compound was generated and quantified by HS analysis and associated 2D fingerprint plots using the Crystal Explorer package [41]. The combination of di and de in the form of a 2D fingerprint provides a summary of intermolecular contacts.
In the HS diagrams, the contacts with distances equal to the sum of the Van der Waals radii are shown in white, and the contacts with distances shorter than or longer than Van der Waals radii are shown in red and blue, respectively.
Figure 5 and Table S3 contain information on the visualization of the Hirschfeld three-dimensional dnorm surface, mapped on the asymmetric unit of a cell (as the electrically neutral chemical basis of a crystal) for our compounds 1 and 2, as well as for their two congener compounds, 1-hydroxy-2-methylpropan-2-aminium hemicarbonate (3) and 1-hydroxy-2-methylpropan-2-aminium carbamate (4). The concentrated red hot spots on the surface, colored according to dnorm, are associated with the interactions involving the oxygen atoms, corresponding to the hydrogen bonds indicated in Tables S1 and S2 (SM). The blue regions correspond to longer contacts with a positive dnorm value, and white regions correspond to the distance of contacts exactly equal to the Van der Waals spaces with a dnorm value of zero.
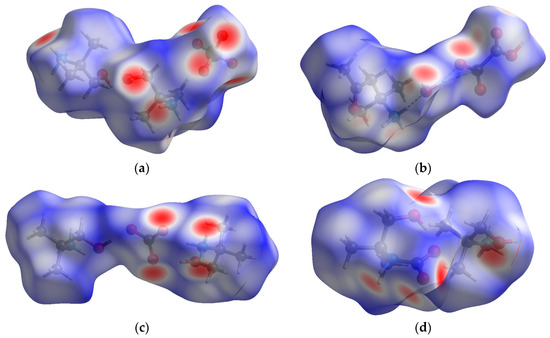
Figure 5.
Hirschfeld surface for the compounds (view along a-axis): (a) 1; (b) 2; (c) 3 [11]; (d) 4 [12].
Figure S15 (SM) illustrates the associated 2D fingerprint plots of the compounds, demonstrating the contributions of the different intermolecular interactions on the Hirschfeld surface. The fingerprint plots can be decomposed to highlight particular close contacts in atom pairs, which are summarized in Table 2. From the latter, it can be clearly seen that Van der Waals interactions caused by the H…H contacts make the highest contributions for the 1-hydroxy-2-methylpropan-2-aminium-based compounds (1, 3 and 4), with a maximal value of 65.2% for compound 3 comprising CO32− as the anion.

Table 2.
Contributions of different interatomic contacts (X…Y) to the Hirschfeld surface (%), calculated from the compounds’ fingerprint plots (see Figure S15, SM).
A slightly lower contribution (64.4%) was found for the H…H contacts in compound 4, in which the anionic part is represented by a singly charged anion of (1-hydroxy-2-methylpropan-2-yl)carbamate. In our opinion, such a significant contribution of the H…H contacts in the aminopropanol compounds is due to the presence of a quaternary carbon atom with a short carbon chain length (only three carbon atoms). The smallest contribution to the interatomic H…H contacts was found for compound 2. This can be explained by the fact that both the cation and the anion for this HOF have larger sizes compared to compounds 1 and 3 with the 1-hydroxy-2-methylpropan-2-aminium cation. It would be interesting to compare the structural motif of the HOF based on 2 with its analog containing a chemically reduced relative, 4-aminium-4-methyl-pentan-2-ol, as the cation. It is possible that the crystallization product of a mixture of 4-amino-4-methyl-pentan-2-ol and oxalic acid from an ethanol solution would be a neutral salt, possibly not including water molecules. However, the latter may not occur if in this case the driving force in the crystallization process is also the formation of an anionic layer in which water molecules bind oxalate anions in a 2D network, and cations only supplement this main network, joining it from below and from above via –NH3+ groups (Figure S12, bottom, SM). In compound 2, the ketone group is short enough to form hydrogen bonds. However, if it were replaced by an alcoholic OH group, the latter would be more likely to form a hydrogen bond. At present, no oxalate salts have been determined for 2-amino-2-methyl-1-propanol, but it is quite possible that a situation similar to that seen for compound 2 may also occur for this bifunctional molecule if the “crystallization template” is the water–hydrogen oxalate anionic layer, unlike the two modifications [11,56] of aminopropanol carbonate, which do not contain water molecules (Figure S10, SM).
The next most represented interactions are related to the O…H contacts, which are the hydrogen bonds between the C–OH (or C=O), NH and HOH fragments of the constituent moieties. The compounds richest in O…H contacts are 1 (47.5%) and 2 (51.7%). The higher value of O…H contacts’ contribution observed for HOF 2 can be attributed to the greater shape–volume complementarity between the cations and anions that make up the crystal of compound 2, as well as the presence of a water molecule. In addition, the hydroxalate anions are hydrogen-bonded to each other. The steric inaccessibility of the ketone oxygen atom prevents the formation of short intermolecular contacts, which also explains the low contribution of O…C (0.7%) and O…O (0.8%) contacts for 2.
It is important to note that three of the four compounds (1, 2, 3) are 2D HOFs, due to the formation of a 2D network of HBs. In compound 4, the differentially charged moieties are locked into a 3D supramolecular system by HBs.
4. Conclusions
A bifunctional compound, 2-amino-2-methyl-propan-1-ol, can be recovered from the products of its interaction with carbonic gas in air (compounds 3 and 4) passing through recrystallization from the H2SO4–ethanol solutions as a differential procedure. During the latter, single crystals of bis(1-hydroxy-2-methylpropane-2-aminium) sulfate (1) were obtained. Another sterically crowded (relative to 1) 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate, 2, was obtained, by a procedure modified from the literature, in a very pure crystalline state with excellent yield. For this commercially available substance, the crystal structure was established for the first time. SCXRD analysis revealed that compounds 1 and 2 are new representatives of a class of ionic HOFs, according to the CCDC database, a search of which showed that only 12 HOFs containing protonated 2-amino-2-methyl-1-propanol have been studied to date. Among these, five species are 3D, and seven are 2D HOFs. For the 2-methyl-4-oxopentane-2-ammonium cation, the statistics are much poorer: only five HOFs have been found. Among these, one is 1D, three are 2D, and one is a 3D HOF, the latter representing the only transition metal complex, trans-bis(2-methyl-4-oxopentan-2-aminium-O)-bis(oxalato-O,O′)-copper(II) [71]. Only one CSD record deals with a close relative of the amino ketone, the iodide of protonated 4-amino-4-methylpentane-2-ol [53], which has a 2D supramolecular structure.
The structural analysis has revealed that due to the abundant hydrogen bonding in both salts, 1 and 2, supramolecular 2D networks are present in the solid state. However, between the other two salts, 3 and 4, which are the congeners of 1, only compound 3 exhibits a layered organization, while the carbamate 4 belongs to the 3D HOFs family. Moreover, the analysis of all the known HOFs involving the bifunctional molecular cations considered in this paper revealed a very fascinating fact, as follows: in only three cases, H2O molecules are present in the crystalline phase of the HOF. Apparently, this is due to the steric hindrance of a hydrocarbon part of the bifunctional moieties, which makes them hydrophobic.
The Hirschfeld surface analysis was used to reveal the relationships between the chemical nature of the bifunctional molecules and the structure of their salts in solids. A quantification was performed to visualize the close intermolecular atomic contacts in crystals of 1 and 2, as well as for solids of their earlier studied relatives—1-hydroxy-2-methylpropan-2-aminium (1-hydroxy-2-methylpropan-2-yl) carbamate, 4, and 1-hydroxy-2-methylpropan-2-aminium hemicarbonate, 3. In the fingerprints calculated for all four analyzed HOFs, there is a predominance of interhydrogen contacts over all others. This is due to the presence of a large number of hydrogen atoms of the sterically crowded group (CH3)2–C–CH2–, which is a common element for cations of 1–4. It was stated that H…H contacts make the highest contributions for the amino alcohol-based compounds 1, 3 and 4, with a maximum value of 65.2% for compound 3 with CO32− as the anion. A slightly lower contribution of H…H contacts (64.4%) was established for compound 4, in which the anionic part is represented by (1-hydroxy-2-methylpropan-2-yl) carbamate. The smallest contribution to interatomic H…H contacts was found for compound 2. This can be explained by the fact that both the cation and the anion for this HOF have larger sizes compared to compounds 1 and 3, where 1-hydroxy-2-methylpropan-2-aminium is the cation.
From the point of view of the use of the two new HOFs studied here, it can be tentatively assumed that they can be tested for the separation of mixtures of organic molecules containing alkane groups with different numbers of carbon atoms, and which are soluble in lower monohydric alcohols. Not being specialists in this field, we can cautiously assume that during crystallization from solution, organic molecules will be located in the interlayer space due to the presence of alkane tails. In addition, by introducing an aromatic fragment into the composition of these bifunctional molecules, it is possible to use them for the purification or separation of mixtures of alkanes and aromatic compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry6050073/s1, Figure S1: Crystals of 2; Figure S2: FTIR spectrum of bis(1-hydroxy-2-methylpropan-2-aminium) sulfate (1); Figure S3: FTIR spectrum of bis(1-hydroxy-2-methylpropan-2-aminium) selenate; Figure S4: FTIR (KBr) spectrum of 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate, 2 (top); FTIR (ATR) of diacetonamine hydrogen oxalate from spectrabase.com/spectrum/F8xjNGWf5DQ (accessed 30.06.2024); Figure S5: Thermal analysis results for 1; Figure S6: Thermal analysis results for 2; Figure S7: The results of the 13C NMR spectrum registered in the D2O solution of 2; Figure S8: The results of 1H NMR spectrum registered in D2O solution of 2; Figure S9: HSQS NMR spectrum registered in the D2O solution of 2; Figure S10: Mediated by hydrogen bonding, the layer formation in the crystals of the two different polymorphs of 1-hydroxy-2-methylpropan-2-aminium hemicarbonate 3: top—C 2/c, (101) view; bottom—P Ī, (100) view; Figure S11: Molecular structure of 1-hydroxy-2-methylpropan-2-aminium (1-hydroxy-2-methylpropan-2-yl)carbamate 4; Figure S12: A layer formation in 2 owing to hydrogen bonding in the 101 plane: top—full view; bottom—anionic motif; Figure S13: The PXRD patterns for bis(1-hydroxy-2-methylpropane-2-ammonium) sulfate, 1. The experimental pattern was recorded at RT, while the theoretical one was calculated from SCXRD data collected at 150 K; Figure S14: The diffractograms for 2-methyl-4-oxopentan-2-aminium hydrogen ethanedioate hydrate, 2, black and red—experimental, green—calculated. The blue pattern—(NH4)2C2O4(H2O). All diffraction data were collected at room temperature; Figure S15: Figure prints for 1, 2 and 3—1-hydroxy-2-methylpropan-2-aminium hemicarbonate 4—1-hydroxy-2-methylpropan-2-aminium (1-hydroxy-2-methylpropan-2-yl)carbamate; Table S1: Hydrogen bonds in 1; Table S2: Hydrogen bonds in 2; Table S3: The views along different crystallographic axes of Hirschfeld surface for the compounds 1–4.
Author Contributions
Conceptualization and methodology, K.E.V.; software, D.G.S.; investigation, all; resources, K.E.V.; data curation, D.G.S. and V.P.K.; writing—original draft preparation, K.E.V. and D.G.S.; writing—review and editing, K.E.V.; visualization, K.E.V.; supervision, K.E.V.; project administration, K.E.V. funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation (project RSF No. 23-23-00437).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The crystallographic data and details of the structure refinements are summarized in Table 1. CCDC 2265962 and 2265963 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center at https://www.ccdc.cam.ac.uk/structures/ accessed on 30 July 2024.
Acknowledgments
K.V.P. thanks the Ministry of Science and Higher Education of the Russian Federation, project No 121031700313-3 for supporting the NMR spectroscopy studies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Harrowfield, J.M. Biological Coordination Chemistry, a Confluence of Chemistry and Biochemistry. Comptes Rendus Chim. 2005, 8, 199–210. [Google Scholar] [CrossRef]
- Apfel, U.-P.; Suntharalingam, K. Bioinspired Reactivity and Coordination Chemistry. Dalt. Trans. 2019, 48, 5859–5860. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.H. Coordination Compounds in Biology. Resonance 1999, 4, 67–77. [Google Scholar] [CrossRef]
- Bergmann, E.D. The Oxazolidines. Chem. Rev. 1953, 53, 309–352. [Google Scholar] [CrossRef]
- Rassat, A.; Rey, P. Nitroxides. 23. Preparation of Amino Acid Free Radicals and Their Complex Salts. Bull. Soc. Chim. Fr. 1967, 3, 815–818. [Google Scholar] [PubMed]
- Keana, J.F.W. Newer Aspects of the Synthesis and Chemistry of Nitroxide Spin Labels. Chem. Rev. 1978, 78, 37–64. [Google Scholar] [CrossRef]
- Rassat, A.; Rey, P. Nitroxydes—LXII: Nitroxydes Oxaziniques: Synthese et Etude Conformationnelle. Tetrahedron 1974, 30, 3315–3325. [Google Scholar] [CrossRef]
- Li, P.; Ryder, M.R.; Stoddart, J.F. Hydrogen-Bonded Organic Frameworks: A Rising Class of Porous Molecular Materials. Acc. Mater. Res. 2020, 1, 77–87. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, Y.; Xiang, S.; Chen, B. Exploring Multifunctional Hydrogen-Bonded Organic Framework Materials. Acc. Chem. Res. 2022, 55, 3752–3766. [Google Scholar] [CrossRef]
- Lin, R.-B.; Chen, B. Hydrogen-Bonded Organic Frameworks: Chemistry and Functions. Chem 2022, 8, 2114–2135. [Google Scholar] [CrossRef]
- Barzagli, F.; Di Vaira, M.; Mani, F.; Peruzzini, M. Improved Solvent Formulations for Efficient CO2 Absorption and Low-Temperature Desorption. ChemSusChem 2012, 5, 1724–1731. [Google Scholar] [CrossRef] [PubMed]
- Hasib-ur-Rahman, M.; Larachi, F. CO2 Capture in Alkanolamine-RTIL Blends via Carbamate Crystallization: Route to Efficient Regeneration. Environ. Sci. Technol. 2012, 46, 11443–11450. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Jhon, Y.H.; Choi, S.B.; Shim, J.-G.; Kim, J.-H.; Lee, J.H.; Lee, I.-Y.; Jang, K.-R.; Kim, J. Crystal Structure and Electronic Properties of 2-Amino-2-Methyl-1-Propanol (AMP) Carbamate. Chem. Commun. 2010, 46, 9158–9160. [Google Scholar] [CrossRef] [PubMed]
- Chambers, L.I.; Yufit, D.S.; Musa, O.M.; Steed, J.W. Understanding the Interaction of Gluconamides and Gluconates with Amino Acids in Hair Care. Cryst. Growth Des. 2022, 22, 6190–6200. [Google Scholar] [CrossRef] [PubMed]
- Hosten, E.C.; Betz, R. The Crystal Structure of Bis(1,3-Dihydroxy-2-Methylpropan-2-Aminium) Carbonate, C9H24N2O7. Z. Krist.-New Cryst. Struct. 2021, 236, 297–299. [Google Scholar] [CrossRef]
- Bai, X.-T.; Cao, L.-H.; Zhao, F.; Li, S.-H. Arylsulfonate Ionic Hydrogen-Bonded Organic Frameworks Enable Highly Stable and Superprotonic Conductivity for Enhancing Direct Methanol Fuel Cells. ACS Mater. Lett. 2024, 6, 3351–3357. [Google Scholar] [CrossRef]
- Xing, G.; Yan, T.; Das, S.; Ben, T.; Qiu, S. Synthesis of Crystalline Porous Organic Salts with High Proton Conductivity. Angew. Chem. Int. Ed. 2018, 57, 5345–5349. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Wu, W.; Zhou, Z.; Gao, H.; Wang, J.; Jiang, Z. Hydrogen-Bonded Organic Framework Membrane with Efficient Proton Conduction. J. Memb. Sci. 2022, 664, 121118. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Huang, J.; Zhang, H.; Lin, S.; Ye, Z.-M.; Xiang, S.; Chen, B.; Zhang, Z. Self-Healing B ← N-Based Hydrogen-Bonded Organic Framework for Exclusive Recognition and Separation of Toluene from Methyl-Cyclohexane. J. Am. Chem. Soc. 2024, 146, 19425–19433. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Song, D.; Ji, Z.; Chen, C.; Wu, M. Robust Two-Dimensional Hydrogen-Bonded Organic Framework for Efficient Separation of C1–C3 Alkanes. Chem Bio Eng. 2024. [Google Scholar] [CrossRef]
- Ma, L.; Xie, Y.; Khoo, R.S.H.; Arman, H.; Wang, B.; Zhou, W.; Zhang, J.; Lin, R.; Chen, B. An Adaptive Hydrogen-Bonded Organic Framework for the Exclusive Recognition of p-Xylene. Chem.—A Eur. J. 2022, 28, e202104269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, C.; Ji, Z.; Krishna, R.; Di, Z.; Yuan, D.; Wu, M. A Layered Hydrogen-Bonded Organic Framework with C3H6-Preferred Pores for Efficient One-Step Purification of Methanol-to-Olefins (MTO) Products. ACS Mater. Lett. 2024, 6, 1388–1395. [Google Scholar] [CrossRef]
- Soleimani Abhari, P.; Gholizadeh, S.; Rouhani, F.; Li, Y.-L.; Morsali, A.; Liu, T.-F. Recent Progress in Gas Separation Platforms Based on Hydrogen-Bonded Organic Frameworks (HOFs). Inorg. Chem. Front. 2023, 10, 6134–6159. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Wang, C.; Wang, D.; Zhuang, G.; Kirlikovali, K.O.; Li, P.; Farha, O.K. Design Rules of Hydrogen-Bonded Organic Frameworks with High Chemical and Thermal Stabilities. J. Am. Chem. Soc. 2022, 144, 10663–10687. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, R.; Xie, L.-H.; Lin, Z.-J.; Zhang, X.; Wang, J.; Huang, H.; Zhang, Z.; Schanze, K.S.; Zhang, J.; et al. Microporous Hydrogen-Bonded Organic Framework for Highly Efficient Turn-Up Fluorescent Sensing of Aniline. J. Am. Chem. Soc. 2020, 142, 12478–12485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, D.; Yin, J.; Shang, Y.; Du, J.; Kang, Z.; Wang, R.; Chen, Y.; Sun, D.; Jiang, J. An Ultrafast Responsive NO2 Gas Sensor Based on a Hydrogen-Bonded Organic Framework Material. Chem. Commun. 2020, 56, 703–706. [Google Scholar] [CrossRef]
- Wang, C.; Song, X.; Wang, Y.; Xu, R.; Gao, X.; Shang, C.; Lei, P.; Zeng, Q.; Zhou, Y.; Chen, B.; et al. A Solution-Processable Porphyrin-Based Hydrogen-Bonded Organic Framework for Photoelectrochemical Sensing of Carbon Dioxide. Angew. Chem. Int. Ed. 2023, 62, e202311482. [Google Scholar] [CrossRef]
- Chen, G.; Tong, L.; Huang, S.; Huang, S.; Zhu, F.; Ouyang, G. Hydrogen-Bonded Organic Framework Biomimetic Entrapment Allowing Non-Native Biocatalytic Activity in Enzyme. Nat. Commun. 2022, 13, 4816. [Google Scholar] [CrossRef]
- Liang, W.; Carraro, F.; Solomon, M.B.; Bell, S.G.; Amenitsch, H.; Sumby, C.J.; White, N.G.; Falcaro, P.; Doonan, C.J. Enzyme Encapsulation in a Porous Hydrogen-Bonded Organic Framework. J. Am. Chem. Soc. 2019, 141, 14298–14305. [Google Scholar] [CrossRef]
- Liu, S.; Sun, Y. Co-encapsulating Cofactor and Enzymes in Hydrogen-Bonded Organic Frameworks for Multienzyme Cascade Reactions with Cofactor Recycling. Angew. Chem. Int. Ed. 2023, 62, e202308562. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, R.; Wang, C.; Song, X.; Wang, R.; Liu, J.; Zhang, M.; Huang, J.; You, T.; Zhang, Y.; et al. In Situ Embedding Hydrogen-Bonded Organic Frameworks Nanocrystals in Electrospinning Nanofibers for Ultrastable Broad-Spectrum Antibacterial Activity. Adv. Funct. Mater. 2023, 33, 2214388. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, K.; Bai, J.; Xu, T.; Han, W.; Wang, C.; Chen, Z.; Kirlikovali, K.O.; Li, P.; Xiao, J.; et al. Chemically Engineered Porous Molecular Coatings as Reactive Oxygen Species Generators and Reservoirs for Long-Lasting Self-Cleaning Textiles. Angew. Chem. Int. Ed. 2022, 61, e202115956. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Zhang, H.; Ren, J.; Qu, X. Hydrogen-Bonded Organic Frameworks: New Horizons in Biomedical Applications. Chem. Soc. Rev. 2023, 52, 7504–7523. [Google Scholar] [CrossRef]
- Huang, W.; Yuan, H.; Yang, H.; Tong, L.; Gao, R.; Kou, X.; Wang, J.; Ma, X.; Huang, S.; Zhu, F.; et al. Photodynamic Hydrogen-Bonded Biohybrid Framework: A Photobiocatalytic Cascade Nanoreactor for Accelerating Diabetic Wound Therapy. JACS Au 2022, 2, 2048–2058. [Google Scholar] [CrossRef]
- Gao, X.; Lu, W.; Wang, Y.; Song, X.; Wang, C.; Kirlikovali, K.O.; Li, P. Recent Advancements of Photo- and Electro-Active Hydrogen-Bonded Organic Frameworks. Sci. China Chem. 2022, 65, 2077–2095. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Mo, G.; Gao, X.; Wu, E.; Li, B.; Bi, Y.; Li, P. Hydration/Dehydration Induced Reversible Transformation between a Porous Hydrogen-Bonded Organic Framework and a Nonporous Molecular Crystal for Highly Efficient Gas Dehydration. Chem Bio Eng. 2024, 1, 283–288. [Google Scholar] [CrossRef]
- Zhai, P.; Wang, C.; Li, Y.; Jin, D.; Shang, B.; Chang, Y.; Liu, W.; Gao, J.; Hou, J. Molecular Engineering of Hydrogen-Bonded Organic Framework for Enhanced Nitrate Electroreduction to Ammonia. Nano Lett. 2024, 24, 8687–8695. [Google Scholar] [CrossRef] [PubMed]
- Zaytseva, E.; Mazhukin, D. Spirocyclic Nitroxides as Versatile Tools in Modern Natural Sciences: From Synthesis to Applications. Part I. Old and New Synthetic Approaches to Spirocyclic Nitroxyl Radicals. Molecules 2021, 26, 677. [Google Scholar] [CrossRef] [PubMed]
- Diacetonamine Hydrogen Oxalate. Org. Synth. 1926, 6, 28. [CrossRef]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirschfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro Software System. Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.43.128a; Rigaku Corporation: Wroclaw, Poland, 2024. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mesityl Oxide. Org. Synth. 1921, 1, 53. [CrossRef]
- Moutin, M.; Rassat, A.; Bordeaux, D.; Lajerowicz-Bonnetau, J. Nitroxydes: LXXIV: Mise En Evidence de Deux Oxazolidines Nitroxydes Derivées Du Norcamphre. J. Mol. Struct. 1976, 31, 275–282. [Google Scholar] [CrossRef]
- Ito, A.; Nakano, Y.; Urabe, M.; Tanaka, K.; Shiro, M. Structural and Magnetic Studies of Copper(II) and Zinc(II) Coordination Complexes Containing Nitroxide Radicals as Chelating Ligands. Eur. J. Inorg. Chem. 2006, 2006, 3359–3368. [Google Scholar] [CrossRef]
- Perfetti, M.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Lanthanide Complexes with a Tripodal Nitroxyl Radical Showing Strong Magnetic Coupling. Inorg. Chem. 2020, 59, 16591–16598. [Google Scholar] [CrossRef]
- Rey, P.; Caneschi, A.; Sukhikh, T.S.; Vostrikova, K.E. Tripodal Oxazolidine-N-Oxyl Diradical Complexes of Dy3+ and Eu3+. Inorganics 2021, 9, 91. [Google Scholar] [CrossRef]
- Brough, P.; Chiarelli, R.; Pécaut, J.; Rassat, A.; Rey, P. A Versatile Synthesis of New Pyrimidinyl Nitronyl Nitroxides. Chem. Commun. 2003, 21, 2722–2723. [Google Scholar] [CrossRef] [PubMed]
- Brough, P.; Pécaut, J.; Rassat, A.; Rey, P. Pyrimidinyl Nitronyl Nitroxides. Chem.—A Eur. J. 2006, 12, 5134–5141. [Google Scholar] [CrossRef] [PubMed]
- Thirunarayanan, S.; Arjunan, V.; Marchewka, M.K.; Mohan, S. Structure, Vibrations and Quantum Chemical Investigations of Hydrogen Bonded Complex of Bis(1–Hydroxy–2–Methylpropan–2–Aminium)Selenate. J. Mol. Struct. 2017, 1134, 6–16. [Google Scholar] [CrossRef]
- Sheikhshoaie, I.; Ghazizadeh, M. A Novel Proton Transfer Compound (a New Molybdate Salt) and Its X-ray Structure. Bull. Chem. Soc. Ethiop. 2012, 27, 69–76. [Google Scholar] [CrossRef]
- Ben Nasr, M.; Aubert, E.; Espinosa, E. A Comparative Study of Two Polymorphs of Bis(1-Hydroxy-2-Methylpropan-2-Aminium) Carbonate. Acta Crystallogr. Sect. C Struct. Chem. 2016, 72, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, D.; Hu, W.; Liu, Q.; Du, Z.; He, C.; Zhang, W.; Chen, X. A Crystalline Supramolecular Rotor Functioned by Dual Ultrasmall Polar Rotators. Chin. J. Chem. 2022, 40, 1917–1923. [Google Scholar] [CrossRef]
- Bagieu-Beucher, M.; Guitel, J.C. Structure of Dimethylethanolammonium Dihydrogenmonophosphate Monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1990, 46, 2382–2384. [Google Scholar] [CrossRef]
- Gharbi, A.; Joini, A. Structural and Physicochemical Characterization of an Organic Dihydrogenodiphosphate: [(CH3)2C(NH3)CH2OH]H2P207. J. Tunis. Chem. Soc. 1996, 3, 813–826. [Google Scholar]
- Schubert, D.M.; Visi, M.Z.; Knobler, C.B. Crystalline Alcoholamine Borates and the Triborate Monoanion. Inorg. Chem. 2008, 47, 2017–2023. [Google Scholar] [CrossRef]
- Cheung, E.Y.; David, S.E.; Harris, K.D.M.; Conway, B.R.; Timmins, P. Structural Properties of a Family of Hydrogen-Bonded Co-Crystals Formed between Gemfibrozil and Hydroxy Derivatives of t-Butylamine, Determined Directly from Powder X-ray Diffraction Data. J. Solid State Chem. 2007, 180, 1068–1075. [Google Scholar] [CrossRef]
- Podjed, N.; Modec, B. Hydrogen Bonding and Polymorphism of Amino Alcohol Salts with Quinaldinate: Structural Study. Molecules 2022, 27, 996. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-F.; Feng, M.-L.; Liu, S.; Yang, H.-Y.; Zhu, H.-J. 1-Hydroxymethyl-1-Methylethanaminium Chloride. Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, o1150. [Google Scholar] [CrossRef] [PubMed]
- Muhonen, H. The Crystal and Molecular Structure of 2-Amino-2-Methyl-1-Propanol Hydrobromide. Finn. Chem. Lett. 1980, 7, 138. [Google Scholar]
- Beckett, M.A.; Horton, P.N.; Hursthouse, M.B.; Knox, D.A.; Timmis, J.L. Structural (XRD) and Thermal (DSC, TGA) and BET Analysis of Materials Derived from Non-Metal Cation Pentaborate Salts. Dalt. Trans. 2010, 39, 3944. [Google Scholar] [CrossRef] [PubMed]
- Croft, K.; Ghisalberti, E.; Jefferies, P.; Mori, T.; Skelton, B.; White, A. The Chemistry of Eremophila spp. XXI. Structural Study of a New Eremane Diterpene. Aust. J. Chem. 1984, 37, 785. [Google Scholar] [CrossRef]
- Knapp, C.E. CCDC 2193537: Experimental Crystal Structure Determinationitle. CSD Commun. 2022. [Google Scholar] [CrossRef]
- Eppel, S.; Bernstein, J. Statistics-Based Design of Multicomponent Molecular Crystals with the Three-Center Hydrogen Bond. Cryst. Growth Des. 2009, 9, 1683–1691. [Google Scholar] [CrossRef]
- André, V.; Martins, I.; Quaresma, S.; Martins, M.; Duarte, M.T. Transforming Aspirin into Novel Molecular Salts of Salicylic Acid. Struct. Chem. 2014, 25, 707–714. [Google Scholar] [CrossRef]
- Rafique, M.; Hussain, G.; Siddiqui, W.A.; Tahir, M.N. 2-Methyl-4-Oxopentan-2-Aminium 2-Sulfamoylbenzoate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o1883. [Google Scholar] [CrossRef]
- Ji, Z.; Wei, S.; Wu, W. Bis(4-ammonio-4-methyl pentan-2-one-κO)dioxalato-κ4O1,O2-copper(II). Acta Crystallogr. Sect. E 2009, 65, m182. [Google Scholar] [CrossRef]
- Schmidt, M.; Bauer, A.; Schmidbaur, H. Beryllium Chelation by Dicarboxylic Acids in Aqueous Solution. Inorg. Chem. 1997, 36, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Küppers, H. The Crystal Structure of Ammonium Hydrogen Oxalate Hemihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 318–327. [Google Scholar] [CrossRef]
- Spackman, M.A.; Byrom, P.G. A Novel Definition of a Molecule in a Crystal. Chem. Phys. Lett. 1997, 267, 215–220. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirschfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirschfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Barbour, L.J. 2.03—Single-Crystal X-Ray Diffraction. In Comprehensive Supramolecular Chemistry II, Vol. 2: Experimental and Computational Methods in Supramolecular Chemistry; Atwood, J.L., Ed.; Elsevier: Oxford, UK, 2017; pp. 23–43. ISBN 978-0-12-803199-5. [Google Scholar]
- Mitchell, A.S.; Spackman, M.A. Molecular surfaces from the promolecule: A comparison with Hartree–Fock ab initio electron density surfaces. J. Comput. Chem. 2000, 21, 933–942. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).