Catalytic Properties of Pd Deposited on Polyaniline in the Hydrogenation of Quinoline
Abstract
1. Introduction
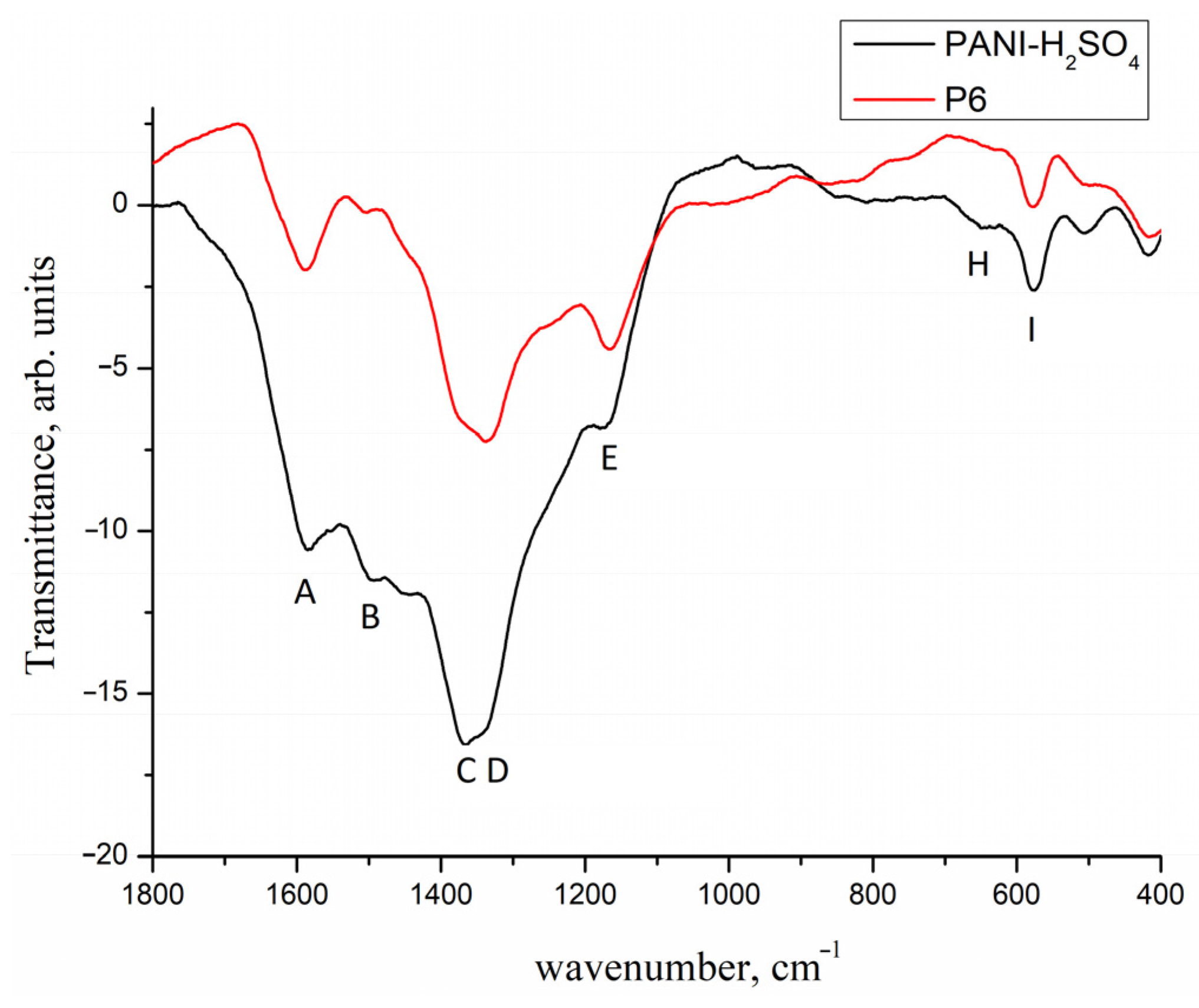
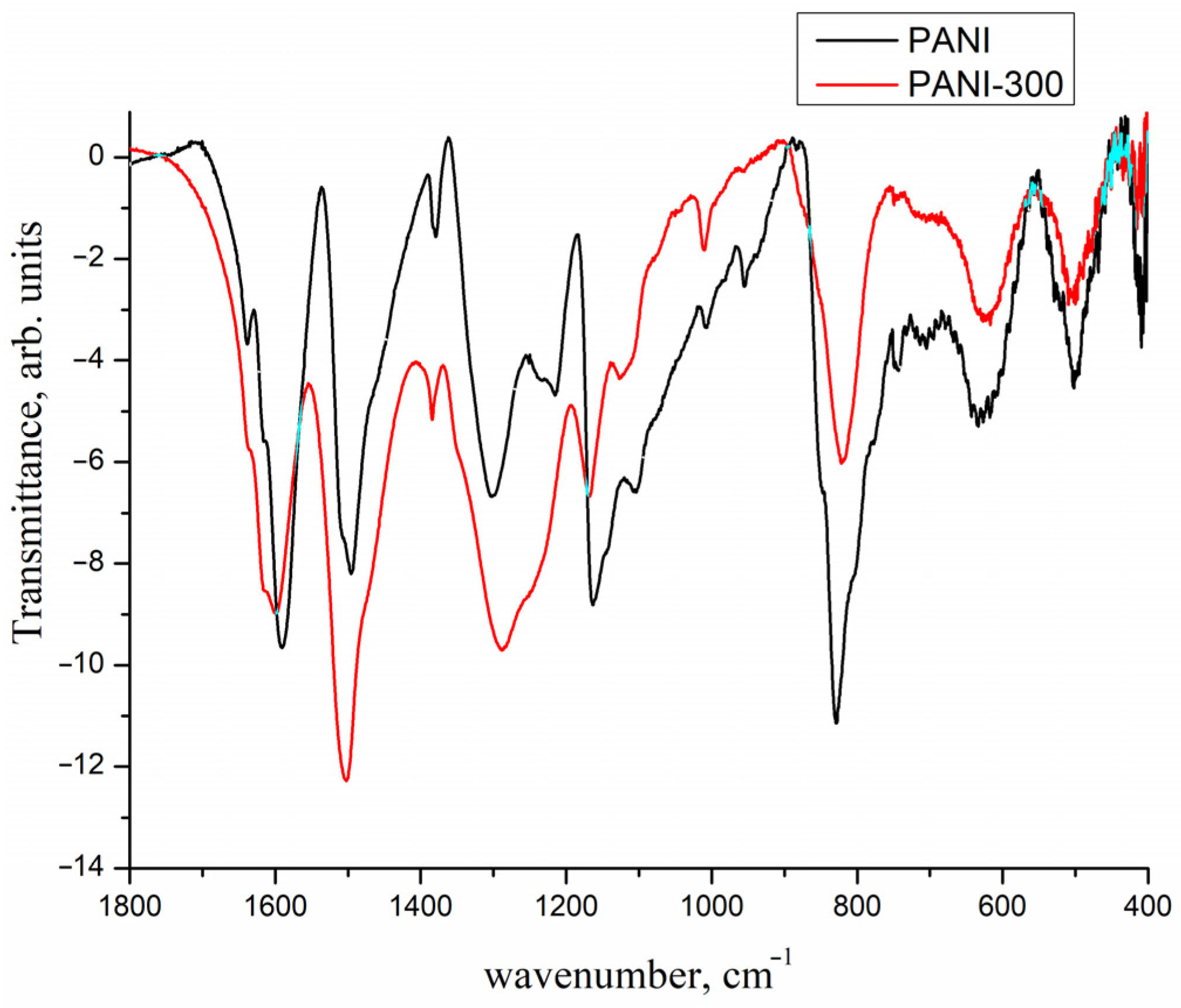
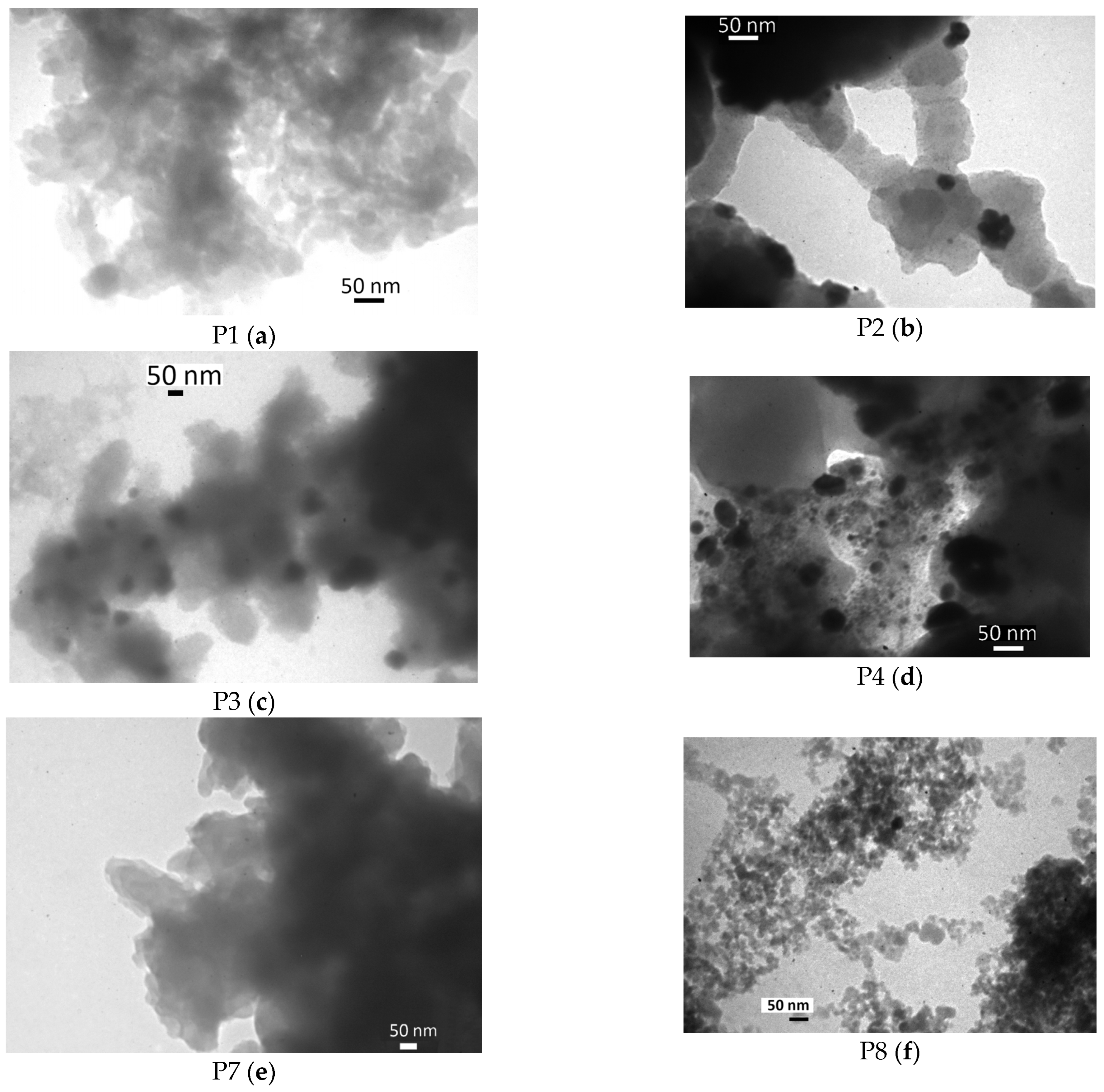
2. Results and Discussion
- ▪
- ▪
- deposition of the metallic Pd on the carrier by decomposition of Pd2(dba)3, similar to the method proposed in [40,41,42,43]. In this case, the following carriers were used:
- -
- PANI (samples P4 and P5, Figure 3);
- -
- the composite of PANI and activated carbon, formed by oxidative polymerization of aniline in the presence of activated carbon Norit GSX (hereinafter referred to as PANI/C samples, Figure 4);
- -
- the composite of PANI and silica, formed by oxidative polymerization of aniline in the presence of Aerosil-300 (hereinafter referred to as PANI/SiO2, Figure 4);
- -
- -
- PANI after heating at 300 °C in an atmosphere of hydrogen (hereinafter referred to as PANI-300, Figure 3).
3. Materials and Methods
3.1. Synthesis of Polyaniline (PANI)
3.2. Synthesis of P1
3.3. Synthesis of P2 and P3
3.4. Synthesis of P4 and P5
3.5. Synthesis of P6
3.6. Synthesis of P7
3.7. Synthesis of P8
3.8. Synthesis of P9
3.9. Synthesis of P10
3.10. Synthesis of P11
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jackson, S.D. (Ed.) Hydrogenation: Catalysts and Processes; De Gruyter: Berlin, Germany; Boston, MA, USA, 2018. [Google Scholar]
- Burke, A.J.; Marques, C.S.; Turner, N.J.; Hermann, G.J. Active Pharmaceutical Ingredients in Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2018; pp. 75–112. [Google Scholar]
- Campbell, M.L. Cyclohexane in Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Gao, R.; Peng, C.; Zou, J.-J. Industrial Arene Chemistry; Wiley-VCH GmbH: Hoboken, NJ, USA, 2023; pp. 1479–1524. [Google Scholar]
- Ivanytsya, M.O.; Subotin, V.V.; Gavrilenko, K.S.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. Advances and challenges in development of transition metal catalysts for heterogeneous hydrogenation of organic compounds. Chem. Rec. 2024, 24, e202300300. [Google Scholar] [CrossRef]
- Kukula, P.; Prins, R. Diastereoselective Hydrogenation in the Preparation of Fine Chemicals. Top. Catal. 2003, 25, 29–42. [Google Scholar] [CrossRef]
- Zhao, X.; Chang, Y.; Chen, W.-J.; Wu, Q.; Pan, X.; Chen, K.; Weng, B. Recent Progress in Pd-Based Nanocatalysts for Selective Hydrogenation. ACS Omega 2022, 7, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.A.; Martins, L.M. Supported Palladium Nanocatalysts: Recent Findings in Hydrogenation Reactions. Processes 2020, 8, 1172. [Google Scholar] [CrossRef]
- Subotin, V.V.; Ivanytsya, M.O.; Terebilenko, A.V.; Yaremov, P.S.; Pariiska, O.O.; Akimov, Y.M.; Kotenko, I.E.; Sabov, T.M.; Kurmach, M.M.; Ryabukhin, S.V.; et al. Air-Stable Efficient Nickel Catalyst for Hydrogenation of Organic Compounds. Catalysts 2023, 13, 706. [Google Scholar] [CrossRef]
- Lipshutz, B.H. Development of Nickel-on-Charcoal as a “Dirt-Cheap” Heterogeneous Catalyst: A Personal Account. Adv. Synth. Catal. 2001, 343, 515–526. [Google Scholar] [CrossRef]
- Sergeev, A.G.; Webb, J.D.; Hartwig, J.F. A Heterogeneous Nickel Catalyst for the Hydrogenolysis of Aryl Ethers without Arene Hydrogenation. J. Am. Chem. Soc. 2012, 134, 20226–20229. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Webb, J.D.; Hartwig, J.F. Chemo- and Regioselective Hydrogenolysis of Diaryl Ether C-O Bonds by a Robust Heterogeneous Ni/C Catalyst: Applications to the Cleavage of Complex Lignin-Related Fragments. Angew. Chem. Int. Ed. 2016, 55, 1474–1478. [Google Scholar] [CrossRef]
- Asaula, V.M.; Lytvynenko, A.S.; Mishura, A.M.; Gavrilenko, K.S.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. In-situ formation of Ni nanoparticles supported by MIL-101 porous coordination polymer for catalytic hydrogenation of quinoline. Inorg. Chem. Commun. 2020, 121, 108203. [Google Scholar] [CrossRef]
- Asaula, V.M.; Shvets, O.V.; Pariiska, O.O.; Bur’yanov, V.V.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. Composites based on nanodispersed nickel, graphene-like carbon, and aerosil for catalytic hydrogenation of furfural and quinoline. Theor. Exp. Chem. 2020, 56, 261–267. [Google Scholar] [CrossRef]
- Westerhaus, F.A.; Jagadeesh, R.V.; Wienhofer, G.; Pohl, M.-M.; Radnik, J.; Surkus, A.-E.; Rabeah, J.; Junge, K.; Junge, H.; Nielsen, M.; et al. Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. Nat. Chem. 2013, 5, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Asaula, V.M.; Buryanov, V.V.; Solod, B.Y.; Tryus, D.M.; Pariiska, O.O.; Kotenko, I.E.; Volovenko, Y.M.; Volochnyuk, D.M.; Ryabukhin, S.V.; Kolotilov, S.V. Catalytic Hydrogenation of Substituted Quinolines on Co-Graphene Composites. Eur. J. Org. Chem. 2021, 2021, 6623–6632. [Google Scholar]
- Subotin, V.V.; Asaula, V.M.; Lishchenko, Y.L.; Ivanytsya, M.O.; Pariiska, O.O.; Ryabukhin, S.V.; Volochnyuk, D.M.; Kolotilov, S.V. Catalytic reductive amination of aromatic aldehydes on Co-containing composites. Chemistry 2023, 5, 281–293. [Google Scholar] [CrossRef]
- Chen, F.; Surkus, A.-E.; He, L.; Pohl, M.-M.; Radnik, J.; Topf, C.; Junge, K.; Beller, M. Selective Catalytic Hydrogenation of Heteroarenes with N-Graphene-Modified Cobalt Nanoparticles (Co3O4–Co/NGr@α-Al2O3). J. Am. Chem. Soc. 2015, 137, 11718–11724. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chen, B.; Zhou, X.; Kang, S.; Xu, Y.; Wei, J. Selective synthesis of furfurylamine by reductive amination of furfural over Raney cobalt. ChemCatChem 2019, 11, 5562–5569. [Google Scholar] [CrossRef]
- Murugesan, K.; Senthamarai, T.; Alshammari, A.S.; Altamimi, R.M.; Kreyenschulte, C.; Pohl, M.-M.; Lund, H.; Jagadeesh, R.V.; Beller, M. Cobalt-Nanoparticles Catalyzed Efficient and Selective Hydrogenation of Aromatic Hydrocarbons. ACS Catal. 2019, 9, 8581–8591. [Google Scholar] [CrossRef]
- Chandrashekhar, V.G.; Senthamarai, T.; Kadam, R.G.; Malina, O.; Kašlík, J.; Zbořil, R.; Gawande, M.B.; Jagadeesh, R.V.; Beller, M. Silica-supported Fe/Fe–O nanoparticles for the catalytic hydrogenation of nitriles to amines in the presence of aluminium additives. Nat. Catal. 2022, 5, 20–29. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, B.; Wang, J.; Zhou, Y.; Huang, X.; Huang, H.; Wang, X.; Li, K. Review of Molybdenum Disulfide Research in Slurry Bed Heavy Oil Hydrogenation. ACS Omega 2023, 8, 18400–18407. [Google Scholar] [CrossRef]
- Bychko, I.B.; Kalishyn, Y.Y.; Strizhak, P.E. Size effect of Fe nanoparticles supported on carbon nanotubes on their activity and selectivity in the hydrogenation of crotonaldehyde. Theor. Exp. Chem. 2012, 48, 194–198. [Google Scholar] [CrossRef]
- Subbotin, V.; Demchenko, P.; Yanko, O.; Kharkova, L.; Gladyshevskii, R.; Volkov, S. Synthesis, structure and some catalytic properties of the new trinuclear rhenium cluster compound Re3Se3S4Br13. Solid State Phenom. 2016, 257, 227–230. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Hasik, M.; Kloc, M. Pd-PANI: Preparation and catalytic properties. Synth. Met. 1999, 102, 1307–1308. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, C.-A.; Gau, H.-M.; Bailey, J.A.; Akhadov, E.; Williams, D.; Wang, H.L. Facile Synthesis of Polyaniline-Supported Pd Nanoparticles and Their Catalytic Properties toward Selective Hydrogenation of Alkynes and Cinnamaldehyde. Chem. Mater. 2008, 20, 2839–2844. [Google Scholar] [CrossRef]
- Yu, L.; Han, Z.; Ding, Y. Gram-Scale Preparation of Pd@PANI: A Practical Catalyst Reagent for Copper-Free and Ligand-Free Sonogashira Couplings. Org. Proc. Res. Dev. 2016, 20, 2124–2129. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, D.; Cao, K.; Yu, L.; Han, J.; Xu, Q. Probing the support effect at the molecular level in the polyaniline-supported palladium nanoparticle-catalyzed Ullmann reaction of aryl iodides. J. Catal. 2018, 360, 250–260. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, S.; Wu, Z.; Liu, W.; Zhan, H.; Liang, Y.; Chen, X.; Ma, B.; Bi, S. Ultra-low-loading palladium nanoparticles stabilized on nanocrystalline Polyaniline (Pd@PANI): A efficient, green, and recyclable catalyst for the reduction of nitroarenes. Appl. Organomet. Chem. 2019, 33, e5159. [Google Scholar] [CrossRef]
- Guo, D.; Jiang, K.; Gan, H.; Ren, Y.; Long, J.; Li, Y.; Yin, B. Template-Oriented Polyaniline-Supported Palladium Nanoclusters for Reductive Homocoupling of Furfural Derivatives. Angew. Chem. Int. Ed. 2023, 62, e202304662. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wei, Z.; Qi, X.; Dong, L.; Guo, Y.-G.; Wan, L.; Shao, Z.; Li, L. Nanostructured Polyaniline-Decorated Pt/C@PANI Core−Shell Catalyst with Enhanced Durability and Activity. J. Am. Chem. Soc. 2012, 134, 13252–13255. [Google Scholar] [CrossRef]
- Tseng, R.J.; Baker, C.O.; Shedd, B.; Huang, J.; Kaner, R.B.; Ouyang, J.; Yang, Y. Charge transfer effect in the polyaniline-gold nanoparticle memory system. Appl. Phys. Lett. 2007, 90, 053101. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- de Albuquerque, J.E.; Mattoso, L.H.C.; Faria, R.M.; Masters, J.G.; MacDiarmid, A.G. Study of the interconversion of polyaniline oxidation states by optical absorption spectroscopy. Synth. Met. 2004, 146, 1–10. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Progr. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Stejskal, J.; Gilbert, R.G. Polyaniline. preparation of a conducting polymer (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 857–867. [Google Scholar] [CrossRef]
- Davied, S.; Nicolau, Y.F.; Melis, F.; Revillon, A. Molecular weight determinaiton of polyaniline. In Proceedings of the International Conference on Science and Technology of Synthetic Metals, Seoul, Republic of Korea, 24–29 July 1994. [Google Scholar] [CrossRef]
- Drelinkiewicz, A.; Hasik, M.; Choczyński, M. Preparation and properties of polyaniline containing palladium. Mater. Res. Bull. 1998, 33, 739–762. [Google Scholar] [CrossRef]
- Yakukhnov, S.A.; Pentsak, E.O.; Galkin, K.I.; Mironenko, R.M.; Drozdov, V.A.; Likholobov, V.A.; Ananikov, V.P. Rapid “Mix-and-Stir” Preparation of Well-Defined Palladium on Carbon Catalysts for Efficient Practical Use. ChemCatChem 2017, 10, 1869–1873. [Google Scholar] [CrossRef]
- Galushko, A.S.; Ilyushenkova, V.V.; Burykina, J.V.; Shaydullin, R.R.; Pentsak, E.O.; Ananikov, V.P. The Fast Formation of a Highly Active Homogeneous Catalytic System upon the Soft Leaching of Pd Species from a Heterogeneous Pd/C Precursor. Inorganics 2023, 11, 260. [Google Scholar] [CrossRef]
- Leonard, D.N.; Franzen, S. Is Pd2(DBA)3 a Feasible Precursor for the Synthesis of Pd Nanoparticles? J. Phys. Chem. C 2009, 113, 12706–12714. [Google Scholar] [CrossRef]
- Arvelos, M.S.; Silva, A.C.; de Souza, A.L.F.; Achete, C.A.; Vasconcelos, T.L.; Robertis, E.; Archanjo, B.S.; Aguiar, L.C.S.; Malta, L.F.B.; Senra, J.D. Revealing Pd Nanoparticles Formation from PEG-Mediated Decomposition of Organometallic Precursor and Their Application as Catalyst for the Synthesis of n-Extended Carbazoles. ChemistrySelect 2018, 3, 9725–9730. [Google Scholar] [CrossRef]
- Picanço, W.M.; Feitosa, B.A.; da Silva, N.G.; Silva, G.T.A.; Giacon, V.M.; Campelo, P.H.; de Souza, S.M.; de Oliveira, K.M.T.; Sanches, E.A. Aniline-oriented polymerization over nano-SiO2 particles. J. Mol. Struct. 2018, 1167, 118–126. [Google Scholar] [CrossRef]
- Reddy, R.R.; Lee, K.-P.; Kim, J.Y.; Lee, Y. Self-assembly and graft polymerization route to Monodispersed Fe3O4@SiO2-polyaniline core-shell composite nanoparticles: Physical properties. J. Nanosci. Nanotechnol. 2008, 11, 5632–5639. [Google Scholar] [CrossRef]
- Kurys, Y.I.; Bychko, I.B.; Pariiska, O.O.; Didenko, O.Z.; Mazur, D.O.; Strizhak, P.E.; Koshechko, V.G.; Pokhodenko, V.D. Carbonized Polyaniline as a Catalyst for Hydrogenation with Molecular Hydrogen of Organic Substrates with C=C Double Bond and Nitro Group. Theor. Exp. Chem. 2023, 59, 193–199. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Ananikov, V.P. Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis. Organometallics 2012, 31, 2302–2309. [Google Scholar] [CrossRef]
- Noack, K.; Zbinden, H.; Schlögl, R. Identification of the state of palladium in various hydrogenation catalysts by XPS. Catal. Lett. 1990, 4, 145–156. [Google Scholar] [CrossRef]
- Teschner, D.; Révay, Z.; Borsodi, J.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; Milroy, D.; Jackson, S.D.; Torres, D.; Sautet, P. Understanding Palladium Hydrogenation Catalysts: When the Nature of the Reactive Molecule Controls the Nature of the Catalyst Active Phase. Angew. Chem. Int. Ed. 2008, 47, 9274–9278. [Google Scholar] [CrossRef] [PubMed]
- Teschner, D.; Pestryakov, A.; Kleimenov, E.; Hävecker, M.; Bluhm, H.; Sauer, H.; Knop-Gericke, A.; Schlögl, R. High-pressure X-ray photoelectron spectroscopy of palladium model hydrogenation catalysts.: Part 1: Effect of gas ambient and temperature. J. Catal. 2005, 230, 186–194. [Google Scholar] [CrossRef]
- Burmeister, R.; Despeyroux, B.; Deller, K.; Seibold, K.; Albers, P. On the XPS-Surface Characterization of Activated Carbons resp. Pd/C Catalysts and a Correlation to the Catalytic Activity. In Heterogeneous Catalysis and Fine Chemicals III; Guisnet, M., Barbier, J., Barrault, J., Bouchoule, C., Duprez, D., Pérot, G., Montassier, C., Eds.; Elsevier Science Publishers: Amsterdam, Netherlands, 1993; p. 361. [Google Scholar]
- Tang, J.; Jing, X.; Wang, B.; Wang, F. Infrared spectra of soluble polyaniline. Synth. Met. 1988, 24, 231–238. [Google Scholar] [CrossRef]
- Trchová, M.; Stejskal, J. Polyaniline: The infrared spectroscopy of conducting polymer nanotubes (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1803–1817. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. The chemical oxidative polymerization of aniline in water: Raman spectroscopy. J. Raman Spectrosc. 2008, 39, 1375–1387. [Google Scholar] [CrossRef]
- He, M.-Q.; Ai, Y.; Hu, W.; Guan, L.; Ding, M.; Liang, Q. Recent Advances of Seed-Mediated Growth of Metal Nanoparticles: From Growth to Applications. Adv. Mater. 2023, 35, 2211915. [Google Scholar] [CrossRef]
- Reed-Berendt, B.G.; Polidano, K.; Morrill, L.C. Recent advances in homogeneous borrowing hydrogen catalysis using earth-abundant first row transition metals. Org. Biomol. Chem. 2019, 17, 1595–1607. [Google Scholar] [CrossRef]
- Hub, S.; Hilaire, L.; Touroude, R. Hydrogenation of But-1-yne and But-1-ene on Palladium Catalysts. Particle Size Effect. Appl. Catal. 1988, 36, 307–322. [Google Scholar] [CrossRef]
- Subotin, V.V.; Vashchenko, B.V.; Asaula, V.M.; Verner, E.V.; Ivanytsya, M.O.; Shvets, O.; Ostapchuk, E.N.; Grygorenko, O.O.; Ryabukhin, S.V.; Volochnyuk, D.M.; et al. Screening of Palladium/Charcoal Catalysts for Hydrogenation of Diene Carboxylates with Isolated-Rings (Hetero)aliphatic Scaffold. Molecules 2023, 28, 1201. [Google Scholar] [CrossRef] [PubMed]
- Mozingo, R. Palladium Catalysts. Org. Synth. 1946, 26, 77. [Google Scholar] [PubMed]
- Pearlman, W.M. Noble metal hydroxides on carbon nonpyrophoric dry catalysts. Tetrahedron Lett. 1967, 8, 1663–1664. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 310, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, I.; Compagnoni, M. Chemical reaction engineering, process design and scale-up issues at the frontier of synthesis: Flow chemistry. Chem. Eng. J. 2016, 296, 56–70. [Google Scholar] [CrossRef]
- Pariiska, O.O.; Mazur, D.O.; Asaula, V.M.; Buryanov, V.V.; Socha, R.; Kurys, Y.I.; Kolotilov, S.V.; Koshechko, V.G.; Pokhodenko, V.D. Influence of the Structure of Nanocomposites Based on Co,N,S-Doped Carbon and Co9S8 on the Catalytic Properties in the Processes of Quinoline and Its Methyl Derivatives Hydrogenation. Theor. Exp. Chem. 2023, 58, 417–426. [Google Scholar] [CrossRef]
- Lan, X.; Wang, T. Highly Selective Catalysts for the Hydrogenation of Unsaturated Aldehydes: A Review. ACS Catal. 2020, 10, 2764–2790. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Yılmaz, F.; Küçükyavuz, Z. Solution properties of polyaniline. Polym. Int. 2009, 59, 552–556. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Sayko, R.; Colby, R.H. Viscosity of Polymer Solutions and Molecular Weight Characterization. ACS Macro Lett. 2023, 12, 773–779. [Google Scholar] [CrossRef]
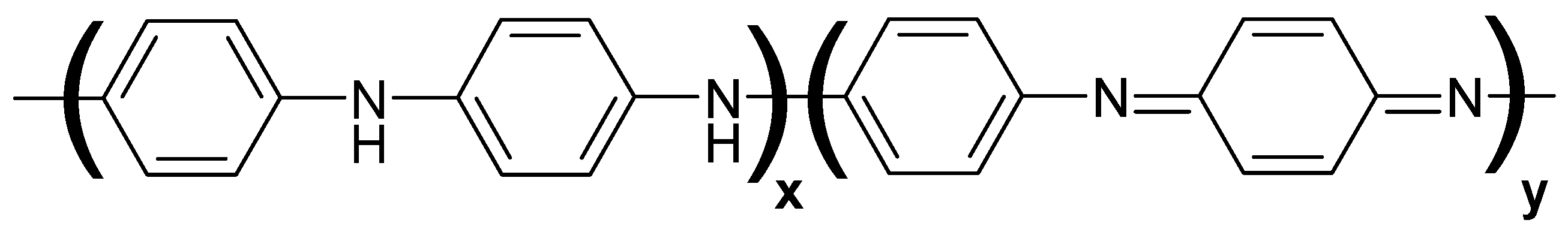
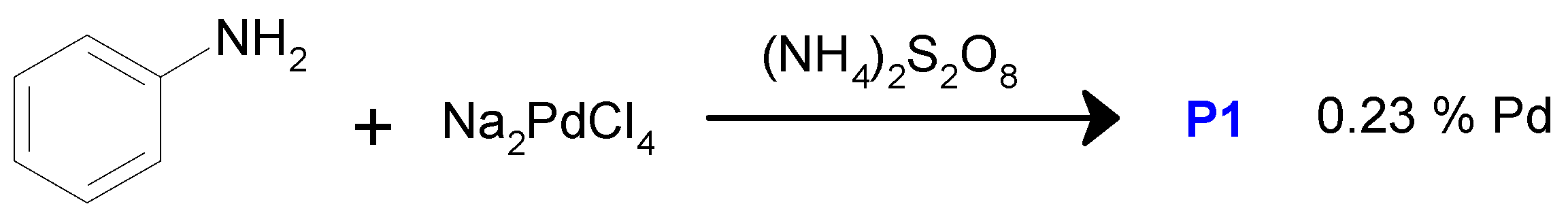
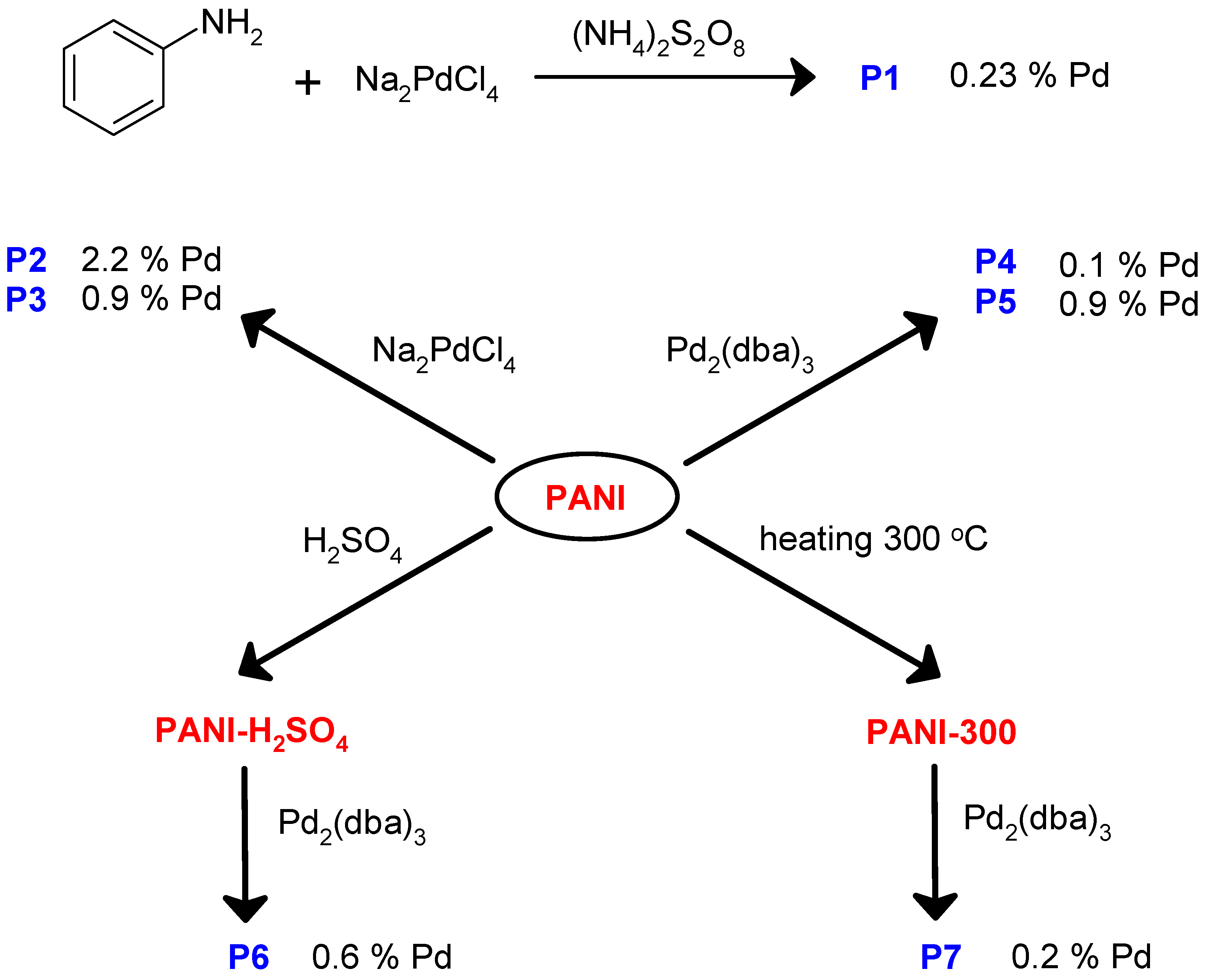


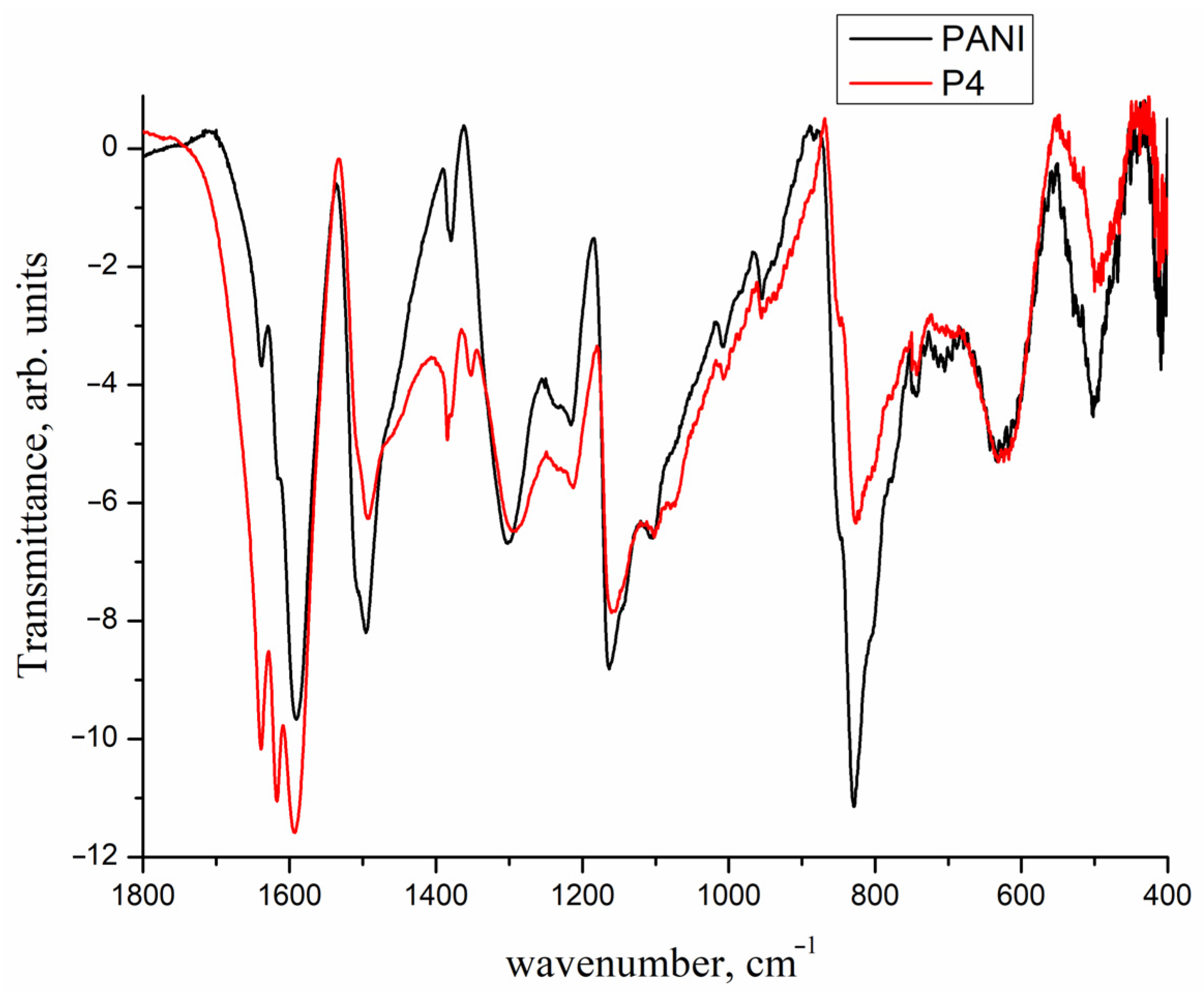


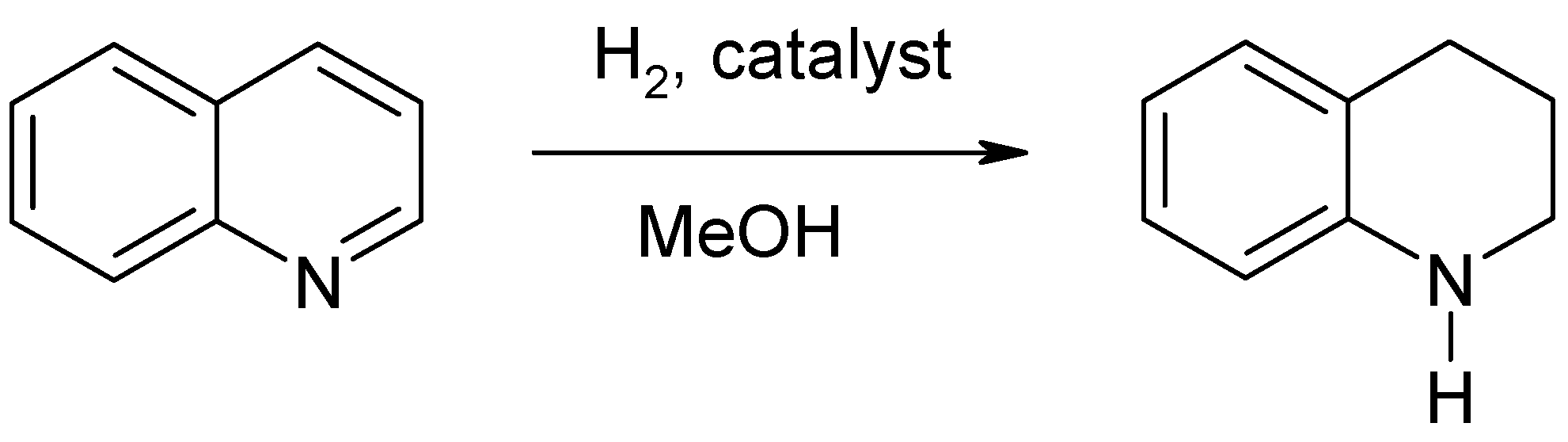
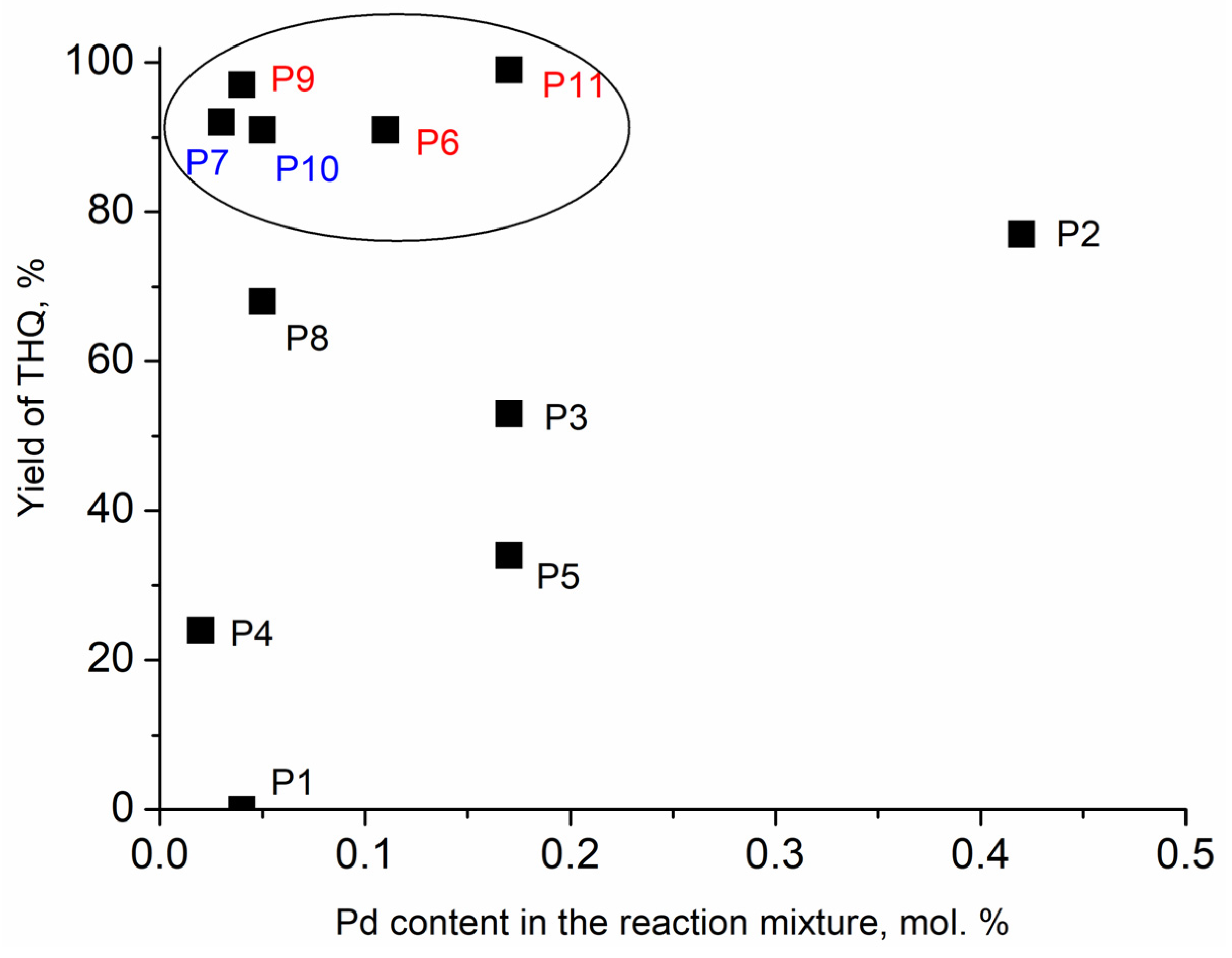
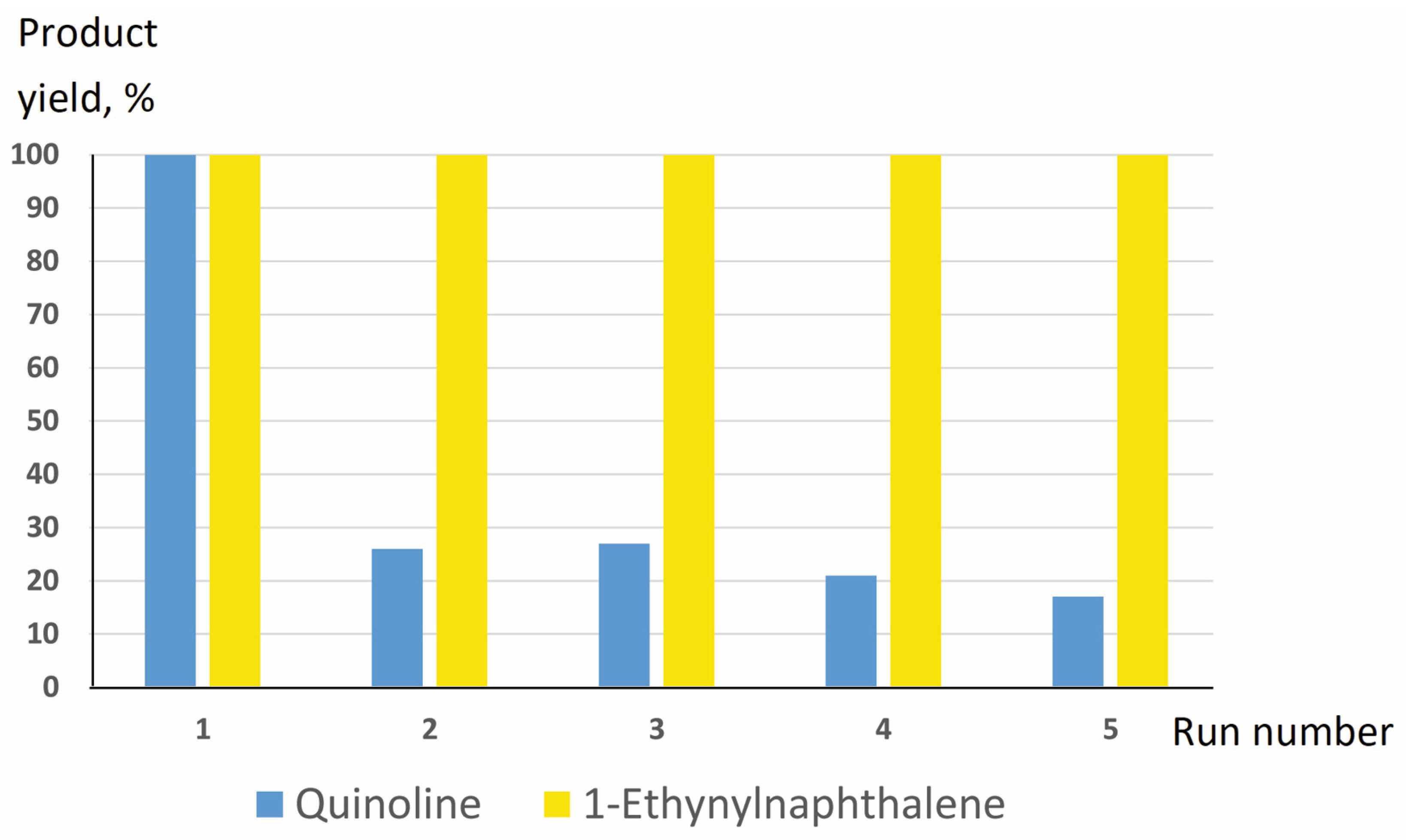
| Entry No. | Catalyst | Pd Content in the Reaction Mixture, mol% (a) | Yield of THQ, % | n(THQ) per 1 Mole of Pd |
|---|---|---|---|---|
| 1 | P1 | 0.04 | 0 | 0 |
| 2 | P2 | 0.42 | 77 | 180 |
| 3 | P3 | 0.17 | 53 | 315 |
| 4 | P4 | 0.02 | 24 | 1272 |
| 5 | P5 | 0.17 | 34 | 195 |
| 6 | P6 | 0.11 | 91 | 830 |
| 7 | P7 | 0.03 | 92 | 2710 |
| 8 | P8 | 0.05 | 68 | 1240 |
| 9 | P9 | 0.04 | 97 | 2285 |
| 10 | P10 | 0.05 | 91 | 1930 |
| 11 | P11 | 0.17 | 99 | 570 |
| Entry No. | Catalyst (a) | Pd Content, mol.% Per 1 mole of Quinoline | Yield of THQ, % | Ref. |
|---|---|---|---|---|
| 1 | P7 | 1 | 100 | this work |
| 2 | P8 | 1 | 60 | this work |
| 3 | P9 | 1 | 29 | this work |
| 4 | P10 | 1 | 61 | this work |
| 5 | P11 | 1 | 100 | this work |
| 6 | Pd/C-1 | 1 | 42 | [58] |
| 7 | Pd/C-2 | 1 | 28 | [58] |
| 8 | Pd/C-3 | 1 | 10 | [58] |
| 9 | Pd/C-4 | 1 | 9 | [58] |
| 10 | Pd(OH)2/C-1 | 1 | 61 | [58] |
| 11 | Pd(OH)2/C-2 | 1 | 67 | [58] |
| 12 | Pd(OH)2/C-3 | 1 | 55 | [58] |
| 13 | PdCl2/MIL-101(Cr) | 2 | 30 | [13] |
| Entry | Starting Compound | Products and Their Yields | |
|---|---|---|---|
| 1 |  | 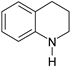 100% | |
| 2 |  |  69% | |
| 3 | 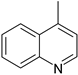 | 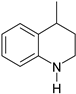 14% | |
| 4 | 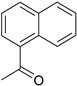 | 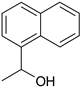 | 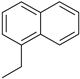 |
| 75% | 17% | ||
| 5 | 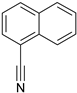 | no reaction | |
| 6 | 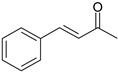 | 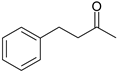 | 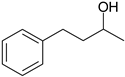 |
| 72% | 28% | ||
| 7 | 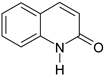 |  16% | |
| 8 | 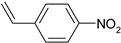 |  95% | |
| 9 | 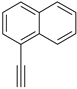 | 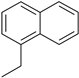 100% | |
| 10 | 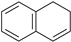 | 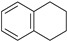 100% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kompaniiets, O.O.; Subotin, V.V.; Poturai, A.S.; Yurchenko, A.A.; Gorlova, A.A.; Bychko, I.B.; Kotenko, I.Y.; Pariiska, O.O.; Ryabukhin, S.V.; Volochnyuk, D.M.; et al. Catalytic Properties of Pd Deposited on Polyaniline in the Hydrogenation of Quinoline. Chemistry 2024, 6, 738-759. https://doi.org/10.3390/chemistry6040044
Kompaniiets OO, Subotin VV, Poturai AS, Yurchenko AA, Gorlova AA, Bychko IB, Kotenko IY, Pariiska OO, Ryabukhin SV, Volochnyuk DM, et al. Catalytic Properties of Pd Deposited on Polyaniline in the Hydrogenation of Quinoline. Chemistry. 2024; 6(4):738-759. https://doi.org/10.3390/chemistry6040044
Chicago/Turabian StyleKompaniiets, Olena O., Vladyslav V. Subotin, Andrii S. Poturai, Aleksandr A. Yurchenko, Alina A. Gorlova, Igor B. Bychko, Igor Ye. Kotenko, Olena O. Pariiska, Serhiy V. Ryabukhin, Dmytro M. Volochnyuk, and et al. 2024. "Catalytic Properties of Pd Deposited on Polyaniline in the Hydrogenation of Quinoline" Chemistry 6, no. 4: 738-759. https://doi.org/10.3390/chemistry6040044
APA StyleKompaniiets, O. O., Subotin, V. V., Poturai, A. S., Yurchenko, A. A., Gorlova, A. A., Bychko, I. B., Kotenko, I. Y., Pariiska, O. O., Ryabukhin, S. V., Volochnyuk, D. M., & Kolotilov, S. V. (2024). Catalytic Properties of Pd Deposited on Polyaniline in the Hydrogenation of Quinoline. Chemistry, 6(4), 738-759. https://doi.org/10.3390/chemistry6040044







