Abstract
Oxygen evolution reactions (OER) are often the decisive step in determining the water electrolysis rate. The first row of transition metals and their derivatives, represented by Ni and Fe, have attracted much attention due to their excellent OER performance. Here, we develop a one-step strategy for preparing oxygen-evolving electrodes, in which the NiFeOOH-modified NiFe layered double hydroxide (NiFe-LDH) nanosheet is supported by nickel foam. At 100 mA·cm−2, the overpotential of NiFeOOH-NiFe-LDH was just 227 mV, and the duration times were over 200 h in 1 mol·L−1 KOH. Furthermore, the co-existence of LDH and hydroxyl oxides helps the oxygen evolution reaction. These results suggest the potential for this synthesis strategy to provide a low-cost, highly active OER electrocatalyst for industrial water splitting.
1. Introduction
Hydrogen energy is a great choice to replace fossil energy because of its advantages of being green, clean, renewable, and easy to transport [1,2,3,4,5]. Among the many ways to produce hydrogen, alkaline electrolytic water is a promising method because of its lower cost and extended stability [6,7,8,9,10]. There are two electrocatalytic reactions, the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER). The OER at the anode side is a four-electron reaction with slow kinetic rates [11,12,13,14,15,16]. The efficiency of water splitting is mainly dependent on the OER. Therefore, decreasing the OER overpotential becomes the bottleneck to promote the process of alkaline electrolytic water.
The first-row transition metal derivatives, including their oxides [17], hydroxides [18], phosphides [19], sulfides [20], selenides [21] and nitrides [22], have demonstrated a catalytic activity for OER reaction. Many studies have shown that electrodes prepared from the transition metal elements of Ni and Fe have good OER catalytic properties and good prospects for application. In particular, Ni- and Fe-based layered double hydroxides (LDHs) have been considered by many researchers as one of the most promising OER electrodes due to their adjustable electronic configuration, flexible chemical composition, and exchangeable interlayer construction [23,24,25,26].
When participating in water electrolysis, the LDH also generates hydroxyl oxides, an intermediate, for the OER catalytic process. Many studies have attempted to prepare hydroxyl oxides as the OER catalysts for efficient electrolytic water. However, the preparation processes of hydroxyl oxides are complicated and often require several reaction steps. Most current preparation methods will first form nano-arrays of metal or metal derivatives on a nickel foam substrate, which are further oxidized to hydroxyl oxides by anodic electro-oxidation or chemical means [2,27,28]. A phase interface exists between the original nano-arrays and the hydroxyl oxides. The electron transfer across the phase interface is also believed to be a decisive factor in promoting electrolytic water catalysis [2]. Therefore, there is a need to develop novel and simple synthesis strategies for the fabrication of hydroxyl oxides.
In this work, hydroxyl oxide-based OER electrodes were prepared by means of a simple one-step method. NiFeOOH-NiFe-LDH nanosheets were synthesized by immersing nickel foam (NF) in a precursor solution containing nickel ions, iron ions and urea, and heated at 75 °C for 12 h at atmospheric pressure. During this process, the NiFeOOH can be generated on the surface layer of NiFe-LDH supported by NF and accelerate up the OER reaction process. The nanosheets of the two substances are laminated to each other, creating a large number of phase interfaces that help electrons transfer faster. With the overpotential of 0.23 V at 100 mA·cm−2, the NiFeOOH-NiFe-LDH exhibits remarkable OER efficiency. The electrode also shows good stability, holding steady in 1.0 mol/L KOH for at least 120 h at 200 mA·cm−2.
2. Materials and Methods
2.1. Materials
The nickel foam (NF) was obtained from Tianjin Leviathan Science & Technology Co., Ltd. (Tianjin, China), with a thickness of 1.0 mm and a porosity of 98%. Ethanol (AR, 99.5%) was purchased from Tianjin Yuanli Chemicals Co., Ltd. (Tianjin, China); Tianjin Hience Optech Technology Company (Tianjin, China) provide Ni(NO3)2·6H2O and CO(NH2)2. Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) offered Fe(NO3)3·9H2O and KOH. Industrial titanium-based iridium oxide electrodes (Ti/IrO2) were purchased from Kunshan Yiwanlin Electronic Technology Co., Ltd. (Kushan, China). Analytical grade reagents were utilized as supplied. Deionized water was used to prepare the aqueous solution.
2.2. Materials Synthesis
NF was used to support NiFeOOH-NiFe-LDH nanosheets via a straightforward one-step water bath process. In brief, a 3 cm × 4 cm area of NF was sonicated in 3 mol/L hydrogen chloride (HCl) solution, and deionized water and ethanol were used to remove the surface nickel oxides and organic molecules. To synthesize NiFeOOH-NiFe-LDH/NF, the solution was made by dissolving 178.0 mg Ni(NO3)2·6H2O, 122.4 mg Fe(NO3)3·9H2O (the atomic ratio of Ni: Fe is 2: 1) and 106.2 mg CO(NH2)2 in 50 mL deionized water under stirring for 30 min. The cleaned NF was added into this beaker and kept at 75 °C over a period of 12 h. After the sample had completely fallen to room temperature, it was taken out of the beaker. After cooling down to room temperature and washing by deionized water and alcohol, the NiFeOOH-NiFe-LDH was obtained. The sample was dried under 60 °C for 3 h, marked as NiFeOOH-NiFe-LDH.
NiFe-LDH/NF was prepared in the same precursor solution, comprising 178.0 mg Ni(NO3)2·6H2O, 122.4 mg Fe(NO3)3·9H2O and 106.2 mg CO(NH2)2 in 50 mL of deionized water. They were kept in a sealed PTFE-lined stainless steel at 120 °C for 12 h. The obtained product was denoted as NiFe-LDH. The empty NF electrode and the purchased industrial Ti/IrO2 electrode were also used as the control groups.
The synthesis methods for electrodes is illustrated in Figure S1.
2.3. Materials Characterization
The structural information of NiFeOOH-NiFe-LDH were studied by an X-ray diffraction (XRD, Bruker D8, Bruker Corporation, Karlsruhe, Germany). Microstructure and elemental distribution are characterized by scanning electron microstructure (SEM with EDS, Apreo S LoVac, Thermo Fisher Scientific, Hillsboro, Oregon, United States) and transmission electron microscopy (TEM, JEM 2100F, JEOL Ltd., Tokyo, Japan). The chemical state data were obtained via X-ray photoelectron spectroscopy (XPS, Thermo 250Xi, Thermo Fisher Scientific, Waltham, MA, USA). The composition of substances was further verified by Raman spectroscopy (Raman spectra, Horiba, Horiba Jobin Yvon, Palaiseau, France).
2.4. Electrochemical Measurements
The OER activity in 1 mol·L−1 KOH was evaluated using an AUTOLAB PGSTAT302N workstation, where NiFe-LDH, NF and Ti/IrO2 were used directly as the working electrode, and the Hg/HgO was the reference one and the Pt foil electrodes were the counter electrode. Reversible hydrogen electrode (RHE) was used to calibrate the potential. The calibrated equation is ERHE = EHg/HgO + 0.0591 × pH + 0.098 V. In addition, the iR drop of each polarization curve was compensated using an electrochemical workstation.
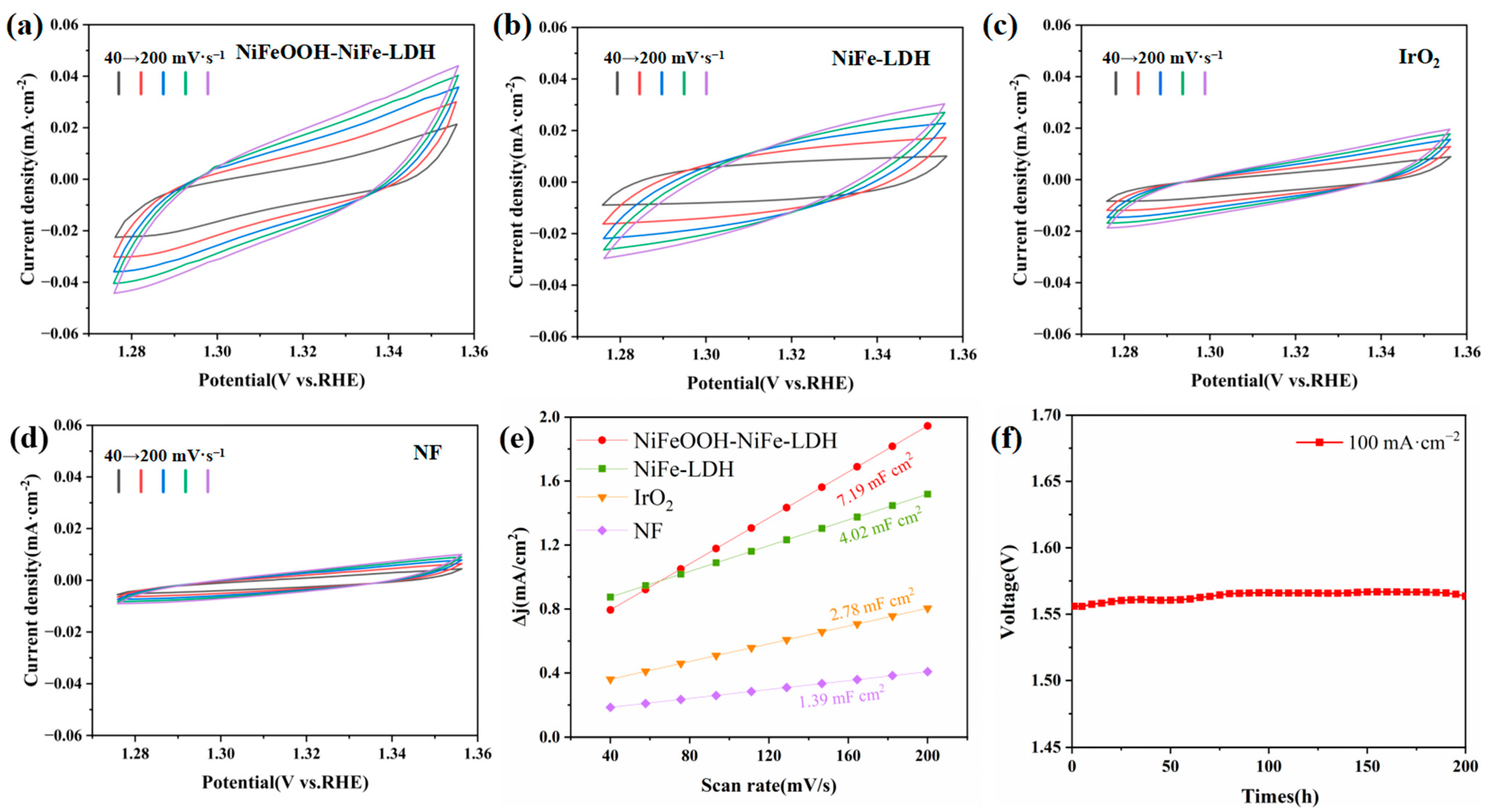
Linear sweep voltammetry (LSV) was collected under the rate of 5 mV·s−1. Electrochemical impedance spectroscopy (EIS) was tested at 1.42 V Versus RHE (VRHE) within the frequency from 105 Hz to 0.01 Hz with the amplitude of 5 mV. Electrochemical surface areas (ECSA) were studied in a non-Faradic potential window. The potentials were between 1.275 VRHE and 1.355 VRHE and the scan rates between 40 and 200 mV·s−1. Chrono potentiometric measurements were made for 120 h at 100 and 200 mA·cm−2 in order to test durability. The geometric area of the NF was used to normalize the current density.
3. Results
3.1. Structural and Morphological Characterizations of the Electrocatalysts
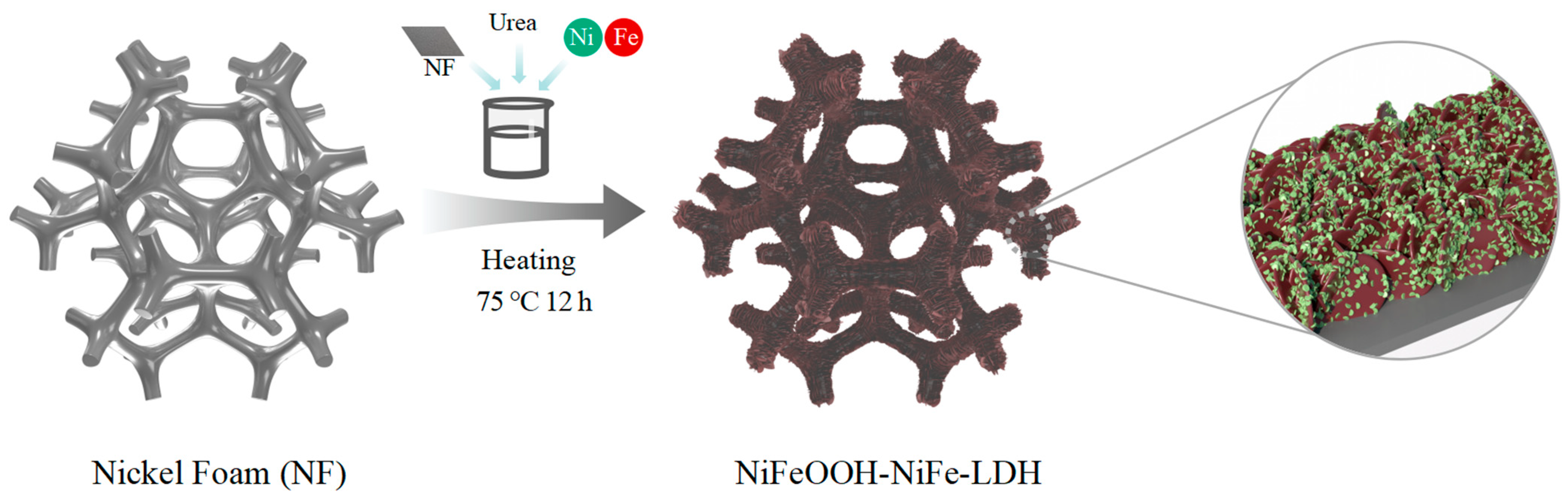
NiFeOOH-NiFe-LDH prepared in a one-step process was obtained by placing the treated nickel foam directly into a beaker with precursor solution and heating the beaker directly in an oven at 75 °C for 12h (Figure 1). As the temperature rises, the dissolved urea slowly decomposes and breaks down to form an alkaline environment. In the early stage, NiFe-LDH crystals nucleate and grow attached to the nickel foam to form nanosheets. Then, with the decomposition of urea, the metal ions foam hydroxyl oxides attached to nanosheets.
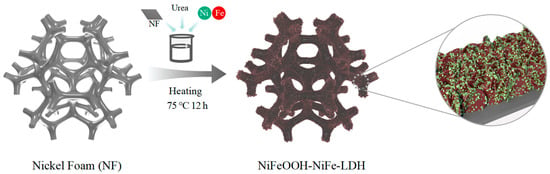
Figure 1.
Diagram of the synthesis procedure for H-(Fe0.67Ni0.33)OOH-NiFe-LDH/NF.
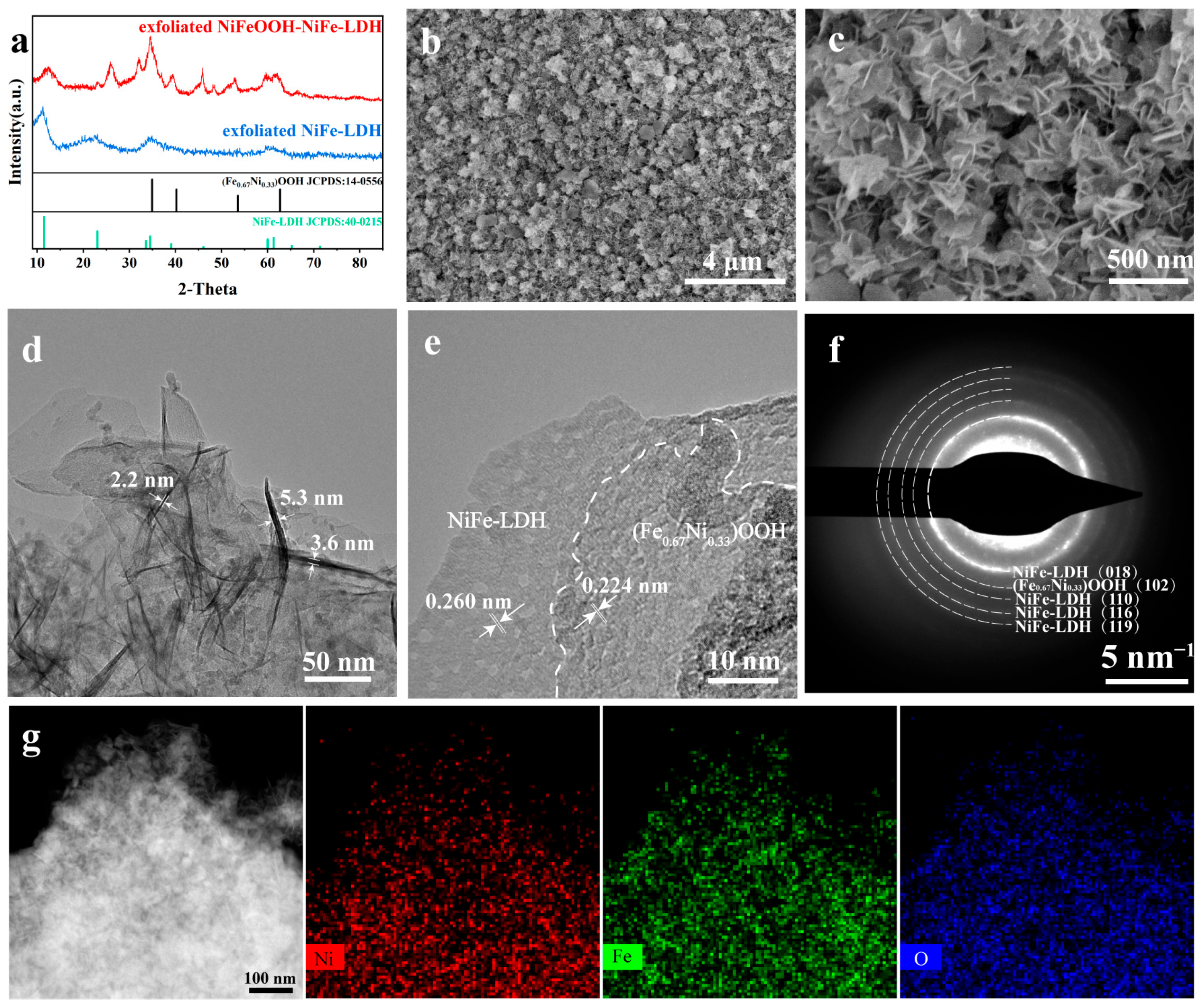
XRD tests were performed on the NiFeOOH-NiFe-LDH and NiFe-LDH samples attached to nickel foam surface (Figure S2). However, due to the low content of the surface catalytic layer, the diffraction peaks of the catalytic layer cannot be observed. Therefore, the ultrasonic oscillation was used to strip the catalytic layer from nickel foam, and the XRD of exfoliated samples are shown in Figure 2a. The diffraction peaks at 11.4°, 22.9°, 38.9° and 61.2° are the (003), (006), (015) and (113) lattice planes of the NiFe-LDH sample, respectively [23,24]. The diffraction peaks at 34.9°, 40.2°, 53.4° and 62.7° are the (110), (101), (102) and (110) facets of (Fe0.67Ni0.33)OOH [28], respectively.
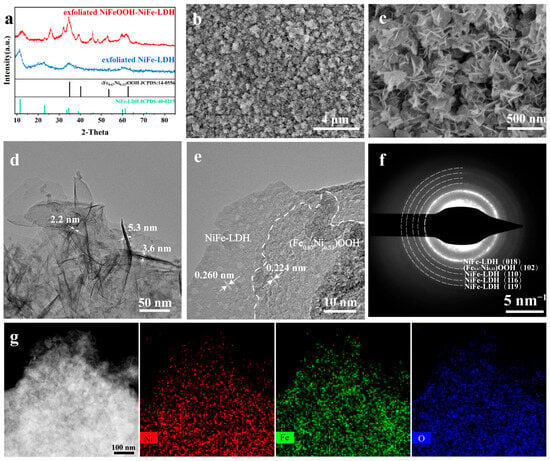
Figure 2.
(a) XRD pattern of exfoliated NiFeOOH-NiFe-LDH and exfoliated NiFe-LDH. (b,c) SEM images, (d,e) TEM images, (f) SAED patterns and (g) TEM images with EDS-mapping images of NiFeOOH-NiFe-LDH.
The surface of catalysts was obtained via the SEM. In Figure 2b,c, the SEM images showed that the interconnected ultrathin nanosheets agglomerated into nanocluster structures, growing vertically on the NF surface. The SEM images at low magnification are shown in Figures S3 and S4. The cross-sectional view of the catalyst layer is shown in Figure S5, and the thickness of the NiFeOOH-NiFe-LDH/NF and NiFe-LDH/NF catalyst layers is approximately 3 μm.
The transmission electron microscopy (TEM) images suggest that the nanosheet thickness is thinner, indicating that more reactive sites can be exposed (Figure 2d). In TEM images (Figure 2e and Figure S6), there are visible lattice stripes with the spacing of 0.224 nm, due to the (101) plane of (Fe0.67Ni0.33)OOH [28]. The lattice spacing of 0.260 nm is due to the (012) lattice plane of NiFe-LDH [29]. Selected area electron diffraction (SAED) patterns showing diffraction rings (Figure 2f) were also consistent with the above findings. The TEM images and the related EDS mapping (Figure 2g) demonstrated that Ni, Fe and O were uniformly distributed on the nanosheets.
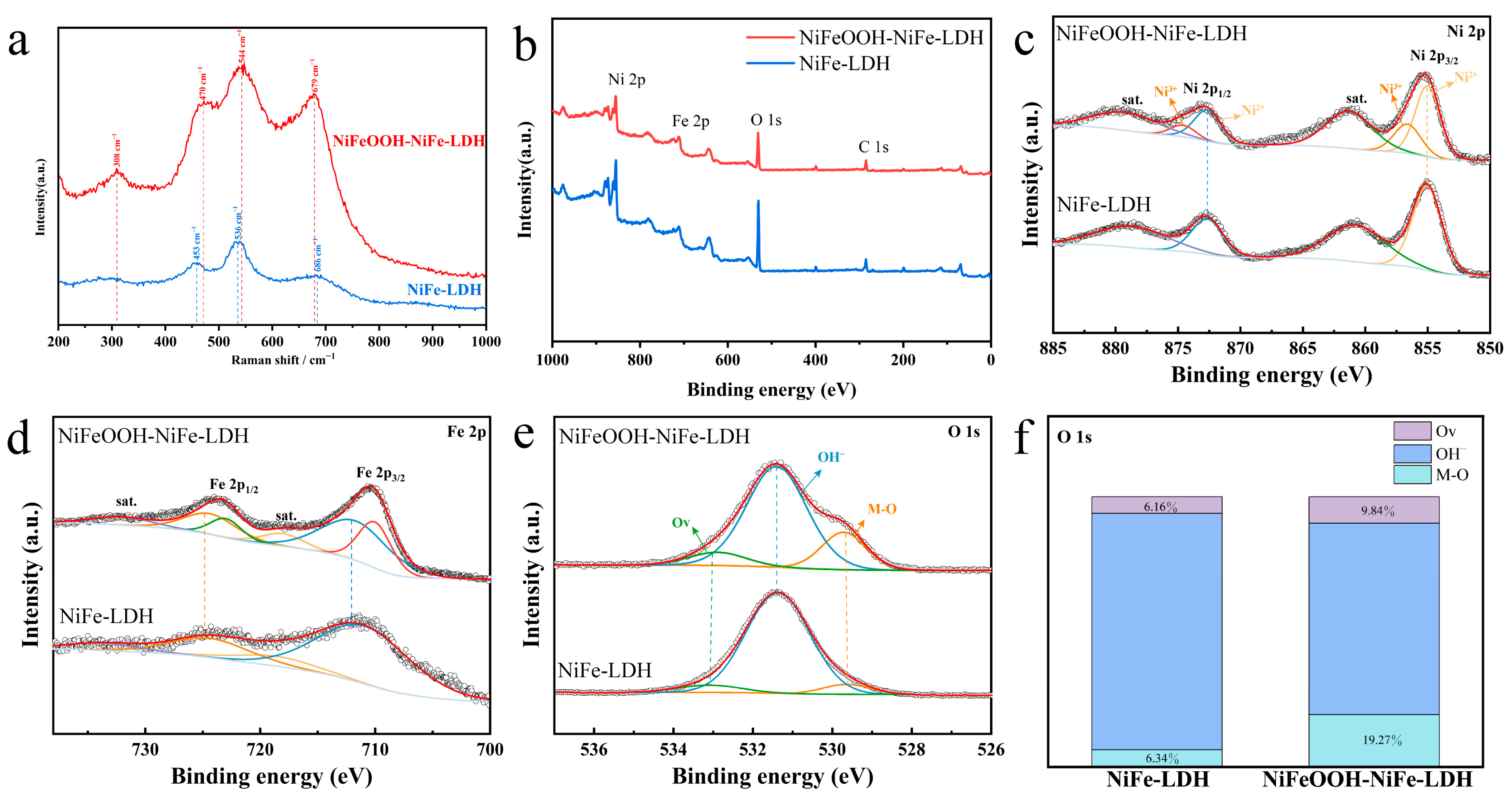
Phase detection was tested using Raman spectroscopy in Figure 3a to verify the substance composition further. The characteristic NiFe-LDH peaks at 453 cm−1 and 536 cm−1 are due to the vibration of the Ni(II)-O bond in Ni(OH)2 [30]. The peaks at the corresponding positions in NiFeOOH-NiFe-LDH are 470 cm−1 and 544 cm−1, which are considered to be the result of the Ni(III)-O in NiOOH and Ni(II)-O in Ni(OH)2 [31,32]. Two strong peaks at 308 cm−1 and 679 cm−1 were detected, corresponding to Fe-O’s bending and stretching vibrations in FeOOH, respectively [33,34].
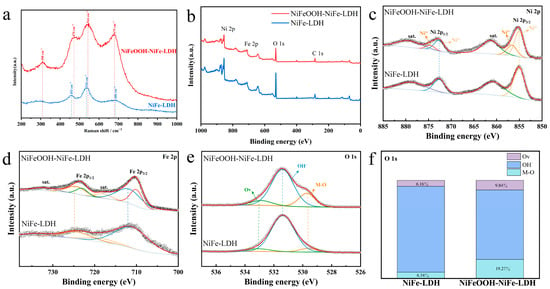
Figure 3.
(a) Raman spectra, (b) XPS survey spectra and single-element spectra, (c) Ni 2p, (d) Fe 2p, (e) O 1s and (f) percentage of O element in different forms of NiFeOOH-NiFe-LDH and NiFe-LDH.
XPS was utilized to study compositions of the NiFeOOH-NiFe-LDH and NiFe-LDH samples. In Figure 3b, the XPS reveals the existence of Ni, Fe and O, which is in accordance with EDS mapping images. The prominent Ni 2p peaks in Figure 3c are at 855.3 eV and 872.7 eV, identified as the Ni 2p3/2 and Ni 2p1/2 peaks, respectively. The typical satellite of Ni 2p3/2 and Ni 2p1/2 are the broad peaks at 861.4 and 879.7 eV [35]. The Ni 2p peak fitting analysis indicates the existence of two states of Ni 2p, Ni2+() and Ni3+ (), which can be attributed to Ni species in Ni(OH)2 and NiOOH, respectively. It is shown in Figure S7 that the prepared sample NiFeOOH-NiFe-LDH contains a high percentage of Ni3+ (30.8%). Many reports have shown that the introduction of high-valent Ni can effectively enhance the electron-withdrawing effect, and the electronic configuration of Ni3+ () is more favorable to the OER by forming σ-bonds with adsorbed oxygen [11,36].
Fe 2p in Figure 3d shows the peaks of NiFeOOH-NiFe-LDH at 710.3 eV and 723.6 eV, with the satellites at 717.0 eV and 732.6 eV, corresponding to the Fe 2p3/2 and Fe 2p1/2, respectively. The peak fitting of NiFeOOH-NiFe-LDH suggests that Fe3+ exists in different compositions. The peak located at 712.1 eV is attributed to FeOOH, and the peak at 710.2 eV is attributed to Fe(OH)3. These results agree with the finding of XRD [37,38,39].
The split-peak fitting of the O 1s spectra in Figure 3e shows that the lattice oxygen (M-O) is at 529.6 eV, the metal hydroxide (M-OH) is at 531.4 eV and the oxygen vacancy (Ov) is at 533.1 eV. It is clear from Figure 3f that the percentage of M-O of NiFeOOH-NiFe-LDH is considerably higher than the percentage of NiFe-LDH, suggesting the generation of hydroxyl oxides in NiFeOOH-NiFe-LDH.
NiFeOOH-NiFe-LDH has more oxygen vacancies than NiFe-LDH (Figure S5), suggesting the presence of high valence Ni in NiFeOOH-NiFe-LDH, which is beneficial to the OER reaction [40,41].
3.2. Electrochemical Test Performance
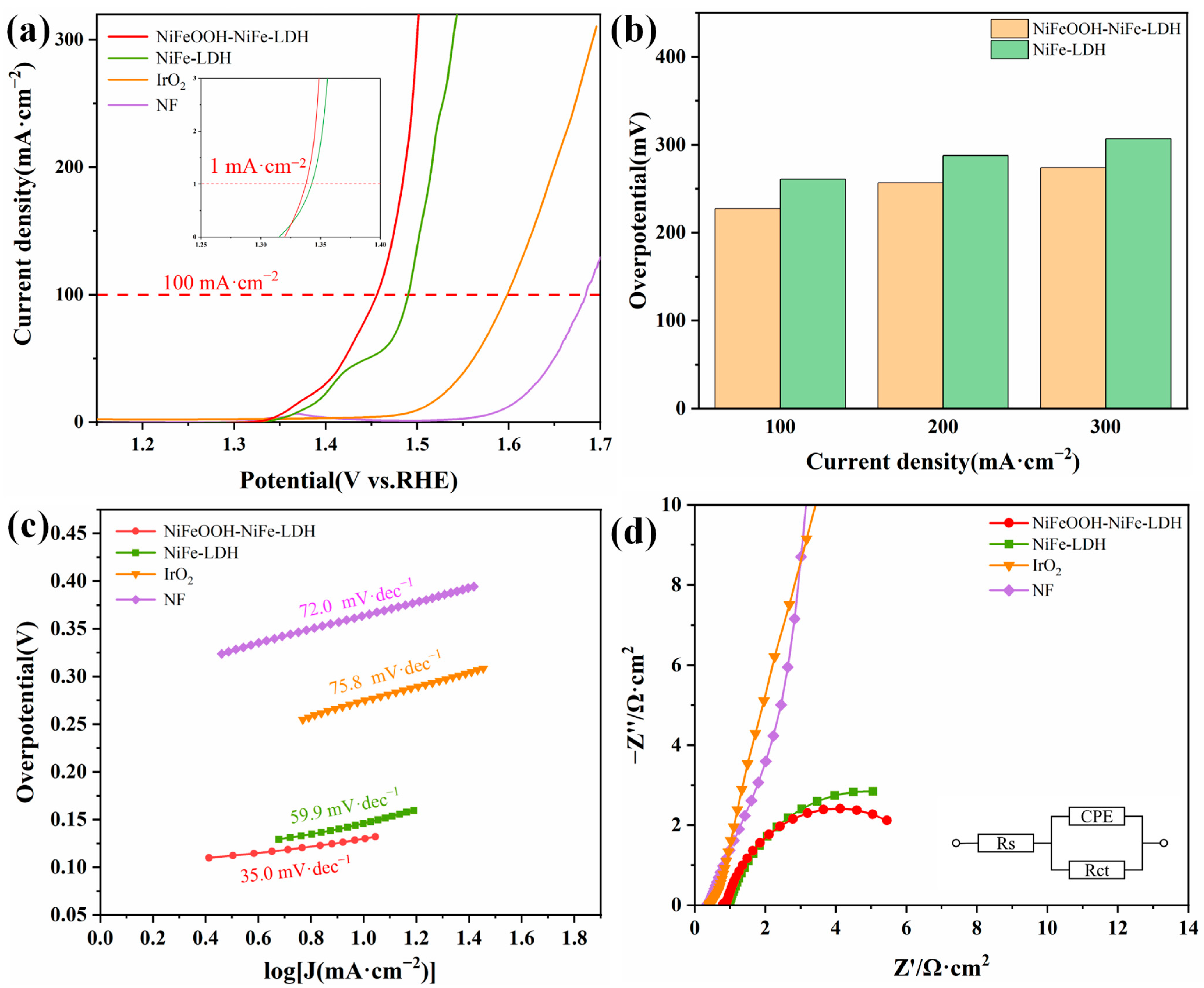
By means of a three-electrode setup, we investigated the electrochemical OER performance of various electrodes in 1 mol·L−1 KOH solution. Commercial IrO2/Ti and NF were also tested for comparison. In Figure 4a, the onset potential of NiFeOOH-NiFe-LDH at 1 mA·cm−2 was 1.337 V. NiFeOOH-NiFe-LDH has an overpotential of only 227 mV at 100 mA·cm−2, which was not only the lowest overpotential among the electrodes (Figure 4b) but also better than recently reported catalysts (Table 1).
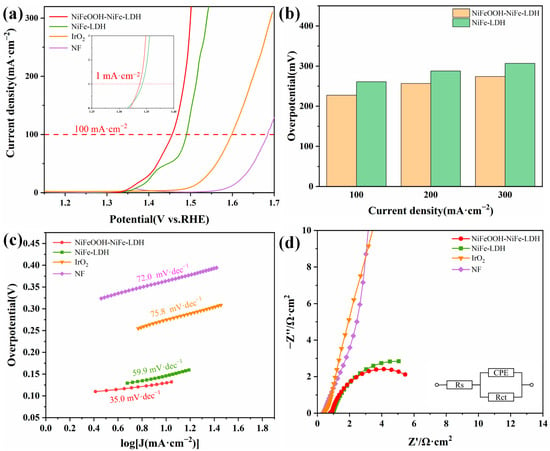
Figure 4.
Electrochemical performance of various catalysts in 1 mol·L−1 KOH. (a) Polarization curves. (b) The overpotentials required for current density of 100, 200 and 300 mA·cm−2. (c) Tafel plots. (d) Nyquist plots at 1.425 VRHE.

Table 1.
Comparison of OER performances between NiFeOOH-NiFe-LDH electrode and recently reported electrocatalysts in alkaline solution.
Additionally, the kinetic of electrodes was evaluated by calculating the Tafel curves based on the polarization curves. In Figure 4c, NiFeOOH-NiFe-LDH has the fastest OER reaction kinetics and lowest Tafel slope of 35.0 mV·dec−1. The enhanced kinetics are verified by the electrochemical impedance (Figure 4d) characterization, with the charge transfer resistance (Rct) of NiFeOOH-NiFe-LDH being the lowest among all of these samples (6.343 Ω·cm2, Table 2).

Table 2.
Equivalent circuit used for EIS data analysis and summary of the parameters obtained from various as-prepared electrodes.
CV curves based on the non-Faraday region of 1.275 V–1.355 V vs. RHE were calculated from the ECSA (Figure 5a–d, Table S1). In this way, the corresponding double-layer capacitance (Cdl) can be calculated for different catalysts. In particular, the Cdl of NiFeOOH-NiFe-LDH is 7.19 mF·cm−2, more significant than those of the other electrodes (Figure 5e). In comparison to NiFe-LDH, the surface area of NiFeOOH-NiFe-LDH is effectively increased. In 1 mol·L−1 KOH, stability tests of NiFeOOH-NiFe-LDH were conducted at 100 mA·cm−2 for 200 h (Figure 5f). It is observed that even after 200 h, the catalytic activity of NiFeOOH-NiFe-LDH remains at a high level, demonstrating its remarkable stability. The excellent catalytic activity of NiFeOOH-NiFe-LDH can be attributed to NiFeOOH serving as an intermediate in the OER mechanism, which promotes faster reaction rates.
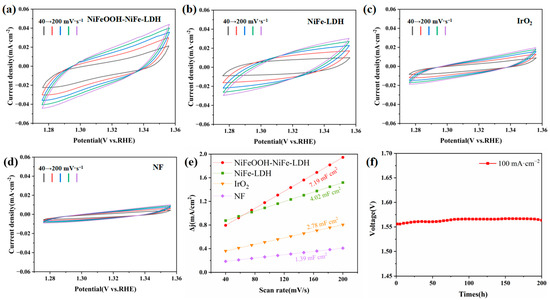
Figure 5.
The Cdl measurement at the non-Faraday region potential window: (a) NiFeOOH-NiFe-LDH, (b) NiFe-LDH, (c) IrO2 and (d) NF. (e) The value of Cdl of different catalysts. (f) Chronoamperometric plots at 100 mA·cm−2 for NiFeOOH-NiFe-LDH.
4. Conclusions
In this study, we explored a strategy to prepare the NiFeOOH/NiFe-LDH electrode by in-site growth of NiFeOOH-decorated NiFe-LDH nanosheets on NF support and compared it with NiFe-LDH (Table S2). Mechanistic analysis showed that the presence of the reaction intermediate NiFeOOH promoted the rapid occurrence of OER. The prepared NiFeOOH-NiFe-LDH has a low overpotential of 227 mV while providing 100 mA·cm−2 current density. It also has a durability of up to 120 h in operation at 200 mA·cm−2. The outstanding electrochemical performance can be attributed to its unique composition and nanostructure and the abundance of phase interfaces that enhance the exposure of catalytically active sites. This work provides a new strategy for preparing NiFe-based electrodes for OER reactions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry6020017/s1. Figure S1: synthesis methods for experimental electrodes; Figure S2: SEM images of NF; Figure S3: (a) SEM images and (b) TEM image of NiFe-LDH/NF; Figure S4: calculation of lattice stripes corresponding to two phase frame selection positions; Figure S5: percentage of Ni2+ and Ni3+ of NiFeOOH-NiFe-LDH; Figure S6: Calculation of lattice stripes corresponding to two phase frame selection positions; Figure S7: Percentage of Ni2+ and Ni3+ of NiFeOOH-NiFe-LDH; Table S1: The ECSA and Cdl values of different catalysts; Table S2: The comparisons between NiFeOOH-NiFe-LDH and NiFe-LDH.
Author Contributions
Conceptualization, J.N. and W.Z.; methodology, J.N., L.X. and W.X.; Validation, J.N. and L.X. and G.L.; formal analysis, J.N. and L.X.; investigation, J.N., L.X. and G.L.; resources, G.L. and W.X.; data curation, J.N.; writing—original draft preparation, J.N. and L.X.; writing—review and editing, W.Z.; visualization, J.N., G.L. and W.X.; supervision, W.Z. and L.X.; project administration, L.X.; funding acquisition, L.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key R&D Program (No. 2021YFB4000303).
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to further research is being carried out.
Conflicts of Interest
Authors L.X., W.X. and G.L. were employed by company Tianjin Mainland Hydrogen Equipment Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, M.; Wang, Y.; Mi, W.; Wu, J.; Zou, J.-J.; Zhu, X.-D.; Gao, J.; Zhang, Y.-C. Surface-modified amorphous FeOOH on NiFe LDHs for high efficiency electrocatalytic oxygen evolution. Electrochim. Acta 2023, 458, 142513. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, J.; Liu, D.; Wu, L.; Li, T.; Yan, S.; Fan, Q.; Zhu, K.; Zou, Z. Ultrafast Fenton-like reaction route to FeOOH/NiFe-LDH heterojunction electrode for efficient oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 21785–21791. [Google Scholar] [CrossRef]
- Wan, Z.; Ma, Z.; Yuan, H.; Liu, K.; Wang, X. Sulfur Engineering on NiFe Layered Double Hydroxide at Ambient Temperature for High Current Density Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2022, 5, 4603–4612. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Wu, D.; Huang, C.; Xiao, D.; Chen, H.; Zheng, S.; Chu, P.K. NiFe-Layered Double Hydroxide Synchronously Activated by Heterojunctions and Vacancies for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2020, 12, 42850–42858. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Y.; Xu, W.; Zhang, W. Ultrafast and Facile Synthesis of (Ni/Fe/Mo)OOH on Ni Foam for Oxygen Evolution Reaction in Seawater Electrolysis. Catalysts 2023, 13, 924. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Dong, Z.-H.; Jiang, Z.; Tang, T.; Yao, Z.-C.; Xue, D.; Niu, S.; Zhang, J.; Hu, J.-S. Rational design of integrated electrodes for advancing high-rate alkaline electrolytic hydrogen production. J. Mater. Chem. A 2022, 10, 12764–12787. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Xu, L.; Yuan, B.; Min, L.; Xu, W.; Zhang, W. Preparation of NiCo-LDH@NiCoV-LDH interconnected nanosheets as high-performance electrocatalysts for overall water splitting. Int. J. Hydrogen Energy 2022, 47, 15583–15592. [Google Scholar] [CrossRef]
- Chen, Y.; Min, L.; Zhang, W.; Xu, L.; Wang, Y. Crown ether as a bifunctional booster in electrochemical water splitting. Int. J. Hydrogen Energy 2024, 51, 1534–1543. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Hu, Y.; Guan, M.; Xu, L.; Li, H.; Bao, J.; Li, H. Cr-doped CoFe layered double hydroxides: Highly efficient and robust bifunctional electrocatalyst for the oxidation of water and urea. Appl. Catal. B Environ. 2020, 272, 118959. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Wang, H.-Y.; Zheng, H.; Zhang, W.; Cao, R. O–O bond formation mechanisms during the oxygen evolution reaction over synthetic molecular catalysts. Chin. J. Catal. 2021, 42, 1253–1268. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Zhong, C.; Zhao, N.; Deng, Y.; Han, X.; Hu, W. Spontaneous Synthesis of Silver-Nanoparticle-Decorated Transition-Metal Hydroxides for Enhanced Oxygen Evolution Reaction. Angew. Chem. Int. Ed. Engl. 2020, 59, 7245–7250. [Google Scholar] [CrossRef]
- Chen, Z.; Qu, Q.; Li, X.; Srinivas, K.; Chen, Y.; Zhu, M. Room-Temperature Synthesis of Carbon-Nanotube-Interconnected Amorphous NiFe-Layered Double Hydroxides for Boosting Oxygen Evolution Reaction. Molecules 2023, 28, 7289. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Y.; Lu, X. Construction of NiFe-Layered Double Hydroxides Arrays as Robust Electrocatalyst for Oxygen Evolution Reaction. Catalysts 2023, 13, 586. [Google Scholar] [CrossRef]
- Guo, D.; Chi, J.; Yu, H.; Jiang, G.; Shao, Z. Self-Supporting NiFe Layered Double Hydroxide “Nanoflower” Cluster Anode Electrode for an Efficient Alkaline Anion Exchange Membrane Water Electrolyzer. Energies 2022, 15, 4645. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Tan, L.; Liu, X.; Wen, Y.; Hou, W.; Zhan, T. Electrodeposition of NiFe-layered double hydroxide layer on sulfur-modified nickel molybdate nanorods for highly efficient seawater splitting. J. Colloid Interface Sci. 2022, 613, 349–358. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Xie, D.; Gu, Y.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H.; Wong, P.K. NiFe-Layered Double Hydroxide Nanosheet Arrays Supported on Carbon Cloth for Highly Sensitive Detection of Nitrite. ACS Appl. Mater. Interfaces 2018, 10, 6541–6551. [Google Scholar] [CrossRef]
- Lai, D.; Kang, Q.; Gao, F.; Lu, Q. High-entropy effect of a metal phosphide on enhanced overall water splitting performance. J. Mater. Chem. A 2021, 9, 17913–17922. [Google Scholar] [CrossRef]
- Zhao, C.-X.; Li, B.-Q.; Zhao, M.; Liu, J.-N.; Zhao, L.-D.; Chen, X.; Zhang, Q. Precise anionic regulation of NiFe hydroxysulfide assisted by electrochemical reactions for efficient electrocatalysis. Energy Environ. Sci. 2020, 13, 1711–1716. [Google Scholar] [CrossRef]
- Zhou, T.; Bai, J.; Gao, Y.; Zhao, L.; Jing, X.; Gong, Y. Selenide-based 3D folded polymetallic nanosheets for a highly efficient oxygen evolution reaction. J. Colloid Interface Sci. 2022, 615, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhao, Y.; Chen, G.; Shang, L.; Shi, R.; Kang, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; Zhang, T. Ni3FeN Nanoparticles Derived from Ultrathin NiFe-Layered Double Hydroxide Nanosheets: An Efficient Overall Water Splitting Electrocatalyst. Adv. Energy Mater. 2016, 6, 1502585. [Google Scholar] [CrossRef]
- Bodhankar, P.M.; Sarawade, P.B.; Singh, G.; Vinu, A.; Dhawale, D.S. Recent advances in highly active nanostructured NiFe LDH catalyst for electrochemical water splitting. J. Mater. Chem. A 2021, 9, 3180–3208. [Google Scholar] [CrossRef]
- Ding, P.; Meng, C.; Liang, J.; Li, T.; Wang, Y.; Liu, Q.; Luo, Y.; Cui, G.; Asiri, A.M.; Lu, S.; et al. NiFe Layered-Double-Hydroxide Nanosheet Arrays on Graphite Felt: A 3D Electrocatalyst for Highly Efficient Water Oxidation in Alkaline Media. Inorg. Chem. 2021, 60, 12703–12708. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hung, S.F.; Zhou, D.; Gao, J.; Yang, C.; Tao, H.; Yang, H.B.; Zhang, L.; Zhang, L.; Xiong, Q.; et al. Layered Structure Causes Bulk NiFe Layered Double Hydroxide Unstable in Alkaline Oxygen Evolution Reaction. Adv. Mater. 2019, 31, e1903909. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jiao, Q.; Chen, W.; Dang, Y.; Dai, Z.; Suib, S.L.; Zhang, J.; Zhao, Y.; Li, H.; Feng, C. Cactus-like NiCo2S4@NiFe LDH hollow spheres as an effective oxygen bifunctional electrocatalyst in alkaline solution. Appl. Catal. B Environ. 2021, 286, 119869. [Google Scholar] [CrossRef]
- Lin, Z.; Bu, P.; Xiao, Y.; Gao, Q.; Diao, P. β- and γ-NiFeOOH electrocatalysts for an efficient oxygen evolution reaction: An electrochemical activation energy aspect. J. Mater. Chem. A 2022, 10, 20847–20855. [Google Scholar] [CrossRef]
- Wang, M.; Cao, K.; Tian, Z.; Sheng, P. Increased charge and mass transfer derived-sheet-like Fe0.67Ni0.33OOH-Fe2O3@NF array for robust oxygen evolution reaction. Appl. Surf. Sci. 2019, 493, 351–358. [Google Scholar] [CrossRef]
- Dang, Y.; Li, X.; Chen, Z.; Zhao, X.; Ma, B.; Chen, Y. Hierarchical MoN@NiFe-LDH Heterostructure Nanowire Array for Highly Efficient Electrocatalytic Hydrogen Evolution. Small 2023, 19, e2303932. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Yuan, M.; Hao, H.; Lv, Z.; Xu, L.; Wei, B. Operando spectroscopies unveil interfacial FeOOH induced highly reactive β-Ni(Fe)OOH for efficient oxygen evolution. Appl. Catal. B Environ. 2022, 318, 121825. [Google Scholar] [CrossRef]
- Durr, R.N.; Maltoni, P.; Tian, H.; Jousselme, B.; Hammarstrom, L.; Edvinsson, T. From NiMoO(4) to gamma-NiOOH: Detecting the Active Catalyst Phase by Time Resolved in Situ and Operando Raman Spectroscopy. ACS Nano 2021, 15, 13504–13515. [Google Scholar] [CrossRef]
- Yan, P.; Liu, Q.; Zhang, H.; Qiu, L.; Wu, H.B.; Yu, X.-Y. Deeply reconstructed hierarchical and defective NiOOH/FeOOH nanoboxes with accelerated kinetics for the oxygen evolution reaction. J. Mater. Chem. A 2021, 9, 15586–15594. [Google Scholar] [CrossRef]
- Luo, W.; Jiang, C.; Li, Y.; Shevlin, S.A.; Han, X.; Qiu, K.; Cheng, Y.; Guo, Z.; Huang, W.; Tang, J. Highly crystallized α-FeOOH for a stable and efficient oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 2021–2028. [Google Scholar] [CrossRef]
- Chemelewski, W.D.; Lee, H.C.; Lin, J.F.; Bard, A.J.; Mullins, C.B. Amorphous FeOOH oxygen evolution reaction catalyst for photoelectrochemical water splitting. J. Am. Chem. Soc. 2014, 136, 2843–2850. [Google Scholar] [CrossRef]
- Wang, F.-L.; Yu Zhang, X.; Zhou, J.-C.; Shi, Z.-N.; Dong, B.; Xie, J.-Y.; Dong, Y.-W.; Yu, J.-F.; Chai, Y.-M. Amorphous–crystalline FeNi2S4@NiFe–LDH nanograsses with molten salt as an industrially promising electrocatalyst for oxygen evolution. Inorg. Chem. Front. 2022, 9, 2068–2080. [Google Scholar] [CrossRef]
- Zhai, Y.; Ren, X.; Sun, Y.; Li, D.; Wang, B.; Liu, S. Synergistic effect of multiple vacancies to induce lattice oxygen redox in NiFe-layered double hydroxide OER catalysts. Appl. Catal. B Environ. 2023, 323, 122091. [Google Scholar] [CrossRef]
- Suliman, M.; Al Ghamdi, A.; Baroud, T.; Drmosh, Q.; Rafatullah, M.; Yamani, Z.; Qamar, M. Growth of ultrathin nanosheets of nickel iron layered double hydroxide for the oxygen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 23498–23507. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.-J.; Yang, Y.-B.; Guo, P.-F.; Zhu, B.; Wang, K.; Wang, W.-T.; He, Z.-H.; Liu, Z.-T. Ru-Doped NiFe Layered Double Hydroxide as a Highly Active Electrocatalyst for Oxygen Evolution Reaction. J. Electrochem. Soc. 2022, 169, 024503. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, S.; Lin, H.; Wang, G.; Zhao, K.; Cai, R.; Tao, K.; Zhang, C.; Sun, M.; Hu, J.; et al. Atomically targeting NiFe LDH to create multivacancies for OER catalysis with a small organic anchor. Nano Energy 2021, 81, 105606. [Google Scholar] [CrossRef]
- Wu, B.; Gong, S.; Lin, Y.; Li, T.; Chen, A.; Zhao, M.; Zhang, Q.; Chen, L. A Unique NiOOH@FeOOH Heteroarchitecture for Enhanced Oxygen Evolution in Saline Water. Adv. Mater. 2022, 34, e2108619. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Gao, G.; Chen, H.; Wang, B.; Zhou, J.; Soo, M.T.; Hong, M.; Yan, X.; Qian, G.; et al. A Heterostructure Coupling of Exfoliated Ni–Fe Hydroxide Nanosheet and Defective Graphene as a Bifunctional Electrocatalyst for Overall Water Splitting. Adv. Mater. 2017, 29, 1700017. [Google Scholar] [CrossRef]
- Liu, G.; Gao, X.; Wang, K.; He, D.; Li, J. Uniformly mesoporous NiO/NiFe2O4 biphasic nanorods as efficient oxygen evolving catalyst for water splitting. Int. J. Hydrogen Energy 2016, 41, 17976–17986. [Google Scholar] [CrossRef]
- Qin, Q.; Jang, H.; Li, P.; Yuan, B.; Liu, X.; Cho, J. A Tannic Acid-Derived N-, P-Codoped Carbon-Supported Iron-Based Nanocomposite as an Advanced Trifunctional Electrocatalyst for the Overall Water Splitting Cells and Zinc-Air Batteries. Adv. Energy Mater. 2019, 9, 1803312. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, Y.; Edvinsson, T. In operando Raman investigation of Fe doping influence on catalytic NiO intermediates for enhanced overall water splitting. Nano Energy 2019, 66, 104118. [Google Scholar] [CrossRef]
- An, L.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.-C.; Cheng, F.; Xi, P. Epitaxial Heterogeneous Interfaces on N-NiMoO4/NiS2 Nanowires/Nanosheets to Boost Hydrogen and Oxygen Production for Overall Water Splitting. Adv. Funct. Mater. 2019, 29, 1805298. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Liu, W.-T.; Li, X.-P.; Ouyang, T.; Liu, Z.-Q. Strong hydrophilicity NiS2/Fe7S8 heterojunctions encapsulated in N-doped carbon nanotubes for enhanced oxygen evolution reaction. Chem. Commun. 2020, 56, 1489–1492. [Google Scholar] [CrossRef]
- Che, Q.; Li, Q.; Chen, X.; Tan, Y.; Xu, X. Assembling amorphous (Fe-Ni)Co -OH/Ni3S2 nanohybrids with S-vacancy and interfacial effects as an ultra-highly efficient electrocatalyst: Inner investigation of mechanism for alkaline water-to-hydrogen/oxygen conversion. Appl. Catal. B Environ. 2020, 263, 118338. [Google Scholar] [CrossRef]
- Park, Y.S.; Jeong, J.-Y.; Jang, M.J.; Kwon, C.-Y.; Kim, G.H.; Jeong, J.; Lee, J.-H.; Lee, J.; Choi, S.M. Ternary layered double hydroxide oxygen evolution reaction electrocatalyst for anion exchange membrane alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 127–134. [Google Scholar] [CrossRef]
- Liu, S.; Wan, R.; Lin, Z.; Liu, Z.; Liu, Y.; Tian, Y.; Qin, D.-D.; Tang, Z. Probing the Co role in promoting the OER and Zn–air battery performance of NiFe-LDH: A combined experimental and theoretical study. J. Mater. Chem. A 2022, 10, 5244–5254. [Google Scholar] [CrossRef]
- Guo, P.-F.; Yang, Y.; Wang, W.-J.; Zhu, B.; Wang, W.-T.; Wang, Z.-Y.; Wang, J.-L.; Wang, K.; He, Z.-H.; Liu, Z.-T. Stable and active NiFeW layered double hydroxide for enhanced electrocatalytic oxygen evolution reaction. Chem. Eng. J. 2021, 426, 130768. [Google Scholar] [CrossRef]
- Xie, X.; Cao, C.; Wei, W.; Zhou, S.; Wu, X.-T.; Zhu, Q.-L. Ligand-assisted capping growth of self-supporting ultrathin FeNi-LDH nanosheet arrays with atomically dispersed chromium atoms for efficient electrocatalytic water oxidation. Nanoscale 2020, 12, 5817–5823. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liang, J.; Zhou, J.; Yin, Z.; Zhang, Z.; Liu, X. Synergistic coupling of FeOOH with Mo-incorporated NiCo LDH towards enhancing the oxygen evolution reaction. New J. Chem. 2022, 46, 7999–8009. [Google Scholar] [CrossRef]
- Zhang, H.; Meng, X.; Zhang, J.; Huang, Y. Hierarchical NiFe Hydroxide/Ni3N Nanosheet-on-Nanosheet Heterostructures for Bifunctional Oxygen Evolution and Urea Oxidation Reactions. ACS Sustain. Chem. Eng. 2021, 9, 12584–12590. [Google Scholar] [CrossRef]
- Xu, X.; Wang, T.; Zheng, M.; Li, Y.; Shi, J.; Tian, T.; Jia, R.; Liu, Y. Metal-organic framework assisted formation of Ni-Fe-based porous nanoflowers for enhanced water splitting. J. Alloys Compd. 2021, 875, 159970. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).