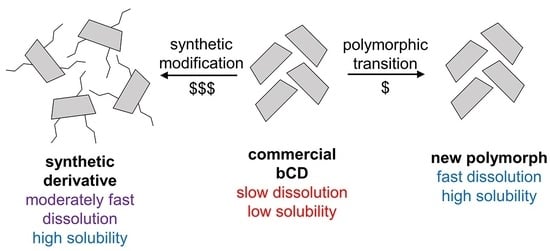
New Polymorph of β-Cyclodextrin with a Higher Bioavailability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
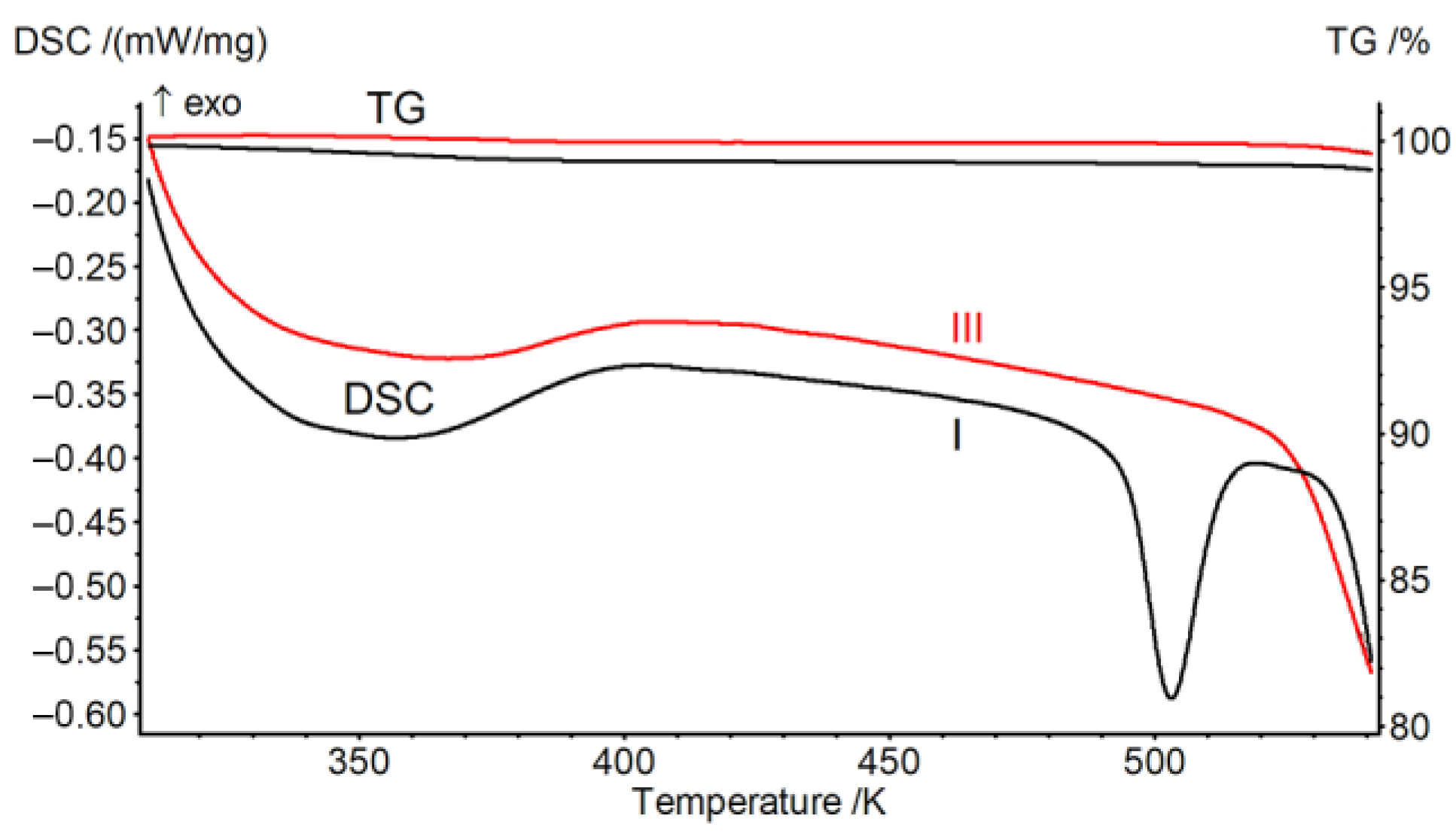
2.2. Thermal Analysis and Thermal Kinetics
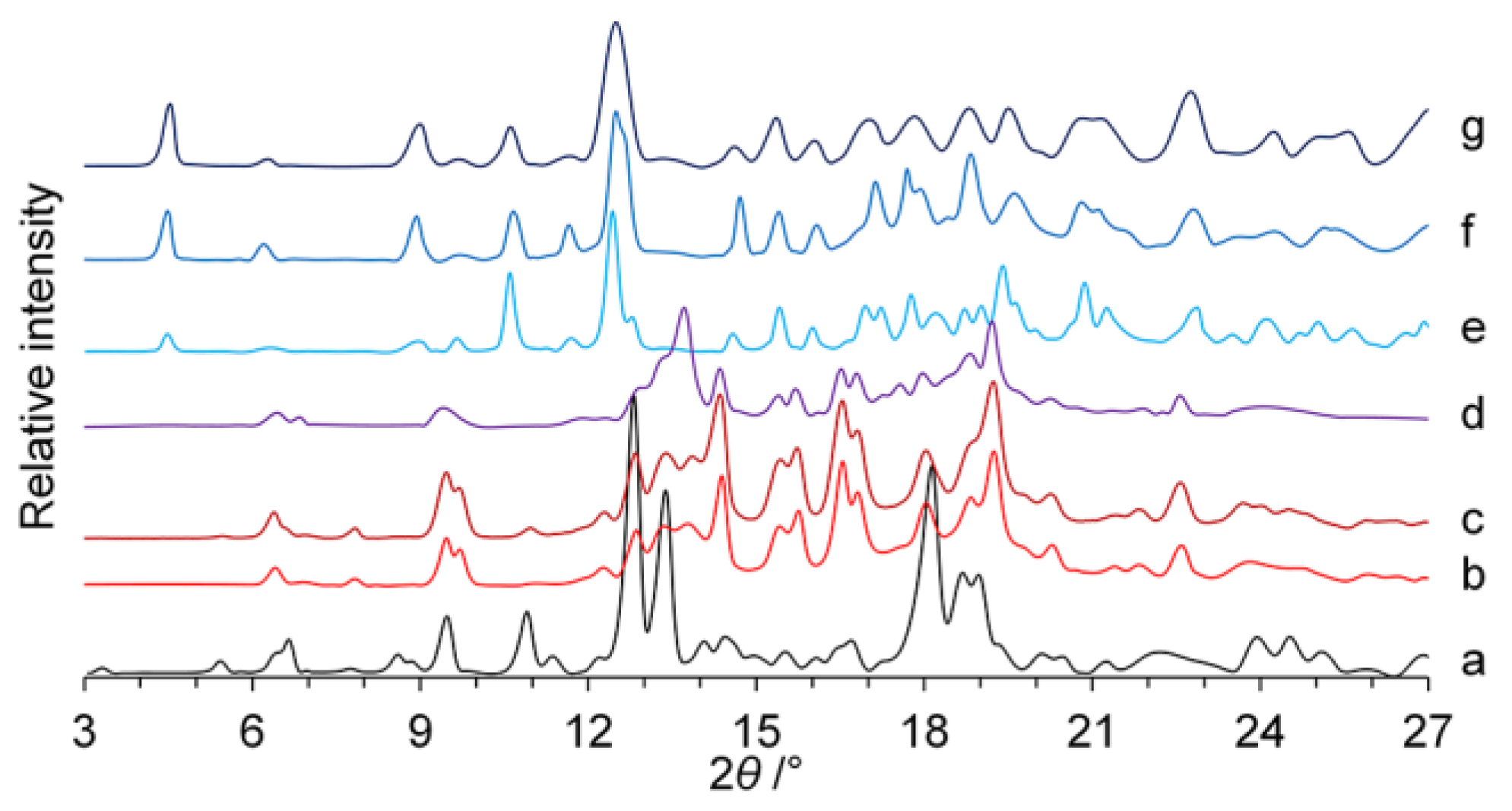
2.3. Powder X-ray Diffraction (PXRD)
2.4. Solution Calorimetry
2.5. Infrared Spectroscopy
3. Results
3.1. Preparation of Polymorph III
3.2. Dissolution Rate
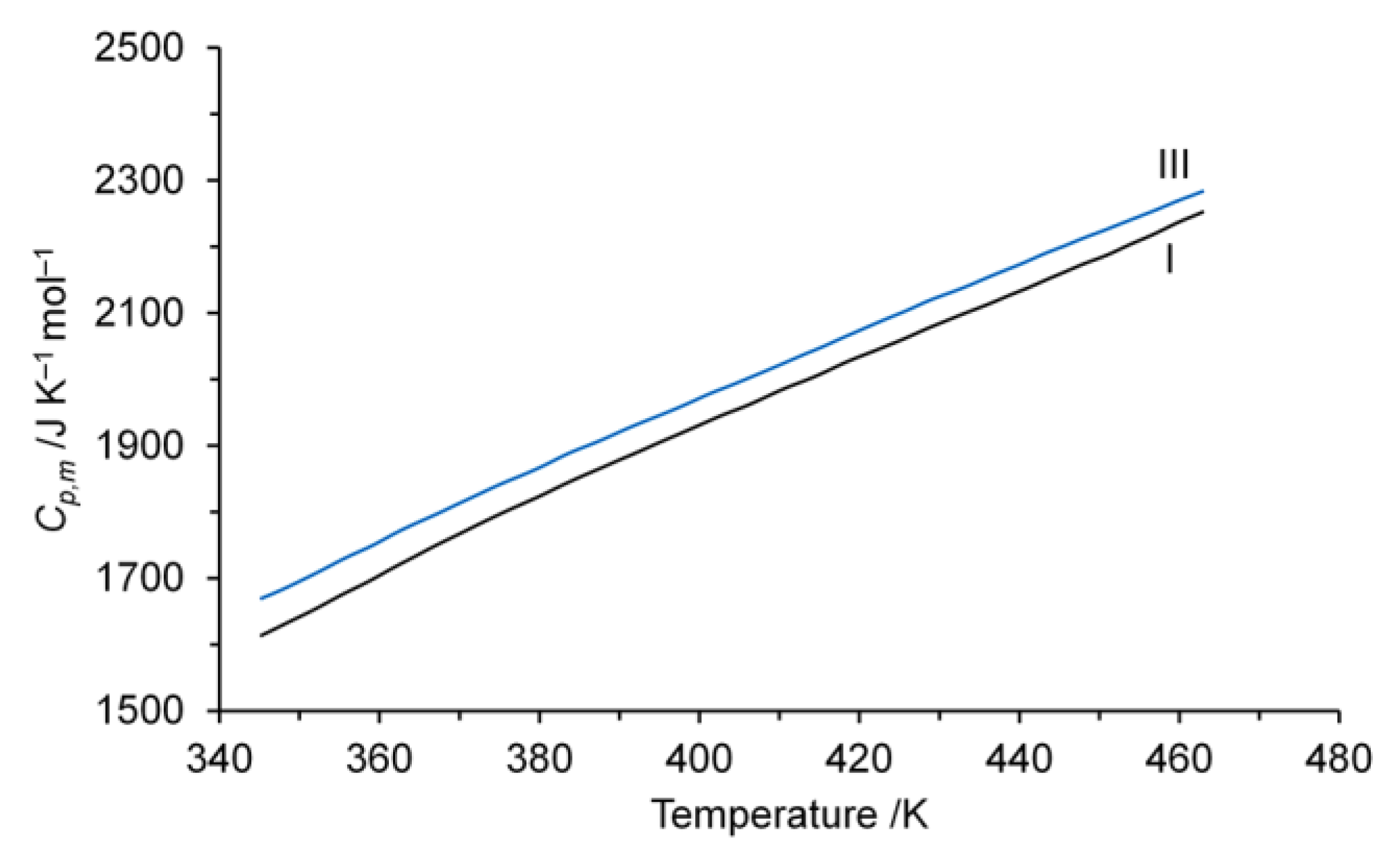
3.3. Thermodynamic Properties
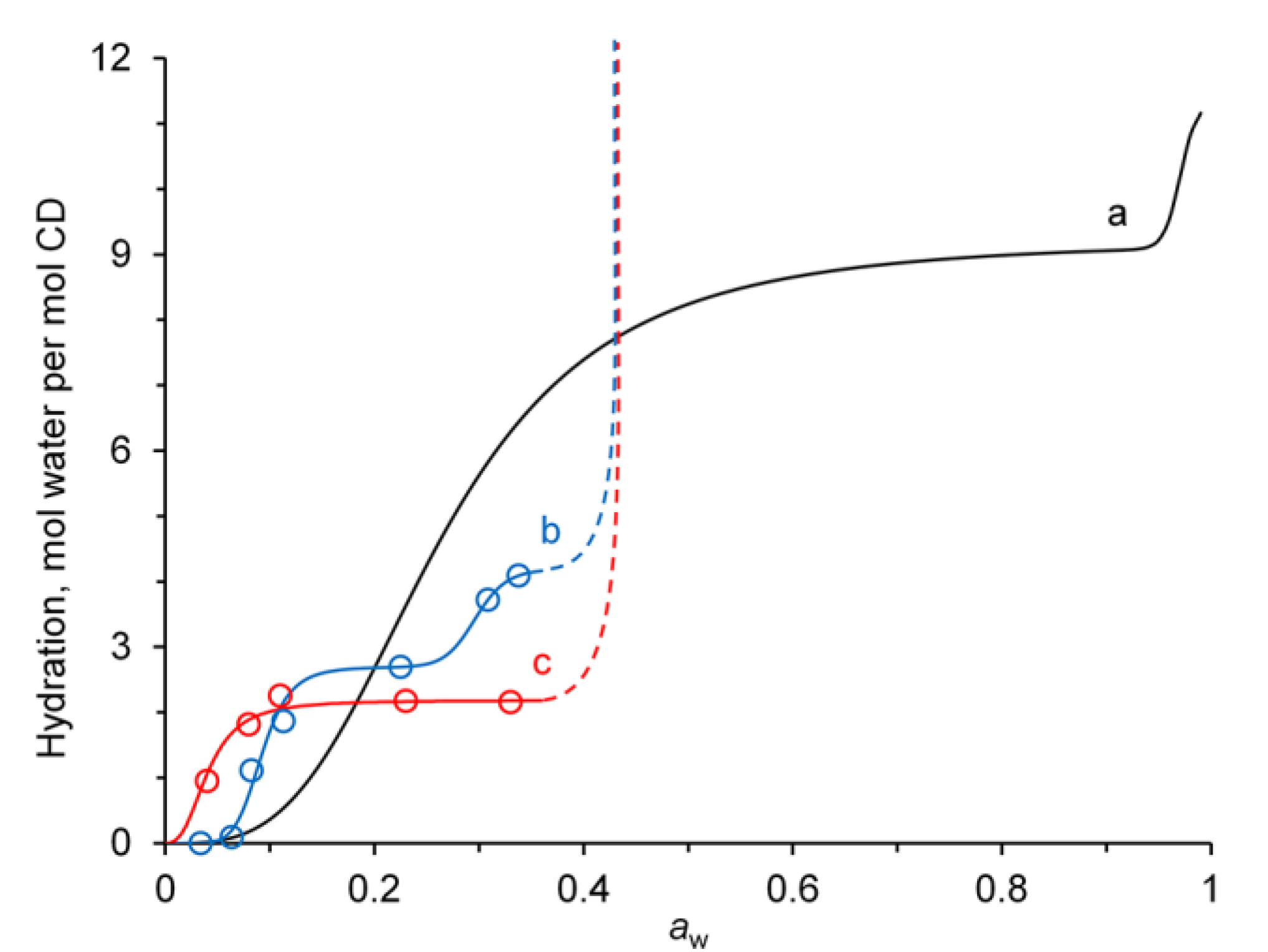
3.4. Affinity for Water
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fenyvesi, E.; Szente, L. Cyclodextrin in Starchy Foods. Acta Aliment. 2021, 50, 417–432. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, S.; Fenyvesi, É.; Lichtfouse, E.; Torri, G.; Fourmentin, M.; Crini, G. 130 Years of Cyclodextrin Discovery for Health, Food, Agriculture, and the Industry: A Review. Environ. Chem. Lett. 2021, 19, 2581–2617. [Google Scholar] [CrossRef]
- Gonzalez-Gaitano, G.; Isasi, J.R.; Velaz, I.; Zornoza, A. Drug Carrier Systems Based on Cyclodextrin Supramolecular Assemblies and Polymers: Present and Perspectives. Curr. Pharm. Des. 2017, 23, 411–432. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Mascarenhas-Melo, F.; Rabaça, S.; Mathur, A.; Sharma, A.; Giram, P.S.; Pawar, K.D.; Rahdar, A.; Raza, F.; Veiga, F.; et al. Cyclodextrin-Based Dermatological Formulations: Dermopharmaceutical and Cosmetic Applications. Colloids Surf. B Biointerfaces 2023, 221, 113012. [Google Scholar] [CrossRef] [PubMed]
- Puskás, I.; Szente, L.; Szőcs, L.; Fenyvesi, É. Recent List of Cyclodextrin-Containing Drug Products. Period. Polytech. Chem. Eng. 2023, 67, 11–17. [Google Scholar] [CrossRef]
- Kravtsova, A.A.; Skuredina, A.A.; Malyshev, A.S.; Le-Deygen, I.M.; Kudryashova, E.V.; Budynina, E.M. The Solubility Studies and the Complexation Mechanism Investigations of Biologically Active Spiro[Cyclopropane-1,3′-Oxindoles] with β-Cyclodextrins. Pharmaceutics 2023, 15, 228. [Google Scholar] [CrossRef]
- Zornoza, A.; Vélaz, I.; González-Gaitano, G.; Martínez-Ohárriz, M.C. A Comprehensive Study of Gemfibrozil Complexation with β-Cyclodextrins in Aqueous Solution Using Different Analytical Techniques. Int. J. Mol. Sci. 2022, 23, 16119. [Google Scholar] [CrossRef]
- Soe, H.M.S.H.; Chamni, S.; Mahalapbutr, P.; Kongtaworn, N.; Rungrotmongkol, T.; Jansook, P. The Investigation of Binary and Ternary Sulfobutylether-β-Cyclodextrin Inclusion Complexes with Asiaticoside in Solution and in Solid State. Carbohydr. Res. 2020, 498, 108190. [Google Scholar] [CrossRef]
- Censi, R.; Di Martino, P. Polymorph Impact on the Bioavailability and Stability of Poorly Soluble Drugs. Molecules 2015, 20, 18759–18776. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.-H.; Diao, K.-S. Improving the Solubility and Bioavailability of Pemafibrate via a New Polymorph Form II. ACS Omega 2020, 5, 26245–26252. [Google Scholar] [CrossRef]
- Loftsson, T.; Fulop, Z. Preparation of Solid Cyclodextrin Complexes for Ophthalmic Active Pharmaceutical Ingredient Delivery. U.S. Patent 1,113,531,1B2, 5 October 2021. [Google Scholar]
- Mentzafos, D.; Bethanis, K. ß-Cyclodextrin Dimeric Inclusion Complexes: A Review of Some Crystal Structure Aspects. In Cyclodextrins: Synthesis, Chemical Applications and Role in Drug Delivery; Ramirez, F.G., Ed.; Nova Science: New York, NY, USA, 2015; Chapter 8. [Google Scholar]
- Ceborska, M. Structural Investigation of Solid State Host/Guest Complexes of Native Cyclodextrins with Monoterpenes and Their Simple Derivatives. J. Mol. Struct. 2018, 1165, 62–70. [Google Scholar] [CrossRef]
- Harata, K. Crystallographic Study of Cyclodextrins and Their Inclusion Complexes. In Cyclodextrins and Their Complexes: Chemistry, Analytical Methods, Applications; Dodziuk, H., Ed.; Wiley-VCH: Weinheim, Germany, 2006; pp. 147–198. [Google Scholar]
- Shih, T.-W.; Hsu, C.-L.; Chen, L.-Y.; Huang, Y.-C.; Chen, C.-J.; Inoue, Y.; Sugiyama, T. Optical Trapping-Induced New Polymorphism of β-Cyclodextrin in Unsaturated Solution. Cryst. Growth Des. 2021, 21, 6913–6923. [Google Scholar] [CrossRef]
- Panova, I.G.; Zhukova, E.K.; Matukhina, E.V.; Topchieva, I.N. Receptor Properties of Nanoporous Structures Based on β-Cyclodextrin. Nanotechnologies Russ. 2010, 5, 304–312. [Google Scholar] [CrossRef]
- Panova, I.G.; Matukhina, E.V.; Gerasimov, V.I.; Topchieva, I.N. Non-Covalent Columnar Cyclodextrin-Based Structures. Colloid J. 2006, 68, 66–78. [Google Scholar] [CrossRef]
- Martini, A.; Torricelli, C.; Muggetti, L.; De Ponti, R. Use of Dehydrated Beta-Cyclodextrin as Pharmaceutical Excipient. Drug Dev. Ind. Pharm. 1994, 20, 2381–2393. [Google Scholar] [CrossRef]
- Gorbatchuk, V.V.; Gatiatulin, A.K.; Ziganshin, M.A.; Gubaidullin, A.T.; Yakimova, L.S. Unusually High Efficiency of β-Cyclodextrin Clathrate Preparation by Water-Free Solid-Phase Guest Exchange. J. Phys. Chem. B 2013, 117, 14544–14556. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.I.; Braga, T.M.; Silva, P.; Fernandes, J.A.; Ribeiro-Claro, P.; Lopes, M.d.F.S.; Paz, F.A.A.; Braga, S.S. Chloramphenicol·cyclodextrin Inclusion Compounds: Co-Dissolution and Mechanochemical Preparations and Antibacterial Action. CrystEngComm 2013, 15, 2822. [Google Scholar] [CrossRef]
- Steiner, T.; Koellner, G. Crystalline β-Cyclodextrin Hydrate at Various Humidities: Fast, Continuous, and Reversible Dehydration Studied by X-Ray Diffraction. J. Am. Chem. Soc. 1994, 116, 5122–5128. [Google Scholar] [CrossRef]
- Sala, A.; Hoossen, Z.; Bacchi, A.; Caira, M.R. Two Crystal Forms of a Hydrated 2:1 β-Cyclodextrin Fluconazole Complex: Single Crystal X-Ray Structures, Dehydration Profiles, and Conditions for Their Individual Isolation. Molecules 2021, 26, 4427. [Google Scholar] [CrossRef]
- Osel’skaya, V.Y.; Gatiatulin, A.K.; Klimovitskii, A.E.; Ziganshin, M.A.; Gorbatchuk, V.V. Competing Role of Water in Inclusion of Indomethacin and Volatile Organic Compounds by Native Cyclodextrins. Macroheterocycles 2022, 15, 174–185. [Google Scholar] [CrossRef]
- Gatiatulin, A.K.; Grishin, I.A.; Buzyurov, A.V.; Mukhametzyanov, T.A.; Ziganshin, M.A.; Gorbatchuk, V.V. Determination of Melting Parameters of Cyclodextrins Using Fast Scanning Calorimetry. Int. J. Mol. Sci. 2022, 23, 13120. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Novak, C.; Moyano, J.R. Thermal Analysis of Cyclodextrins and Their Inclusion Compounds. Thermochim. Acta 2001, 380, 123–151. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, Physicochemical Properties and Pharmaceutical Applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Lan Pham, T.; Usacheva, T.R.; Alister, D.A.; Thu Ha Nguyen, T.; Tukumova, N.V.; Kuranova, N.N.; Minh Vu, X.; My Hanh Le, T.; Tung Nguyen, Q.; Lam Tran, D. Thermodynamic Parameters and Quantum Chemical Calculations of Complex Formation between Rutin and 2-Hydroxypropyl-β-Cyclodextrin in Water-Ethanol Solvents. J. Mol. Liq. 2022, 366, 120324. [Google Scholar] [CrossRef]
- Nagrimanov, R.N.; Samatov, A.A.; Buzyurov, A.V.; Kurshev, A.G.; Ziganshin, M.A.; Zaitsau, D.H.; Solomonov, B.N. Thermochemical Properties of Mono- and Di-Cyano-Aromatic Compounds at 298.15 K. Thermochim. Acta 2018, 668, 152–158. [Google Scholar] [CrossRef]
- Altomare, A.; Cuocci, C.; Giacovazzo, C.; Moliterni, A.; Rizzi, R.; Corriero, N.; Falcicchio, A. EXPO2013: A Kit of Tools for Phasing Crystal Structures from Powder Data. J. Appl. Crystallogr. 2013, 46, 1231–1235. [Google Scholar] [CrossRef]
- McCusker, L.B.; Von Dreele, R.B.; Cox, D.E.; Louër, D.; Scardi, P. Rietveld Refinement Guidelines. J. Appl. Crystallogr. 1999, 32, 36–50. [Google Scholar] [CrossRef]
- Varfolomeev, M.A.; Abaidullina, D.I.; Solomonov, B.N.; Verevkin, S.P.; Emel’yanenko, V.N. Pairwise Substitution Effects, Inter- and Intramolecular Hydrogen Bonds in Methoxyphenols and Dimethoxybenzenes. Thermochemistry, Calorimetry, and First-Principles Calculations. J. Phys. Chem. B 2010, 114, 16503–16516. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin/Heidelberg, Germany, 2015; p. 239. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Gatiatulin, A.K.; Ziganshin, M.A.; Yumaeva, G.F.; Gubaidullin, A.T.; Suwińska, K.; Gorbatchuk, V.V. Using Water-Mimic Organic Compounds to Activate Guest Inclusion by Initially Dry Beta-Cyclodextrin. RSC Adv. 2016, 6, 61984–61995. [Google Scholar] [CrossRef]
- Kayaert, P.; Li, B.; Jimidar, I.; Rombaut, P.; Ahssini, F.; Van den Mooter, G. Solution Calorimetry as an Alternative Approach for Dissolution Testing of Nanosuspensions. Eur. J. Pharm. Biopharm. 2010, 76, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; de Brauer, C.; Claudy, P.; Germain, P.; Letoffe, J. β-Cyclodextrin hydration: A calorimetric and gravimetric study. Thermochim. Acta 1995, 249, 63–73. [Google Scholar] [CrossRef]
- Lindner, K.; Saenger, W. Crystal and Molecular Structure of Cyclohepta-Amylose Dodecahydrate. Carbohydr. Res. 1982, 99, 103–115. [Google Scholar] [CrossRef]
- Briggner, L.-E.; Wadsö, I. Heat Capacities of Maltose, Maltotriose, Maltotetrose and α-, β-, and γ-Cyclodextrin in the Solid State and in Dilute Aqueous Solution. J. Chem. Thermodyn. 1990, 22, 1067–1074. [Google Scholar] [CrossRef]
- Burger, A.; Ramberger, R. On the Polymorphism of Pharmaceuticals and Other Molecular Crystals. I. Mikrochim. Acta 1979, 72, 259–271. [Google Scholar] [CrossRef]
- Gorbatchuk, V.V.; Tsifarkin, A.G.; Antipin, I.S.; Solomonov, B.N.; Konovalov, A.I. Influence of the Guest Molecular Size on the Thermodynamic Parameters of Host–Guest Complexes between Solid Tert-Butylcalix[4]Arene and Vapours of Organic Compounds. Mendeleev Commun. 1999, 9, 11–13. [Google Scholar] [CrossRef]
- Ogata, F.; Hayabuchi, R.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Adsorption Behavior of Water on Virgin and Modified Cyclodextrin. Yakugaku Zasshi 2020, 140, 1165–1173. [Google Scholar] [CrossRef]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous Properties of Cyclodextrins and Their Complexes in Aqueous Solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef]

| Polymorph | I (Cage-Type) | II (Channel-Type) |
|---|---|---|
| Preparation method | Drying of βCD hydrate (form I) | Precipitation from an acetone-water solution [16]; treatment of polymer inclusion complexes with organic solvents [17] |
| Characteristic PXRD peaks (2θ), ° | 5.5, 6.5, 9.6, 11.0, 12.9, 13.5, 14.1, 16.9, 18.2, 22.3 [18,19] | 7.2, 11.9, 18.1 [17] |
| Crystal system | Monoclinic [23] | Hexagonal [17] |
| Onset point of thermal degradation, K | 540 a [24] | 478–483 b [17] |
| Polymorph | I | II | III a |
|---|---|---|---|
| Crystal system | Monoclinic | Hexagonal | Triclinic |
| Cell parameters | a = 13.703 Å b = 16.065 Å c = 13.163 Å β = 92.69° [23] | a = 15.34 Å c = 15.06 Å [17] | a = 17.09 Å b = 15.12 Å c = 11.76 Å, α = 109.5° β = 95.8° γ = 70.6° |
| Cell volume, Å3 | 2894 | 3069 | 2704 |
| Dissolution time tsoln/s | Solution enthalpy ΔHsoln/kJ mol−1 | |
|---|---|---|
| Polymorph I | 190 ± 15 | −86 ± 1 |
| Polymorph III | 43 ± 4 | −94 ± 1 |
| Amorphous βCD a | 43 ± 4 (90 ± 10) b | −111 ± 1 |
| HPβCD a | 100 ± 10 | −144 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatiatulin, A.K.; Balakhontsev, I.S.; Talashmanova, S.M.; Ziganshin, M.A.; Gorbatchuk, V.V. New Polymorph of β-Cyclodextrin with a Higher Bioavailability. Chemistry 2024, 6, 51-61. https://doi.org/10.3390/chemistry6010003
Gatiatulin AK, Balakhontsev IS, Talashmanova SM, Ziganshin MA, Gorbatchuk VV. New Polymorph of β-Cyclodextrin with a Higher Bioavailability. Chemistry. 2024; 6(1):51-61. https://doi.org/10.3390/chemistry6010003
Chicago/Turabian StyleGatiatulin, Askar K., Ilya S. Balakhontsev, Sofia M. Talashmanova, Marat A. Ziganshin, and Valery V. Gorbatchuk. 2024. "New Polymorph of β-Cyclodextrin with a Higher Bioavailability" Chemistry 6, no. 1: 51-61. https://doi.org/10.3390/chemistry6010003
APA StyleGatiatulin, A. K., Balakhontsev, I. S., Talashmanova, S. M., Ziganshin, M. A., & Gorbatchuk, V. V. (2024). New Polymorph of β-Cyclodextrin with a Higher Bioavailability. Chemistry, 6(1), 51-61. https://doi.org/10.3390/chemistry6010003