Low-Temperature Properties of the Sodium-Ion Electrolytes Based on EC-DEC, EC-DMC, and EC-DME Binary Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Preparation
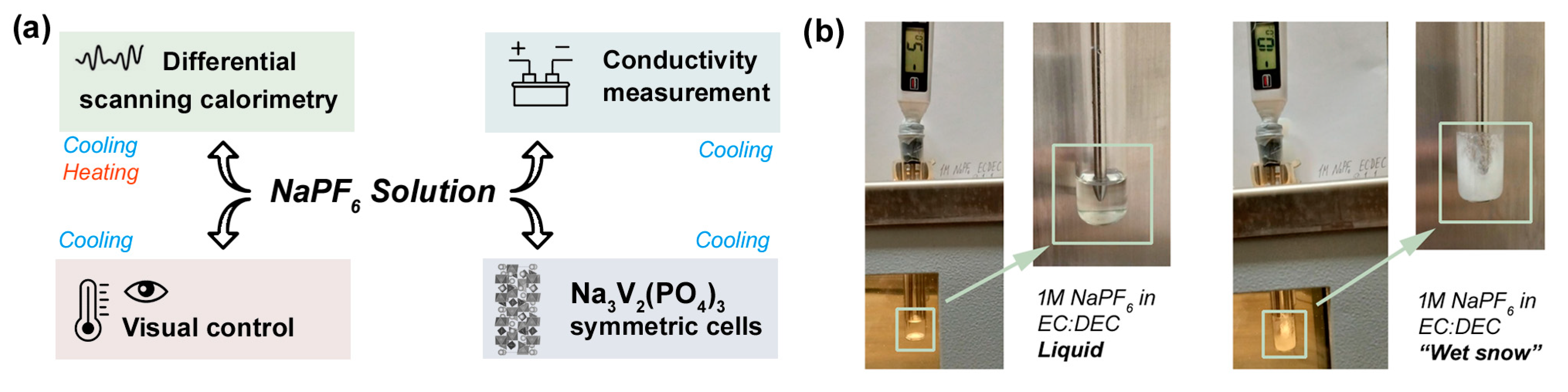
2.2. Characterization
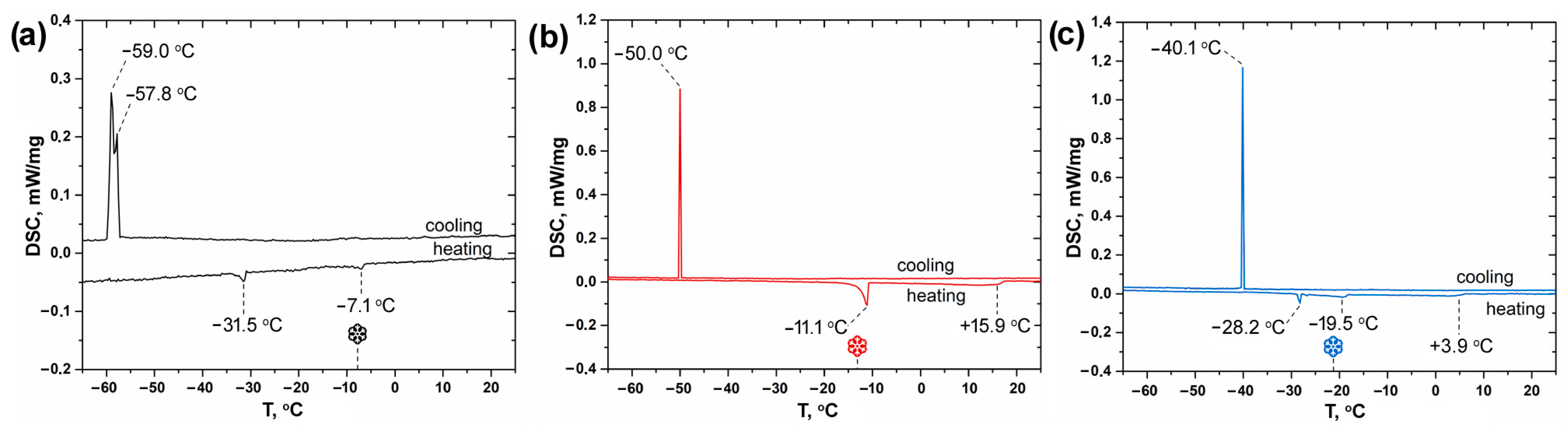
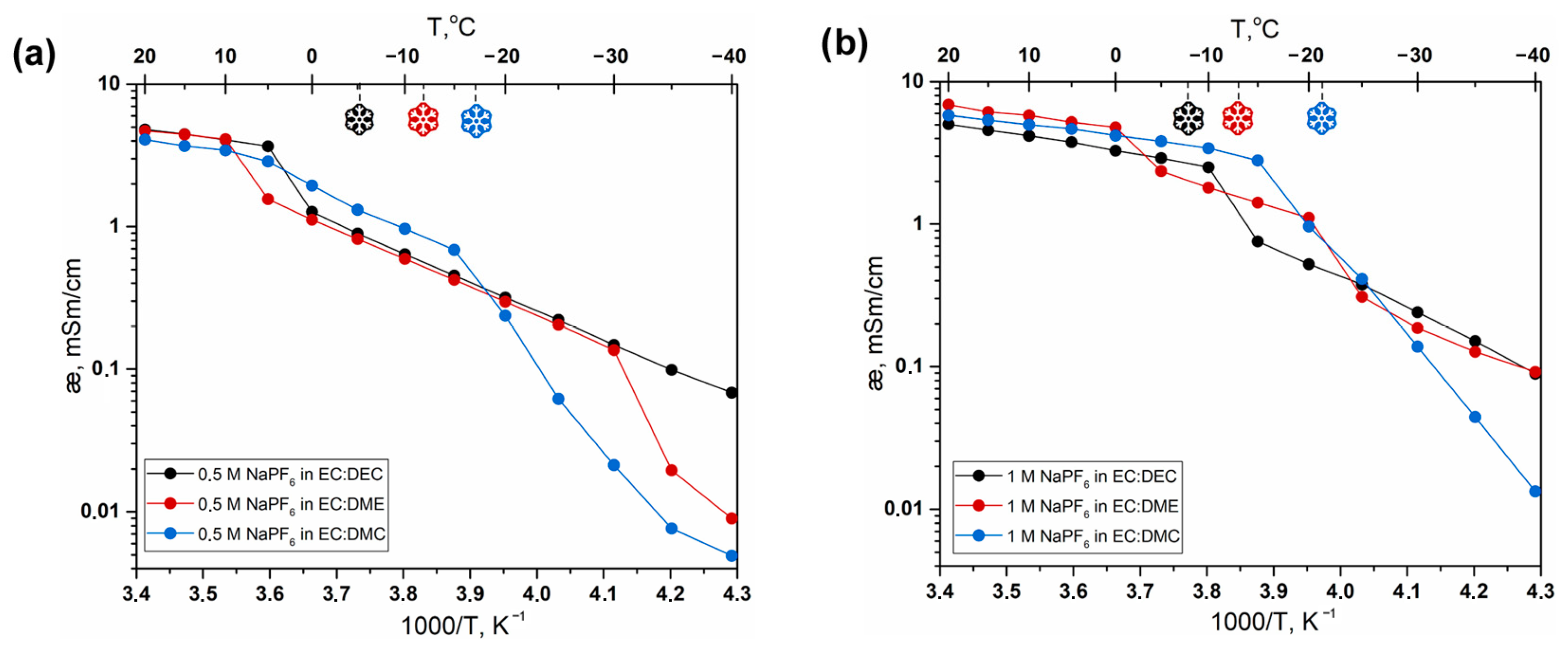
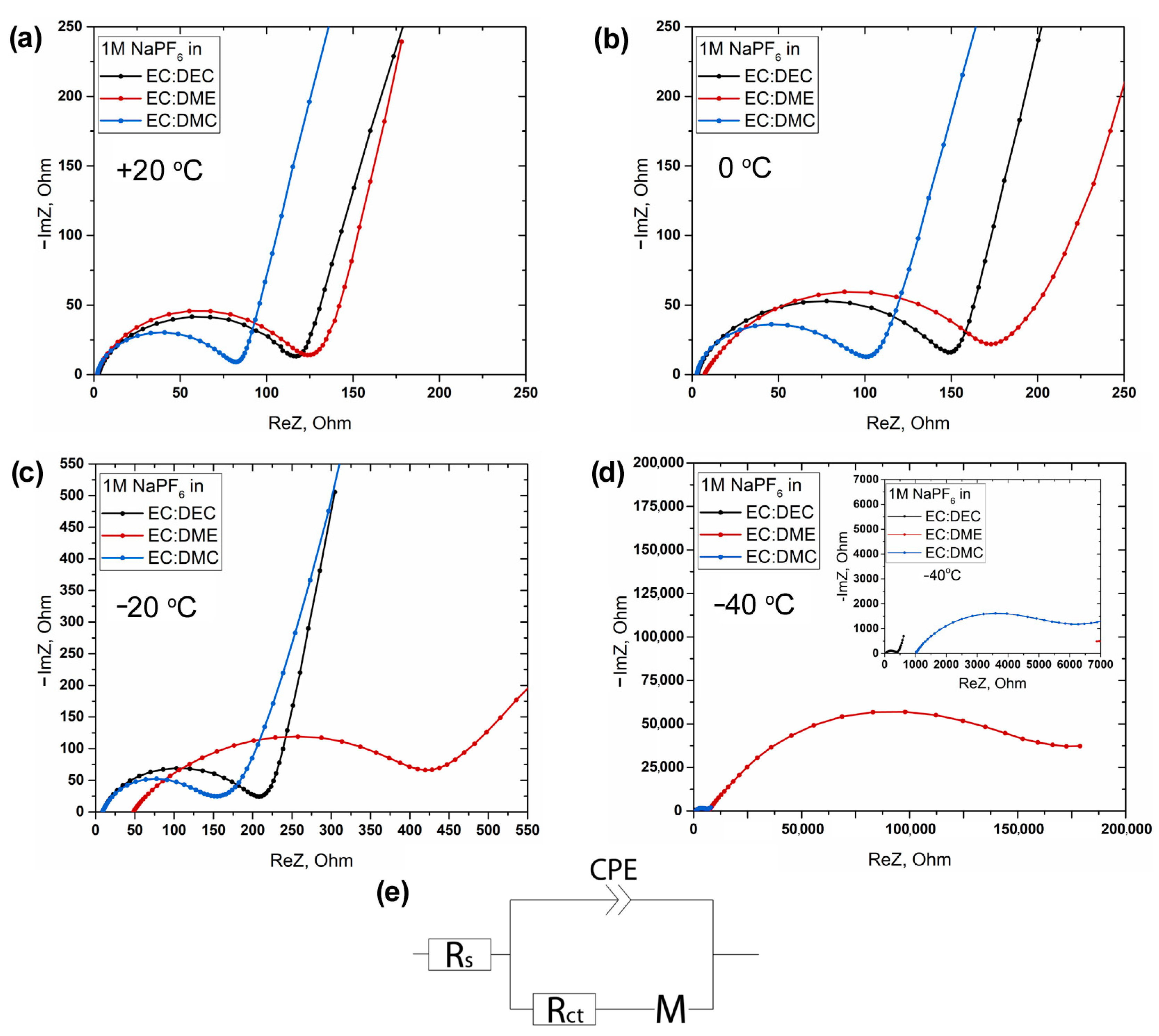
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Ruiz, N.; Armstrong, A.R.; Alptekin, H.; Amores, M.A.; Au, H.; Barker, J.; Boston, R.; Brant, W.R.; Brittain, J.M.; Chen, Y.; et al. 2021 roadmap for sodium-ion batteries. J. Phys. Energy 2021, 3, 031503. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-ion batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.; Tan, D.H.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-ion batteries paving the way for grid energy storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Pesaran, A.; Santhanagopalan, S.; Kim, G.H. Addressing the Impact of Temperature Extremes on Large Format Li-Ion Batteries for Vehicle Applications. In Proceedings of the 30th International Battery Seminar, Ft. Lauderdale, FL, USA, 11–14 March 2013. [Google Scholar]
- Luo, H.; Wang, Y.; Feng, Y.-H.; Fan, X.-Y.; Han, X.; Wang, P.-F. Lithium-Ion Batteries under Low-Temperature Environment: Challenges and Prospects. Materials 2022, 15, 8166. [Google Scholar] [CrossRef]
- Rodrigues, M.-T.F.; Babu, G.; Gullapalli, H.; Kalaga, K.; Sayed, F.N.; Kato, K.; Joyner, J.; Ajayan, P.M. A materials perspective on Li-ion batteries at extreme temperatures. Nat. Energy 2017, 2, 17108. [Google Scholar] [CrossRef]
- Zhu, G.; Wen, K.; Lv, W.; Zhou, X.; Liang, Y.; Yang, F.; Chen, Z.; Zou, M.; Li, J.; Zhang, Y.; et al. Materials insights into low-temperature performances of lithium-ion batteries. J. Power Sources 2015, 300, 29–40. [Google Scholar] [CrossRef]
- Kulova, T.L.; Skundin, A.M. Problems of low-temperature lithium-ion batteries. Electrochem. Energy 2017, 17, 61–88. [Google Scholar]
- Li, Q.; Liu, G.; Cheng, H.; Sun, Q.; Zhang, J.; Ming, J. Low-Temperature Electrolyte Design for Lithium-Ion Batteries: Prospect and Challenges. Chem. Eur. J. 2021, 27, 15842. [Google Scholar] [CrossRef]
- Hubble, D.; Brown, D.E.; Zhao, Y.; Fang, C.; Lau, J.; McCloskey, B.D.; Liu, G. Liquid electrolyte development for low-temperature lithium-ion batteries. Energy Environ. Sci. 2022, 15, 550–578. [Google Scholar] [CrossRef]
- Zhang, N.; Deng, T.; Zhang, S.; Wang, C.; Chen, L.; Wang, C.; Fan, X. Critical Review on Low-Temperature Li-Ion/Metal Batteries. Adv. Mater. 2022, 34, 2107899. [Google Scholar] [CrossRef]
- Laforgue, A.; Yuan, X.Z.; Platt, A.; Brueckner, S.; Perrin-Sarazin, F.; Toupin, M.; Huot, J.-Y.; Mokrini, A. Effects of fast charging at low temperature on a high energy Li-ion battery. J. Electrochem. Soc. 2020, 167, 140521. [Google Scholar] [CrossRef]
- Jow, R.; Zhang, S.S.; Xu, K.; Allen, J. Electrolytes for Low Temperature Operations of Li-Ion Batteries. ECS Trans. 2007, 3, 51. [Google Scholar] [CrossRef]
- Smart, M.C.; Ratnakumar, B.V.; Surampudi, S. Electrolytes for low-temperature lithium batteries based on ternary mixtures of aliphatic carbonates. J. Electrochem. Soc. 1999, 146, 486–492. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Che, H.; Yang, X.; Yu, Y.; Pan, C.; Wang, H.; Deng, Y.; Li, L.; Ma, Z.F. Engineering optimization approach of nonaqueous electrolyte for sodium ion battery with long cycle life and safety. Green Energy Environ. 2021, 6, 212–219. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, S.; Luo, L.; Ding, M.S.; Zheng, J.; Cartmell, S.S.; Wang, C.-M.; Xu, K.; Zhang, J.-G.; Xu, W. Wide-temperature electrolytes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 18826–18835. [Google Scholar] [CrossRef]
- Lin, Z.; Xia, Q.; Wang, W.; Li, W.; Chou, S. Recent research progresses in ether-and ester-based electrolytes for sodium-ion batteries. InfoMat 2019, 1, 376–389. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, L.; Xie, F.; Li, Y.; Chen, Z.; Lu, Y.; Hu, Y.-S. Part IV Electrolytes.Ester- and Ether-Based Electrolytes for Na-Ion Batteries. In Sodium-Ion Batteries: Materials, Characterization, and Technology, 2 Volumes; Titirici, M.M., Adelhelm, P., Hu, Y.S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 333–356. ISBN 978-3-527-34709-4. [Google Scholar]
- Shakourian-Fard, M.; Kamath, G.; Smith, K.; Xiong, H.; Sankaranarayanan, S.K. Trends in Na-ion solvation with alkyl-carbonate electrolytes for sodium-ion batteries: Insights from first-principles calculations. J. Phys. Chem. C 2015, 119, 22747–22759. [Google Scholar] [CrossRef]
- Ponrouch, A.; Monti, D.; Boschin, A.; Steen, B.; Johansson, P.; Palacín, M.R. Non-aqueous electrolytes for sodium-ion batteries. J. Mater. Chem. A 2015, 3, 22–42. [Google Scholar] [CrossRef]
- Vidal-Abarca, C.; Lavela, P.; Tirado, J.L.; Chadwick, A.V.; Alfredsson, M.; Kelder, E. Improving the cyclability of sodium-ion cathodes by selection of electrolyte solvent. J. Power Sources 2012, 197, 314–318. [Google Scholar] [CrossRef]
- Ponrouch, A.; Marchante, E.; Courty, M.; Tarascon, J.M.; Palacin, M.R. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 2012, 5, 8572–8583. [Google Scholar] [CrossRef]
- Lakienko, G.P.; Bobyleva, Z.V.; Apostolova, M.O.; Sultanova, Y.V.; Dyakonov, A.K.; Zakharkin, M.V.; Sobolev, N.A.; Alekseeva, A.M.; Drozhzhin, O.A.; Abakumov, A.M.; et al. Sosnowskyi Hogweed-Based Hard Carbons For Sodium-Ion Batteries. Batteries 2022, 8, 131. [Google Scholar] [CrossRef]
- Ding, M.S. Liquid-solid phase diagrams of ternary and quaternary organic carbonates. J. Electrochem. Soc. 2004, 151, A731–A738. [Google Scholar] [CrossRef]
- Ding, M.S. Liquid− solid phase equilibria and thermodynamic modeling for binary organic carbonates. J. Chem. Eng. Dat. 2004, 49, 276–282. [Google Scholar] [CrossRef]
- Bülow, M.; Ascani, M.; Held, C. ePC-SAFT advanced-Part I: Physical meaning of including a concentration-dependent dielectric constant in the born term and in the Debye-Hückel theory. Fluid Ph. Equilibria 2021, 535, 112967. [Google Scholar] [CrossRef]
- Ding, S.P.; Xu, K.; Zhang, S.S.; Jow, T.R.; Amine, K.; Henriksen, G.L. Diminution of supercooling of electrolytes by carbon particles. J. Electrochem. Soc. 1999, 146, 3974–3980. [Google Scholar] [CrossRef]
- Weingarth, D.; Drumm, R.; Foelske-Schmitz, A.; Kötz, R.; Presser, V. An electrochemical in situ study of freezing and thawing of ionic liquids in carbon nanopores. Phys. Chem. Chem. Phys. 2014, 16, 21219–21224. [Google Scholar] [CrossRef]
- Tatara, R.; Nishimura, S.; Okamoto, Y.; Ueno, K.; Watanabe, M.; Dokko, K. Structures and electrochemistry of γ-butyrolactone solvates of Na salts. J. Phys. Chem. C 2020, 124, 15800–15811. [Google Scholar] [CrossRef]
- Ding, M.S.; Xu, K.; Jow, T.R. Phase Diagram of EC–DMC Binary System and Enthalpic Determination of Its Eutectic Composition. J. Therm. Anal. 2000, 62, 177–186. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, X.; Tu, Z.; Hu, X.; Wu, Y. Novel Deep Eutectic Electrolyte Induced by Na⋯N Interactions for Sodium Batteries. Ind. Eng. Chem. Res. 2023, 62, 51–61. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. Charge and discharge characteristics of a commercial LiCoO2-based 18650 Li-ion battery. J. Power Sources 2006, 160, 1403. [Google Scholar] [CrossRef]
- Nikitina, V.A.; Zakharkin, M.V.; Vassiliev, S.Y.; Yashina, L.V.; Antipov, E.V.; Stevenson, K.J. Lithium Ion Coupled Electron-Transfer Rates in Superconcentrated Electrolytes: Exploring the Bottlenecks for Fast Charge-Transfer Rates with LiMn2O4 Cathode Materials. Langmuir 2017, 33, 9378–9389. [Google Scholar] [CrossRef]
- Drozhzhin, O.A.; Shevchenko, V.A.; Bobyleva, Z.V.; Alekseeva, A.M.; Antipov, E.V. Rational Screening of High-Voltage Electrolytes and Additives for Use in LiNi0.5Mn1.5O4-Based Li-Ion Batteries. Molecules 2022, 27, 3596. [Google Scholar] [CrossRef]
- Xu, K.; von Wald Cresce, A. Li+-solvation/desolvation dictates interphasial processes on graphitic anode in Li ion cells. J. Mater. Res. 2012, 27, 2327–2341. [Google Scholar] [CrossRef]
- Xu, K.; von Cresce, A.; Lee, U. Differentiating Contributions to “Ion Transfer” Barrier from Interphasial Resistance and Li+ Desolvation at Electrolyte/Graphite Interface. Langmuir 2010, 26, 11538–11543. [Google Scholar] [CrossRef]
- Abe, T.; Fukuda, H.; Iriyama, Y.; Ogumi, Z. Solvated Li-Ion Transfer at Interface Between Graphite and Electrolyte. J. Electrochem. Soc. 2004, 151, A1120–A1123. [Google Scholar] [CrossRef]
- Li, Q.; Lu, D.; Zheng, J.; Jiao, S.; Luo, L.; Wang, C.M.; Xu, K.; Zhang, J.G.; Xu, W. Li+−Desolvation Dictating Lithium-Ion Battery’s Low-Temperature Performances. ACS Appl. Mater. Interfaces 2017, 9, 42761–42768. [Google Scholar] [CrossRef]

| Solvent | Structural Formula | Tm, °C | Tb, °C | Viscosity, mPa·s (25 °C) | Dielectric Constant (25 °C) | Density, g/cm3 (25 °C) |
|---|---|---|---|---|---|---|
| EC | 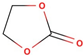 | 36.4 | 248.0 | 1.90 (40 °C) | 89.780 | 1.321 |
| DEC |  | −74.3 | 126.0 | 0.75 | 2.800 | 0.969 |
| DMC | 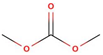 | 4.6 | 91.0 | 0.59 (20 °C) | 3.107 | 1.063 |
| DME |  | −58.0 | 84.0 | 0.46 | 7.200 | 0.860 |
| Solvent Mixture | C(NaPF6), M | T, °C |
|---|---|---|
| EC:DEC (V1:V2 = 1:1) | 0.0 | 3.3 ± 1 |
| 0.5 | −5.2 ± 1 | |
| 1.0 | −7.8 ± 1 | |
| EC:DME (V1:V2 = 1:1) | 0.0 | 14.0 ± 1 |
| 0.5 | −11.9 ± 1 | |
| 1.0 | −13.1 ± 1 | |
| EC:DMC (V1:V2 = 1:1) | 0.0 | −2.7 ± 1 |
| 0.5 | −17.1 ± 1 | |
| 1.0 | −21.2 ± 1 |
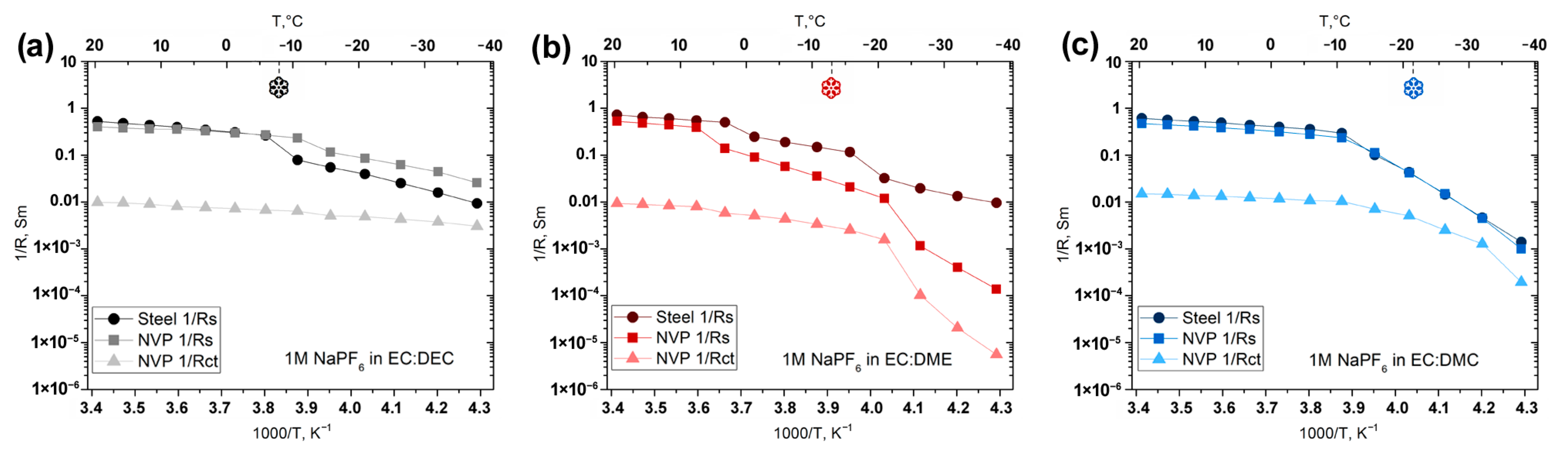
| T, °C | Rs, Ohm | Rct, Ohm | ||||
|---|---|---|---|---|---|---|
| In EC:DEC | In EC:DME | In EC:DMC | In EC:DEC | In EC:DME | In EC:DMC | |
| +20 | 2.8 | 1.9 | 2.1 | 101.7 | 107.0 | 66.9 |
| +10 | 3.1 | 2.3 | 2.4 | 110.6 | 119.0 | 72.5 |
| 0 | 3.1 | 7.2 | 2.9 | 131.3 | 171.8 | 81.0 |
| −10 | 3.7 | 17.5 | 3.6 | 148.3 | 229.8 | 92.5 |
| −20 | 8.7 | 47.6 | 9.0 | 197.4 | 396.0 | 141.9 |
| −30 | 16.0 | 864.4 | 67.0 | 231.7 | 9707.0 | 398.2 |
| −40 | 38.8 | 7244.0 | 1002.0 | 329.1 | 179,666.0 | 5150.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lutsenko, D.S.; Belova, E.V.; Zakharkin, M.V.; Drozhzhin, O.A.; Antipov, E.V. Low-Temperature Properties of the Sodium-Ion Electrolytes Based on EC-DEC, EC-DMC, and EC-DME Binary Solvents. Chemistry 2023, 5, 1588-1598. https://doi.org/10.3390/chemistry5030109
Lutsenko DS, Belova EV, Zakharkin MV, Drozhzhin OA, Antipov EV. Low-Temperature Properties of the Sodium-Ion Electrolytes Based on EC-DEC, EC-DMC, and EC-DME Binary Solvents. Chemistry. 2023; 5(3):1588-1598. https://doi.org/10.3390/chemistry5030109
Chicago/Turabian StyleLutsenko, Denis S., Ekaterina V. Belova, Maxim V. Zakharkin, Oleg A. Drozhzhin, and Evgeny V. Antipov. 2023. "Low-Temperature Properties of the Sodium-Ion Electrolytes Based on EC-DEC, EC-DMC, and EC-DME Binary Solvents" Chemistry 5, no. 3: 1588-1598. https://doi.org/10.3390/chemistry5030109
APA StyleLutsenko, D. S., Belova, E. V., Zakharkin, M. V., Drozhzhin, O. A., & Antipov, E. V. (2023). Low-Temperature Properties of the Sodium-Ion Electrolytes Based on EC-DEC, EC-DMC, and EC-DME Binary Solvents. Chemistry, 5(3), 1588-1598. https://doi.org/10.3390/chemistry5030109






