Abstract
A synthetic strategy based on reactions of cyclic imine oxides, namely 2H-imidazole 1-oxides, with thiophenols mediated by acetyl chloride was successfully applied as a convenient tool to obtain a series of novel azaheterocyclic molecules, including water-soluble hydrochloride forms. Optimized reaction conditions found herein for the nucleophilic substitution of hydrogen (SNH) in non-aromatic azaheterocyclic substrates via the “addition-elimination” (SNH AE) scheme enabled 15 arylthiolated 2H-imidazoles to be prepared in yields of up to 90%. The developed methodology discloses an original synthetic way to obtain numerous azaheterocyclic molecules, which are of interest in the field of medicinal chemistry and materials science.
1. Introduction
The arylthiol and imidazole moieties are known to be key structural motifs of organic compounds with various types of pharmaceutical activities and functional materials [1,2,3,4,5]. In particular, the molecules bearing imidazole rings also provide a huge number of biological activities and are used as active pharmaceutical ingredients in many drugs. For example, Losartan is an angiotensin II receptor agonist that is used as an antihypertensive agent [6]. The imidazole-containing compounds also demonstrate antifungal activity; for instance, clotrimazole is used successfully for treating systemic Candida infections, pseudallescheriasis, and some refractory cases of cryptococcal meningitis [7]. Besides, im-idazole substrates are also used as antiparasitic drugs (tinidazole, secnidazole, etc.) (Figure 1, top) [8,9]. Moreover, there are known imidazole-based structures characterized by anticancer effects as well. Dacarbazine is used for the treatment of metastatic malignant melanoma, Hodgkin lymphoma, sarcoma, and islet cell pancreatic carcinoma [10]. Therefore, the elaboration of new ways and approaches for the design and synthesis of azaheterocyclic systems, especially with imidazole moiety, appears to be a key task for modern organic and medicinal chemistry.
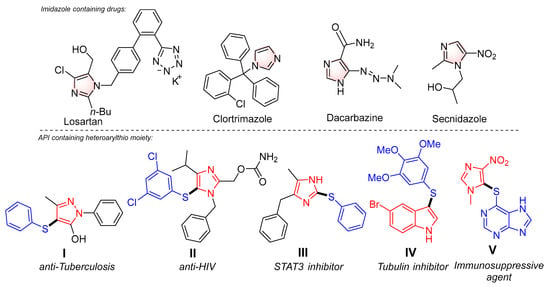
Figure 1.
Active pharmaceutical ingredients (APIs) based on imidazole (top) and azaheterocyclic sulfenylated derivatives (bottom: red color indicates the azaheterocyclic fragment; blue color shows the arylthio moiety).
Among sulfenyl-derived compounds, the molecules that contain the C-S bond linking the arylthio and the azaheterocyclic moieties are of increasing interest. Substances of this type have found several applications as pharmaceutically active compounds (Figure 1, bottom), particularly as anti-tuberculosis (I) and anti-HIV (II) agents [11,12]. In addition, benzyl-modified imidazole III possesses inhibitory activity regarding the biological target STAT3 associated with the pathogenesis of oncological diseases, namely breast cancer [13]. Also, arylthioindole derivative IV is a tubulin inhibitor related to oncogenesis progression [14]. Azathioprine V is used for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, and other diseases [15].
Currently, a limited number of synthetic methods to obtain arylthio(hetero)cycles, particularly imidazoles, have been reported [16,17,18,19,20,21,22,23]. Arylation of thiol or thion group in the imidazole moiety by the reaction with aryl halides is commonly used (Scheme 1a) [13,24]. There are also C-I/S-H couplings of imidazole halides with thiophenols or disulfides (Scheme 1b) [25]. Previously, our group reported the transitional metal-free C-H/C-H coupling reactions of 2H-imidazole 1-oxides with various nucleophiles (pyrroles, indoles, and phenols) [26,27]. The latter reactions were developed according to the basic principles of green chemistry, particularly using nontoxic solvents, reducing the number of formed by-products, etc. [28,29,30]. Following the green chemistry-oriented C-H functionalization synthetic strategy [31,32,33], namely, reactions of nucleophilic substitution of hydrogen (SNH) can be successfully used to modify various organic substrates [34]. At the same time, the application of this approach to the direct modification of heterocyclic substrates by S-nucleophiles has not been thoroughly studied yet.
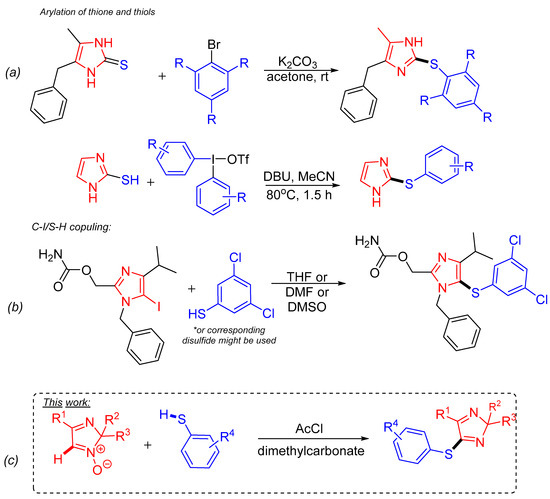
Scheme 1.
Synthetic strategies towards arylthiolated-imidazoles: (a) arylation of thiole or thione; (b) direct C-I/S-H coupling method; (c) the present work (red color indicates the imidazole ring; blue color shows the arylthio moiety).
This work deals with the first systematic study of eliminative arylthiolation of 2H-imidazole N-oxides by coupling with thiophenols. Furthermore, the reaction conditions optimization, scope, and limitation for the developed method are highlighted (Scheme 1c).
2. Materials and Methods
2.1. Experimental Procedure
Nuclear magnetic resonance (NMR) spectra were recorded on the Bruker Avance II (400 MHz) and Bruker Avance II (600 MHz) spectrometers. All 1H NMR experiments were reported in δ units, parts per million (ppm), and were measured relative to residual chloroform CDCl3 (7.26 ppm), DMSO (2.50 ppm), or CF3COOD + CD3COOD (2.04 ppm) signals in the deuterated solvent. All 13C NMR spectra were reported in parts per million (ppm) relative to CDCl3 (77.16 ppm), DMSO-d6 (39.52 ppm), or CF3COOD + CD3COOD (20.0 ppm), and all spectra were obtained with 1H decoupling. All coupling constants J were reported in Hertz (Hz). The following abbreviations were used to describe peak splitting patterns (s = singlet, d = doublet, t = triplet, dd = doublet of doublet, m = multiplet, and br s = broadened singlet). Copies of NMR spectra are illustrated in Supplementary Materials. The mass spectra were recorded on a mass spectrometer, SHIMADZU GCMS-QP2010 Ultra, with sample ionization by electron impact (EI). The IR spectra were recorded using a Fourier-transform infrared spectrometer (Bruker Corporation, 40 Manning Rd, Billerica, MA, USA) equipped with a diffuse reflection attachment. The elemental analysis was carried out on a Perkin Elmer Instrument (PerkinElmer, Waltham, MA, USA) equipped with CHN PE 2400 II analyzer. The course of the reactions was monitored by TLC on 0.25 mm silica gel plates (60F 254, MACHEREY-NAGEL Inc., 924 Marcon Blvd, Allentown, PA 18109, USA).
Thiophenol, 4-hydroxythiophenol, 3,5-difluorothiophenol, thiosalicylic acid, 4-methoxythiophenol, 2,6-dichlorothiophenol, toluene, ethyl acetate, acetone, hexachloroacetone, chlorobenzene, dimethyl carbonate, hexane, chloroform, acetyl chloride, trichloroacetyl chloride, benzoyl chloride, chlorotrimethylsilane, trifluoroacetyc anhydride, acetic anhydride, and sodium bicarbonate were purchased and used as received.
Additionally, 2,2-Dimethyl-4-phenyl-2H-imidazole 1-oxide [35], 3-phenyl-1,4-diazaspiro[4.5]deca-1,3-diene 1-oxide [36], 4-(4-bromophenyl)-2,2-dimethyl-2H-imidazole 1-oxide, 4-(4-bromophenyl)-2-ethyl-2-methyl-2H-imidazole 1-oxide [37], 2,2-dimethyl-4-(p-tolyl)-2H-imidazole 1-oxide [38], and 2,2-dimethyl-4-(naphthalen-2-yl)-2H-imidazole 1-oxide [27] were used as starting materials and were prepared according to the literature procedures.
2.2. General Procedure for the Synthesis of Hydrochloride Salts of Sulfenyl-Imidazole Derivatives (3a-k)
To a vigorously stirred mixture of 2H-imidazole 1-oxide 1a-f (0.5 mmol) and thiophenolic substrate 2a-d (0.5 mmol) in dimethyl carbonate (4 mL) at 0 °C, acetyl chloride (0.5 mmol) was added. Subsequently, the resulting mixture was allowed to warm up to room temperature and was stirred continuously for 6 h. Then, the resulting precipitate 3 was filtered off, washed with hexane (10 mL), and dried under air.
2,2-Dimethyl-4-phenyl-5-(phenylthio)-2H-imidazole hydrochloride (3a). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Colorless solid. Yield: 0.37 mmol (117 mg, 74%), mp = 98–99 °C. Rf 0.18 (hexane/EtOAc, 6:4). 1H NMR (CDCl3): δ 8.26 (d, J = 7.3 Hz, 2H); 7.72 (t, J = 7.5 Hz, 1H); 7.64–7.60 (m, 4H); 7.56–7.50 (m, 3H); 1.78 (s, 6H) ppm. 13C {1H} NMR (CDCl3, BB mode): δ 165.5; 162.9; 134.8; 134.7; 131.6; 130.6; 130.5; 129.7; 125.6; 124.4; 100.4; 24.2 ppm. IR (DRA): ν 1734, 1625, 1519, 1438, 1361, 1281, 994, 889, 832, 755, 724, 708, 684, 570, 515 cm−1. MS calcd: m/z 316 [M]+. Found (EI) m/z 280 [M]+. Elemental analysis calcd for: C17H17ClN2S: C, 64.44; H, 5.51; N, 8.84. Found: C, 64.42; H, 5.52; N, 8.81.
2-Phenyl-3-(phenylthio)-1,4-diazaspiro[4.5]deca-1,3-diene hydrochloride (3b). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Light-yellow solid. Yield: 0.27 mmol (96 mg, 54%), mp = 112–113 °C. Rf 0.14 (hexane/EtOAc, 6:4). 1H NMR (CDCl3): δ 8.28 (d, J = 7.7 Hz, 2H); 7.71 (t, J = 7.4 Hz, 1H); 7.63–7.59 (m, 4H); 7.53–7.45 (m, 3H); 2.16–2.04 (m, 2H); 1.98–183 (m, 3H); 1.73–1.58 (m, 5H) ppm. 13C {1H} NMR (CDCl3, BB mode): δ 162.9; 161.0; 134.5; 131.1; 130.7; 130.3; 129.7; 129.5; 129.1; 128.9; 103.3; 34.9; 24.5; 23.5. ppm. IR (DRA): ν 2935, 1698, 1629, 1519, 1473, 1373, 1300, 1140, 1066, 1001, 850, 817, 747, 686, 581 cm−1. MS calcd: m/z 356 [M]+. Found (EI): m/z 320 [M - HCl]+. Elemental analysis calcd for: C20H21ClN2S: C, 67.31; H, 5.93; N, 7.85. Found: C, 67.28; H, 5.94; N, 7.86.
4-(4-Bromophenyl)-2,2-dimethyl-5-(phenylthio)-2H-imidazole hydrochloride (3c). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Light-yellow solid. Yield: 0.26 mmol (103 mg, 52%), mp = 128–129 °C. Rf 0.26 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 7.82–7.81 (m, 2H); 7.78–7.76 (m, 2H); 7.65–7.63 (m, 2H); 7.48–7.44 (m, 3H); 1.39 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 161.0 (C); 160.7 (C); 133.6 (CH); 131.9 (CH); 130.4 (CH); 130.1 (C); 129.5 (CH); 129.4 (CH); 128.3 (C); 124.7 (C); 102.40 (C); 24.1 (CH3) ppm. IR (DRA): ν 1755, 1627, 1585, 1514, 1362, 1291, 1183, 1126, 1069, 995, 892, 728, 704, 689, 570, 523 cm−1. MS calcd: m/z 394 [M]+; 396 [M + 2]+. Found (EI): m/z 358 [M - HCl]+; 360 [M + 2 - HCl]+. Elemental analysis calcd for: C17H16BrClN2S: C, 51.60; H, 4.08; N, 7.08. Found: C, 51.64; H, 4.07; N, 7.09.
4-(4-Bromophenyl)-2-ethyl-2-methyl-5-(phenylthio)-2H-imidazole hydrochloride (3d). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Light-yellow solid. Yield: 0.29 mmol (108 mg, 58%), mp = 111–112 °C. Rf 0.23 (hexane/EtOAc, 6:4). 1H NMR (CDCl3): δ 8.08–8.06 (m, 2H); 7.75–7.73 (m, 2H); 7.62–7.60 (m, 2H); 7.57–7.48 (m, 3H); 2.36–2.22 (m, 2H); 1.73 (s, 3H); 0.72 (t, J = 7.3 Hz, 3H) ppm. 13C {1H} NMR (CDCl3, BB mode): δ 162.5; 160.8; 134.6; 133.0; 132.2; 131.5; 130.6; 129.5; 129.2; 103.6; 82.6; 31.3, 22.8, 8.1 ppm. IR (DRA): ν 2978, 1828, 1708, 1625, 1584, 1515, 1478, 1280, 1068, 1005, 834, 755, 663, 579, 554 cm−1. MS calcd: m/z 408 [M]+; 410 [M + 2]+. Found (EI): m/z 372 [M - HCl]+; 374 [M + 2 - HCl]+. Elemental analysis calcd for: C18H18BrClN2S: C, 52.76; H, 4.43; N, 6.84. Found: C, 52.75; H, 4.43; N, 6.85.
2,2-Dimethyl-4-(phenylthio)-5-(p-tolyl)-2H-imidazole hydrochloride (3e). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Colorless solid. Yield: 0.21 mmol (70 mg, 42%), mp = 132–133 °C. Rf 0.15 (hexane/EtOAc, 6:4). 1H NMR (CDCl3): δ 8.25 (d, J = 8.0 Hz, 2H); 7.62–7.60 (m, 2H); 7.55–7.47 (m, 3H); 7.43 (d, J = 8.0 Hz, 2H); 2.48 (s, 3H); 1.76 (s, 6H) ppm. 13C {1H} NMR (CDCl3, BB mode): δ 164.1; 163.0; 147.0; 134.6; 131.1; 131.0; 130.5; 130.3; 125.4; 122.3; 100.0; 24.3; 22.1 ppm. IR (DRA): ν 1825, 1624, 1604, 1520, 1439, 1359, 1183, 1132, 993, 891, 757, 740, 688, 570, 517 cm−1. MS calcd: m/z 330 [M]+. Found (EI): m/z 294 [M - HCl]+. Elemental analysis calcd for: C18H19ClN2S: C, 65.34; H, 5.79; N, 8.47. Found: C, 65.32; H, 5.80; N, 8.45.
2,2-Dimethyl-4-(naphthalen-2-yl)-5-(phenylthio)-2H-imidazole hydrochloride (3f). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Light-yellow solid. Yield: 0.275 mmol (101 mg, 55%), mp = 117–118 °C. Rf 0.25 (hexane/EtOAc, 6:4). 1H NMR (CDCl3): δ 8.87 (s, 1H); 8.30 (d, J = 8.6 Hz, 1H); 8.06 (d, J = 8.7 Hz, 2H); 7.95–7.92 (m, 1H), 7.71–7.62 (m, 4H), 7.56–7.52 (m, 3H); 1.82 (s, 6H) ppm. 13C {1H} NMR (CDCl3, BB mode): δ 162.9; 160.8; 136.0; 134.8; 132.5; 131.5; 130.8; 130.6; 129.9; 129.5; 129.2; 128.2; 128.0; 127.9; 125.7; 125.4; 100.4; 24.4 ppm. IR (DRA): ν 2156, 1854, 1617, 1523, 1472, 1438, 1389, 1289, 1170, 1018, 985, 941, 848, 684, 641, 583, 564 cm−1. MS calcd: m/z 366 [M]+. Found (EI): m/z 330 [M - HCl]+. Elemental analysis calcd for: C21H19ClN2S: C, 65.75; H, 5.22; N, 7.64. Found: C, 65.79; H, 5.21; N, 7.62.
4-((2,2-Dimethyl-5-phenyl-2H-imidazol-4-yl)thio)phenol hydrochloride (3g). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Bright-yellow solid. Yield: 0.4 mmol (133 mg, 80%), mp = 218–219 °C. Rf 0.28 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 9.97 (br s, 1H); 8.32–8.30 (m, 2H); 7.67–7.53 (m, 1H); 7.49 (t, J = 7.4 Hz, 2H); 7.27–7.25 (m, 2H); 6.77–6.76 (m, 2H); 1.44 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 163.9 (C); 160.2 (C); 158.4 (C); 133.1 (CH); 131.4 (CH); 130.7 (C); 128.5 (CH); 127.9 (CH); 125.0 (C); 116.3 (CH); 79.6 (C); 27.0 (CH3) ppm. IR (DRA): ν 3156, 2393, 1598, 1581, 1545, 1494, 1330, 1314, 1277, 1229, 1164, 996, 912, 831, 777, 569, 529 cm−1. MS calcd: m/z 332 [M]+. Found (EI): m/z 296 [M - HCl]+. Elemental analysis calcd for: C17H17ClN2OS: C, 61.35; H, 5.15; N, 8.42. Found: C, 61.38; H, 5.14; N, 8.41.
4-((3-Phenyl-1,4-diazaspiro[4.5]deca-1,3-dien-2-yl)thio)phenol hydrochloride (3h). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Bright-green solid. Yield: 0.435 mmol (162 mg, 87%), mp = 225–226 °C. Rf 0.23 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 10.25 (br s, 1H); 7.87–7.85 (m, 2H); 7.57–7.53 (m, 3H); 7.27–7.25 (m, 2H); 6.78–6.76 (m, 2H); 1.79–1.47 (m, 10H) ppm. 13C {1H} NMR (DMSO-d6, BB mode): δ 164.2; 160.3; 158.4; 133.1; 131.3; 130.9; 128.5; 127.9; 125.0; 116.3; 104.0; 34.4; 24.6; 23.7 ppm. IR (DRA): ν 3117, 2951, 2413, 1597, 1548, 1495, 1441, 1341, 1284, 1118, 817, 779, 726, 696, 545 cm−1. MS calcd: m/z 372 [M]+. Found (EI): m/z 336 [M - HCl]+. Elemental analysis calcd for: C20H21ClN2OS: C, 64.42; H, 5.68; N, 7.51. Found: C, 64.42; H, 5.67; N, 7.53.
4-((5-(4-Bromophenyl)-2,2-dimethyl-2H-imidazol-4-yl)thio)phenol hydrochloride (3i). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Bright-green solid. Yield: 0.45 mmol (185 mg, 90%), mp = 236–237 °C. Rf 0.3 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 10.02 (br s, 1H); 7.82–7.80 (m, 2H); 7.77–7.76 (m, 2H); 7.39–7.38 (m, 2H); 6.86–6.85 (m, 2H); 1.37 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 162.0 (C); 161.0 (C); 158.9 (C); 135.9 (CH); 131.9 (CH); 130.4 (CH); 130.2 (C); 124.6 (C); 116.6 (CH); 116.0 (C); 102.0 (C); 24.1 (CH3) ppm. IR (DRA): ν 3120, 2406, 1584, 1542, 1496, 1400, 1331, 1279, 1212, 1116, 1069, 889, 833, 566 cm−1. MS calcd: m/z 410 [M]+; 412 [M + 2]+. Found (EI): m/z 374 [M - HCl]+; 376 [M + 2 - HCl]+. Elemental analysis calcd for: C17H16BrClN2OS: C, 49.59; H, 3.92; N, 6.80. Found: C, 49.60; H, 3.92; N, 6.81.
2-((2,2-Dimethyl-5-phenyl-2H-imidazol-4-yl)thio)benzoic acid hydrochloride (3j). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Colorless solid. Yield: 0.325 mmol (117 mg, 65%), mp = 176–177 °C. Rf 0.34 (hexane/EtOAc, 6:4). Note: the hydrogen from -COOH group is not revealed in CF3COOD 1H NMR (CF3COOD + CD3COOD): δ 8.29–8.27 (m, 1H); 7.92–7.90 (m, 2H); 7.83–7.90 (m, 1H); 7.76–7.74 (m, 2H); 7.67–7.63 (m, 1H); 7.56–7.52 (m, 2H); 1.70 (s, 6H) ppm. 13C {1H} NMR (CF3COOD + CD3COOD): δ 182.1; 175.9; 171.6; 165.7; 139.7; 137.5; 136.2; 135.6; 134.4; 131.4; 130.8; 127.9; 124.4; 102.0; 24.5 ppm. IR (DRA): ν 2811, 2458, 1714, 1632, 1522, 1456, 1384, 1237, 1180, 1117, 1050, 986, 840, 762, 727, 688, 641, 575, 520 cm−1. MS calcd: m/z 360 [M]+. Found (EI): m/z 324 [M - HCl]+. Elemental analysis calcd for: C18H17ClN2O2S: C, 59.91; H, 4.75; N, 7.76. Found: C, 59.81; H, 4.75; N, 7.75.
4-((3,5-Difluorophenyl)thio)-2,2-dimethyl-5-phenyl-2H-imidazole hydrochloride (3k). Note: In case of hydrochloride compound, m/z = m/z compound − m/z hydrochloride. Colorless solid. Yield: 0.26 mmol (92 mg, 52%), mp = 105–106 °C. Rf 0.38 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 7.84–7.82 (m, 2H); 7.61–7.55 (m, 3H); 7.54–7.50 (m, 2H); 7.39–7.35 (m, 1H); 1.44 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, BB mode): δ 162.1 (dd, J = 248.5, 13.7 Hz); 161.1; 159.8; 132.4 (t, J = 11.0 Hz); 131.0; 130.6; 128.8; 128.2; 116.2 (dd, J = 20.6, 3.5 Hz); 105.1 (t, J = 26.1 Hz); 102.7; 24.0 ppm. 19F NMR (DMSO-d6): -108.40 (s, 2F) ppm. IR (DRA): ν 3011, 1815, 1593, 1547, 1434, 1331, 1284, 1211, 1166, 1120, 934, 871, 725, 693, 672, 655, 594, 571 cm−1. MS calcd: m/z 352 [M]+. Found (EI): m/z 316 [M - HCl]+. Elemental analysis calcd for: C17H15ClF2N2S: C, 57.87; H, 4.29; N, 7.94. Found: C, 57.84; H, 4.28; N, 7.96.
2.3. General Procedure for the Synthesis of Sulfenyl-Imidazole Derivatives (4a,i)
A mixture of the corresponding hydrochloride of 3a or 3i (0.3 mmol) and NaHCO3 (0.45 mmol) in chloroform (5 mL) was refluxed for 30 min. Then, the reaction mixture was cooled to room temperature, filtered off, and the precipitate was washed with 5 mL of chloroform. The filtrate was combined and evaporated in vacuo to obtain compounds 4a or 4h as solids.
2,2-Dimethyl-4-phenyl-5-(phenylthio)-2H-imidazole (4a). Gray crystals. Yield: 0.35 mmol (98 mg, 100%), mp = 90–91 °C. Rf 0.45 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 7.87–7.85 (m, 2H); 7.65–7.63 (m, 2H); 7.58–7.53 (m, 3H); 7.48–7.42 (m, 3H); 1.39 (s, 6H) ppm. Note: one carbon (C) atom has overlapped with carbon (C) on 128.5 ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 161.9 (C); 161.0 (C); 133.7 (CH); 130.9 (CH); 129.5 (CH); 129.3 (CH); 128.8 (CH); 128.5 (C); 128.3 (CH); 102.3 (C); 24.2 (CH3) ppm. IR (DRA): ν 3060, 2977, 2930, 1697, 1630, 1605, 1562, 1489, 1437, 1214, 1163, 1105, 1025, 982, 775, 750, 687, 568 cm−1. MS calcd: m/z 280 [M]+. Found (EI): m/z 280 [M]+. Elemental analysis calcd for: C17H16N2S: C, 72.82; H, 5.75; N, 9.99. Found: C, 72.80; H, 5.75; N, 10.00.
4-((5-(4-Bromophenyl)-2,2-dimethyl-2H-imidazol-4-yl)thio)phenol (4i). Gray crystals. Yield: 0.35 mmol (131.6 mg, 100%), mp = 229–230 °C. Rf 0.4 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 9.70 (br s, 1H); 7.84–7.82 (m, 2H); 7.70–7.68 (m, 2H); 7.33–7.31 (m, 2H); 6.82–6.80 (m, 2H); 1.39 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 161.8 (C); 161.0 (C); 158.9 (C); 135.8 (CH); 131.8 (CH); 130.3 (CH); 130.2 (C); 124.5 (C); 116.5 (CH); 116.1 (C); 102.0 (C); 24.1 (CH3) ppm. IR (DRA): ν 3053, 2982, 2931, 1706, 1599, 1576, 1526, 1429, 1359, 1283, 1218, 1164, 1099, 1067, 1009, 988, 827, 740, 678, 569 cm−1. MS calcd: m/z 374 [M]+; 376 [M + 2]+. Found (EI): m/z 374 [M]+; 376 [M + 2]+. Elemental analysis calcd for: C17H15BrN2OS: C, 54.41; H, 4.03; N, 7.46. Found: C, 54.40; H, 4.03; N, 7.47.
4-((4-Methoxyphenyl)thio)-2,2-dimethyl-5-phenyl-2H-imidazole (4l). Note: This compound was additionally purified by manual column chromatography (SiO2, Hexane/EtOAc (7/3)) Light-brown crystals. Yield: 0.14 mmol (44 mg, 28%), mp = 118–119 °C. Rf 0.35 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 7.88–7.86 (m, 2H); 7.59–7.52 (m, 5H); 7.04–7.02 (m, 2H); 3.80 (s, 3H); 1.38 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 161.8 (C); 161.7 (C); 160.2 (C); 135.7 (CH); 131.0 (C); 130.9 (CH); 128.8 (CH); 128.2 (CH); 118.6 (C); 115.1 (CH); 101.9 (C); 55.3 (CH3); 24.2 (CH3) ppm. IR (DRA): ν 2925, 2852, 1590, 1521, 1490, 1443, 1243, 1167, 1092, 1022, 983, 922, 830, 812, 777, 639, 571 cm−1. MS calcd m/z 310 [M]+. Found (EI): m/z 310 [M]+. Elemental analysis calcd for: C18H18N2OS: C, 69.65; H, 5.85; N, 9.02. Found: C, 69.67; H, 5.85; N, 9.00.
4-((2,6-Dichlorophenyl)thio)-2,2-dimethyl-5-phenyl-2H-imidazole (4m). Note: This compound was additionally purified by manual column chromatography (SiO2, Hexane/EtOAc (7/3)) Light-yellow crystals. Yield: 0.175 mmol (61 mg, 35%), mp = 132–133 °C. Rf 0.35 (hexane/EtOAc, 6:4). 1H NMR (DMSO-d6): δ 7.89 (dd, J = 7.7, 1.9 Hz, 2H); 7.67 (s, 1H); 7.65 (s, 1H); 7.62–7.57 (m, 3H); 7.55–7.51 (m, 1H); 1.37 (s, 6H) ppm. 13C {1H} NMR (DMSO-d6, APT mode): δ 161.2 (C); 158.3 (C); 139.9 (C); 132.6 (CH); 131.2 (CH); 130.6 (C); 129.2 (CH); 129.0 (CH); 128.0 (CH); 127.3 (CH); 102.3 (C); 24.1 (CH3) ppm. IR (DRA): ν 2928, 1613, 1529, 1488, 1258, 1213, 1159, 1107, 1024, 980, 871, 773, 718, 692, 572 cm−1. MS calcd m/z 348 [M]+; 350 [M + 2]+. Found (EI): m/z 348 [M]+; 350 [M + 2]+. Elemental analysis calcd for: C17H14Cl2N2S: C, 58.46; H, 4.04; N, 8.02. Found: C, 58.44; H, 4.05; N, 8.02.
3. Results and Discussion
Novel arylthioimidazoles were prepared by transition metal-free C-H arylthiolation of 2H-imidazole 1 with thiophenols 2. This reaction can be considered as a special case of nucleophilic substitution of hydrogen (SNH) to be proceeded via the “addition-elimination” (SNH AE) pathway, with N-oxide moiety acting as a leaving group. As a result, the desired compounds have been obtained as hydrochloride salts; the latter can be easily converted into their corresponding bases (Scheme 2).
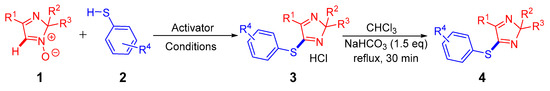
Scheme 2.
Transition metal-free C-H arylthiolation of 2H-imidazole 1-oxides 1 (red) with thiophenol 2 (blue).
To determine the optimal conditions for these couplings, a reaction between 2H-imidazole-1-oxide 1a and thiophenol 2a was chosen as the model (Scheme 3). The effect of solvent, activator, temperature, and reaction time has been investigated. For the first time, the desired compound 3a was obtained with a yield of 15% by stirring the reaction mixture from 0 °C to ambient temperature in toluene for 6 h, followed by the addition of acetyl chloride (Table 1, Entry 1). The further iteration resulted in a yield of 56% when acetone was used as a solvent (Table 1, Entry 3). Finally, a more thorough choice of solvent, temperature, and reaction time allowed us to obtain a product with 74% yield under the following conditions: in dimethyl carbonate (DMC) from 0°C to room temperature and stirring for 6 h (Table 1, Entries 4–13). All attempts to replace acetyl chloride as an activator were found to lead to a decrease in yield (Table 1, Entry 14) or to isolated starting materials (Table 1, Entries 15–18). It should be mentioned that DMC is one of the preferable green solvents for synthesis due to its low toxicity, biodegradability, and absence of irritable and mutagenic effects [39].
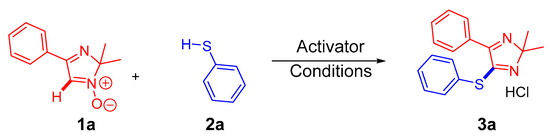
Scheme 3.
Model reaction for optimization of C-H arylthiolation of 2H-imidazole 1-oxide 1a (red) with thiophenol 2a (blue).

Table 1.
Optimization of the C-H arylthiolation of 2H-imidazole 1-oxide 1a with thiophenol 2a (bold for the best result of optimization).
In our previously reported studies of the SNH methodology, we investigated reactions of 2H-imidazole 1-oxides 1 with various substrates of aromatic and heteroaromatic nature [8,9,10]. The current work deals with the formation of C-S bonds in contrast to published C-C couplings. The S-nucleophilicity of thiophenols is obviously much higher than the C-nucleophilic properties of carbon centers of pyrroles, indoles, and phenols. Thereby, this reactivity feature affects the regioselectivity for the studied reaction and thus results exclusively in the C-S coupling products. It is also worth noting that the analogues reactions with polyphenols did not lead to C-O bond formation products following the C-S coupling logic. This observation could account for the greater electronegativity (lower nucleophilicity) of the oxygen atom compared with sulfur.
Finally, we have managed to obtain 11 arylthio-2H-imidazoles 3a-k as hydrochloride salts in yields of 42–90%, as well as four compounds 4a, 4i, 4l, 4m as bases in 28–90% yields (Scheme 4). Pure compounds 4l and 4m in the forms of hydrochloride have not been able to be isolated solely and thus require further extra purification by column chromatography.
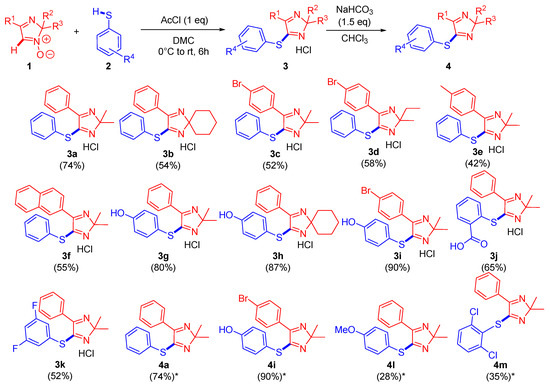
Scheme 4.
The developed arylthio-2H-imidazoles (red color indicates the imidazole ring; blue color shows the thioaryl moiety; * yield of two steps).
According to the plausible reaction mechanism (Scheme 5), at the first stage, acetyl chloride is attached to the N-oxide group of 2H-imidazole 1-oxide 1 to obtain a structure 1.1, which is equal to 1.2 with a positive charge on the C(5) atom. This form is likely to undergo a nucleophilic attack from the active S-H bond of thiophenol 2 with the formation of intermediate 1.3 to be stabilized by the positive charge on the sulfur atom by the chloride anion. As a result of the acetic acid elimination, a new C-S bond is formed, with sulfenylated imidazole derivatives in the form of hydrochloride 3 being formed.
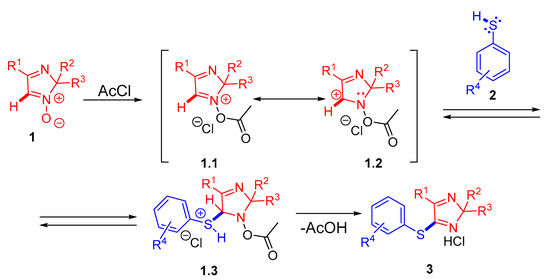
Scheme 5.
Plausible mechanism for C-H arylthiolation of 2H-imidazole 1-oxide 1 (red) with thiophenols 2 (blue).
4. Conclusions
In summary, 15 novel arylthioimidazoles of various architectures, including water-soluble hydrochloride forms, were synthesized in yields of up to 90%. In particular, the strategy of nucleophilic substitution of hydrogen (SNH) was first applied in reactions of 2H-imidazole-1-oxides with thiophenols. The elaborated synthetic method demonstrated a high level of regioselectivity, thus providing only C-S coupling products in the absence of C-C coupling by-products. The synthesized arylthiolated 2H-imidazoles could be considered challenging molecules in the field of medicinal chemistry and advanced materials, as well as valuable intermediates for further chemical modifications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5030100/s1, Figure S1–S31: Copies of NMR spectra for 3a-k, 4a,i,l,m.
Author Contributions
Conceptualization, O.N.C., V.N.C., and M.V.V.; methodology, E.A.N. and T.D.M.; investigation, E.A.N., N.F.V., and T.D.M.; writing—original draft preparation, E.A.N. and T.D.M.; writing—review and editing, M.V.V. and V.N.C.; visualization, N.F.V.; supervision, M.V.V.; project administration, V.N.C. and O.N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Science Foundation (RSF), project № 23-63-10011.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author and co-authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Beaupre, D.M.; Weiss, R.G. Thiol- and Disulfide-Based Stimulus-Responsive Soft Materials and Self-Assembling Systems. Molecules 2021, 26, 3332. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, A.R.; Beltz, J.; King, E.; Ercal, N. Medicinal Thiols: Current Status and New Perspectives. Mini-Rev. Med. Chem. 2020, 20, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, S.S.; Suliman, R.S.; Almutairi, K.; Kahtani, K.; Aljatli, D. Imidazole as a Promising Medicinal Scaffold: Current Status and Future Direction. Drug Des. Dev. Ther. 2021, 15, 3289–3312. [Google Scholar] [CrossRef] [PubMed]
- Tolomeu, H.V.; Fraga, C.A.M. Imidazole: Synthesis, Functionalization and Physicochemical Properties of a Privileged Structure in Medicinal Chemistry. Molecules 2023, 28, 838. [Google Scholar] [CrossRef]
- Ye, S.; Zhuang, S.; Pan, B.; Guo, R.; Wang, L. Imidazole Derivatives for Efficient Organic Light-Emitting Diodes. J. Inf. Disp. 2020, 21, 173–196. [Google Scholar] [CrossRef]
- Goa, K.L.; Wagstaff, A.J. Losartan Potassium. Drugs 1996, 51, 820–845. [Google Scholar] [CrossRef]
- Sawyer, P.R.; Brogden, R.N.; Pinder, R.M.; Speight, T.M.; Avery, G.S. Clotrimazole. Drugs 1975, 9, 424–447. [Google Scholar] [CrossRef]
- Sawyer, P.R.; Brogden, R.N.; Pinder, R.M.; Speight, T.M.; Avery, G.S. Tinidazole. Drugs 1976, 11, 423–440. [Google Scholar] [CrossRef]
- Gillis, J.C.; Wiseman, L.R. Secnidazole. Drugs 1996, 51, 621–638. [Google Scholar] [CrossRef]
- Iradyan, M.A.; Iradyan, N.S.; Stepanyan, G.M.; Arsenyan, F.G.; Garibdzhanyan, B.T. Antitumor Activity of Imidazole Derivatives: Dacarbazine and the New Alkylating Agent Imidazene (Review). Pharm. Chem. J. 2010, 44, 175–182. [Google Scholar] [CrossRef]
- Evans, J.C.; Murugesan, D.; Post, J.M.; Mendes, V.; Wang, Z.; Nahiyaan, N.; Lynch, S.L.; Thompson, S.; Green, S.R.; Ray, P.C.; et al. Targeting Mycobacterium Tuberculosis CoaBC through Chemical Inhibition of 4′-Phosphopantothenoyl- l -Cysteine Synthetase (CoaB) Activity. ACS Infect. Dis. 2021, 7, 1666–1679. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, T.; Sato, A.; El-Farrash, M.; Miki, S.; Abe, K.; Isaka, Y.; Kodama, M.; Wu, Y.; Chen, L.B.; Harada, H.; et al. S-1153 Inhibits Replication of Known Drug-Resistant Strains of Human Immunodeficiency Virus Type 1. Antimicrob. Agents Chemother. 1998, 42, 1340–1345. [Google Scholar] [CrossRef] [PubMed]
- Beshay, B.Y.; Abdellatef, A.A.; Loksha, Y.M.; Fahmy, S.M.; Habib, N.S.; Bekhit, A.E.-D.A.; Georghiou, P.E.; Hayakawa, Y.; Bekhit, A.A. Design and Synthesis of 2-Substituted-4-Benzyl-5-Methylimidazoles as New Potential Anti-Breast Cancer Agents to Inhibit Oncogenic STAT3 Functions. Bioorg. Chem. 2021, 113, 105033. [Google Scholar] [CrossRef]
- La Regina, G.; Edler, M.C.; Brancale, A.; Kandil, S.; Coluccia, A.; Piscitelli, F.; Hamel, E.; De Martino, G.; Matesanz, R.; Díaz, J.F.; et al. Arylthioindole Inhibitors of Tubulin Polymerization. 3. Biological Evaluation, Structure−Activity Relationships and Molecular Modeling Studies. J. Med. Chem. 2007, 50, 2865–2874. [Google Scholar] [CrossRef]
- Maltzman, J.S.; Koretzky, G.A. Azathioprine: Old Drug, New Actions. J. Clin. Investig. 2003, 111, 1122–1124. [Google Scholar] [CrossRef]
- Saroha, M.; Bartwal, G.; Khurana, J.M. Transition Metal Free K2CO3 Mediated Thioarylation, Selenoarylation and Arylation of 2-Aminomaleimides at Ambient Temperature. Tetrahedron 2019, 75, 130486. [Google Scholar] [CrossRef]
- Lv, F.; Tang, B.; Hao, E.; Liu, Q.; Wang, H.; Jiao, L. Transition-Metal-Free Regioselective Cross-Coupling of BODIPYs with Thiols. Chem. Commun. 2019, 55, 1639–1642. [Google Scholar] [CrossRef]
- Dodds, A.C.; Sutherland, A. Regioselective C–H Thioarylation of Electron-Rich Arenes by Iron(III) Triflimide Catalysis. J. Org. Chem. 2021, 86, 5922–5932. [Google Scholar] [CrossRef]
- Dodds, A.C.; Puddu, S.; Sutherland, A. Thioarylation of Anilines Using Dual Catalysis: Two-Step Synthesis of Phenothiazines. Org. Biomol. Chem. 2022, 20, 5602–5614. [Google Scholar] [CrossRef] [PubMed]
- Vara, B.A.; Li, X.; Berritt, S.; Walters, C.R.; Petersson, E.J.; Molander, G.A. Scalable Thioarylation of Unprotected Peptides and Biomolecules under Ni/Photoredox Catalysis. Chem. Sci. 2018, 9, 336–344. [Google Scholar] [CrossRef]
- Sharma, P.; Jain, N. S-Aryl Arenesulfonothioate and Copper Acetate Mediated Arylthiolation of 2-Arylpyridines and Heteroarenes. J. Org. Chem. 2019, 84, 13045–13052. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yang, H.; Zhu, C.; Fu, H. Arylthiolation of Arylamine Derivatives with (Arylthio)- Pyrrolidine-2,5-Diones. Adv. Synth. Catal. 2015, 357, 481–488. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Yang, S.; An, Y.; Wu, W.; Jiang, H. Assembly of 3-Sulfenylbenzofurans and 3-Sulfenylindoles by Palladium-Catalyzed Cascade Annulation/Arylthiolation Reaction. J. Org. Chem. 2016, 81, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Wojciechowska, N.; Rajkiewicz, A.A.; Kalek, M. Synthesis of Aryl Sulfides by Metal-Free Arylation of Thiols with Diaryliodonium Salts under Basic Conditions. Eur. J. Org. Chem. 2022, 2, e202101408. [Google Scholar] [CrossRef]
- Hirohiko, S.; Fujiwara, T. Imidazole Derivative. WO Patent 1996010019A1, 24 June 1994. [Google Scholar]
- Varaksin, M.; Moseev, T.; Chupakhin, O.; Charushin, V.; Trofimov, B. Metal-Free C–H Functionalization of 2H-Imidazole 1-Oxides with Pyrrolyl Fragments in the Design of Novel Azaheterocyclic Ensembles. Org. Biomol. Chem. 2017, 15, 8280–8284. [Google Scholar] [CrossRef]
- Moseev, T.D.; Nikiforov, E.A.; Varaksin, M.V.; Charushin, V.N.; Chupakhin, O.N. Metal-Free C–H/C–H Coupling of 2H -Imidazole 1-Oxides with Polyphenols toward Imidazole-Linked Polyphenolic Compounds. J. Org. Chem. 2021, 86, 13702–13710. [Google Scholar] [CrossRef]
- Vaccaro, L. Green Shades in Organic Synthesis. Eur. J. Org. Chem. 2020, 2020, 4273–4283. [Google Scholar] [CrossRef]
- De Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of Green Chemistry and Its Multidimensional Impacts: A Review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Gujral, S.S.; Sheela, M.A.; Khatri, S.; K Singla, R. A Focus & Review on the Advancement of Green Chemistry. Indo Glob. J. Pharm. Sci. 2012, 02, 397–408. [Google Scholar] [CrossRef]
- Dhawa, U.; Kaplaneris, N.; Ackermann, L. Green Strategies for Transition Metal-Catalyzed C–H Activation in Molecular Syntheses. Org. Chem. Front. 2021, 8, 4886–4913. [Google Scholar] [CrossRef]
- Dalton, T.; Faber, T.; Glorius, F. C–H Activation: Toward Sustainability and Applications. ACS Cent. Sci. 2021, 7, 245–261. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Panja, S.; Sahoo, S.R.; Chatterjee, S.; Maiti, D. Enroute Sustainability: Metal Free C–H Bond Functionalisation. Chem. Soc. Rev. 2023, 52, 2391–2479. [Google Scholar] [CrossRef] [PubMed]
- Charushin, V.N.; Chupakhin, O.N. Nucleophilic C—H Functionalization of Arenes: A Contribution to Green Chemistry. Russ. Chem. Bull. 2019, 68, 453–471. [Google Scholar] [CrossRef]
- Kirilyuk, I.A.; Grigor’ev, I.A.; Volodarskii, L.B. Synthesis of 2H-Imidazole 1-Oxides and Stable Nitroxyl Radicals Based on Them. Bull. Acad. Sci. USSR Div. Chem. Sci. 1991, 40, 1871–1879. [Google Scholar] [CrossRef]
- Varaksin, M.V.; Utepova, I.A.; Chupakhin, O.N.; Charushin, V.N. Palladium(II)-Catalyzed Oxidative C–H/C–H Coupling and Eliminative SNH Reactions in Direct Functionalization of Imidazole Oxides with Indoles. J. Org. Chem. 2012, 77, 9087–9093. [Google Scholar] [CrossRef]
- Smyshliaeva, L.A.; Varaksin, M.V.; Slepukhin, P.A.; Chupakhin, O.N.; Charushin, V.N. Transition Metal-Free Oxidative and Deoxygenative C–H/C–Li Cross-Couplings of 2H-Imidazole 1-Oxides with Carboranyl Lithium as an Efficient Synthetic Approach to Azaheterocyclic Carboranes. Beilstein J. Org. Chem. 2018, 14, 2618–2626. [Google Scholar] [CrossRef]
- Moseev, T.D.; Varaksin, M.V.; Gorlov, D.A.; Charushin, V.N.; Chupakhin, O.N. Transition-Metal-Free C–H/C–Li Coupling of Nonaromatic 2H-Imidazole 1-Oxides with Pentafluorophenyl Lithium in the Design of Novel Fluorophores with Intramolecular Charge Transfer Effect. J. Org. Chem. 2020, 85, 11124–11133. [Google Scholar] [CrossRef]
- Aricò, F.; Tundo, P. Dimethyl Carbonate as a Modern Green Reagent and Solvent. Russ. Chem. Rev. 2010, 79, 479–489. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).