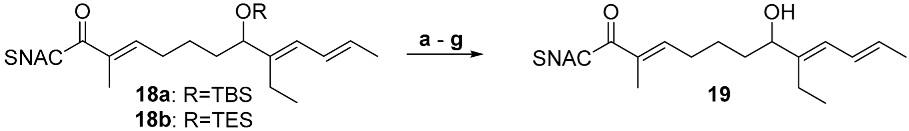Abstract
Biomimetic N-acetylcysteamine thioesters are essential for the study of polyketide synthases, non-ribosomal peptide synthetases and fatty acid synthases. The chemistry for their preparation is, however, limited by their specific functionalization and their susceptibility to undesired side reactions. Here we report a method for the rapid preparation of N-acetylcysteamine (SNAC) 7-hydroxy-2-enethioates, which are suitable for the study of various enzymatic domains of megasynthase enzymes. The method is based on a one-pot sequence of hydroboration and the Suzuki–Miyaura reaction. The optimization of the reaction conditions made it possible to suppress potential side reactions and to introduce the highly functionalized SNAC methacrylate unit in a high yield. The versatility of the sequence was demonstrated by the synthesis of the complex polyketide-SNAC thioesters 12 and 33. Brown crotylation followed by the hydroboration to Suzuki–Miyaura reaction sequence enabled the introduction of the target motif in significantly fewer steps and with a higher overall yield and stereoselectivity than previously described approaches. This is the first report of a transition-metal-catalyzed cross-coupling reaction in the presence of an SNAC thioester.
1. Introduction
Thioesters are an important functional group in many biosynthetic systems. They often serve to link biosynthetic acyl intermediates to carrier thiols, which can be free molecules such as coenzyme A (CoA) or proteins. The important systems working with protein-bound metabolites are so-called megasynthase enzymes, such as fatty acid synthases, polyketide synthases (PKS) and non-ribosomal peptide synthetases (NRPS) and their hybrids [1,2]. They are responsible for the formation of polyketide and peptide natural products, including some of the most important small-molecule drugs in clinical use, such as erythromycin, rapamycin or epothilone. The availability of suitable substrate surrogates is essential for the functional study of such biosynthetic systems (Figure 1A). N-Acetylcysteamine (SNAC) thioesters are of particular importance for this as they effectively mimic the protein attachment of the substrate via the 4′-phosphopantetheine arm and thus allow simplified studies with active enzymes (Figure 1B) [3,4,5,6,7,8,9,10].
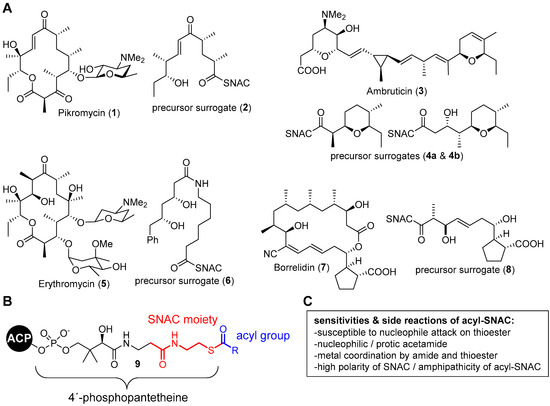
Figure 1.
(A) Structure of the 4′-phosphopantetheine prosthetic group of acyl carrier proteins and the partial structure that is mimicked by SNAC. (B) Susceptibilities and side reactions of acyl-SNACs. (C) Prominent natural products and the structures of biomimetic SNAC thioesters that have been used for their biosynthesis studies.
Acylated SNACs contain an acetamide and a thioester as conserved reactive functional groups, which afford them problematic properties (Figure 1C) [7,11]. The thioester can undergo side reactions with external or internal nucleophiles, resulting in the irreversible loss of substance. Due to its polarity, the acetamide can cause problems during substance purification and can, as a nucleophilic/protic group, cause undesired side reactions. The functionalization distance between the acetamide and thioester carries the risk that they act as a chelate ligand and interact with metals. The synthesis of the SNAC thioester surrogates of late-stage biosynthetic intermediates is as challenging as the synthesis of natural products of similar structural complexity but, for the above-mentioned reasons, has the challenge of an additional problematic functional group. A useful strategy to overcome this problem would be to introduce the SNAC moiety at a late stage of synthesis along with a larger fraction of the polyketide moiety.
Improving the specific methodology for the synthesis of complex polyketide–SNAC thioesters is therefore of great interest to the biosynthetic research community. Transition metal-mediated reactions are well suited to late-stage attachment in the convergent synthesis of complex biosynthetic thioester surrogates, but have only very rarely been described in the presence of SNAC thioesters. To the best of our knowledge, the literature currently only contains a report about olefin cross-metathesis between SNAC–acrylates and hydroxyolefins catalyzed by the second-generation Grubbs catalyst [12].
The Suzuki–Miyaura reaction (SMR) is a highly versatile Pd-catalyzed cross-coupling reaction. It allows couplings between halides and non-toxic boronic acid derivatives under relatively mild conditions (Figure 2) [13,14]. In addition to sp2–sp2 bond formations, it is now possible to carry out couplings between sp2 and sp3 centers, as well as between two sp3 centers. Two aspects of the SMR could be problematic when applied to SNAC thioesters. On the one hand, the use of a base is necessary to accelerate the essential group transfer from the boronic acid to the Pd during the catalytic cycle (step 3). Moreover, Pd can also be inserted into the C–S bond of the thioester instead of the C–halide bond (step 1) [15]. This reactivity is so pronounced that it forms the basis of the Liebeskind–Srogl reaction, a modification of the SMR for the direct synthesis of ketones from thioesters [16,17].
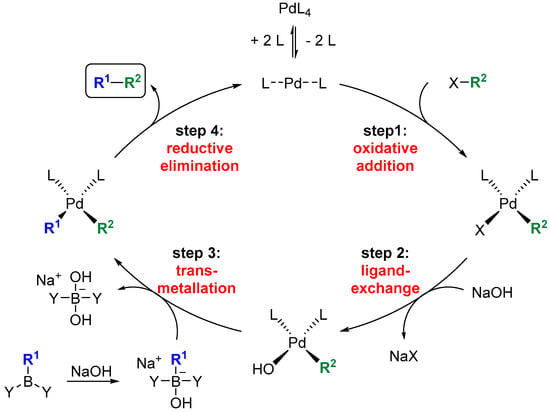
Figure 2.
Mechanism of the SMR.
Among the diverse enzymatic PKS domains, cyclases that form saturated oxygen heterocycles via intramolecular oxa-Michael addition (IMOMA) stand out due to the synthetical value of this transformation (Figure 3A) [18,19]. It has been shown that they catalyze a ring formation with exceptional stereoselectivity and therefore represent a potential new type of biocatalyst [20,21,22,23,24,25,26,27,28,29]. For the study of such enzymes, SNAC-7-hydroxy-2-enethioates are required as substrate surrogates. The synthetic methodology used for the selective installation of this structural motif is, however, not well developed, making the generation of precursor libraries a difficult task. The multi-step routes described in the literature are either highly elaborate, are not stereoselective or lack flexibility, and are therefore narrow in their applicability [20,21,22,23]. For example, the synthesis of the SNAC surrogate of 10 in stereochemical pure form was accomplished in eight steps and required multiple purification procedures [21,22]. Furthermore, a lack of convergence makes it necessary to carry out the largest part of this sequence using different starter building blocks to access derivatives with variations in the eastern part of the molecule. Other reported routes are shorter, but also less flexible due to the choice of larger starting building blocks or the introduction reaction chosen for the SNAC thioester. Olefin cross-metathesis, for example, is only possible with SNAC–acrylthioates and not with SNAC–methacrylthioates. Therefore, we set out to develop a flexible, straightforward and broadly applicable method for the preparation of SNAC-7-hydroxy-2-enethioates.
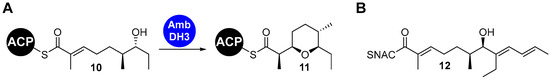
Figure 3.
(A) IMOMA cyclases catalyze the intramolecular oxa-Michael addition to oxygen heterocycles. The natural reaction of AmbDH3 is shown as an example. (B) Structure of the target compound required for our biosynthetic studies.
As a solution, we turned to a sequence of hydroboration and SMR to assemble the backbone and directly introduce the SNAC moiety. The specific challenge was to effectively perform the SMR in the presence of the SNAC thioester, which has not been achieved before to the best of our knowledge. The versatility of the method should be shown on the example of the synthesis of 12 (Figure 3B). This compound was, on the one hand, specifically required for our enzymatic studies on new IMOMA cyclases (Figure 3A). On the other hand, it represents a particularly challenging substrate during whose preparation various detrimental side reactions occur; it is thus a reasonable benchmark.
2. Materials and Methods
2.1. General Methods and Materials
All chemicals and solvents were obtained from Abcr (Karlsruhe, Germany), Acros Organics (Geel, Belgium), BLD Pharm (Kaiserslautern, Germany), Carbolution (St. Ingbert, Germany), Eurisotop (Saarbrücken, Germany), Fluorochem (Hadfield, UK), Grüssing (Filsum, Germany), Roth (Karlsruhe, Germany), Sigma-Aldrich (Schnelldorf, Germany), TCI (Zwijndrecht, Belgium) Thermo Fisher Chemical (Schwerte, Germany), and VWR (Rednor, DE, USA) and were, unless otherwise stated, used without further purification. Dry solvents were obtained from Acros Organics. All reactions were performed under argon gas using dry solvents and reagents. Light-sensitive substances were handled in brown glass- or aluminum-foil-wrapped flasks. The reactions were monitored via TLC using Alugram SilG/UV254 TLC foils from Macherey-Nagel (Düren, Germany). The substances were detected using UV light and a KMnO4 stain (1.50 g of KMnO4, 10.0 g of K2CO3, 2.50 mL of 5% NaOH, 200 mL of H2O). Products were purified via flash chromatography on SiO2 (Macherey-Nagel MN Kieselgel 60, 40–63 μm). Semi-preparative HPLC was performed using a Waters HPLC (600 controller, 2487 Dual wavelength absorbance detector) and a C18-SP stationary phase (H2O:MeCN = 95:5 {5 min}, Gradient H2O:MeCN 95:5 → 5:95 {20 min}, H2O:MeCN = 5:95 {5 min}, 20 mL/min).
All NMR spectra were recorded using Bruker Avance III HD 500 (Rheinstetten, Germany) with the residual solvent signal as an internal standard: CDCl3 7.26 ppm for 1H and 77.16 ppm for 13C.2 Signal multiplicities are stated, using the following abbreviations: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet and br = broad. For 13C-NMR, the following abbreviations were used: q = quarternary, t = tertiary, s = secondary and p = primary. The chemical shifts are reported as values of the δ-scale in [ppm] and the coupling constants J in [Hz]. Signal assignments were made with 2D NMR spectra (COSY, HSQC, HMBC, NOESY). High-resolution mass spectra (HRMS) were obtained using a Thermo Fisher Scientific Q Exactive (Orbitrap) mass spectrometer. Optical rotation was recorded on a Jasco P-1020 polarimeter (10 cm cell) from Portman Instruments (Biel-Benken, Switzerland) using the sodium D line (589 nm). The given value of [α]D represents the average of 50 individual measurements and is stated as deg∙mL∙g−1∙dm−1. Elemental analyses were carried out using a 2400 CHN elemental analyzer from Perkin-Elmer (Waltham, MA, USA).
2.2. General Procedures
2.2.1. General Procedure 1: Steglich Esterification for SNAC Thioester Formation
A solution of carboxylic acid (GP2, 1.0 eq.) and N-acetylcysteamine (GP1, 1.5 eq.) in CH2Cl2 (0.2 M) was cooled to 0 °C. Subsequently, DMAP (0.1 eq.) and EDC*HCl (1.5 eq.) were added. After warming to room temperature, the solution was stirred for 2 h, before diluting with saturated aqueous NH4Cl solution. The resulting phases were separated and the aqueous one was extracted three times with CH2Cl2. The combined organic phases were washed with brine, dried over MgSO4 and filtrated. The solvent was removed in vacuo and the crude thioester (GP3) was purified via flash chromatography.
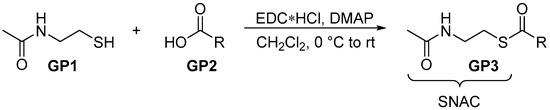
Scheme 1.
General procedure for thioesterification.
2.2.2. General Procedure 2: Protection of Hydroxyls as Silylethers
To a stirred solution of secondary alcohol (GP4, 1.0 eq.) in DMF (1 M), silylchloride (1.5 eq.) and imidazole (2.5 eq.) were added. After stirring the mixture at room temperature overnight, pentane and water were added. The resulting phases were separated and the aqueous one was extracted three times with pentane. Subsequently, the combined organic phases were washed with brine, dried over MgSO4 and filtrated. The solvent was removed in vacuo and the crude silylether (GP5) was purified via filtration over a short plug of silica (pentane).
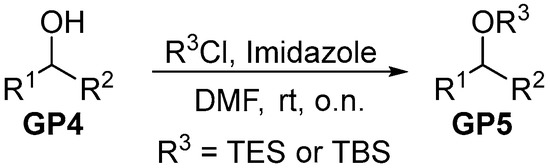
Scheme 2.
General procedure for silylether protection.
2.2.3. General Procedure 3: Hydroboration-Suzuki-Miyauri Reaction
A solution of terminal alkene (GP7, 1.5 eq.) in freshly degassed THF (1 M) was cooled to 0 °C and 9-BBN (1.5 eq., 0.5 M in THF) was added dropwise. The mixture was stirred overnight while being allowed to slowly warm to room temperature. Subsequently, freshly degassed DMF (total 0.2 M), thioester vinylhalogenide (GP6, 1.0 eq.), PdCl2 (dppf) (5 mol%), AsPh3 (5 mol%) and Cs2CO3 (2.0 eq.) were added, and the suspension was heated to 50 °C. After the complete consumption of the starting material (TLC), EtOAc was added, and the mixture was transferred to a separating funnel containing aqueous LiCl solution (10% wt). After the separation and extraction of the aqueous phase using EtOAc (3×), the combined organics were washed with brine and dried over MgSO4. After concentration in vacuo, the crude product (GP8) was purified via flash chromatography.
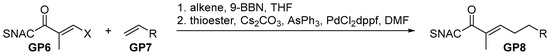
Scheme 3.
General procedure for SMR.
2.2.4. General Procedure 4: Deprotection
The silylether (GP9, 1.0 eq.) was dissolved in HF-containing stock solution (70% HF*pyridine/pyridine/THF (1:2:8)) at 0 °C. After warming to room temperature and the complete consumption of the starting material according to TLC, the saturated aqueous NaHCO3 solution was added dropwise until no more formation of CO2 was observed. Subsequently, the phases were separated and the aqueous one was extracted three times using EtOAc. The combined organics were washed with brine and dried over MgSO4. After concentration in vacuo, the crude product (GP7) was purified via semi-preparative HPLC.
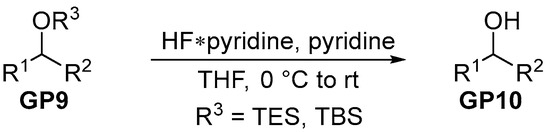
Scheme 4.
General procedure for silylether deprotection.
3. Results
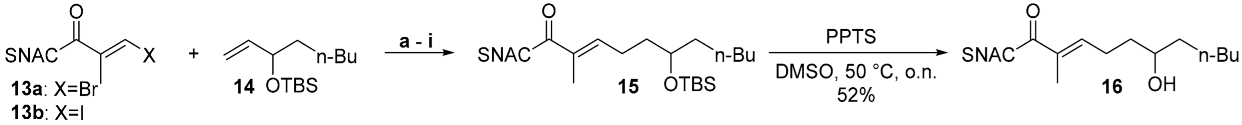
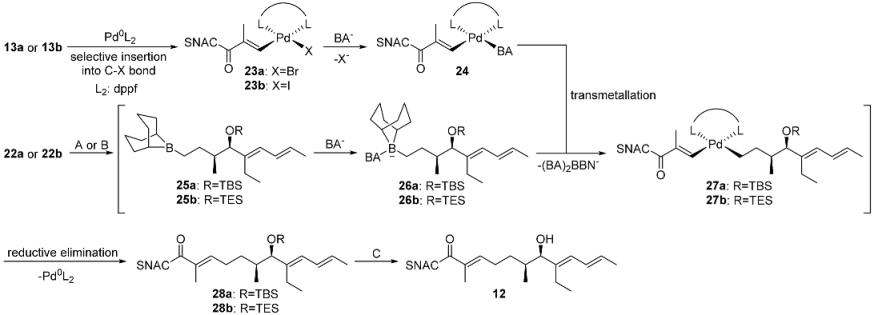
Thioester-halides are rare substrates in SMRs. The literature, however, contains an example of the coupling reactions of simple 4-bromothiophenols with 4-tolyl-boronic acid, in which the bulky acyl unit of the thioesters served as a protecting group for the thiol [15]. We used the reported conditions for the synthesis of an ethylenoate that was sensitive to hydrolysis and a base described by Suzuki et al. as the starting point for our studies [30]. These were carried out using the SNAC (E)-3-bromo-2-methylprop-2-enethioate 13a and the OTBS-protected 3-hydroxyolefin 14 (1.0 equiv. of 13a, 1.1 equiv. of 14, 1.1 equiv. 9-BBN, 5mol% PdCl2(dppf) (dppf: 1,1′-bis(diphenylphosphino)ferrocene) and 2.0 equiv. K2CO3) (Scheme 1 and Scheme 2). Although a basic coupling reactivity was observed, the yields of the reactions varied hardly reproducibly over a wide range and showed a strong dependence on even small variations in the amounts of thioester, alkene, borane and Pd catalyst. This suggests that several side reactions might proceed at rates similar to the desired pathway. We therefore carried out a systematic optimization study (Table 1, see Supplementary Materials pages 3–7).

Table 1.
Optimization of the conditions for the coupling of the SNAC thioester halides 13a/13b and TBS-protected olefin 14.
For this, we varied the individual reaction parameters. Since we assumed that the side reactions of the 3-bromoacryl thioate 13a were a particular problem, we worked with an excess of 1.5 equiv. of alkene 14 and 9-BBN. Different thioester halides (Br and I), bases (K2CO3 and Cs2CO3), additives (P(o-furyl)3 and AsPh3) and temperatures (20 °C, 50 °C and 65 °C) were tested. All reactions were carried out on a scale of 90–100 µmol of 13a/13b and compared based on the isolated yield after column chromatography. The yields in the basic experiment with an excess of 1.5 equiv. of 14 and 9-BBN (entry a) were fortunately stable upon repetition in a range slightly above 50%. The variation in the individual parameters did not lead to a marked increase in this value, whereas the addition of P(o-furyl)3 and the decrease in the reaction temperature even significantly reduced the yield (entries b and e). Fortunately, the combined change in several parameters led to a significantly improved result (entry i). Using 3-iodoacryl thioate 13b, Cs2CO3, AsPh3 and carrying out the reaction at room temperature gave 15 a yield of 78%. The TBS deprotection of 16 achieved a 52% yield using PPTS under conditions that we identified as successful in the synthesis of other SNAC-7-hydroxy-2-enethioates [21,22,23].
Side products that could not be isolated and fully analyzed were regularly detected in the low-yielding hydroboration SMRs in Table 1. According to TLC, these were highly polar compounds whose migration behavior suggests that they were derived from SNAC. We assume that a major part of this is the homocoupling product of the thioester acrylates 13a/13b and the 2-(N-acetamidyl)-ethylketone resulting after the C–S bond insertion of the Pd, a side reaction described previously for low-functionalized thioesters [15]. The yield improvement observed in the optimization study is consistent with the suppression of these side reactions. Cs2CO3 is much more soluble in DMF than K2CO3, leading to a much higher effective concentration of carbonate. This should significantly improve the activation of the ate complex for alkyl group transfer to the Pd (step 3 in Figure 2) and accelerate the heterocoupling reaction. The iodoacrylate is more reactive towards Pd insertion than the bromoacrylate, thus favoring this productive reaction (step 1) compared to the insertion of Pd into the C–S bond. This selectivity is expected to be even more pronounced at room temperature than at 50 °C. The addition of AsPh3 supports these effects by accelerating both the formation of the active Pd(0) from the Pd(II) species and transmetallation, due to its lower σ-donor effect than PPh3 [31,32].
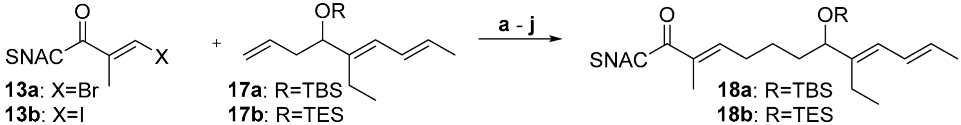
We now turn to the coupling between the thioesters 13a/13b and the olefins 17a and 17b, which resemble the sensitive 5-hydroxy-tri-1,3,7-ene in target molecule 12 (Table 2, see SI pages 7–13). Their higher degree of functionalization makes them more susceptible to side reactions during the introduction and removal of the protecting group and during the coupling cascade. In addition to screening the same thioester halides (13a/13b) as in Table 2, a broader panel of bases (Cs2CO3, K2CO3 and K3PO4) and hydroxyl protection groups (TBS and TES) on the olefinic coupling partner were examined. Due to the superiority of the previous optimization, only AsPh3 was applied as an additive and only 20 °C and 50 °C were tested as reaction temperatures.

Table 2.
Optimization of the conditions for the coupling between 13a/13b and protected trienes 17a/17b, varying protecting group, halogenide, base, additives and temperature.
Compared to the basic experiments (entries a and c, Table 2), a decrease in the yield of 18 was observed when the reaction was carried out at 20 °C instead of 50 °C, or when K3PO4 was employed as a base (entries a–e). In contrast to the experiments using the simpler coupling partner 14 (Table 1), the change in one or two reaction parameters led to an increase in the yield of up to 80% (entries f–i). When these measures were combined and the reaction was carried out at 20 °C, a further increase to an 87% yield of 18b was achieved (entry j, Scheme 3). The TES group was expected to be more easily removable than the TBS group (vide infra). As the former demonstrated stability under the conditions tested, and as both protecting groups gave similar yields in the comparable entries a–d, the optimization in entries f–j was conducted using the TES protection group. The results summarized in Table 2 are in agreement with those observed in Table 1 and confirm the conclusions/interpretations drawn from them.
Numerous side reactions were conceivable during the deprotection of the silyl ethers 18a and 18b, such as eliminations, intramolecular oxa-Michael additions or interferences with the thioester. The slightly acidic conditions of the standard deprotection protocol with PPTS (see Table 1, step 2) resulted in the elimination of the alcohol/silylether (entry a, Table 3, see SI page 14). With TBAF, the formation of the desired product was also not observed in any case. No reaction of the TBS ether 18a was found after 1 h at 0 °C (entry b). Decomposition occurred for the TBS ether 18b after overnight reaction at 20 °C and for the TES ether after only 1 h at 0 °C (entries c and d). Standard HF*pyridine treatment also resulted in decomposition (entry e). A successful procedure was finally adopted from a protocol previously reported by Carreira et al., which relied on using a premixed stock solution of HF*pyridine in THF supplemented with additional pyridine at 0 °C [33]. Deprotection was successful for both silylethers and led to the attainment of the desired alcohol 19 in pure form after column chromatography (entries f and g, Scheme 4). The reactions were continuously monitored by TLC and stopped before noticeable decomposition occurred. The yield for the TES ether was significantly better than that of the TBS ether, suggesting that the former is the preferable protecting group for the synthesis of 12.

Table 3.
Testing conditions for silylether deprotection.
The reaction sequences to 12 were carried out starting from aldehyde 20 (see Supplementary Materials pages 14–16). Brown crotylation first afforded the highly sensitive hydroxytriene 21 in a yield of 71% and an 86% e.e., which was immediately transformed into the isolatable TBS and TES ethers 22a and 22b, with yields of 91% and 84% (Scheme 5). This was followed by the established one-pot two-step cascade of hydroboration and SMR to give 28a and 28b, which were deprotected under the optimized conditions to give 7-hydroxy-2-ene-SNAC thioate 12 in overall yields of 16–60% (Table 4, see Supplementary Materials pages 17–19). These results confirm, on the one hand, that TES is the preferable protecting group compared to TBS as it leads to higher yields in both the coupling and deprotection reaction (entries a and b). On the other hand, they show the positive effect of optimizing the SMR conditions, which led to an improvement in the yield from 51% to 74% in the coupling step (entries b and c).

Table 4.
Two-pot three-step reaction sequence for thioester 12 starting from 13a/13b and 22a/22b. The coupling step is formulated under the assumption that the SMR proceeds via a Pd(0)/Pd(II) mechanism.
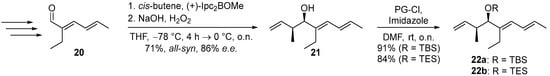
Scheme 5.
Synthesis of protected hydroxytrienes 22a/22b from aldehyde 20. See SI for the steps leading to precursor aldehyde 20.
To illustrate the broader synthetic utility of the synthesis of chiral 7-hydroxy-2-ene-SNAC thioates, we additionally applied the method to the synthesis of octaketide 33 (Scheme 6, see SI pages 20–24). In comparison to 12, this compound exhibits a highly hydrophobic heptyl chain, an additional chiral secondary alcohol and a relative anti-configuration at the vicinal stereocenters at C-6 and C-7. Olefin 31 was obtained from aldehyde 29 via Brown crotylation and TBS protection. Despite the presence of two sterically demanding TBS groups, the hydroboration to SMR sequence proceeded similarly well, as in the synthesis of 28b, giving 32 a yield of 79% starting from 31. The deprotection of 32 was achieved in a 93% yield under the conditions optimized for the synthesis of the sensitive allylic alcohols 12 and 19. These conditions thus also provide an advantage for the deprotection of SNAC 2-enethioate silyl ethers devoid of critical (poly)enes, as is evident from the comparison made with the deprotection of 15 to 16 that was carried out using PPTS and that led to a yield of only 52%.
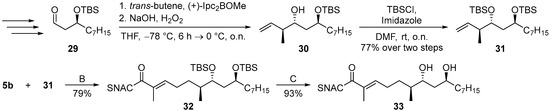
Scheme 6.
Synthesis of chiral hydroxythioate 33. See SI for the steps leading to precursor aldehyde 29.
4. Discussion
In summary, a useful method for the rapid assembly of SNAC hydroxyenethioates that makes use of a cascade of hydroboration to SMR, followed by optimized silylether deprotection, was developed. The four SNAC hydroxyenethioates 12, 16, 19 and 33 were synthesized using this strategy. The chiral 12 and 33 were obtained in four synthetic operations using the aldehydes 20 and 29, respectively, with overall yields of 36% and 57% and high stereoisomeric purity. This represents a significant improvement over previously described routes to similar compounds, which either required significantly more steps or gave lower overall yields (eight steps, 10% overall yield for the SNAC thioester analog of 10 starting from propionaldehyde) [21]. Other routes gave SNAC 7-hydroxy-2-ene thioates in five steps from TBS-protected 1,5-hexanediol with a total yield of 23% [20]. The latter, however, only provided access to racemic products, which were also not branched in the α-position. It did also not offer the flexibility in backbone installation that the presented method does.
The SMR-based coupling method presented here is compatible with the presence of SNAC thioesters and can be used in the future for the flexible and efficient preparation of substrate surrogates for studies of IMOMA cyclases and other enzymatic megasynthase domains that act on similar functionalization patterns as those present at C-1–C-6 in 12, 16, 19 and 33. The method should also be of interest for the synthesis of precursors of non-enzymatic IMOMA reactions. It has been shown for chemically catalyzed IMOMA reactions that cis-THP stereoselectivity can be more reliably achieved using enethioates rather than enoates, meaning that the former are attractive precursors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5020096/s1, The Supplementary Materials contain detailed synthetic procedures and analytical data including 1H and 13C NMR spectra in Figures S1–S50. References [34,35,36,37] are cited in Supplementary Materials.
Author Contributions
Conceptualization, S.D. and F.H.; investigation, S.D. and L.S.; resources, F.H.; writing—original draft preparation, F.H.; writing—review and editing, S.D., L.S. and F.H.; supervision, F.H.; project administration, F.H.; funding acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG), grant numbers HA 5841/5-1 and HA 5841/7-1.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (Frank Hahn).
Acknowledgments
We thank Central Analytics of the Department of Chemistry, as well as the North Bavarian NMR Centre (NBNC) at the University of Bayreuth.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weissman, K.J.; Müller, R. Protein–Protein Interactions in Multienzyme Megasynthetases. ChemBioChem 2008, 9, 826–848. [Google Scholar] [CrossRef]
- Grininger, M. Enzymology of Assembly Line Synthesis by Modular Polyketide Synthases. Nat. Chem. Biol. 2023, 19, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.-M.; Huang, T.; Rudolf, J.D.; Lohman, J.R.; Huang, S.-X.; Guo, X.; Shen, B. Enediyne Polyketide Synthases Stereoselectively Reduce the β-Ketoacyl Intermediates to β-d-Hydroxyacyl Intermediates in Enediyne Core Biosynthesis. Org. Lett. 2014, 16, 3958–3961. [Google Scholar] [CrossRef] [PubMed]
- Sahner, J.H.; Sucipto, H.; Wenzel, S.C.; Groh, M.; Hartmann, R.W.; Müller, R. Advanced Mutasynthesis Studies on the Natural α-Pyrone Antibiotic Myxopyronin from Myxococcus Fulvus. ChemBioChem 2015, 16, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Wang, M.; Horsman, M.; Boddy, C.N. 6-Deoxyerythronolide B Synthase Thioesterase-Catalyzed Macrocyclization Is Highly Stereoselective. Org. Lett. 2012, 14, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.A.; Rath, C.M.; Eisman, E.B.; Narayan, A.R.H.; Kittendorf, J.D.; Mortison, J.D.; Yoon, Y.J.; Sherman, D.H. Biocatalytic Synthesis of Pikromycin, Methymycin, Neomethymycin, Novamethymycin, and Ketomethymycin. J. Am. Chem. Soc. 2013, 135, 11232–11238. [Google Scholar] [CrossRef]
- Franke, J.; Hertweck, C. Biomimetic Thioesters as Probes for Enzymatic Assembly Lines: Synthesis, Applications, and Challenges. Cell Chem. Biol. 2016, 23, 1179–1192. [Google Scholar] [CrossRef]
- Hahn, F.; Kandziora, N.; Friedrich, S.; Leadlay, P.F. Synthesis of Complex Intermediates for the Study of a Dehydratase from Borrelidin Biosynthesis. Beilstein J. Org. Chem. 2014, 10, 634–640. [Google Scholar] [CrossRef]
- Berkhan, G.; Merten, C.; Holec, C.; Hahn, F. The Interplay between a Multifunctional Dehydratase Domain and a C-Methyltransferase Effects Olefin Shift in Ambruticin Biosynthesis. Angew. Chem. Int. Ed. 2016, 55, 13589–13592. [Google Scholar] [CrossRef]
- Schröder, M.; Roß, T.; Hemmerling, F.; Hahn, F. Studying a Bottleneck of Multimodular Polyketide Synthase Processing: The Polyketide Structure-Dependent Performance of Ketoreductase Domains. ACS Chem. Biol. 2022, 17, 1030–1037. [Google Scholar] [CrossRef]
- Wunderlich, J.; Roß, T.; Schröder, M.; Hahn, F. Step-Economic Synthesis of Biomimetic β-Ketopolyene Thioesters and Demonstration of Their Usefulness in Enzymatic Biosynthesis Studies. Org. Lett. 2020, 22, 4955–4959. [Google Scholar] [CrossRef]
- Sundaram, S.; Kim, H.J.; Bauer, R.; Thongkongkaew, T.; Heine, D.; Hertweck, C. On-Line Polyketide Cyclization into Diverse Medium-Sized Lactones by a Specialized Ketosynthase Domain. Angew. Chem. Int. Ed. 2018, 57, 11223–11227. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent Advances in the Suzuki–Miyaura Cross-Coupling Reaction Using Efficient Catalysts in Eco-Friendly Media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Zeysing, B.; Gosch, C.; Terfort, A. Protecting Groups for Thiols Suitable for Suzuki Conditions. Org. Lett. 2000, 2, 1843–1845. [Google Scholar] [CrossRef]
- Liebeskind, L.S.; Srogl, J. Thiol Ester−Boronic Acid Coupling. A Mechanistically Unprecedented and General Ketone Synthesis. J. Am. Chem. Soc. 2000, 122, 11260–11261. [Google Scholar] [CrossRef]
- Cheng, H.-G.; Chen, H.; Liu, Y.; Zhou, Q. The Liebeskind–Srogl Cross-Coupling Reaction and Its Synthetic Applications. Asian J. Org. Chem. 2018, 7, 490–508. [Google Scholar] [CrossRef]
- Meng, S.; Tang, G.-L.; Pan, H.-X. Enzymatic Formation of Oxygen-Containing Heterocycles in Natural Product Biosynthesis. ChemBioChem 2018, 19, 2002–2022. [Google Scholar] [CrossRef]
- Hemmerling, F.; Hahn, F. Biosynthesis of Oxygen and Nitrogen-Containing Heterocycles in Polyketides. Beilstein J. Org. Chem. 2016, 12, 1512–1550. [Google Scholar] [CrossRef] [PubMed]
- Pöplau, P.; Frank, S.; Morinaka, B.I.; Piel, J. An Enzymatic Domain for the Formation of Cyclic Ethers in Complex Polyketides. Angew. Chem. Int. Ed. 2013, 52, 13215–13218. [Google Scholar] [CrossRef]
- Berkhan, G.; Hahn, F. A Dehydratase Domain in Ambruticin Biosynthesis Displays Additional Activity as a Pyran-Forming Cyclase. Angew. Chem. Int. Ed. 2014, 53, 14240–14244. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, T.; Berkhan, G.; Wagner, L.; Sung, K.H.; Kolb, S.; Geise, H.; Hahn, F. Biocatalysts from Biosynthetic Pathways: Enabling Stereoselective, Enzymatic Cycloether Formation on a Gram Scale. ACS Catal. 2020, 10, 4973–4982. [Google Scholar] [CrossRef]
- Wagner, L.; Stang, J.; Derra, S.; Hollmann, T.; Hahn, F. Towards Understanding Oxygen Heterocycle-Forming Biocatalysts: A Selectivity Study of the Pyran Synthase PedPS7. Org. Biomol. Chem. 2022, 20, 9645–9649. [Google Scholar] [CrossRef]
- Sung, K.H.; Berkhan, G.; Hollmann, T.; Wagner, L.; Blankenfeldt, W.; Hahn, F. Insights into the Dual Activity of a Bifunctional Dehydratase-Cyclase Domain. Angew. Chem. Int. Ed. 2018, 57, 343–347. [Google Scholar] [CrossRef]
- Wagner, L.; Roß, T.; Hollmann, T.; Hahn, F. Cross-Linking of a Polyketide Synthase Domain Leads to a Recyclable Biocatalyst for Chiral Oxygen Heterocycle Synthesis. RSC Adv. 2021, 11, 20248–20251. [Google Scholar] [CrossRef]
- Wagner, D.T.; Zhang, Z.; Meoded, R.A.; Cepeda, A.J.; Piel, J.; Keatinge-Clay, A.T. Structural and Functional Studies of a Pyran Synthase Domain from a Trans-Acyltransferase Assembly Line. ACS Chem. Biol. 2018, 13, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Ueoka, R.; Uria, A.R.; Reiter, S.; Mori, T.; Karbaum, P.; Peters, E.E.; Helfrich, E.J.N.; Morinaka, B.I.; Gugger, M.; Takeyama, H.; et al. Metabolic and Evolutionary Origin of Actin-Binding Polyketides from Diverse Organisms. Nat. Chem. Biol. 2015, 11, 705–712. [Google Scholar] [CrossRef]
- Luhavaya, H.; Dias, M.V.B.; Williams, S.R.; Hong, H.; de Oliveira, L.G.; Leadlay, P.F. Enzymology of Pyran Ring A Formation in Salinomycin Biosynthesis. Angew. Chem. Int. Ed. 2015, 54, 13622–13625. [Google Scholar] [CrossRef]
- Woo, A.J.; Strohl, W.R.; Priestley, N.D. Nonactin Biosynthesis: The Product of NonS Catalyzes the Formation of the Furan Ring of Nonactic Acid. Antimicrob. Agents Chemother. 1999, 43, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Ishiyama, T.; Sasaki, H.; Ishikawa, M.; Sato, M.; Suzuki, A. Palladium-Catalyzed Inter- and Intramolecular Cross-Coupling Reactions of B-Alkyl-9-Borabicyclo [3.3.1] Nonane Derivatives with 1-Halo-1-Alkenes or Haloarenes. Syntheses of Functionalized Alkenes, Arenes, and Cycloalkenes via a Hydroboration-Coupling Sequence. J. Am. Chem. Soc. 1989, 111, 314–321. [Google Scholar] [CrossRef]
- Farina, V.; Krishnan, B. Large Rate Accelerations in the Stille Reaction with Tri-2-Furylphosphine and Triphenylarsine as Palladium Ligands: Mechanistic and Synthetic Implications. J. Am. Chem. Soc. 1991, 113, 9585–9595. [Google Scholar] [CrossRef]
- Chishiro, A.; Konishi, M.; Inaba, R.; Yumura, T.; Imoto, H.; Naka, K. Tertiary Arsine Ligands for the Stille Coupling Reaction. Dalton Trans. 2021, 51, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Carreira, E.M.; Du Bois, J. (+)-Zaragozic Acid C: Synthesis and Related Studies. J. Am. Chem. Soc. 1995, 117, 8106–8125. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zhang, H.; Tian, W.; Wu, L.; Wang, S.; Zheng, M.; Zhang, J.; Sun, C.; Deng, Z.; et al. Structural Basis of a Broadly Selective Acyltransferase from the Polyketide Synthase of Splenocin. Angew. Chem. 2018, 130, 5925–5929. [Google Scholar] [CrossRef]
- Campbell, N.E.; Sammis, G.M. Single-Electron/Pericyclic Cascade for the Synthesis of Dienes. Angew. Chem. Int. Ed. 2014, 53, 6228–6231. [Google Scholar] [CrossRef]
- Whittaker, A.M.; Lalic, G. Monophasic Catalytic System for the Selective Semireduction of Alkynes. Org. Lett. 2013, 15, 1112–1115. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Moloney, M.; Parsons, A.F. An intramolecular cobalt cyclisation for the construction of substituted pyrrolidines. Tetrahedron 1992, 48, 9373–9384. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).