Abstract
Cyanide ions are known to be lethal for insects and mammals and harmful for the environment, and new methods for their selective detection are in high demand. Herein, the mechanosynthesis of simple Schiff’s bases-based probes S1–S3 for visual detection of CN− anion is reported. These probes were obtained by means of a reaction between isomeric 4,4-, 3,3- and 2,2-diaminobiphenyls and 4-nitrobenzaldehyde under ball milling conditions. The probes showed high selectivity and sensitivity toward CN− anion via a dramatic “yellow-to-dark purple” color change with a detection limit of 26 × 103, 8.7 × 103 and 14 × 103 ppm for S1–S3, respectively. The proposed mechanism of the detection suggests the deprotonation of a proton from an imine moiety, followed by the formation of charge transfer complexes (CTC).
1. Introduction
CN− ion donors are heavily involved in a wide variety of industrial processes, for instance, for the manufacturing of synthetic fibers, resins, and herbicides in mining and metallurgical branches such as gold extraction and in galvanic processes, accompanied by potential environmental contamination [1,2]. In nature, cyanides are produced by cyanobacteria, certain fungi, and algae [3]. In the form of cyanogenic glycosides cyanides are presented as antifeedants in a number of plants and plant seeds, such as cassava, seeds of bitter almonds, apples, and apricots [4,5]. According to the World Health Organization (WHO, Geneva, Switzerland), the allowed level of cyanide in drinking water should not exceed 2 µg/L [6]. The high toxicity of cyanide is associated with its ability to inhibit the enzyme cytochrome-c oxidase, which prevents the transport of electrons from cytochrome-c to oxygen and interrupts the generation of aerobically produced ATP to cause histotoxic hypoxia [7].
Based on all of the above, there is a demand for analytical systems for CN− detection and/or monitoring at potential pollution sources for early warning of seepage of hazardous wastes. Nowadays, numerous methods and equipment have found an application in the purpose of analyzing soil, water, and air [8]. The instrumental methods have several drawbacks; for instance, they are bulky, expensive, require specific qualification, and are inappropriate for prompt analysis. However, colorimetric chemosensor tests are the most widespread because of their fast response, high sensitivity and selectivity, visual/naked-eye detection, ease of use, high selectivity, cheapness, and wide range of target analytes [9]. In addition to a large number of reports on chemical sensors that are able to detect cyanide by means of reversible binding to a receptor site to cause the generation of a visual signal via fluorescence or color change [10], many reports are published on the rational design of various probes that are able to detect cyanide via nucleophilic addition to a receptor site. Recently, many molecular probes for the visual cyanide detection were reported [11], including indoles [12] or bisindoles [13], 2-(trifluoroacetylamino)anthraquinone [14], cyanine dyes and related indoliminium NIR dyes [15], acyltriazene [16], squarane [17], acridinium salt [18], benzils [19], (het)aryl-substituted dienes [20,21], etc. Even though some of these chemosensors/probes could be very efficient and selective, their preparation requires multistep synthesis, which sometimes involves expensive metal catalysts or reagents and/or tedious methods for the purification.
(Aza) Schiff’s bases [22,23] provide different mechanisms for the cyanide detection. In the most common scenario, the deprotonation of (aza) Schiff’s base molecules [24,25,26] takes place to cause a dramatic color change. The mechanism involved hydrogen bonding formation followed by hydrogen abstraction by CN−. To ensure efficient hydrogen abstraction in most cases the strong hydrogen bond donors, such as hydroxy- or NH-moieties, as well as strong acceptors, such nitro-group(s) need to be introduced into the structure of Schiff’s bases [27,28,29,30,31]. In most cases, the above-mentioned Schiff’s bases could be prepared via multi-step synthesis by using organic solvents and column chromatography. In addition, the most probes exhibited an almost identical colorimetric response towards both CN− and F− ions.
In this manuscript, we wish to report a method for the preparation of Schiff’s bases, such as bis(4-nitrobenzylidene)-[1,1′-biphenyl]-4,4′-diamine S1, bis(4-nitrobenzylidene)-[1,1′-biphenyl]-3,3′-diamine S2, and bis(4-nitrobenzylidene)-[1,1′-biphenyl]-2,2′-diamine S3 (also available from several vendors) as simple highly selective colorimetric probes for the CN− anion, by using both traditional solvent-based and mechanochemical (under ball milling conditions) synthesis.
2. Materials and Methods
Unless otherwise indicated, all common reagents and solvents were used from commercial suppliers without further purification. Benzidine, 2-nitroaniline, 3-nitroaniline, Pd(OAc)2, Pd/C, 4-nitrobenzaldehyde were purchased from Sigma-Aldrich, Merck. Iodo-2-nitrobenzene (m.p. 48–49 °C [32]), and iodo-3-nitrobenzene [32], 3,3′-dinitro-1,1′-biphenyl [33], m.p. 178–180 °C, and corresponding [1,1′-biphenyl]-diamines B2 and B3 [34] were synthesized according to the reported procedures. Mechanochemical reactions were performed in the ball mill Retsch PM100 by using a 25 mL stainless steel jar with 4 stainless steel 10 mm-diameter milling balls at 500 rpm. TLC and column chromatography were carried out on SiO2. 1H NMR and 13C NMR spectra were recorded at room temperature at 400 and 100 MHz, respectively, on a Bruker DRX-400 spectrometer using CDCl3 or DMSO-d6 as the solvents. Hydrogen chemical shifts were referenced to the hydrogen resonance of the corresponding solvent (DMSO-d6, δ = 2.50 ppm, or CDCl3, δ = 7.26 ppm). Carbon chemical shifts were referenced to the carbon resonances of the solvent (CDCl3, δ = 77.16 ppm). Peaks were labeled as singlet (s), doublet (d), triplet (t), and multiplet (m). Melting points were measured by the Stuart SMP10 melting point apparatus. Mass spectra were recorded on a SHIMADZU GCMS-QP2010 Ultra with electronic ionization (EI). UV–Vis absorption spectra were recorded on a Shimadzu UV-1800 spectrophotometer by using quartz cells with a 1 cm path length at room temperature. For UV/Vis titration experiments, tetrabutylammonium cyanide (TBACN) and tetrabutylammonium hydroxide (TBAOH) purchased from Sigma-Aldrich were used.
2.1. General Methods for the Synthesis of Probes S1–S3
2.1.1. Method A
A mixture of corresponding diamino-biphenylene (450 mg, 1 mmol, 1 eq), 4-nitrobenzaldehyde (302 mg, 2 mmol, 2 eq), and 3 drops of acetic acid in methanol (10 mL) was stirred at 68 °C for 8 h. Then ethyl acetate (10 mL) and water (20 mL) were added to the reaction mixture, and the product was extracted. The organic layer was dried over sodium sulfate and concentrated under reduced pressure. Product yields for S1, S2, and S3 were 70.0%, 65.4%, and 60.3%, respectively.
2.1.2. Method B
The mixture of the corresponding diamino-biphenylene (225 mg, 0.5 mmol, 1 eq) and 4-nitrobenzaldehyde (151 mg, 1 mmol, 2 eq) and 3 drops of acetic acid was ball milled at 500 rpm for 1 h at room temperature. The reaction mixture was dissolved in ethyl acetate (20 mL) and washed with water (3 × 10 mL). The organic layer was dried over sodium sulfate, filtered, and concentrated under reduced pressure. Product yields for S1, S2, and S3 were 94.8%, 87.5%, and 76.7%, respectively.
N4,N4′-bis(4-nitrobenzylidene)biphenyl-4,4′-diamine S1: 1H NMR (DMSO-d6, 400 MHz) δ: 8.92 (s, 2 H, N=CH), 8.38–8.24 (m, 8 H, Ph-NO2), 7.85–7.50 (m, 8 H, biphenyl). IR (KBr, cm−1) ν: 1342 (-NO2), 1531 (C=C), 1683 (C=N). 13C NMR (DMSO-d6, 100 MHz) δ 158.72, 149.73, 148.86, 141.56, 137.97, 129.65, 127.38, 124.01, 122.05. MS (m/z, %): 450 (M+, 100), 434 (7.36), 433 (22.92), 402 (7.90), 356 (7.98), 317 (6.78), 254 (11.37), 241 (11.56), 225 (13.49), 178 (14.39), 152 (37.65), 127 (17.09), 113 (6.00), 77 (12.34), 65 (7.10), 51 (7.31), 39 (2.71). m.p. 246–248 °C. Yellow powder.
N3,N3′-bis(4-nitrobenzylidene)biphenyl-3,3′-diamine S2: 1H NMR (DMSO-d6, 400 MHz) δ: 8.95 (s, 2 H, N=CH), 8.39 (d, 3J = 8.0 Hz, 4 H, Ph-NO2), 8.24–8.20 (d, 3J = 8.0 Hz, 8 H, Ph-NO2), 7.73–7.69 (d, 4 H, biphenyl), 7.57 (m, 2 H, biphenyl), 7.39–7.37 (m, 2 H, biphenyl). 13C NMR (DMSO-d6, 100 MHz) δ 159.50, 151.26, 148.87, 141.49, 140.83, 129.85, 125.25, 123.99, 121.00, 119.19. MS (m/z, %): 450 (M+, 100), 434 (7.36), 433 (22.92), 402 (7.90), 356 (7.98), 317 (6.78), 254 (11.37), 241 (11.56), 225 (13.49), 178 (14.39), 152 (37.65), 127 (17.09), 113 (6.00), 77 (12.34), 65 (7.10), 51 (7.31), 39 (2.71). m.p. > 250 °C. Yellow powder.
N2,N2′-bis(4-nitrobenzylidene)biphenyl-2,2′-diamine S3: 1H NMR (DMSO-d6, 400 MHz) δ: 8.41(s, 2 H, N=CH), 8.21 (d, 3J = 8.0 Hz, 4 H, Ph-NO2), 7.64 (d, 3J = 8.0 Hz, 4 H, Ph-NO2), 7.51–7.37 (m, 6 H, biphenyl), 7.19 (d, 3J = 8.0 Hz, 2 H, biphenyl). 13C NMR (DMSO-d6, 100 MHz) δ 158.17, 149.79,148.60, 141.33, 134.35, 130.97, 129.09, 128.83, 126.24, 123.88, 118.08. MS (m/z, %): 450 (M+, 100), 434 (7.36), 433 (22.92), 402 (7.90), 356 (7.98), 317 (6.78), 254 (11.37), 241 (11.56), 225 (13.49), 178 (14.39), 152 (37.65), 127 (17.09), 113 (6.00), 77 (12.34), 65 (7.10), 51 (7.31), 39 (2.71). m.p. 227–229 °C. Yellow powder.
Limits of detection (LOD) for the CN− by the probes S1–S3 were calculated according to Equation (1) [35]:
Equation (1): The calculation of LOD, where σ is the standard deviation of the chromophore intensity in the absence of an analyte (TBACN) and k is the slope of the linear calibration curve.
3. Results and Discussion
Since the first report of H. Schiff [36], the most common way (a conventional method) for their preparation [37] is the acid-catalyzed reaction of nucleophilic addition of aliphatic or aromatic amines to carbonyl compounds in solution upon heating to result in hemiaminals, followed by dehydration. Compared to the conventional methods, the ball milling under solvent-free conditions for the Schiff’s base preparation looks more attractive due to the shorter reaction times [38,39].
It should be noted that the synthesis of N4,N4′-bis(4-nitrobenzylidene)biphenyl-4,4′-diamine S1 was reported previously [40,41], however, with much prolonged reaction time (48 h) or in absolute ethanol with comparable reaction yields.
Based on the above, to prepare the probes S1–S3, we used solvent-free conditions under ball milling as the most preferable method. The probes S1–S3 were synthesized by means of the reaction between the corresponding isomeric 4,4- (B1), 3,3- (B2), and 2,2- (B3) diamino-biphenyls and 4-nitrobenzaldehyde (Scheme 1).
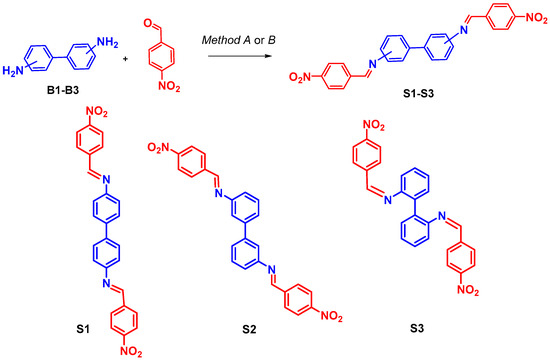
Scheme 1.
Reagents and conditions: Method A: MeOH, TFA (3 drops), reflux, 12 h, up to 70%; Method B: ball-milling, TFA (3 drops), 500 rpm, 1 h, up to 95%.
For comparison, we carried out the same reaction in methanol under reflux. As we expected, the ball milling conditions proved to be more efficient in many respects, such as reaction time (1 h), reaction yield (up to 95%), reduced solvent consumption (only for purification), and room temperature conditions, while for the solvent-based reaction (in our case) a more prolonged reaction time (8 h) was required and much lower reaction yields (70%) were observed.
The structures of the products S1–S3 were confirmed by 1H, 13C NMR, and IR-spectroscopy (see Supporting Information for details).
As a next step, the visual response of the probes S1–S3 to CN− in solution was studied. Thus, in a DMSO solution of S1–S3 (1.1 × 10−3 M) upon the addition of tetrabutylammonium cyanide (TBACN, 1.4 × 10−2 M) a dramatic color change from yellow to orange-purple was observed (Figure 1). Depending on the structure of S1–S3, the colors varied from dark orange (S1) to dark purple (S3). The plausible mechanism for this interaction is presented below (Figure 2). Thus, the interaction involves the initial partial deprotonation of the CH=N moiety of the probes S1–S3 by the CN− ion. This deprotonation was observed by means of a 1H NMR experiment in the case of S3 (Figure S30 ESI†). Thus, in DMSO-d6, the addition of 3.0 eq. of TBACN results in the complete disappearance of the proton signal of CH=N moiety at 8.45 ppm, which confirms the deprotonation of this moiety. Most probably, the presence of strong electron-withdrawing NO2 group in the para-position of an aromatic moiety in the structure of S1–S3 polarizes strongly the imine bond, which makes this imine proton more acidic. After that, the formation of deeply colored charge-transfer complexes with another molecule of S1–S3 takes place. This concept was confirmed by a similar color change after the addition of 0–1.35 μM of tetrabutylammonium hydroxide (TBAOH) as a strong base in DMSO as well as UV-titration studies (Figures S31–S34 ESI†).

Figure 1.
Visible detection of CN− ion (1.4 × 10−2 M) by the probes S1–S3 (1.1 × 10−3 M).
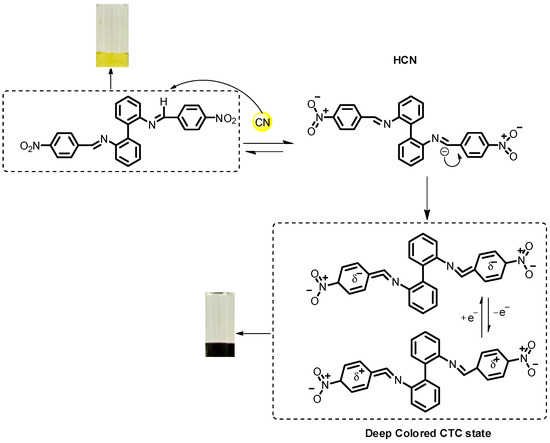
Figure 2.
Plausible mechanism for the CN− ion detection by probes S1–S3.
To gain more evidence, the photophysical studies of S1–S3 were carried out. Thus, in UV spectra of DMSO solution, probe S1 (22µM) exhibits an absorption band with a maximum at 295 nm, and the addition of TBACN in concentrations 0–1.35 mM results in the appearance of a new absorption band at 575 nm, which can be attributed to the formation of the charge-transfer complex (Figure S35 ESI†). In the case of probe S2, one absorption peak at 275 nm was observed, and a new growing peak at 475 nm was detected upon the addition of TBACN (Figure S36 ESI†). For the probe S3, one absorption peak at 295 nm was observed, and a new peak at 555 nm was observed upon the addition of TBACN (Figure 3a). For the most representative probe, S3, we attempted to carry out Job plot experiment in order to determine the stoichiometry of the complex and the binding mode between S3 and the CN- ion. However, the increasing hypochromic shift with the increasing concentration of the TBACN never reached a steady state, and the mathematical representation (A = ex) where A—absorbance; X—concentration (see Figure S37 ESI†) can be seen as an exponential dependence. The observed result corresponds to the literature data [42,43,44,45,46] according to which the job plot method could not be fully validated in the case of supramolecular assemblies with low to moderate association constants. In addition, for the probes S1–S3, the CN- detection limit (LOD) was calculated according to Equation (1), which was found to be 26 × 103, 8.7 × 103 and 14 × 103 ppm, respectively.
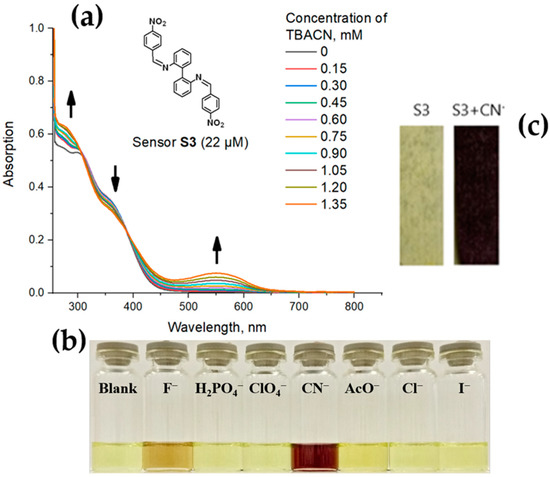
Figure 3.
(a) UV-titration of S3 (22 μM) with TBACN (0–1.35 mM) in DMSO; (b) The selective response of S3 (1 × 10−3 M) to CN−3 vs. other anions (1 × 10−3 M); (c) S3-impregnated test stripe before (left) and after (right) the treatment with CN−3 ion-containing solution.
It is worth mentioning that the addition of other anions, such as Cl−, HPO4−, I−, ClO4−, CH3CO2−, and even F−, to the solution of the most representative probe S3 in DMSO results in no color change or only subtle color changes (Figure 3b), while the presence of CN− can be easily distinguished by the naked eye due to the dramatic color change, which is detectable in the UV/Vis scale. All of the above-mentioned confirms the high selectivity of S3 toward the CN− anion among other anions.
As a final step, to explore the potential application of the herein reported probes for environmental monitoring, the test strips for the CN− ion detection were prepared by means of impregnation of filter paper with the solution of the most representative probe S3 in DMSO (0.014 M) with the following air-drying. As a result, the S3-impregnated test strips demonstrated the selectivity and sensitivity to CN− (14 mM) ions via a dramatic color change (Figure 3c).
4. Conclusions
In summary, simple Schiff’s base probes S1–S3 for the detection of cyanide ions were prepared by using a reaction between isomeric 4,4-, 3,3-, and 2,2-diaminobiphenyls and 4-nitrobenzaldehyde under ball milling conditions. The obtained probes demonstrated a selective visual response to the CN- ion over other anions in a solution of DMSO via a dramatic color change with a detection limit of 26 × 103, 8.7 × 103 and 14 × 103 ppm for S1–S3, respectively. The plausible mechanism of the reaction suggests a deprotonation of CH=N moieties of S1–S3 with the following formation of a deeply colored charge transfer complex with another molecule of S1–S3, which is detectable by means of 1H NMR and UV/vis-methods. To confirm the practical application of the obtained probes for the detection of CN−, S3-impregnated test strips were prepared, which demonstrated the selective detection of CN− via a dramatic color change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5020066/s1, Figures S1–S30: 1H and 13C NMR spectra, mass spectra, IR spectra of the chemosensors S1–S3 and the corresponding precursors, Figures S31–S33: UV-Vis spectra of chemosensors S1–S3 with various concentration of TBAOH, Figure S34: a photo of chemosensor S1 with and without TBAOH, Figures S35 and S36: UV-Vis spectrum of S1 and S2 with various concentration of TBACN, Figure S37: Relationship between the absorption of S3 and the concentration of TBACN.
Author Contributions
Conceptualization, G.V.Z. and B.C.R.; methodology, W.K.A.A.-I., I.L.N., G.V.Z., D.S.K., I.S.K., V.A.P., S.S. and A.F.K.; investigation, W.K.A.A.-I., G.V.Z., I.L.N., D.S.K., I.S.K., V.A.P., S.S. and A.F.K.; data curation, W.K.A.A.-I., I.L.N., D.S.K., I.S.K., S.S. and A.F.K.; writing—original draft preparation, G.V.Z., W.K.A.A.-I., I.L.N., D.S.K., I.S.K., S.S., A.F.K. and B.C.R.; writing—review and editing, G.V.Z., W.K.A.A.-I., I.L.N., D.S.K., I.S.K., S.S., A.F.K. and B.C.R.; supervision, G.V.Z. and B.C.R.; project administration, G.V.Z. and B.C.R.; funding acquisition, G.V.Z. and B.C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation (Agreement # 075-15-2022-1118 accessed on 29 June 2022).
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors are grateful for the collaboration of who provided financial support to the students involved in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dash, R.R.; Gaur, A.; Balomajumde, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mat. 2009, 163, 1–11. [Google Scholar] [CrossRef]
- Jaszczak, E.; Polkowska, Z.; Narkowicz, S.; Namieśnik, J. Cyanides in the environment-analysis-problems and challenges. Environ. Sci. Pollut. Res. Int. 2017, 24, 15929–15948. [Google Scholar] [PubMed]
- Stewart, I.; Webb, P.M.; Schluter, P.J.; Shaw, G.R. Recreational and occupational field exposure to freshwater cyanobacteria—A review of anecdotal and case reports, epidemiological studies and the challenges for epidemiologic assessment. Environ. Health 2006, 5, 6. [Google Scholar] [CrossRef]
- Vetter, J. Plant cyanogenic glycosides. Toxicon 2000, 38, 11–36. [Google Scholar] [CrossRef]
- Ballhorn, D.J. Cyanogenic glycosides in nuts and seeds. In Nuts & Seeds in Health and Disease Prevention, 1st ed.; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: London, UK, 2011; pp. 129–136. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality; WHO: Geneva, Switzerland, 1996; Volume 2, pp. 6–8. [Google Scholar]
- Biller, J. Chapter 163. In Interface of Neurology and Internal Medicine (Illustrated Ed.); Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; p. 939. ISBN 978-0-7817-7906-7. [Google Scholar]
- Ma, J.; Dasgupta, P.K. Recent developments in cyanide detection: A review. Anal. Chim. Acta 2010, 673, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Anslyn, E.V. (Eds.) Chemosensors: Principles, Strategies, and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Kima, H.N.; Yoon, J. Sensors for the optical detection of cyanide ion. Chem. Soc. Rev. 2010, 39, 127–137. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Cyanide-sensitive fluorescent probes. Dye. Pigment. 2005, 64, 49–55. [Google Scholar] [CrossRef]
- Yin, C.; Huo, F.; Xu, M.; Barnes, C.L.; Glass, T.E. A NIR, special recognition on HS−/CN−colorimetric and fluorescent imaging material for endogenous H2S based on nucleophilic addition. Sens. Actuators B Chem. 2017, 252, 592–599. [Google Scholar] [CrossRef]
- Kumari, N.; Jha, S.; Bhattacharya, S. An efficient probe for rapid detection of cyanide in water at parts per billion levels and naked-eye detection of endogenous cyanide. Chem. Asian J. 2014, 9, 830–837. [Google Scholar] [CrossRef]
- Niu, H.T.; Su, D.; Jiang, X.; Yang, W.; Yin, Z.; He, J.; Cheng, J.P. A simple yet highly selective colorimetric sensor for cyanide anion in an aqueous environment. Org. Biomol. Chem. 2008, 6, 3038–3040. [Google Scholar] [CrossRef]
- Barare, B.; Babahan, I.; Hijji, Y.M.; Bonyi, E.; Tadesse, S.; Aslan, K. A highly selective sensor for cyanide in organic media and on solid surfaces. Sensors 2016, 16, 271. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, W.; Hui, Y.; Sun, X.; Xu, L.; Feng, L.; Xie, Z. A new highly selective fluorescent turn-on chemosensor for cyanide anion. Talanta 2015, 137, 38–42. [Google Scholar] [CrossRef]
- Chung, S.Y.; Nam, S.W.; Lim, J.; Park, S.; Yoon, J. A highly selective cyanide sensing in water via fluorescence change and its application to in vivo imaging. Chem. Commun. 2009, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-K.; Tae, J. Acridinium salt based fluorescent and colorimetric chemosensor for the detection of cyanide in water. Org. Lett. 2006, 8, 5721–5723. [Google Scholar] [CrossRef]
- Sessler, J.L.; Cho, D.-G. The benzil rearrangement reaction: Trapping of a hitherto minor product and its application to the development of a selective cyanide anion indicator. Org. Lett. 2008, 10, 73–75. [Google Scholar] [CrossRef]
- Li, H.; Chen, T.; Jin, L.; Kan, Y.; Yin, B. Colorimetric and fluorometric dual-modal probes for cyanide detection based on the doubly activated Michael acceptor and their bioimaging applications. Anal. Chim. Acta 2014, 852, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, C.; Kumar, P.S.; Shanmugapriya, R.; Satheeshkumar, A.; Vennila, K.N.; Elango, K.P. A multi-site probe for selective detection of cyanide and sulphite ions via different mechanisms with concomitant different fluorescent behaviors. Results Chem. 2022, 4, 100312. [Google Scholar] [CrossRef]
- Junaid, H.M.; Batool, M.; Harun, F.W.; Akhter, M.S.; Shabbir, N. Naked Eye Chemosensing of Anions by Schiff Bases. Crit. Rev. Anal. Chem. 2022, 52, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Moon, J.H.; Swamy, K.M.K.; Jeong, Y.; Kim, G.; Choi, J.; Lee, J.Y.; Yoon, J. A new bis-pyrene derivative as a selective colorimetric and fluorescent chemosensor for cyanide and fluoride and anion-activated CO2 sensing. Sens. Actuators B Chem. 2014, 199, 369–376. [Google Scholar] [CrossRef]
- Orojloo, M.; Amani, S. Naked-eye detection of cyanide ions in aqueous media based on an azo-azomethine chemosensor. Comptes Rendus Chim. 2017, 20, 415–423. [Google Scholar] [CrossRef]
- Dey, S.; Sen, C.; Sinha, C. Chromogenic Hydrazide Schiff Base Reagent: Spectrophotometric Determination of CN-Ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 225, 117471. [Google Scholar] [CrossRef]
- Kodlady, S.N.; Narayana, B.; Sarojini, B.K.; Gauthama, B.U. Aromatic aldehyde based chemosensors for fluoride and cyanide detection in organic and aqueous media: As certained by characterization, spectroscopic and DFT studies. Inorg. Chim. Acta 2019, 494, 245–255. [Google Scholar] [CrossRef]
- Elsafy, A.G.; Al-Easa, H.S.; Hijji, Y.M. Substituted 2-aminobenzothiazoles salicylidenes synthesis and characterization as cyanide sensors in aqueous medium. Sensors 2018, 18, 2219. [Google Scholar] [CrossRef]
- Yildirim, N.; Yildiz, M. A Schiff base sensor selective to anions, biological activity and spectral studies. J. Turk. Chem. Soc. Sec. A Chem. 2018, 5, 1271–1278. [Google Scholar] [CrossRef]
- Dini, S.; Khanmohammadi, H. A new azo-azomethine sensor for detection of CN- and AcO- anions: Highly selective chemosensor for naked eye detection of sodium diclofenac. Spectrochim. Acta Part A 2019, 222, 117157. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Guo, Y.; Xu, J.; Shao, S. Novel indole based colorimetric and “turn on” fluorescent sensors for biologically important fluoride anion sensing. J. Photochem. Photobiol. B Biol. 2011, 103, 140–144. [Google Scholar] [CrossRef]
- Moghadam, F.N.; Amirnasr, M.; Meghdadi, S.; Eskandari, K.; Buchholz, A.; Plass, W. A new fluorene derived schiff-base as a dual selective fluorescent probe for Cu2+ and CN−. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 6–15. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Dong, C.; Nakamura, K.; Taniguchi, T.; Mita, S.; Kodama, S.; Kawaguchi, S.; Nomoto, A.; Ogawa, A.; Mizuno, T. Synthesis of Aryl Iodides from Arylhydrazines and Iodine. ACS Omega 2018, 3, 9814–9821. [Google Scholar] [CrossRef]
- Roy, P.-P.; D’Souza, K.; Cuperlovic-Culf, M.; Kienesberger, P.C.; Touaibia, M. New Atglistatin Closely Related Analogues: Synthesis and Structure-Activity Relationship towards Adipose Triglyceride Lipase Inhibition. Eur. J. Med. Chem. 2016, 118, 290–298. [Google Scholar] [CrossRef]
- Wang, L.; Lu, W. Preparation of Unsymmetrical Biaryls by Pd(II)-Catalyzed Cross-Coupling of Aryl Iodides. Org. Lett. 2009, 11, 1079–1082. [Google Scholar] [CrossRef] [PubMed]
- Schiff, H. Mittheilungen aus dem Universitäts-laboratorium in Pisa: 2. Eine neue Reihe organischer Basen. Ann. Chem. Pharm. 1864, 131, 118–119. [Google Scholar] [CrossRef]
- Qin, W.; Long, S.; Panunzio, M.; Biondi, S. Schiff bases: A short survey on an evergreen chemistry tool. Molecules 2013, 18, 12264–12289. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.A.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- El-Sayed, T.H.; Aboelnaga, A.; Hagar, M. Ball milling assisted solvent and catalyst free synthesis of benzimidazoles and their derivatives. Molecules 2016, 21, 1111. [Google Scholar] [CrossRef]
- Iqbal, A.; Siddiqui, H.L.; Ashraf, C.M.; Bukhari, M.H.; Akram, C.M. Synthesis, Spectroscopic and Cytotoxic Studies of Biologically Active New Schiff Bases Derived from p-Nitrobenzaldehyde. Chem Pharm. Bull. 2007, 55, 1070–1072. [Google Scholar] [CrossRef]
- Eshghi, H.; Rahimizadeh, M.; Eshkil, F.; Hosseini, M.; Bakavoli, M.; Sanei-Ahmadabad, M. Synthesis of novel bis(β-aminocarbonyl) compounds and some β-aminocarbonyls by catalyst-free multicomponent Mannich reactions. J. Iran. Chem. Soc. 2014, 11, 685–692. [Google Scholar] [CrossRef]
- Ingham, K.C. On the application of Job’s method of continuous variation to the stoichiometry of protein-ligand complexes. Anal. Biochem. 1975, 68, 660–663. [Google Scholar] [CrossRef]
- Gil, V.M.S.; Oliveira, N.C. On the use of the method of continuous variations. J. Chem. Educ. 1990, 67, 473. [Google Scholar] [CrossRef]
- Landy, D.; Tetart, F.; Truant, E.; Blach, P.; Fourmentin, S.; Surpateanu, G. Development of a competitive continuous variation plot for the determination of inclusion compounds stoichiometry. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 409–413. [Google Scholar] [CrossRef]
- Olson, E.J.; Bühlmann, P. Getting more out of a job plot: Determination of reactant to product stoichiometry in cases of displacement reactions and n:n complex formation. J. Org. Chem. 2011, 76, 8406–8412. [Google Scholar] [CrossRef] [PubMed]
- Ulatowski, F.; Dąbrowa, K.; Bałakier, T.; Jurczak, J. Recognizing the limited applicability of job plots in studying host–guest interactions in supramolecular chemistry. J. Org. Chem. 2016, 81, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).