Room Temperature Surfactant-Free Synthesis of Gold Nanoparticles in Alkaline Ethylene Glycol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
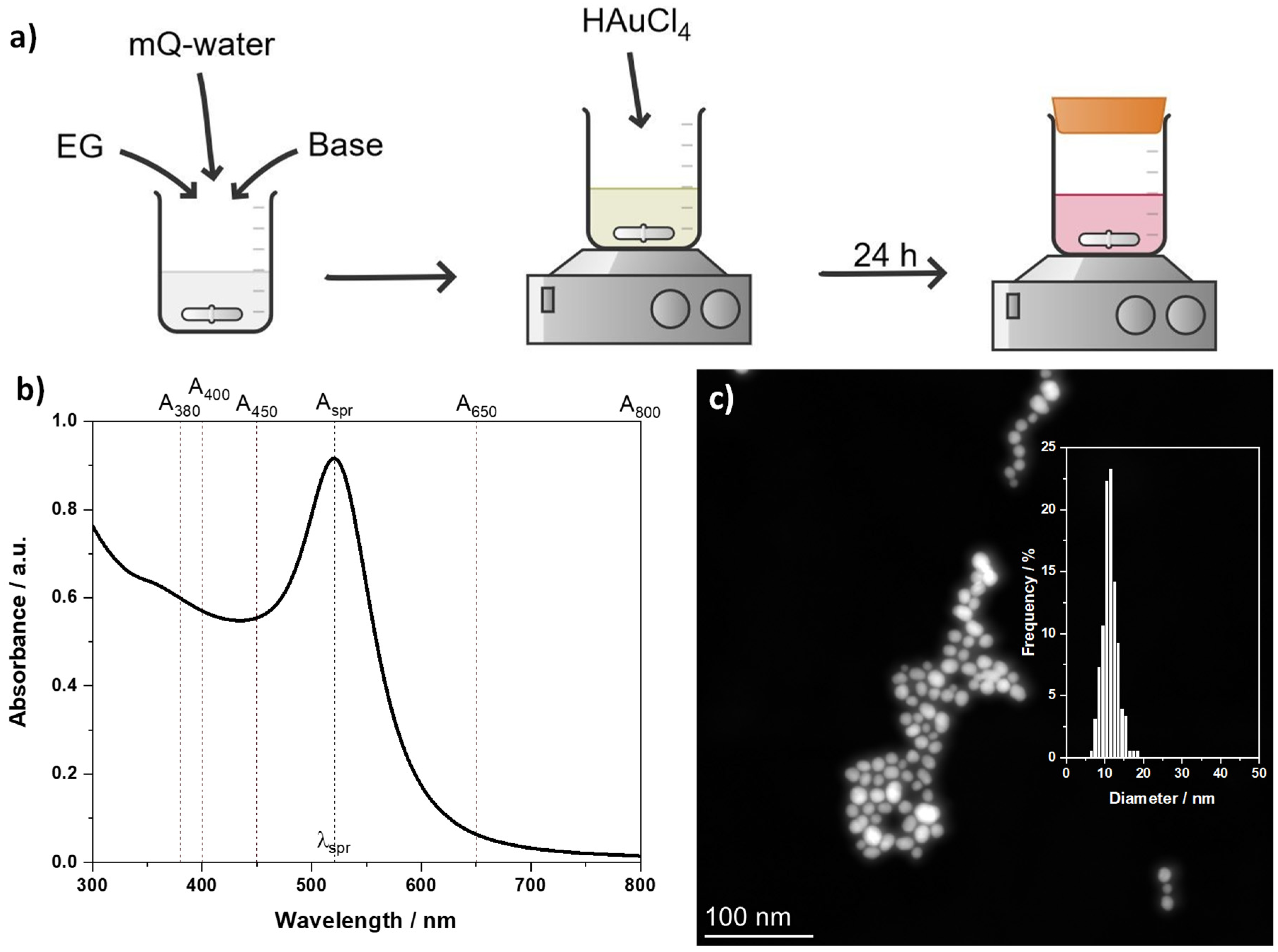
2.2. Synthesis of Au NPs
2.3. Characterization
2.3.1. UV-vis Spectroscopy
2.3.2. Scanning Transmission Electron Microscopy (STEM)
3. Results
3.1. Ethylene Glycol as Reducing Agent for Au NP Synthesis
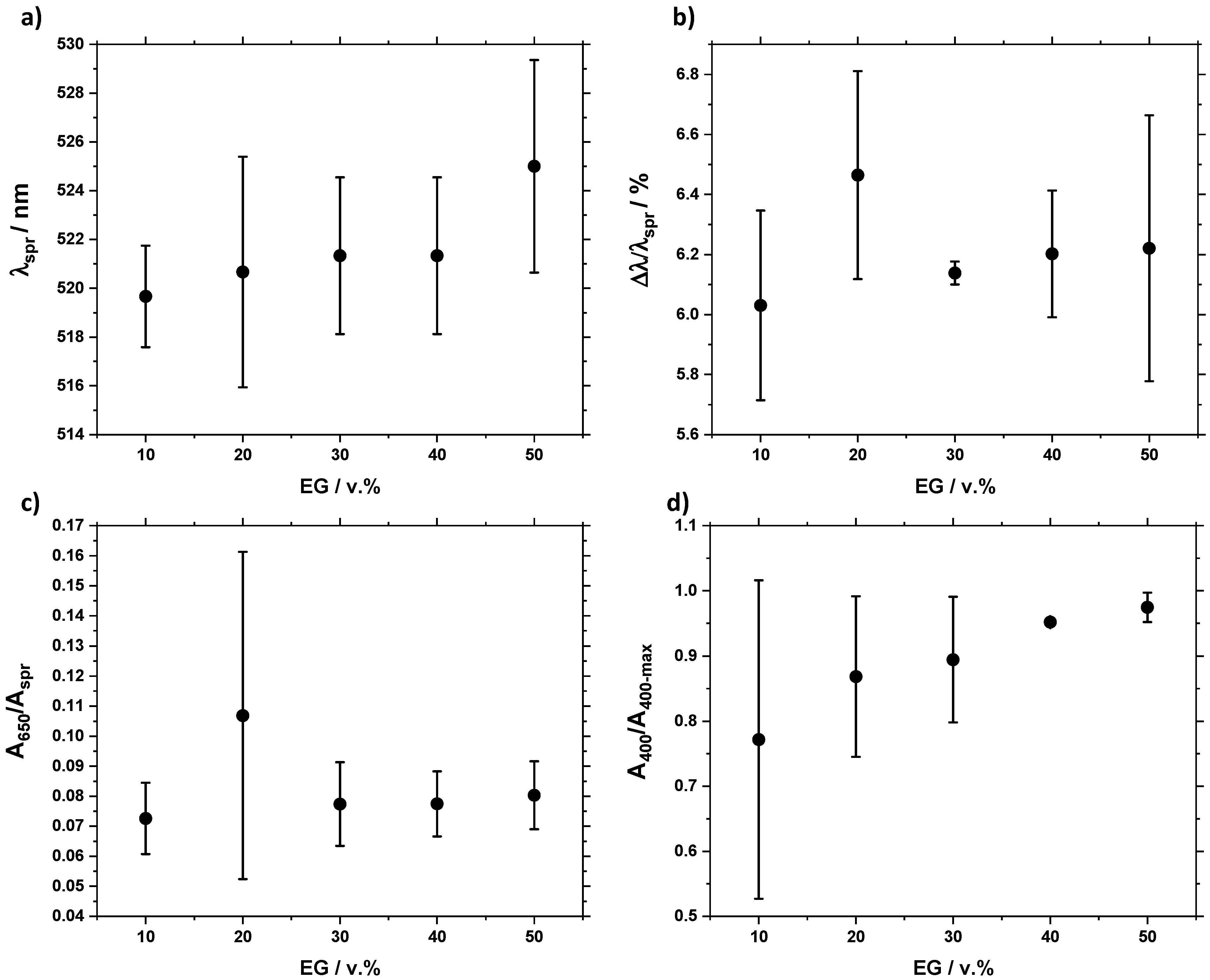
3.2. Influence of the Base/HAuCl4 Ratio and EG Content
4. Discussion
4.1. Trends and Optimal Synthesis Conditions
4.2. Reproducibility
4.3. Long Term Stability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alshammari, A.S. Heterogeneous Gold Catalysis: From Discovery to Applications. Catalysts 2019, 9, 402. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Condens. Matter Phys. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.W.; Yong, K.T.; Roy, I.; Dinh, X.Q.; Yu, X.; Luan, F. A Review on Functionalized Gold Nanoparticles for Biosensing Applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Maccora, D.; Dini, V.; Battocchio, C.; Fratoddi, I.; Cartoni, A.; Rotili, D.; Castagnola, M.; Faccini, R.; Bruno, I.; Scotognella, T.; et al. Gold Nanoparticles and Nanorods in Nuclear Medicine: A Mini Review. Appl. Sci. 2019, 9, 3232. [Google Scholar] [CrossRef]
- De Souza, C.D.; Nogueira, B.R.; Rostelato, M. Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J. Alloys Compd. 2019, 798, 714–740. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Wuithschick, M.; Birnbaum, A.; Witte, S.; Sztucki, M.; Vainio, U.; Pinna, N.; Rademann, K.; Emmerling, F.; Kraehnert, R.; Polte, J. Turkevich in New Robes: Key Questions Answered for the Most Common Gold Nanoparticle Synthesis. ACS Nano 2015, 9, 7052–7071. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatized gold nanoparticles in a 2-phase liquid-liquid system. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Duan, H.H.; Wang, D.S.; Li, Y.D. Green chemistry for nanoparticle synthesis. Chem. Soc. Rev. 2015, 44, 5778–5792. [Google Scholar] [CrossRef]
- Gilbertson, L.M.; Zimmerman, J.B.; Plata, D.L.; Hutchison, J.E.; Anastas, P.T. Designing nanomaterials to maximize performance and minimize undesirable implications guided by the Principles of Green Chemistry. Chem. Soc. Rev. 2015, 44, 5758–5777. [Google Scholar] [CrossRef]
- Hutchison, J.E. The Road to Sustainable Nanotechnology: Challenges, Progress and Opportunities. ACS Sustain. Chem. Eng. 2016, 4, 5907–5914. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Olugbeko, S.C.; Folorunso, A.S. A review on synthesis, optimization, characterization and antibacterial application of gold nanoparticles synthesized from plants. Int. Nano Lett. 2020, 10, 237–248. [Google Scholar] [CrossRef]
- Khan, T.; Ullah, N.; Khan, M.A.; Mashwani, Z.U.R.; Nadhman, A. Plant-based gold nanoparticles; a comprehensive review of the decade-long research on synthesis, mechanistic aspects and diverse applications. Adv. Colloid Interface Sci. 2019, 272, 102017. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Annu; Ikram, S.; Yudha, S.S. Biosynthesis of gold nanoparticles: A green approach. J. Photochem. Photobiol. B Biol. 2016, 161, 141–153. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M.; Kagan, C.R.; Millstone, J.E. Reproducibility in Nanocrystal Synthesis? Watch Out for Impurities! ACS Nano 2020, 14, 6359–6361. [Google Scholar] [CrossRef]
- Fievet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Chahdoura, F.; Favier, I.; Gomez, M. Glycerol as Suitable Solvent for the Synthesis of Metallic Species and Catalysis. Chem. Eur. J. 2014, 20, 10884–10893. [Google Scholar] [CrossRef]
- Garcia, A.G.; Lopes, P.P.; Gomes, J.F.; Pires, C.; Ferreira, E.B.; Lucena, R.G.M.; Gasparotto, L.H.S.; Tremiliosi-Filho, G. Eco-friendly synthesis of bimetallic AuAg nanoparticles. New J. Chem. 2014, 38, 2865–2873. [Google Scholar] [CrossRef]
- Parveen, R.; Tremiliosi, G. A step ahead towards the green synthesis of monodisperse gold nanoparticles: The use of crude glycerol as a greener and low-cost reducing agent. RSC Adv. 2016, 6, 95210–95219. [Google Scholar] [CrossRef]
- Gasparotto, L.H.S.; Garcia, A.C.; Gomes, J.F.; Tremiliosi-Filho, G. Electrocatalytic performance of environmentally friendly synthesized gold nanoparticles towards the borohydride electro-oxidation reaction. J. Power Sources 2012, 218, 73–78. [Google Scholar] [CrossRef]
- Gomes, J.F.; Garcia, A.C.; Ferreira, E.B.; Pires, C.; Oliveira, V.L.; Tremiliosi-Filho, G.; Gasparotto, L.H.S. New insights into the formation mechanism of Ag, Au and AgAu nanoparticles in aqueous alkaline media: Alkoxides from alcohols, aldehydes and ketones as universal reducing agents. Phys. Chem. Chem. Phys. 2015, 17, 21683–21693. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Parveen, R.; Ullah, S.; Sgarbi, R.; Tremiliosi, G. One-pot ligand-free synthesis of gold nanoparticles: The role of glycerol as reducing-cum-stabilizing agent. Colloids Surf. A Physicochem. Eng. Asp. 2019, 565, 162–171. [Google Scholar] [CrossRef]
- Quinson, J. Surfactant-free precious metal colloidal nanoparticles for catalysis. Front. Nanotechnol. 2021, 3, 770281. [Google Scholar] [CrossRef]
- Arminio Ravelo, J.A.; Quinson, J.; Pedersen, M.A.; Kirkensgaard, J.J.K.; Arenz, M.; Escudero Escribano, M. Synthesis of iridium nanocatalysts for water oxidation in acid: Effect of the surfactant. ChemCatChem 2020, 12, 1282–1287. [Google Scholar] [CrossRef]
- Niu, Z.Q.; Li, Y.D. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Ferreira, A.G.M.; Egas, A.P.V.; Fonseca, I.M.A.; Costa, A.C.; Abreu, D.C.; Lobo, L.Q. The viscosity of glycerol. J. Chem. Thermodyn. 2017, 113, 162–182. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J.W.; Deng, K.; Gui, L.L.; Tang, Y.Q. Preparation of tractable platinum, rhodium, and ruthenium nanoclusters with small particle size in organic media. Chem. Mater. 2000, 12, 1622–1627. [Google Scholar] [CrossRef]
- Bonet, F.; Delmas, V.; Grugeon, S.; Urbina, R.H.; Silvert, P.Y.; Tekaia-Elhsissen, K. Synthesis of monodisperse Au, Pt, Pd, Ru and Ir nanoparticles in ethylene glycol. Nanostruct. Mater. 1999, 11, 1277–1284. [Google Scholar] [CrossRef]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Kuhn, L.T.; Kirkensgaard, J.J.K.; Jensen, K.M.O.; Oezaslan, M.; et al. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Neumann, S.; Grotheer, S.; Tielke, J.; Schrader, I.; Quinson, J.; Zana, A.; Oezaslan, M.; Arenz, M.; Kunz, S. Nanoparticles in a box: A concept to isolate, store and re-use colloidal surfactant-free precious metal nanoparticles. J. Mater. Chem. A 2017, 5, 6140–6145. [Google Scholar] [CrossRef]
- Jerome, F.S.; Tseng, J.T.; Fan, L.T. Viscosities of aqueous glycol solutions. J. Chem. Eng. Data 1968, 13, 496. [Google Scholar] [CrossRef]
- Quinson, J.; Kunz, S.; Arenz, M. Beyond Active Site Design: A Surfactant-Free Toolbox Approach for Optimized Supported Nanoparticle Catalysts. ChemCatChem 2021, 13, 1692–1705. [Google Scholar] [CrossRef]
- Nalawade, P.; Mukherjee, T.; Kapoor, S. Green Synthesis of Gold Nanoparticles Using Glycerol as a Reducing Agent. Adv. Nanopart. 2013, 2, 76–86. [Google Scholar] [CrossRef]
- Inaba, M.; Zana, A.; Quinson, J.; Bizzotto, F.; Dosche, C.; Dworzak, A.; Oezaslan, M.; Simonsen, S.B.; Kuhn, L.T.; Arenz, M. The Oxygen Reduction Reaction on Pt: Why Particle Size and Interparticle Distance Matter. ACS Catal. 2021, 11, 7144–7153. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Hendel, T.; Wuithschick, M.; Kettemann, F.; Birnbaum, A.; Rademann, K.; Polte, J. In Situ Determination of Colloidal Gold Concentrations with UV-vis Spectroscopy: Limitations and Perspectives. Anal. Chem. 2014, 86, 11115–11124. [Google Scholar] [CrossRef]
- Larm, N.E.; Essner, J.B.; Thon, J.A.; Bhawawet, N.; Adhikari, L.; St Angelo, S.K.; Baker, G.A. Single Laboratory Experiment Integrating the Synthesis, Optical Characterization, and Nanocatalytic Assessment of Gold Nanoparticles. J. Chem. Educ. 2020, 97, 1454–1459. [Google Scholar] [CrossRef]
- Ye, Y.J.; Lv, M.X.; Zhang, X.Y.; Zhang, Y.X. Colorimetric determination of copper(II) ions using gold nanoparticles as a probe. RSC Adv. 2015, 5, 102311–102317. [Google Scholar] [CrossRef]
- Agarwal, S.; Mishra, P.; Shivange, G.; Kodipelli, N.; Moros, M.; de la Fuente, J.M.; Anindya, R. Citrate-capped gold nanoparticles for the label-free detection of ubiquitin C-terminal hydrolase-1. Analyst 2015, 140, 1166–1173. [Google Scholar] [CrossRef]
- Merk, V.; Rehbock, C.; Becker, F.; Hagemann, U.; Nienhaus, H.; Barcikowski, S. In Situ Non-DLVO Stabilization of Surfactant-Free, Plasmonic Gold Nanoparticles: Effect of Hofmeister’s Anions. Langmuir 2014, 30, 4213–4222. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Siiman, O.; Hsu, W.P. Surface-enhanced Raman-scaterring (SERS) enhancements and excitation profiles for 3,5-pyridinedicarboxylate and dabsyl aspartate on colloidal gold. J. Chem. Soc. Faraday Trans. I 1986, 82, 851–867. [Google Scholar] [CrossRef]
- Quinson, J.; Aalling-Frederiksen, O.; Dacayan, W.L.; Bjerregaard, J.D.; Jensen, K.D.; Jørgensen, M.R.V.; Kantor, I.; Sørensen, D.R.; Theil Kuhn, L.; Johnson, M.S.; et al. Surfactant-free colloidal syntheses of gold-based nanomaterials in alkaline water and mono-alcohol mixtures. Chem. Mater. 2023, in press. [Google Scholar] [CrossRef]
- El Amri, N.; Roger, K. Polyvinylpyrrolidone (PVP) impurities drastically impact the outcome of nanoparticle syntheses. J. Colloid Interface Sci. 2020, 576, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Bucher, J.; Simonsen, S.B.; Kuhn, L.T.; Kunz, S.; Arenz, M. Monovalent Alkali Cations: Simple and Eco-Friendly Stabilizers for Surfactant-Free Precious Metal Nanoparticle Colloids. ACS Sustain. Chem. Eng. 2019, 7, 13680–13686. [Google Scholar] [CrossRef]
- Strmcnik, D.; Kodama, K.; van der Vliet, D.; Greeley, J.; Stamenkovic, V.R.; Markovic, N.M. The role of non-covalent interactions in electrocatalytic fuel-cell reactions on platinum. Nat. Chem. 2009, 1, 466–472. [Google Scholar] [CrossRef]


| EG v.% in Water | LiOH/HAuCl4 Molar Ratio |
|---|---|
| 10 | 2.5, 3.0, 3.5, 4.0, 4.5, 6.0, 9.0, 12.0, 15.0 |
| 20 | 2.5, 3.0, 3.5, 4.0, 4.5, 6.0, 9.0, 12.0, 15.0 |
| 30 | 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 9.0, 12.0, 15.0 |
| 40 | 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 9.0, 12.0, 15.0 |
| 50 | 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 9.0, 12.0, 15.0 |
| Values | Property | Indicates |
|---|---|---|
| λspr | spr | Size (lower values correspond to smaller sizes) |
| Δλ/λspr | Broadness of the peak at 90% of Aspr | Size distribution (higher values correspond to larger size distributions) |
| Aspr/A450 | Ratio of absorbances at λspr and 450 nm | Size (lower values correspond to smaller sizes) |
| A650/Aspr | Ratio of absorbances at 650 nm and λspr | Aggregation (lower values correspond to less aggregated samples) |
| A380/A800 | Ratio of absorbances at 380 and 800 nm | Stability (higher values correspond to more stable colloids) |
| A400 | Absorbance at 400 nm | Relative yields |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasmussen, D.R.; Nielsen, M.F.; Quinson, J. Room Temperature Surfactant-Free Synthesis of Gold Nanoparticles in Alkaline Ethylene Glycol. Chemistry 2023, 5, 900-911. https://doi.org/10.3390/chemistry5020061
Rasmussen DR, Nielsen MF, Quinson J. Room Temperature Surfactant-Free Synthesis of Gold Nanoparticles in Alkaline Ethylene Glycol. Chemistry. 2023; 5(2):900-911. https://doi.org/10.3390/chemistry5020061
Chicago/Turabian StyleRasmussen, Ditte Røjkjær, Malthe Friis Nielsen, and Jonathan Quinson. 2023. "Room Temperature Surfactant-Free Synthesis of Gold Nanoparticles in Alkaline Ethylene Glycol" Chemistry 5, no. 2: 900-911. https://doi.org/10.3390/chemistry5020061
APA StyleRasmussen, D. R., Nielsen, M. F., & Quinson, J. (2023). Room Temperature Surfactant-Free Synthesis of Gold Nanoparticles in Alkaline Ethylene Glycol. Chemistry, 5(2), 900-911. https://doi.org/10.3390/chemistry5020061







