Abstract
Electrocatalytic reduction of nitrite (NO2−) is a sustainable and carbon-neutral approach to producing green ammonia (NH3). We herein report the first work on building defects on PrOx for electrochemical NO2− reduction to NH3, and demonstrate a high NH3 yield of 2870 μg h−1 cm−2 at the optimal potential of –0.7 V with a faradaic efficiency (FE) of 97.6% and excellent FEs of >94% at a wide given potential range (−0.5 to −0.8 V). The kinetic isotope effect (KIE) study suggested that the reaction involved promoted hydrogenation. Theoretical calculations clarified that there was an accelerated rate-determining step of NO2− reduction on PrOx. The results also indicated that PrOx could be durable for long-term electrosynthesis and cycling tests.
1. Introduction
Ammonia (NH3) is not only one of the most essential chemicals, but also a promising high-density energy carrier contributing to carbon neutrality [1,2,3,4,5,6]. However, current NH3 industrial production based on the traditional Haber–Bosch process suffers from harsh conditions and high CO2 emissions [7,8,9]. Consequently, exploring a sustainable and carbon-neutral approach to green NH3 is of great importance [10,11,12]. Recently, electrochemical N2 reduction reaction (NRR) with H2O as the proton source has caused worldwide concern as an alternating method for ambient NH3 synthesis using clean energy [13,14]. Nevertheless, low N2 solubility in aqueous electrolytes, hard N≡N bond (with an ultra-high bond energy of 941 kJ mol−1) activation, and undesired hydrogen evolution reaction (HER) are becoming the main factors hindering the further application of NRR [15]. Compared with N2, nitrite (NO2−) has a higher solubility and lower dissociation energy of N=O bond (204 kJ mol−1), coherently decreasing the thermodynamic limit of its conversion to NH3 [16,17]. In addition, NO2− is a widespread N-pollutant causing water pollution and a public health issue, and has also recently been reported as one of the main N2 derivatives in eco-friendly plasmatic air oxidation [18,19,20]. Hence, electrocatalytic NO2− reduction to NH3 provides opportunities to remove NO2− from contaminated water, utilization of renewable nitrogen sources, and production of green NH3 through renewable energy-driven pathways.
Rare earth, the strategic source known as a modern industrial vitamin, was regarded as a key component in catalysts for many emerging reactions [21,22]. Its unique ground-state electronic configurations and unpaired 4f orbital electrons are expected to be promising electrocatalysts for NO2− conversion. For instance, rare earth oxides such as CeO2 could achieve an excellent performance for NH3 electrosynthesis [23,24,25]. However, the reports on rare earth-based catalysts are still very limited, and the catalytic activity needs to be improved further. Introducing defects to the surface of oxide catalysts was proved to be an efficient strategy to modulate the electron configuration of the catalytic sites and thus promote the formation and conversion of key intermediates during the reaction [26,27,28,29]. As a result, constructing defect structures could be a promising methodology to develop novel rare earth-based catalysts in the enhancement of NO2− reduction.
Herein, we have demonstrated the first work on building defects on praseodymium oxide (PrOx) for electrochemical NO2− reduction to NH3. When tested using 0.01 M KNO2 as the nitrogen source and 0.5 M K2SO4 as the supporting electrolyte, PrOx could achieve a high NH3 yield of 2870 μg h−1 cm−2 at –0.7 V, which is 8 times larger than the pristine Pr6O11. In addition, PrOx could exhibit an excellent faradaic efficiency (FE) of >94% in a wide given potential range of −0.5 V to −0.8 V. The kinetic isotope effect (KIE) study indicates there is promoted hydrogenation during NO2− reduction on PrOx. The NH3 products were identified using isotope labelling. PrOx also showed robust durability for long-term bulk electrosynthesis and cycling tests.
2. Results and Discussion
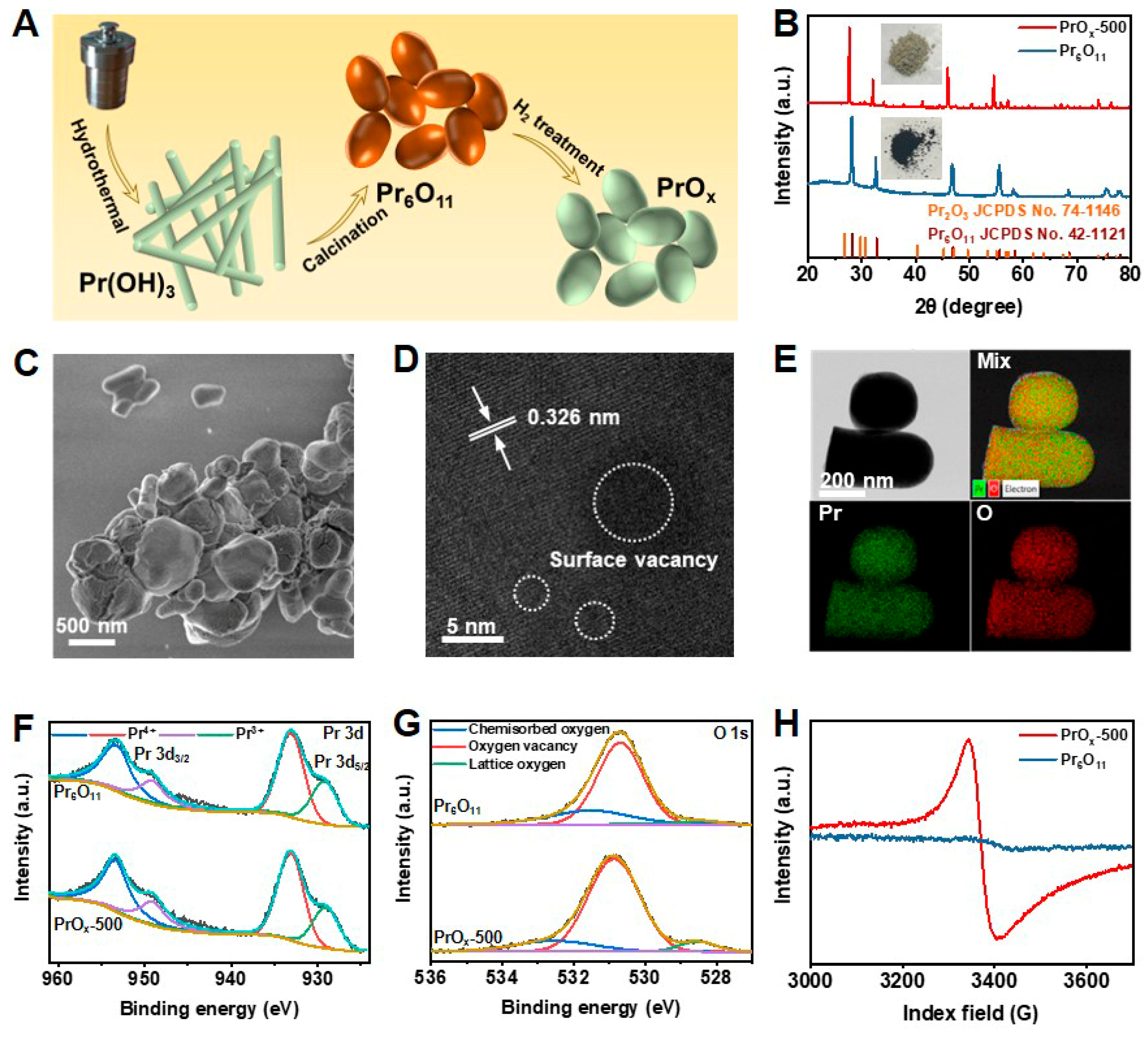
The process of preparing defective PrOx catalysts is illustrated in Figure 1A. The intermediate products obtained from the hydrothermal method were proven to be Pr(OH)3 nanorods by X-ray diffraction (XRD, JCPDS No. 83-2304) patterns, scanning electron microscopy (SEM), and transmission electron microscopy (TEM) in Figures S1–S4, which could transfer to Pr6O11 through calcining in air [30]. Thermal treatment of Pr6O11 in an H2 atmosphere at different temperatures (300 °C, 500 °C, and 700 °C) was utilized to construct oxygen vacancies (Vo) on its surface. The obtained samples were donated as PrOx-T (T = 300, 500, and 700). The XRD patterns of Pr6O11 and PrOx-500 are exhibited in Figure 1B. Similar peaks presented by Pr6O11 and PrOx-500 at 27.7, 32.1, 46.1, 54.6° are well indexed to the (111), (200), (220), and (311) crystallographic planes of Pr6O11 (JCPDS No. 42-1121). Very little Pr2O3 phase (JCPDS No. 74-1146) was observed. The gradual color change was found from the optical photograph (Figure 1B inset and Figure S5) from Pr6O11 to PrOx powders, which was characteristic of Pr3+ and indicated the increase of Pr3+ with the increasing temperature of PrOx preparation [31]. The results of XRD as well as SEM and low-resolution TEM images shown in Figure 1C and Figures S6–S8, indicated that the introduction of defects could not result in a significant change of crystal phase, morphology, and particle size of ca. 200 nm. High-resolution TEM (HR-TEM) images in Figure 1D and Figure S9 revealed that PrOx-500 posed distinct lattice fringes and a lattice spacing of 0.326 nm, which could be consistent with the cubic fluorite structure of the Pr6O11(111) plane. In addition, PrOx-500 exhibited disrupted fringes due to the presence of dot defects which was not observed in Pr6O11 samples (Figure S10), thereby confirming the existence of defects introduced by the H2 treatment. Figure 1E displays the energy-dispersive X-ray (EDX) elemental mapping images of PrOx-500, suggesting there was uniform distribution of Pr and O elements.
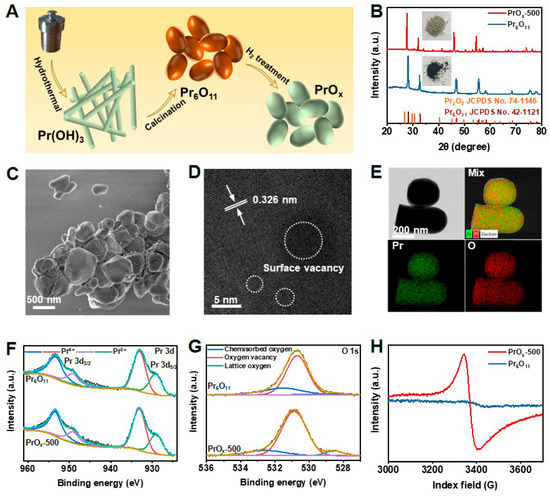
Figure 1.
(A) Schematic illustration of the synthesis of PrOx electrocatalyst. (B) XRD patterns and optical photographs (inset) of Pr6O11 and PrOx-500. (C) SEM image of PrOx-500. (D) HR-TEM image and (E) corresponding EDX mapping images of PrOx-500. XPS spectra of Pr6O11 and PrOx-500 in the regions of (F) Pr 3d and (G) O 1s. (H) EPR curves of Pr6O11 and PrOx-500.
Further analysis of the PrOx surface was performed using X-ray photoelectron spectroscopy (XPS,AXIS ULTRA DLD, UK). The Pr 3d spectra in Figure 1F and Figure S11 were observed in the PrOx-500 sample, with peaks at 953.1 eV (Pr 3d3/2) and 933.1 eV (Pr 3d5/2) being attributed to Pr4+ and peaks at 949.1 eV (Pr 3d3/2) and 929.2 eV (Pr 3d5/2) being assigned to Pr3+ [32,33]. As the temperature increased, the intensity of the Pr3+ peaks increased, indicating that more Pr4+ was reduced to Pr3+. The O 1s spectra in Figure 1G and Figure S12 indicated the presence of different chemical environments for oxygen atoms in the PrOx catalysts. The concentration of Vo increased with the increasing calcination temperature. The peaks at 531.5, 530.7, and 528.3 eV corresponded to chemisorbed oxygen, oxygen deficiency, and lattice oxygen, respectively [34,35,36]. As the temperature increased, the peak of oxygen vacancy was found to increase due to more Vo on the surface of PrOx-500 compared with Pr6O11. The slight shifts of Pr 3d and O1s peaks between Pr6O11 and PrOx samples were also attributed to the introduced defects. Electron paramagnetic resonance (EPR) spectra were used to confirm the presence of Vo in Pr6O11 and PrOx catalysts (Figure 1H). Compared with Pr6O11, PrOx had a distinct EPR signal of Vo at g = 2.004, demonstrating again the successful preparation of PrOx with Vo [31,32,33,34,35,36,37].
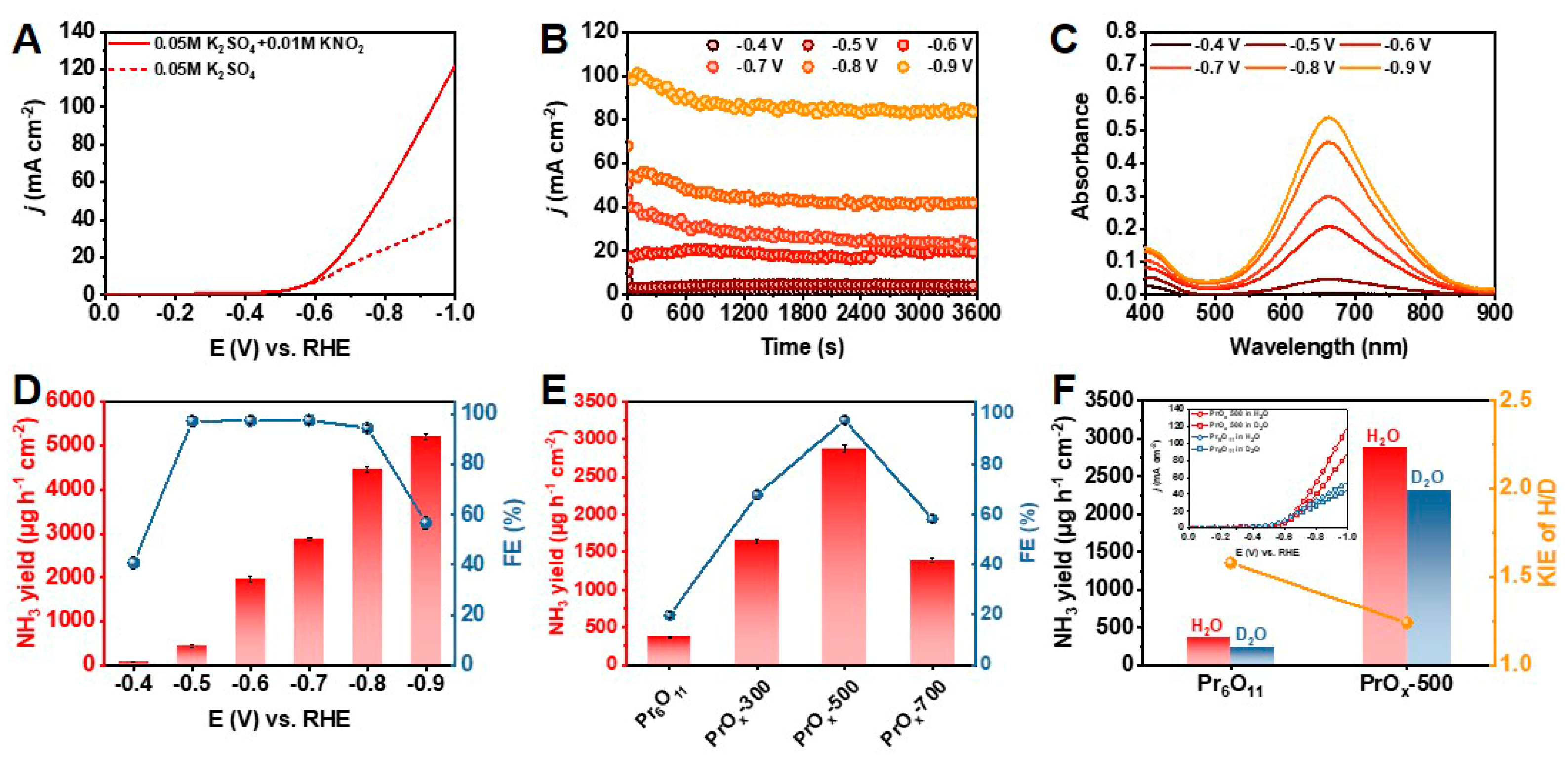
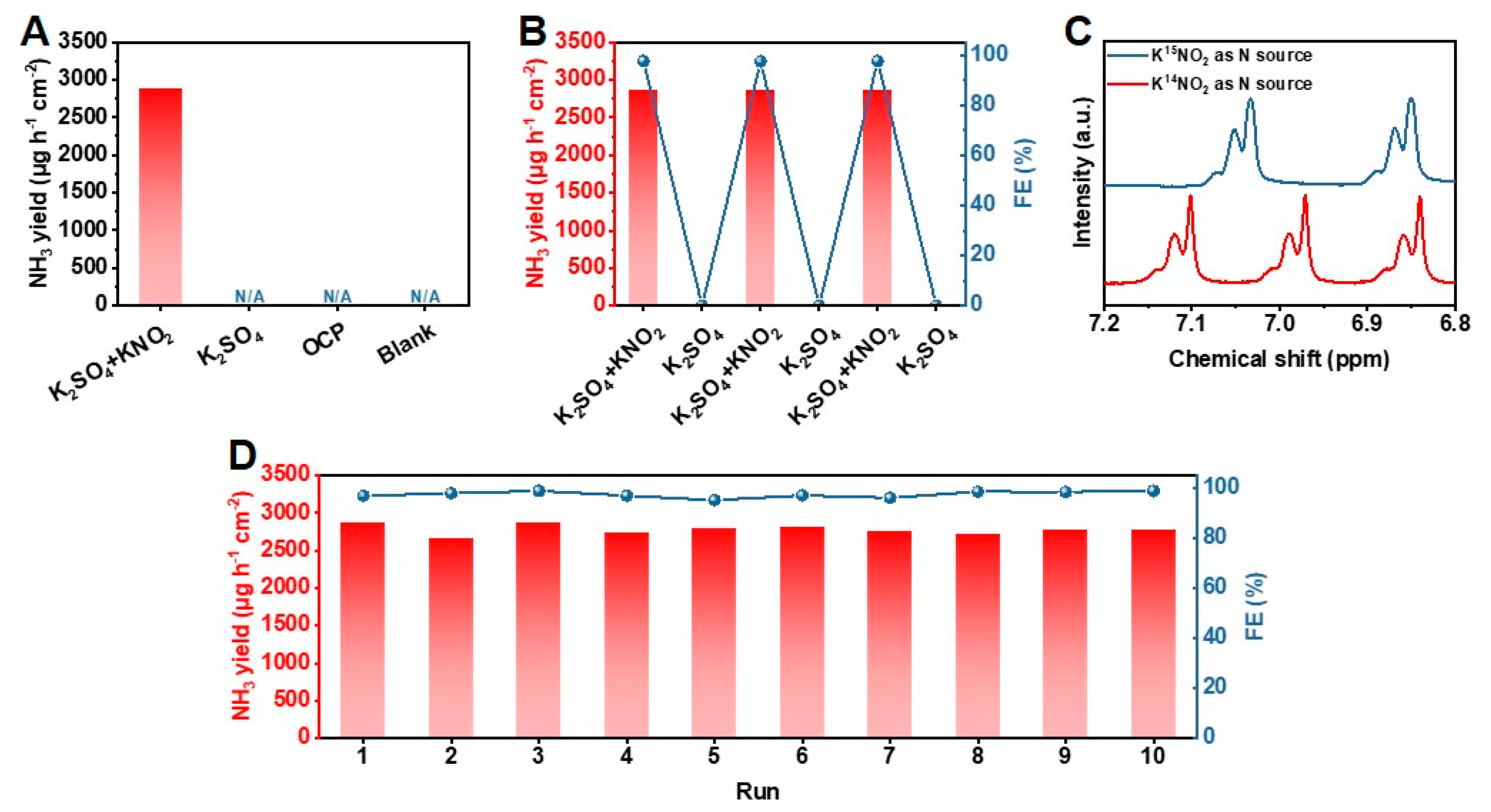
The electrochemical performance of PrOx-500 for NH3 synthesis was evaluated in 0.5 M K2SO4 aqueous solution with 0.01 M KNO2 saturated by Ar, utilizing an H-type cell with a three-electrode configuration (Figure S13). All potentials reported in this paper were converted to the relative hydrogens electrode (RHE). The performances reported in this paper are verified by three repeated experiments, and the average results with error bars are given. A catalyst ink of PrOx-500 powder was prepared and loaded on a carbon paper evenly (1 mg cm−2), which served as the working electrode. As presented in Figure 2A, the electrochemical catalytic activity of PrOx-500 for NO2− reduction to NH3 was firstly investigated using the linear scanning voltammetry (LSV) curves (scan rate = 10 mV s−1). Adding 0.01 M KNO2 promoted the current density (j) remarkably, indicating there was excellent activity of NO2− reduction over PrOx-500 in neutral media. To investigate the optimal efficiency of NH3 production, chronoamperometry tests were carried out by applying various potentials ranging from –0.4 to –0.9 V. As shown in Figure 2B, these chronoamperometry curves remained stable during electrochemical tests for 3600 s. The corresponding UV-vis spectra showed that the peak of absorption curves increased with the applied potential, meaning there was an increase in the NH3 yield with growing potential (Figure 2C). The NH3 yield and FE calculated from calibration curves in Figure S14 are presented in Figure 2D. PrOx-500 exhibited the highest FE of 97.6% at –0.7 V with a NH3 yield of 2870 μg h−1 cm−2. In addition, PrOx-500 had an ideal performance with an FE of >94% from the range of applied potential from –0.5 to –0.8 V, which is essential for further application. Since –0.7 V was chosen as the optimal potential for NH3 production, we further studied different Pr-based catalysts on –0.7 V. The results in Figure 2E indicate that Pr6O11, PrOx-300, and PrOx-700 exhibited lower NH3 yields (372, 1643, and 1387 μg h−1 cm−2) and poor FEs (19.7%, 67.8%, and 58.2%), implying that the construction of defects under optimal temperature is a useful strategy to boost NO2− reduction reaction for NH3 electrosynthesis. Further experiments were conducted to clarify the acceleration of the kinetics process during the NO2− reduction by examining the KIE of H/D (H2O/D2O) over the Pr6O11 and PrOx-500 catalysts. The KIE values, which serve as a descriptor of proton transfer rate, were calculated and compared in Figure 2F. The results showed a significant decrease in KIE value from 1.58 in the PrOx-500 sample to 1.24 in the PrOx-500 catalyst, indicating there was a faster rate of hydrogenation kinetics [38,39,40]. Additionally, the onset potential of the Pr6O11 and PrOx 500 samples depicted in Figure 2F (inset) suggested that their performance was enhanced in H2O solvent as compared to D2O solvent, which is consistent with the variation in NH3 yield obtained using different catalysts and solvents. To eliminate the influence of other nitrogen sources, control experiments were performed. The results of these experiments, as presented in Figure 3A, indicated that our electrodes, electrolyte, and reagents were not contaminated by N-impurities, as there was an absence of NH3 production in the cathode solution after electrolysis at the open circuit potential (OCP) with a blank electrolyte. The NH3 production of PrOx-500 was further evaluated by alternately conducting experiments in electrolytes with and without NO2− for three cycles at −0.7 V (Figure 3B). The results showed that NH3 was only detected in electrolytes containing NO2−. Next, 15N isotope labelling was performed using 15NO2− as an additive electrolyte. Figure 3C showed two peaks of 15NH4+ and three peaks of 14NH4+ in the corresponding 1H Nuclear magnetic resonance (NMR) spectra (signals of standard samples were shown in Figure S15), obtained from experiments with 15NO2− and 14NO2− as additive electrolytes, further confirming that the NH3 came from the reduction of NO2−. The stability of electrocatalysts is critical for industrial applications because it determines the longevity and efficiency of the electrochemical reactions [41]. To assess the remarkable catalytic stability of PrOx-500, we performed cycling experiments using the same working electrode and refreshed the electrolyte for each cycle. The results showed that the NH3 yields and FEs for the ten cycles remained stable with negligible fluctuations. In addition, the similarity in color (Figure S16), XRD patterns (Figure S17), SEM images (Figure S18), TEM images (Figure S19), EDS elemental mapping images (Figure S20), XPS spectra (Figures S21 and S22), and LSV curves (Figure S23) of PrOx-500, both prior to and after extended electrolysis, further supports the excellent electrochemical and structural stability of PrOx-500 as a catalyst in the reduction of NO2− for the synthesis of NH3.
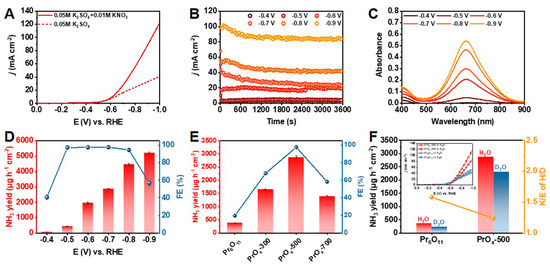
Figure 2.
(A) LSV curves of PrOx-500 in 0.5 M K2SO4 aqueous solution in the presence and absence of 0.01 M KNO2. (B) Chronoamperometry curves of NO2− reduction from –0.4 V to –0.9 V over PrOx-500 catalyst. (C) Corresponding UV-vis spectra of PrOx-500 catalyzed NO2− reduction. (D) Potential-dependent NH3 yields and FE of PrOx-500. (E) Comparison of the performance of various Pr-based catalysts. (F) KIE study of H/D over Pr6O11 and PrOx-500 and LSV curves (inset) of Pr6O11 and PrOx-500 in H2O and D2O solvents.
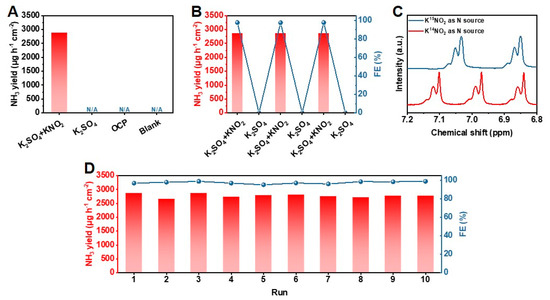
Figure 3.
(A) NH3 yields of PrOx-500 for NO2− reduction at various conditions. (B) NH3 yields and FE of NO2− reduction on PrOx-500 during the alternating cycling test between 0.5 M K2SO4 with/without additional 0.01 M KNO2. (C) 1H NMR spectra of NH3 products in the electrolytes after the reduction of K14NO2 and K15NO2 at −0.7 V for 3600 s. (D) NH3 yields and FE of PrOx-500 during consecutive recycling tests.
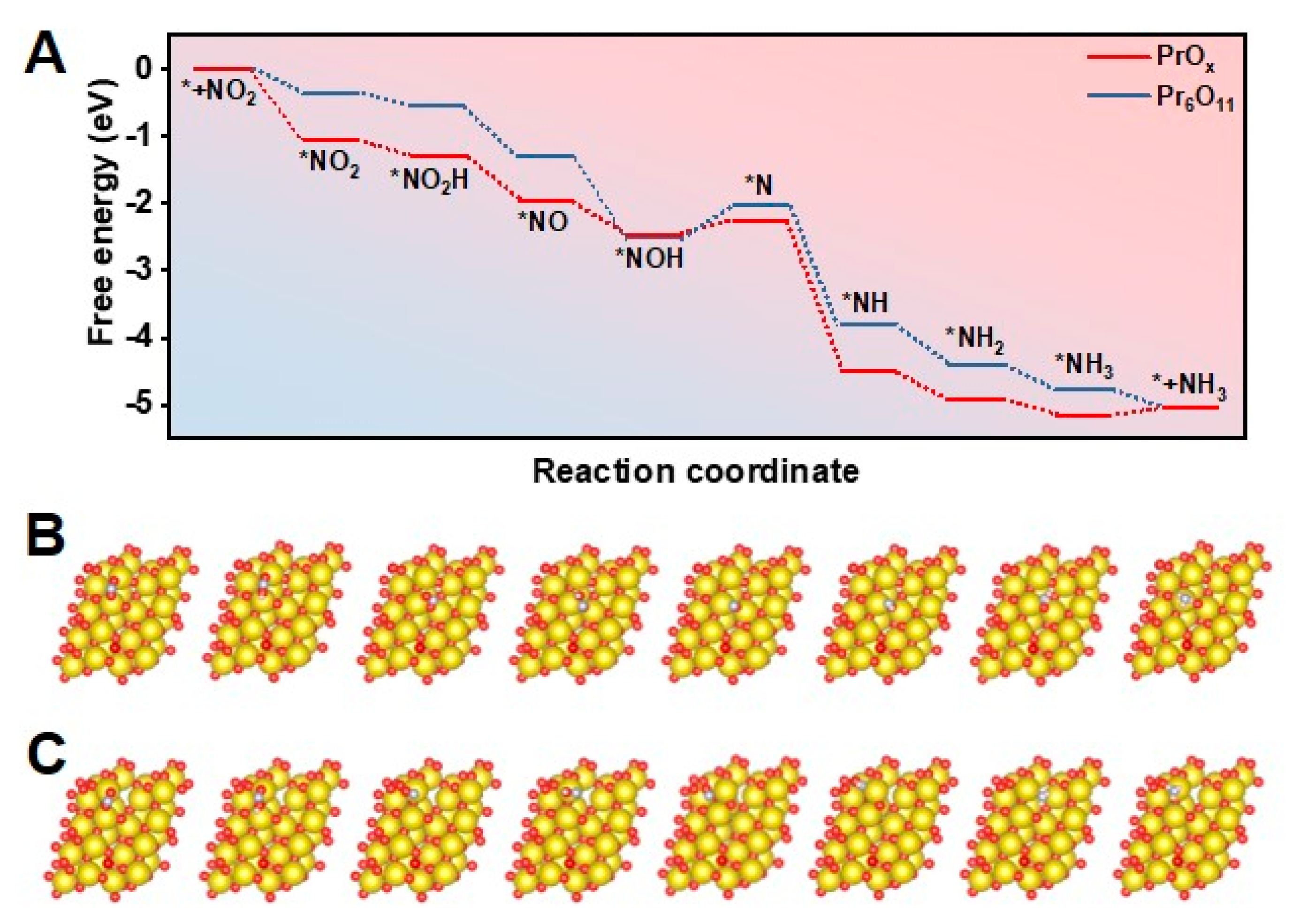
A density functional theory (DFT) study was then carried out to investigate the reaction pathways of NO2− reduction on Pr-based catalysts with different defects and thus modified electronic structure. The (111) facet was found to be the main plane in Pr6O11 and PrOx samples according to XRD patterns and HR-TEM images shown in Figure 1B and 1D. The catalyst models of PrOx(111) were thus built by constructing Vo on the perfect Pr6O11(111) facet. Figure 4A presented the reaction-free energy levels of various intermediates for NO2− reduction on PrOx and Pr6O11, revealing that the rate-determining step (RDS) on the PrOx and Pr6O11 surface was *NOH plus H to generate *N. Additionally, the corresponding structures of NO2− reduction intermediates are illustrated in Figure 4B,C. On the surface of PrOx, the coordination number of Pr near the defect was lower, thus increasing the adsorption capacity of *N, and lowering the energy barrier of the PDS. Hence, the free energy of the final RDS on PrOx is reduced compared to that on the perfect Pr6O11 surface. Hence, constructing defects on PrOx catalysts could significantly accelerate the RDS, leading to the better performance of electrochemical NO2− reduction.
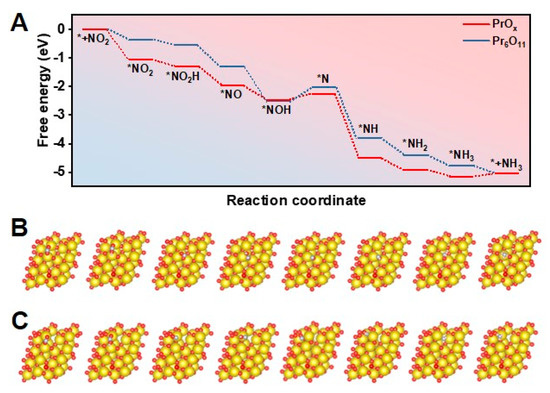
Figure 4.
(A) Free energy of various intermediates generated during NO2− reduction on PrOx(111) and Pr6O11(111). Atomic configurations of the intermediates on (B) PrOx(111) and (C) Pr6O11(111) during the electrochemical progress (Pr: gold, O: red, N: purple, and H: pink).
3. Conclusions
In summary, this work has demonstrated the highly efficient electrochemical reduction of NO2− to NH3 utilizing PrOx catalysts with defects. Electrocatalysis tests showed a high yield of 2870 μg h−1 cm−2 at an optimal potential of −0.7 V and FE of >94% in a wide applied potential range. A KIE study confirmed the promotion of hydrogenation during the reduction process, and the products were identified using isotope labelling. Additionally, PrOx demonstrated robust durability for long-term electrosynthesis and cycling tests. DFT calculations demonstrated that PrOx could accelerate the RDS of NO2− reduction, resulting in the enhanced performance of NH3 production. The work opens up new avenues for the development of ambient, efficient, and sustainable NH3 synthesis processes and lays a foundation for the development of next-generation electrochemical systems for environmental protection, energy conversion, and chemical manufacturing.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry5020053/s1, Figure S1. Optical photograph of Pr(OH)3 after hydrothermal treatment, Figure S2. XRD pattern of Pr(OH)3 obtained from hydrothermal method, Figure S3. SEM images of synthesized Pr(OH)3 nanorods, Figure S4. TEM image of Pr(OH)3 nanorods, Figure S5. Optical photograph of (A) PrOx-300 and (B) PrOx-700 samples, XRD patterns of (A) PrOx-300 and (B) PrOx-700, Figure S7. SEM images of the as-synthesized (A) Pr6O11, (B) PrOx-300, and (C) PrOx-700 samples, Figure S8. TEM images of the as-synthesized (A) Pr6O11, (B) PrOx-300, and (C) PrOx-700, Figure S9. Well-resolved lattice fringe of PrOx-500 in Figure 1D, Figure S10. HR-TEM image of Pr6O11, Figure S11. XPS curves of Pr 3d orbital of PrOx-300 and PrOx-700 surface, Figure S12. XPS curves of O 1s orbital of PrOx-300 and PrOx-700 surface, Figure S13. Illustration of H-cell used in this study, Figure S14. UV-vis absorption curves of indophenol assays kept with samples with different [NH4+] for at least 2 h at 25 ℃. (b) Calibration curve to estimate unknown [NH4+], Figure S15. 1H NMR spectra of 15NH4+ and 14NH4+ standard samples, Figure S16. Optical photograph of working electrodes loading with PrOx-500 catalysts before and after reactions, Figure S17. XRD pattern of PrOx-500 after electrolysis, Figure S18. SEM image of PrOx-500 after electrolysis, Figure S19. TEM image of PrOx-500 after electrolysis, Figure S20. EDX mappings of PrOx-500 after electrolysis, Figure S21. XPS curves of Pr 3d orbital of PrOx-500 surface after reduction, Figure S22. XPS curves of O 1s orbital of PrOx-500 surface after reduction, Figure S23. LSV curves of PrOx-500 before and after reduction. References [30,42,43,44,45] are cited in the supplementary materials.
Author Contributions
Conceptualization, S.J., X.S. and B.H.; methodology, S.J., X.S. and B.H.; formal analysis, X.T., L.W., J.F., L.Z., L.X. and R.W.; writing—original draft preparation, S.J.; writing—review and editing, X.S. and B.H.; supervision, X.S. and B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by National Natural Science Foundation of China (22002172, 22121002 and 22203099) and China Postdoctoral Science Foundation (2022M713200).
Data Availability Statement
The data presented in this study are available on request from the corresponding authors (Prof. Xiaofu Sun and Prof. Buxing Han).
Acknowledgments
The authors thank the staff at Centre for Physiochemical Analysis & Measurement of ICCAS for material characterizations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Wang, B.; Ni, M.; Jiao, K. Green ammonia as a fuel. Sci. Bull. 2022, 67, 1530–1534. [Google Scholar] [CrossRef]
- He, M.; Zhang, K.; Guan, Y.; Sun, Y.; Han, B. Green carbon science: Fundamental aspects. Natl. Sci. Rev. 2023, nwad046. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Scientific Basis for Integrating Carbon Resource Processing, Utilization, and Recycling. Angew. Chem. Int. Ed. 2013, 52, 9620–9633. [Google Scholar] [CrossRef]
- He, M.; Sun, Y.; Han, B. Green Carbon Science: Efficient Carbon Resource Processing, Utilization, and Recycling towards Carbon Neutrality. Angew. Chem. Int. Ed. 2022, 61, e202112835. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Bullock, R.M.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K.; et al. Beyond fossil fuel-driven nitrogen transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Overa, S.; Jiao, F. Emerging Electrochemical Processes to Decarbonize the Chemical Industry. JACS Au 2022, 2, 1054–1070. [Google Scholar] [CrossRef]
- Song, X.; Jia, S.; Xu, L.; Feng, J.; He, L.; Sun, X.; Han, B. Towards sustainable CO2 electrochemical transformation via coupling design strategy. Mater. Today Sustain. 2022, 19, 100179. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Wang, F.; Harindintwali, J.D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; et al. Technologies and perspectives for achieving carbon neutrality. Innovation 2021, 2, 100180. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Wang, Y.; Guo, Y.; Xie, X.; Yu, Y.; Zhang, B. Recent advances in electrocatalytic nitrite reduction. Chem. Commun. 2022, 58, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Guo, W.; Sun, X.; Han, B. Rational design of nanocatalysts for ambient ammonia electrosynthesis. Pure Appl. Chem. 2021, 93, 777–797. [Google Scholar] [CrossRef]
- Jia, S.; Wu, L.; Xu, L.; Sun, X.; Han, B. Multicomponent catalyst design for CO2/N2/NOx electroreduction. Ind. Chem. Mater. 2023, 1, 93–105. [Google Scholar] [CrossRef]
- Iriawan, H.; Andersen, S.Z.; Zhang, X.; Comer, B.M.; Barrio, J.; Chen, P.; Medford, A.J.; Stephens, I.E.L.; Chorkendorff, I.; Shao-Horn, Y. Methods for nitrogen activation by reduction and oxidation. Nat. Rev. Methods Prim. 2021, 1, 56. [Google Scholar] [CrossRef]
- Stirling, A.; Papai, I.; Mink, J.; Salahub, D.R. Density functional study of nitrogen oxides. J. Chem. Phys. 1994, 100, 2910–2923. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Liu, S.; Feng, J.; Xu, L.; Tan, X.; Ma, X.; Sun, X. Promoting ambient ammonia electrosynthesis on modulated Cuδ+ catalysts by B-doping. J. Mater. Chem. A 2023, 11, 5520–5526. [Google Scholar] [CrossRef]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef]
- Li, L.; Tang, C.; Cui, X.; Zheng, Y.; Wang, X.; Xu, H.; Zhang, S.; Shao, T.; Davey, K.; Qiao, S.-Z. Efficient Nitrogen Fixation to Ammonia through Integration of Plasma Oxidation with Electrocatalytic Reduction. Angew. Chem. Int. Ed. 2021, 60, 14131–14137. [Google Scholar] [CrossRef]
- Ren, Y.; Yu, C.; Wang, L.; Tan, X.; Wang, Z.; Wei, Q.; Zhang, Y.; Qiu, J. Microscopic-Level Insights into the Mechanism of Enhanced NH3 Synthesis in Plasma-Enabled Cascade N2 Oxidation–Electroreduction System. J. Am. Chem. Soc. 2022, 144, 10193–10200. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Xu, Y.; Du, Y.; Yan, C. Ultrathin 2D Rare-Earth Nanomaterials: Compositions, Syntheses, and Applications. Adv. Mater. 2020, 32, 1806461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Saji, S.E.; Yin, Z.; Zhang, H.; Du, Y.; Yan, C.-H. Rare-Earth Incorporated Alloy Catalysts: Synthesis, Properties, and Applications. Adv. Mater. 2021, 33, 2005988. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, Z.; Ouyang, L.; Fan, X.; Zhang, L.; Sun, S.; Liu, Q.; Alshehri, A.A.; Luo, Y.; Kong, Q.; et al. CeO2 nanoparticles with oxygen vacancies decorated N-doped carbon nanorods: A highly efficient catalyst for nitrate electroreduction to ammonia. Nano Res. 2022, 15, 8914–8921. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Guan, L.; Li, K.; Lin, Y. Oxygen Vacancy Regulation Strategy Promotes Electrocatalytic Nitrogen Fixation by Doping Bi into Ce-MOF-Derived CeO2 Nanorods. J. Phys. Chem. C 2020, 124, 18003–18009. [Google Scholar] [CrossRef]
- Liu, G.; Cui, Z.; Han, M.; Zhang, S.; Zhao, C.; Chen, C.; Wang, G.; Zhang, H. Ambient Electrosynthesis of Ammonia on a Core–Shell-Structured Au@CeO2 Catalyst: Contribution of Oxygen Vacancies in CeO2. Chem. Eur. J. 2019, 25, 5904–5911. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Li, H.; Chen, C.; Zou, Y.; Wang, S. Defect Engineering Strategies for Nitrogen Reduction Reactions under Ambient Conditions. Small Methods 2019, 3, 1800331. [Google Scholar] [CrossRef]
- Yang, C.; Lu, Y.; Zhang, L.; Kong, Z.; Yang, T.; Tao, L.; Zou, Y.; Wang, S. Defect Engineering on CeO2-Based Catalysts for Heterogeneous Catalytic Applications. Small Struct. 2021, 2, 2100058. [Google Scholar] [CrossRef]
- Xu, L.; Feng, J.; Wu, L.; Song, X.; Tan, X.; Zhang, L.; Ma, X.; Jia, S.; Du, J.; Chen, A.; et al. Identifying the optimal oxidation state of Cu for electrocatalytic reduction of CO2 to C2+ products. Green Chem. 2023, 25, 1326–1331. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, J.; Liu, S.; Tan, X.; Wu, L.; Jia, S.; Xu, L.; Ma, X.; Song, X.; Ma, J.; et al. Atomically Dispersed Ni–Cu Catalysts for pH-Universal CO2 Electroreduction. Adv. Mater. 2023, 2209590. [Google Scholar] [CrossRef]
- Huang, P.X.; Wu, F.; Zhu, B.L.; Li, G.R.; Wang, Y.L.; Gao, X.P.; Zhu, H.Y.; Yan, T.Y.; Huang, W.P.; Zhang, S.M.; et al. Praseodymium Hydroxide and Oxide Nanorods and Au/Pr6O11 Nanorod Catalysts for CO Oxidation. J. Phys. Chem. B 2006, 110, 1614–1620. [Google Scholar] [CrossRef]
- Su, L.; Zhang, Y.; Zhan, X.; Zhang, L.; Zhao, Y.; Zhu, X.; Wu, H.; Chen, H.; Shen, C.; Wang, L. Pr6O11: Temperature-Dependent Oxygen Vacancy Regulation and Catalytic Performance for Lithium–Oxygen Batteries. ACS Appl. Mater. Interfaces 2022, 14, 40975–40984. [Google Scholar] [CrossRef]
- Tankov, I.; Arishtirova, K.; Bueno, J.M.C.; Damyanova, S. Surface and structural features of Pt/PrO2–Al2O3 catalysts for dry methane reforming. App. Catal. A Gen. 2014, 474, 135–148. [Google Scholar] [CrossRef]
- Guo, M.; Lu, J.; Wu, Y.; Wang, Y.; Luo, M. UV and Visible Raman Studies of Oxygen Vacancies in Rare-Earth-Doped Ceria. Langmuir 2011, 27, 3872–3877. [Google Scholar] [CrossRef]
- Sanivarapu, S.R.; Lawrence, J.B.; Sreedhar, G. Role of Surface Oxygen Vacancies and Lanthanide Contraction Phenomenon of Ln(OH)3 (Ln = La, Pr, and Nd) in Sulfide-Mediated Photoelectrochemical Water Splitting. ACS Omega 2018, 3, 6267–6278. [Google Scholar] [CrossRef] [PubMed]
- Lei, F.; Sun, Y.; Liu, K.; Gao, S.; Liang, L.; Pan, B.; Xie, Y. Oxygen Vacancies Confined in Ultrathin Indium Oxide Porous Sheets for Promoted Visible-Light Water Splitting. J. Am. Chem. Soc. 2014, 136, 6826–6829. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, D.; Harle, G.; Qin, Q.; Guo, L.; Zheng, T.; Yin, X.; Du, J.; Zhao, Y. Ammonia removal in selective catalytic oxidation: Influence of catalyst structure on the nitrogen selectivity. J. Hazard. Mater. 2021, 416, 125782. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, S.; Tan, X.; Wu, R.; Yan, X.; Chen, C.; Zhu, Q.; Zheng, L.; Ma, J.; Zhang, J.; et al. Highly Efficient CO2 Electroreduction to Methanol through Atomically Dispersed Sn Coupled with Defective CuO Catalysts. Angew. Chem. Int. Ed. 2021, 60, 21979–21987. [Google Scholar] [CrossRef]
- Yang, Y.; Agarwal, R.G.; Hutchison, P.; Rizo, R.; Soudackov, A.V.; Lu, X.; Herrero, E.; Feliu, J.M.; Hammes-Schiffer, S.; Mayer, J.M.; et al. Inverse kinetic isotope effects in the oxygen reduction reaction at platinum single crystals. Nat. Chem. 2022, 15, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Xie, W.; Li, J.; Sun, Y.; Xu, P.; Tang, Y.; Li, Z.; Shao, M. Active hydrogen boosts electrochemical nitrate reduction to ammonia. Nat. Commun. 2022, 13, 7958. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Sang, X.; Yang, B.; Li, Z.; Zheng, S.; Zhang, Q.; Yao, S.; Yang, X.; Lei, L.; et al. Atomically Dispersed Zinc(I) Active Sites to Accelerate Nitrogen Reduction Kinetics for Ammonia Electrosynthesis. Adv. Mater. 2022, 34, 2103548. [Google Scholar] [CrossRef]
- Jia, S.; Ma, X.; Sun, X.; Han, B. Electrochemical Transformation of CO2 to Value-Added Chemicals and Fuels. CCS Chem. 2022, 4, 3213–3229. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lin, Z.; Liang, Y.; Wang, H. Direct electrosynthesis of methylamine from carbon dioxide and nitrate. Nat. Sustain. 2021, 4, 725–730. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B Condens. Matter. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).