Ni-Mg/Al Mixed Oxides Prepared from Layered Double Hydroxides as Catalysts for the Conversion of Furfural to Tetrahydrofurfuryl Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Catalysts
2.2. Characterization of the Catalysts
2.3. Catalytic Activity
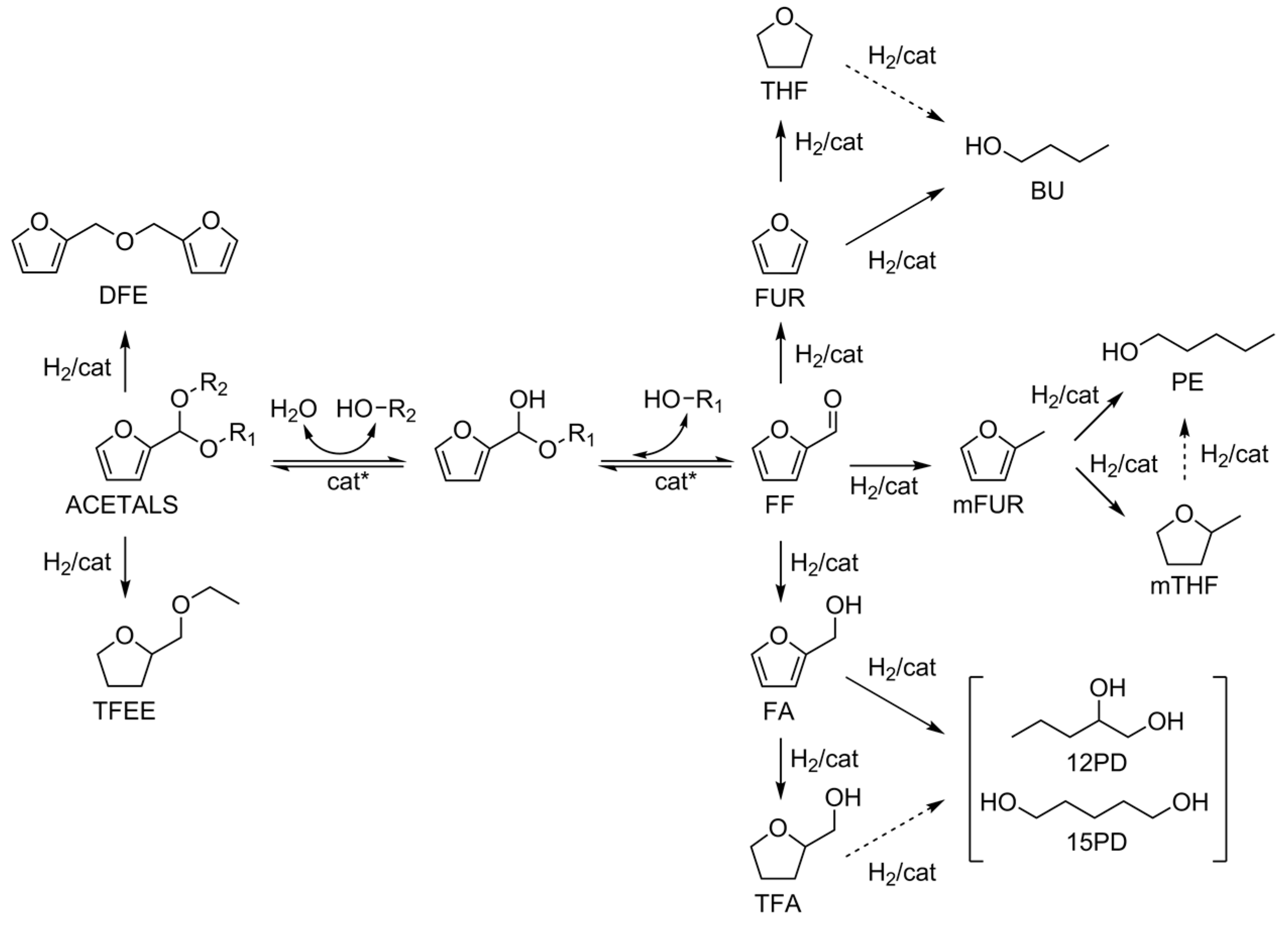
3. Results and Discussion
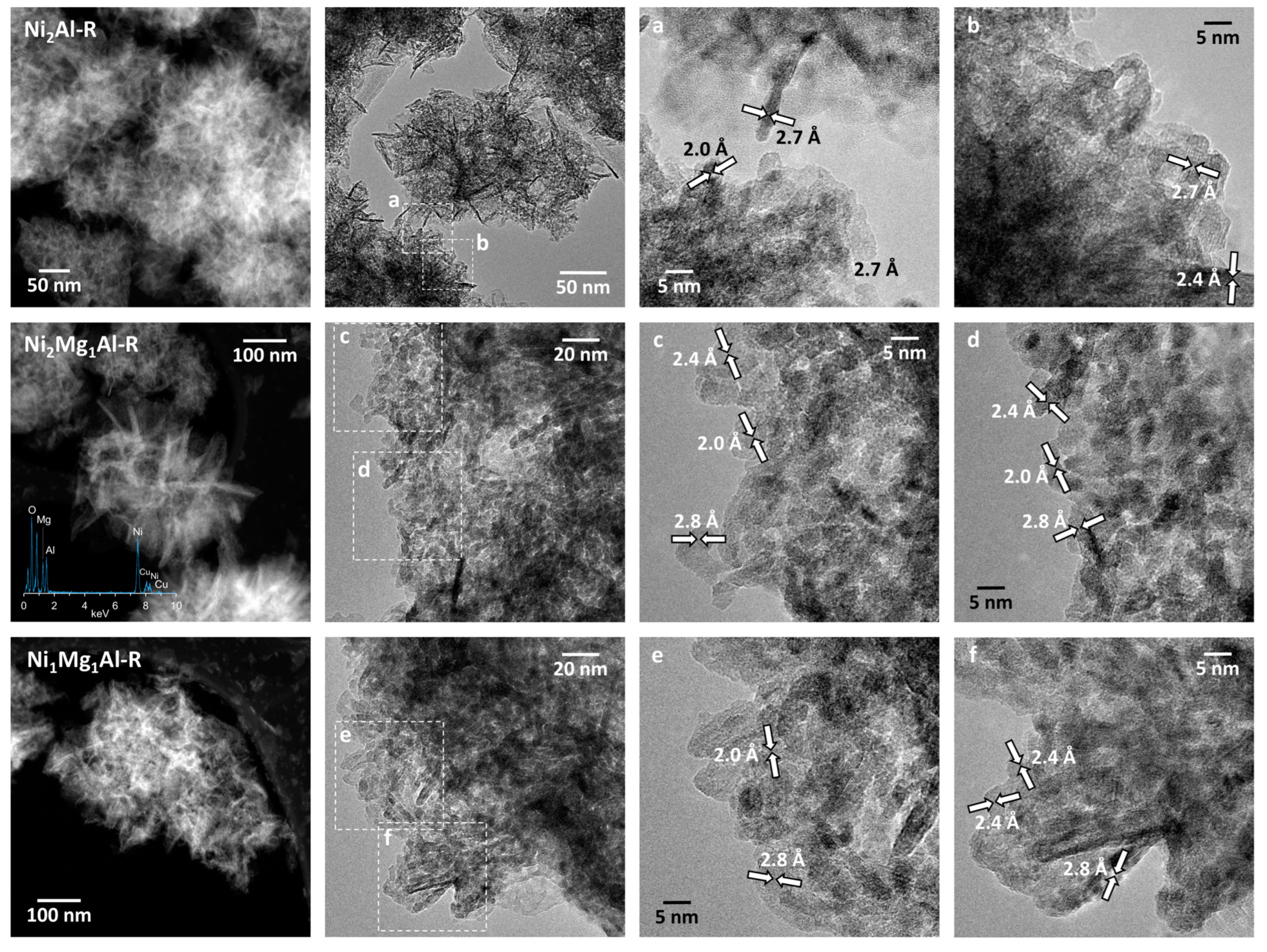
3.1. Catalyst Characterization
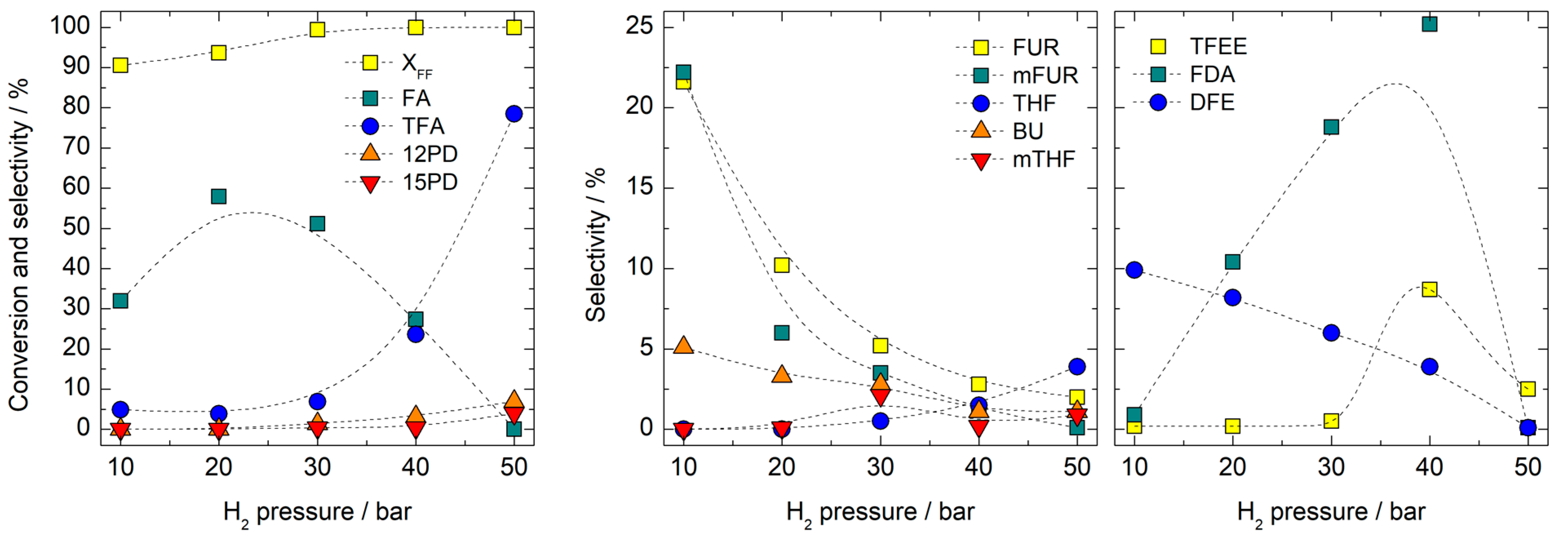
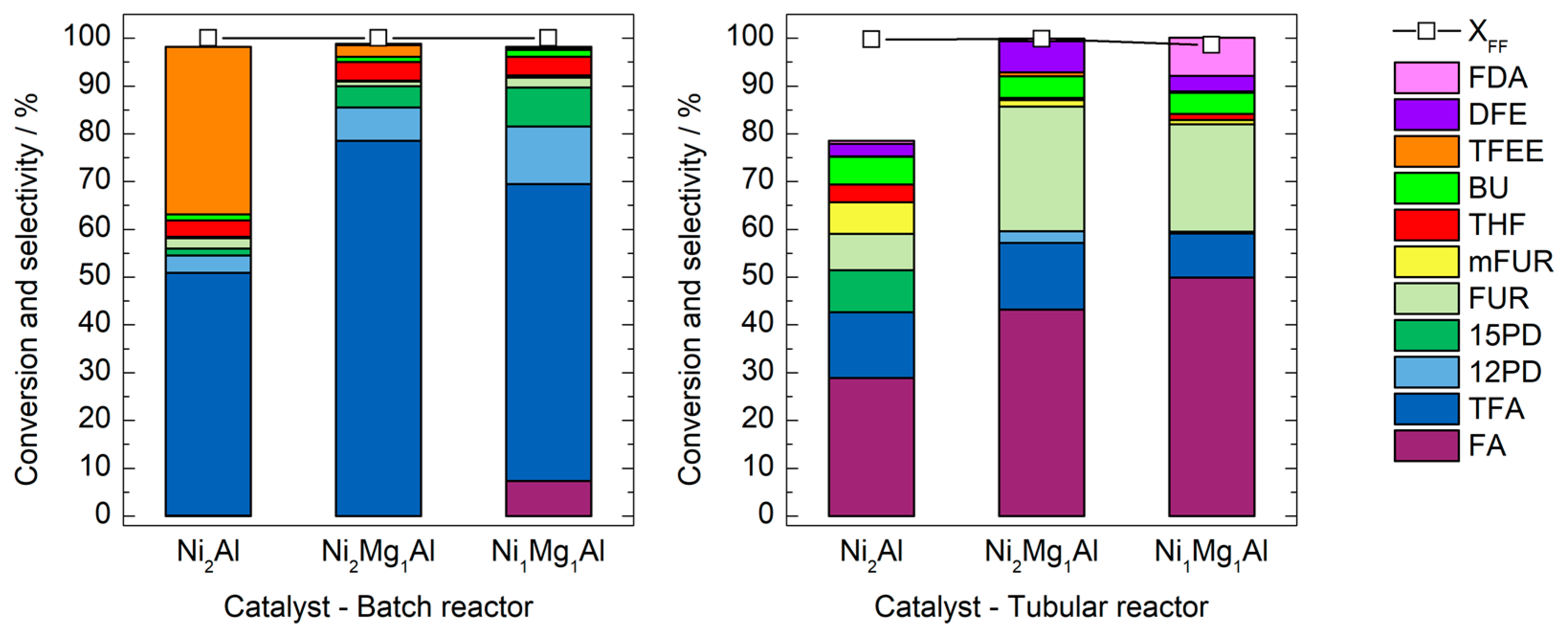
3.2. Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier: New York, NY, USA, 2000; ISBN 9780080528991. [Google Scholar]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 16, pp. 285–313. [Google Scholar]
- Biradar, N.S.; Hengne, A.M.; Birajdar, S.N.; Niphadkar, P.S.; Joshi, P.N.; Rode, C.V. Single-pot formation of THFAL via catalytic hydrogenation of FFR over Pd/MFI catalyst. ACS Sustain. Chem. Eng. 2014, 2, 272–281. [Google Scholar] [CrossRef]
- Li, C.; Xu, G.; Liu, X.; Zhang, Y.; Fu, Y. Hydrogenation of biomass-derived furfural to tetrahydrofurfuryl alcohol over hydroxyapatite-supported Pd catalyst under mild conditions. Ind. Eng. Chem. Res. 2017, 56, 8843–8849. [Google Scholar] [CrossRef]
- Wang, C.; Wang, A.; Yu, Z.; Wang, Y.; Sun, Z.; Kogan, V.M.; Liu, Y.-Y. Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol over Pd/UiO-66. Catal. Commun. 2021, 148, 106178. [Google Scholar] [CrossRef]
- Albilali, R.; Douthwaite, M.; Heb, Q.; Taylor, S.H. The selective hydrogenation of furfural over supported palladium nanoparticle catalysts prepared by sol-immobilisation: Effect of catalyst support and reaction conditions. Catal. Sci. Technol. 2018, 8, 252–267. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Chen, Q.; Chen, L.; Liu, Q.; Wang, C.; Ma, L. One-pot hydrogenation of furfural into tetrahydrofurfuryl alcohol under ambient conditions over PtNi alloy catalyst. Energy Fuels 2020, 34, 2178–2184. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Hsu, C.-Y.; Chen, S.S.; Ahamad, T.; Alshehri, S.M.; Tsang, D.C.W.; Wu, K.C.-W. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over a Rh-loaded carbon catalyst in aqueous solution under mild conditions. Sustain. Energy Fuels 2020, 4, 293–301. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total hydrogenation of furfural over a silica-supported nickel catalyst prepared by the reduction of a nickel nitrate precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Jia, X.; Du, Z.; Duan, Y.; Xu, J. Aqueous phase hydrogenation of furfural to tetrahydrofurfuryl alcohol on alkaline earth metal modified Ni/Al2O3. RSC Adv. 2016, 6, 51221–51228. [Google Scholar] [CrossRef]
- Parikh, J.; Srivastava, S.; Jadeja, G.C. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol using supported nickel−cobalt catalysts. Ind. Eng. Chem. Res. 2019, 58, 16138–16152. [Google Scholar] [CrossRef]
- Sunyol, C.; Owen, R.E.; González, M.D.; Salagre, P.; Cesteros, Y. Catalytic hydrogenation of furfural to tetrahydrofurfuryl alcohol using competitive nickel catalysts supported on mesoporous clays. Appl. Catal. A 2021, 611, 117903. [Google Scholar] [CrossRef]
- Kumar, A.; Shivhare, A.; Bal, R.; Srivastava, R. Metal and solvent-dependent activity of spinel- based catalysts for the selective hydrogenation and rearrangement of furfural. Sustain. Energy Fuels 2021, 5, 3191–3204. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Lauwaert, J.; Van Der Voort, P.; Verberckmoes, A. Recent advances on the utilization of layered double hydroxides (LDHs) and related heterogeneous catalysts in a lignocellulosic feedstock biorefinery scheme. Green Chem. 2017, 19, 5269–5302. [Google Scholar] [CrossRef]
- Kaneda, K.; Mizugaki, T. Design of high-performance heterogeneous catalysts using hydrotalcite for selective organic transformations. Green Chem. 2019, 21, 1361–1389. [Google Scholar] [CrossRef]
- Sels, B.F.; De Vos, D.E.; Jacobs, P.A. Hydrotalcite-like anionic clays in catalytic organic reactions. Catal. Rev. Sci. Eng. 2001, 43, 443–488. [Google Scholar] [CrossRef]
- Kaneda, K.; Ebitani, K.; Mizugaki, T.; Mori, K. Design of high-performance heterogeneous metal catalysts for green and sustainable chemistry. Bull Chem. Soc. Jpn. 2006, 79, 981–1016. [Google Scholar] [CrossRef]
- Kannan, S. Catalytic applications of hydrotalcite-like materials and their derived forms. Catal. Surv. Asia 2006, 10, 117–137. [Google Scholar] [CrossRef]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, Y.; Liu, Y.; Du, Y.; Li, D. Supported catalysts based on layered double hydroxides for catalytic oxidation and hydrogenation: General functionality and promising application prospects. Chem. Soc. Rev. 2015, 44, 5291–5319. [Google Scholar] [CrossRef] [PubMed]
- Sulmonetti, T.P.; Pang, S.H.; Claure, M.T.; Lee, S.; Cullen, D.A.; Agrawal, P.K.; Jones, C.W. Vapor phase hydrogenation of furfural over nickel mixed metal oxide catalysts derived from layered double hydroxides. Appl. Catal. A 2016, 517, 187–195. [Google Scholar] [CrossRef]
- Wu, J.; Gao, G.; Li, J.; Sun, P.; Long, X.; Li, F. Efficient and versatile CuNi alloy nanocatalysts for the highly selective hydrogenation of furfural. Appl. Catal. B 2017, 203, 227–236. [Google Scholar] [CrossRef]
- Meng, X.; Yang, Y.; Chen, L.; Xu, M.; Zhang, X.; Wei, M. A control over hydrogenation selectivity of furfural via tuning exposed facet of Ni catalysts. ACS Catal. 2019, 9, 4226–4235. [Google Scholar] [CrossRef]
- Rao, T.U.; Suchada, S.; Choi, C.; Machida, H.; Huo, Z.; Norinaga, K. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol in 2-butanol over an equimolar Ni-Cu-Al catalyst prepared by the co-precipitation method. Energy Convers. Manag. 2022, 265, 115736. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Leont’eva, N.N.; Kobzar, E.O.; Salanov, A.N.; Gulyaeva, T.I.; Trenikhin, M.V.; Likholobov, V.A. Study of the properties of the catalysts based on Ni(Mg)Al-Layered Hydroxides for the reaction of furfural hydrogenation. Mater. Chem. Phys. 2021, 263, 124091. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Balzar, D. Voigt-function model in diffraction line-broadening analysis. In Defect and Microstructure Analysis by Diffraction; Snyder, R.L., Fiala, J., Bunge, H.J., Eds.; International Union of Crystallography Monographs on Crystallography Num. 10; Oxford University Press: New York, NY, USA, 1999; pp. 94–124. [Google Scholar]
- Hill, R.J.; Howard, C.J. Quantitative phase analysis from neutron powder diffraction data using the Rietveld method. J. Appl. Crystallogr. 1987, 20, 467–474. [Google Scholar] [CrossRef]
- Abelló, S.; Bolshak, E.; Montané, D. Ni-Fe catalysts derived from hydrotalcite-like precursors for hydrogen production by ethanol steam reforming. Appl. Catal. A 2013, 450, 261–274. [Google Scholar] [CrossRef]
- Jorgensen, A.D.; Picel, K.C.; Stamoudis, V.C. Prediction of gas chromatography flame ionization detector response factors from molecular structures. Anal. Chem. 1990, 62, 683–689. [Google Scholar] [CrossRef]
- Saint Laumer, J.-Y.; Leocata, S.; Tissot, E.; Baroux, L.; Kampf, D.M.; Merle, P.; Boschung, A.; Seyfried, M.; Chaintreau, A. Prediction of response factors for gas chromatography with flame ionization detection: Algorithm improvement, extension to silylated compounds, and application to the quantification of metabolites. J. Sep. Sci. 2015, 38, 3209–3217. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Brown, I.D. Bond valence theory. In Bond Valences; Brown, I., Poeppelmeier, K.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3, pp. 11–58. [Google Scholar]
- Vegard, L. Die konstitution der mischkristalle und die raumfüllung der atome. Z. Physik 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Clause, O.; Coelho, M.G.; Gazzano, M.; Matteuzzi, D.; Trifirò, F.; Vaccari, A. Synthesis and thermal reactivity of nickel-containing anionic clays. Appl. Clay Sci. 1993, 8, 169–186. [Google Scholar] [CrossRef]
- Mas, V.; Baronetti, G.; Amadeo, N.; Laborde, M. Ethanol steam reforming using Ni(II)-Al(III) layered double hydroxide as catalyst precursor. Kinetic study. Chem. Eng. J. 2008, 138, 602–667. [Google Scholar]
- Abelló, D.; Berrueco, C.; Gispert-Guirado, F.; Montané, D. Synthetic natural gas by direct CO2 hydrogenation on activated takovites: Effect of Ni/Al molar ratio. Catal. Sci. Technol. 2016, 6, 2305–2317. [Google Scholar] [CrossRef]
- Puxley, D.C.; Kitchener, I.J.; Komodromos, C.; Parkyns, N.D. The effect of preparation method upon the structures, stability and metal/support interactions in nickel/alumina catalysts. Stud. Surf. Sci. Catal. 1983, 16, 237–271. [Google Scholar]
- Gavilà, L.; Lähde, A.; Jokiniemi, J.; Constanti, M.; Medina, F.; del Río, E.; Tichit, D.; Álvarez, M.G. Insights on the One-Pot Formation of 1,5-Pentanediol from Furfural with Co–Al Spinel-based Nanoparticles as an Alternative to Noble Metal Catalysts. ChemCatChem 2019, 11, 4944–4953. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Q.; Guan, Y.; Wu, P. Facile synthesis of furfuryl ethyl ether in high yield via the reductive etherification of furfural in ethanol over Pd/C under mild conditions. Green Chem. 2018, 20, 2110–2117. [Google Scholar] [CrossRef]
- He, J.; Nielsen, M.R.; Hansen, T.W.; Yang, S.; Riisager, A. Hierarchically constructed NiO with improved performance for catalytic transfer hydrogenation of biomass-derived aldehydes. Catal. Sci. Technol. 2019, 9, 1289–1300. [Google Scholar] [CrossRef]
- Ramos, R.; Peixoto, A.F.; Arias-Serrano, B.I.; Soares, O.S.G.P.; Pereira, M.F.R.; Kubička, D.; Freire, C. Catalytic transfer hydrogenation of furfural over Co3O4-Al2O3 hydrotalcite-derived catalyst. ChemCatChem 2020, 12, 1467–1475. [Google Scholar] [CrossRef]
- Jorge, E.Y.; Lima, T.D.M.; Lima, C.G.; Marchini, L.; Castelblanco, W.N.; Rivera, D.G.; Urquieta-Gonzalez, E.A.; Varma, R.S.; Paixão, M.W. Metal-exchanged magnetic β-zeolites: Valorization of lignocellulosic biomass-derived compounds to platform chemicals. Green Chem. 2017, 19, 3856–3868. [Google Scholar] [CrossRef]
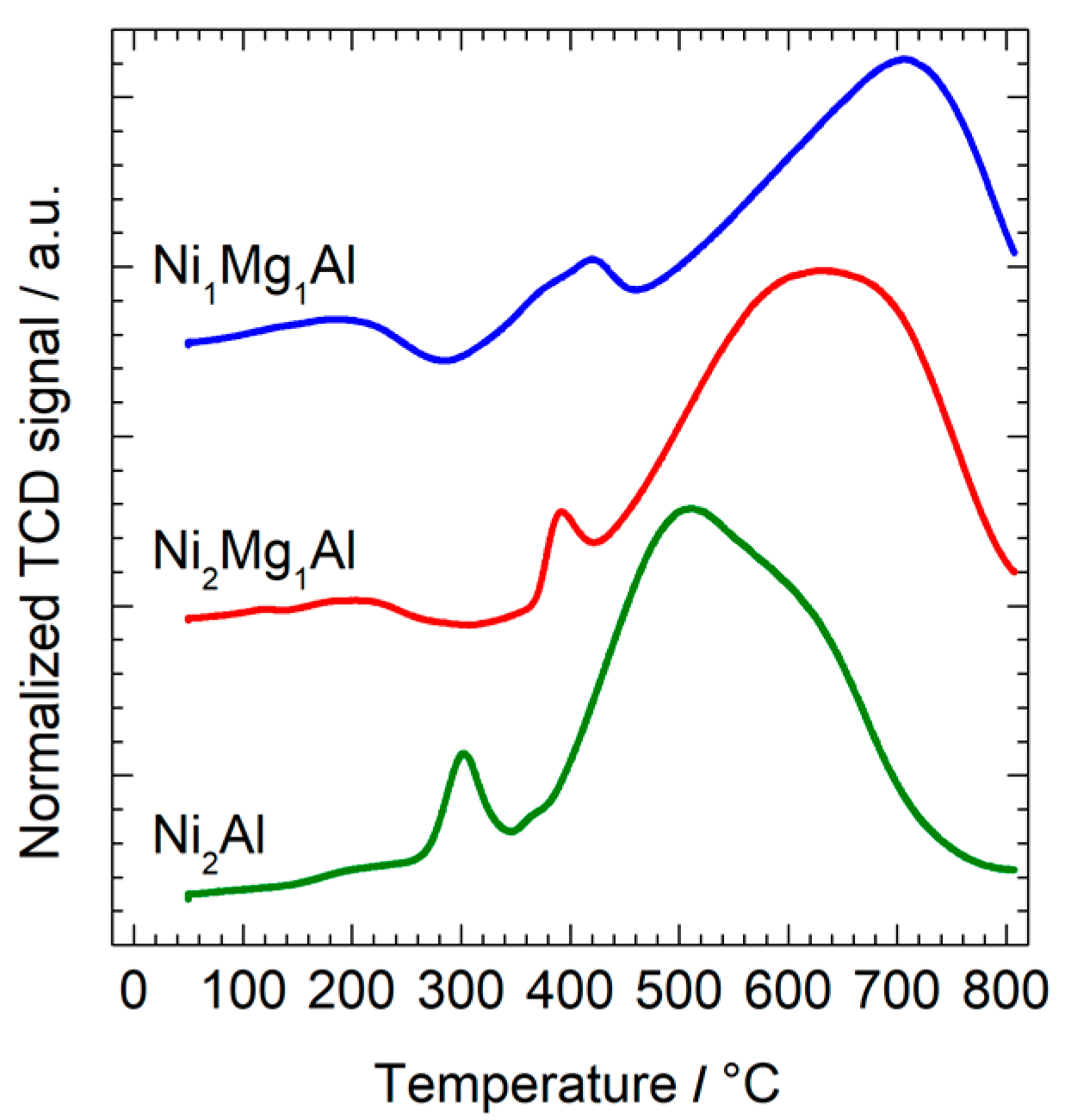
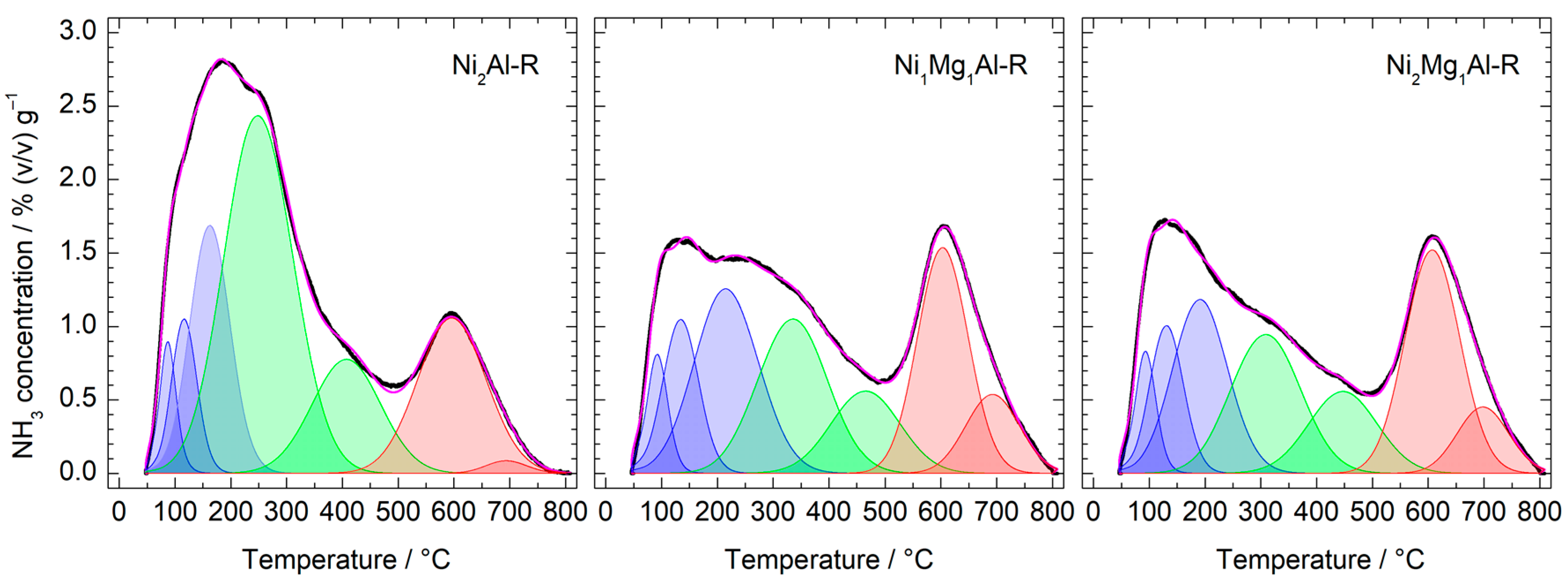
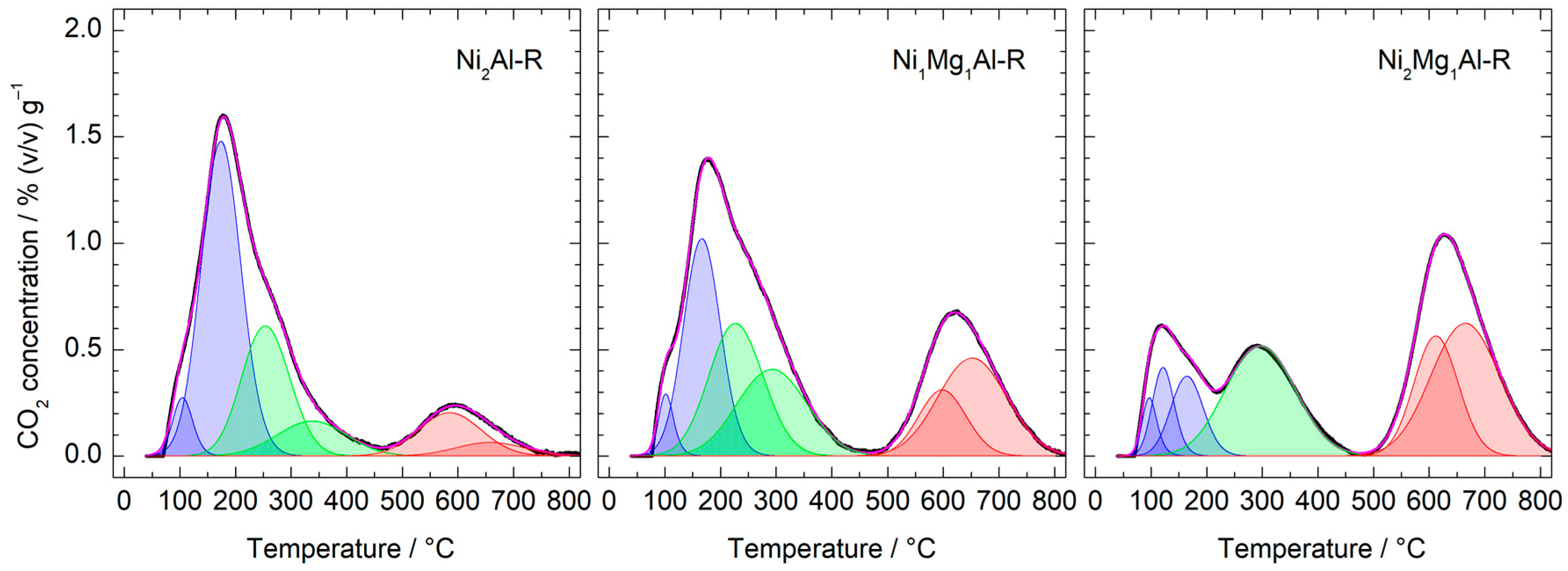


| Calcined Material | ICP-EOS | FESEM-EDX | ||
|---|---|---|---|---|
| Ni/Al | Mg/Al | Ni/Al | Mg/Al | |
| Ni2Al | 1.83 | - | 2.05 | - |
| Ni2Mg1Al | 1.90 | 1.11 | 1.27 | 0.82 |
| Ni1Mg1Al | 0.90 | 0.98 | 0.96 | 1.02 |
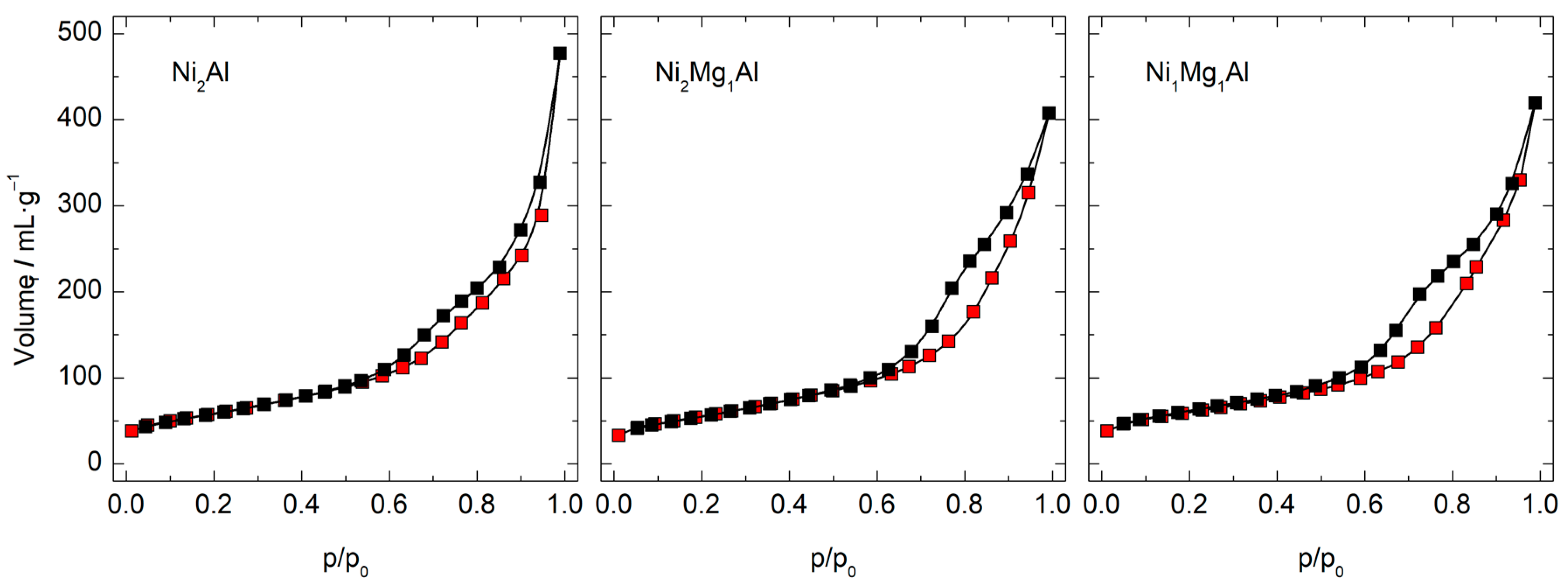
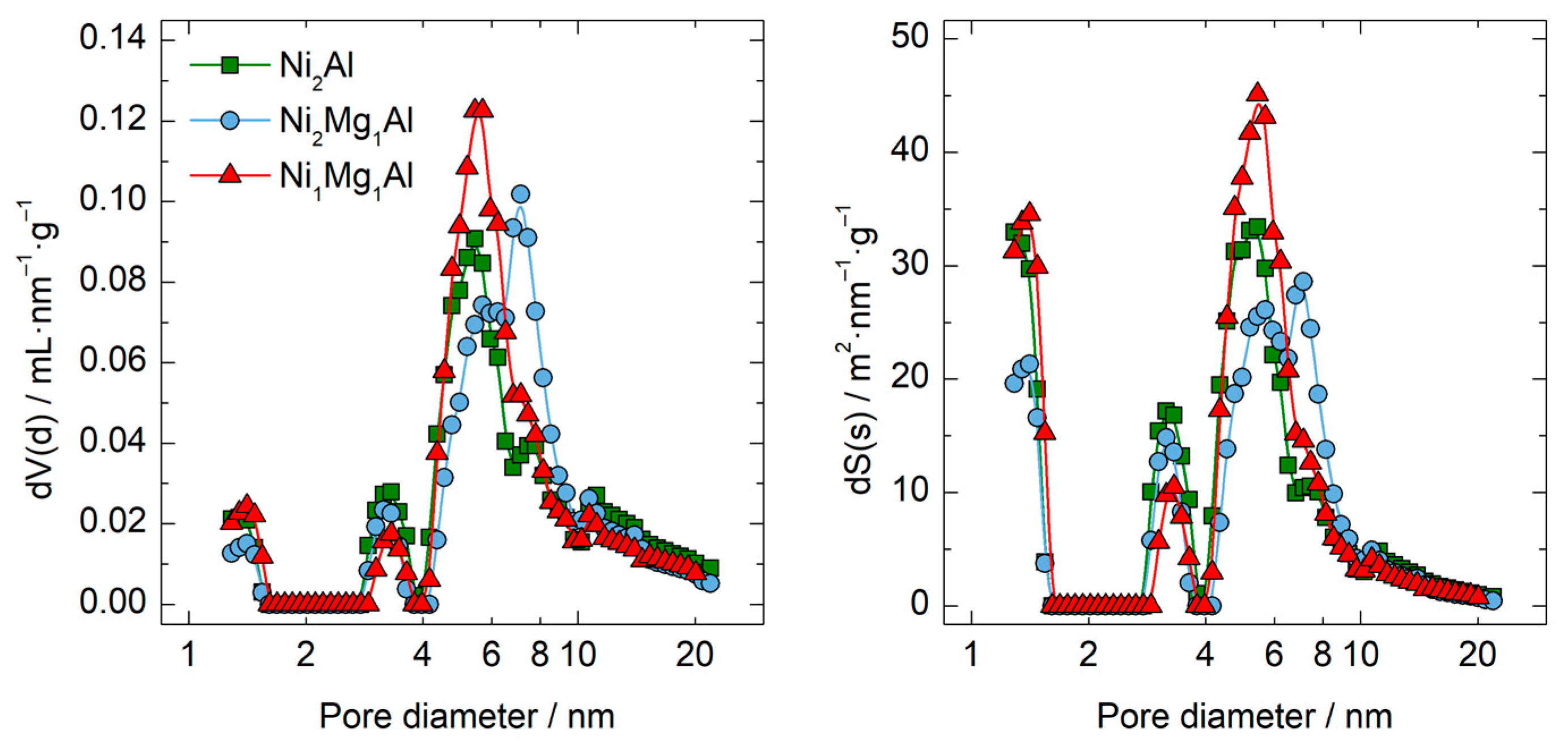
| Calcined Material | BET Surface Area (m2·g−1) | DFT Pore Volume (mL·g−1) | DFT Pore Diameter (nm) |
|---|---|---|---|
| Ni2Al | 210 | 0.49 | 5.4 |
| Ni2Mg1Al | 200 | 0.50 | 7.1 |
| Ni1Mg1Al | 212 | 0.48 | 5.4 |
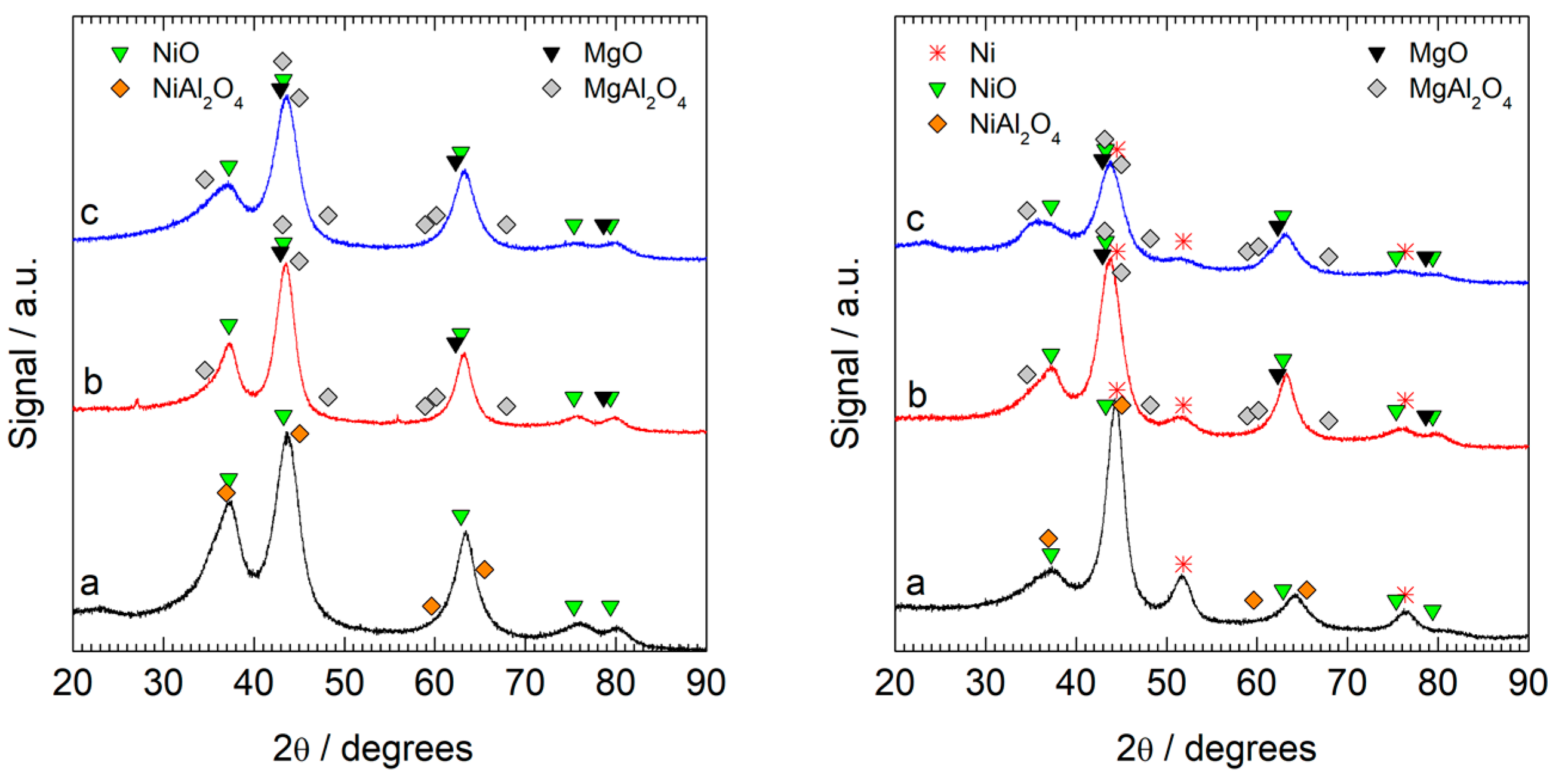
| Composition (XRD %) | Phases | |||||
|---|---|---|---|---|---|---|
| Samples | Al | Mg | Ni | Identified | wt.% | Crystallite Size (nm) |
| Ni2Al | 13.6 | - | 57.6 | (Al0.85Ni0.15)[Al0.55Ni0.45]2O4 | 46 | 1.3 |
| NiO | 54 | 2.2 | ||||
| Ni2Mg1Al | 10.5 | 13.6 | 33.0 | Ni0.3Mg0.7[Al]2O4 | 42 | 1.2 |
| Ni0.49Mg0.51O | 58 | 2.8 | ||||
| Ni1Mg1Al | 7.7 | 13.0 | 18.8 | Ni0.5Mg0.5[Al]2O4 | 46 | 0.7 |
| Ni0.31Mg0.69O | 54 | 2.1 | ||||
| Ni2Al-R | 18.3 | - | 59.0 | (Al0.90Ni0.10)[Al0.99Ni0.01]2O4 | 35 | 1.3 |
| NiO | 35 | 1.8 | ||||
| Ni | 30 | 3.4 | ||||
| Ni2Mg1Al-R | 15.1 | 16.8 | 37.1 | Mg[Al]2O4 | 40 | 1.3 |
| Ni0.51Mg0.49O | 48 | 2.8 | ||||
| Ni | 12 | 2.8 | ||||
| Ni1Mg1Al-R | 16.5 | 18.8 | 30.3 | Mg[Al]2O4 | 44 | 1.3 |
| Ni0.44Mg0.56O | 50 | 1.5 | ||||
| Ni | 6.2 | 3.6 | ||||
| Calcined Material | Peak 1 | Peak 2 | Total | ||
|---|---|---|---|---|---|
| T (°C) | H2 (mmol·g−1) | T (°C) | H2 (mmol·g−1) | H2 (mmol·g−1) | |
| Ni2Al | 302 | 0.31 | 513 | 6.77 | 7.08 |
| Ni2Mg1Al | 392 | 0.31 | 630 | 5.67 | 5.98 |
| Ni1Mg1Al | 422 | 0.58 | 706 | 4.29 | 4.87 |
| Catalyst | Concentration (mmol NH3·g−1) | Surface Density (µmol NH3·m−2) | ||||
|---|---|---|---|---|---|---|
| Weak (α) | Medium (β) | Total | Weak (α) | Medium (β) | Total | |
| Ni2Al-R | 0.34 | 0.66 | 1.00 | 1.62 | 3.14 | 4.76 |
| Ni1Mg1Al-R | 0.41 | 0.33 | 0.74 | 1.93 | 1.56 | 3.49 |
| Ni2Mg1Al-R | 0.36 | 0.31 | 0.67 | 1.80 | 1.55 | 3.35 |
| Catalyst | Concentration (mmol CO2·g−1) | Surface Density (µmol CO2·m−2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Weak (α) | Medium (β) | Strong (γ) | Total | Weak (α) | Medium (β) | Strong (γ) | Total | |
| Ni2Al-R | 0.20 | 0.12 | 0.05 | 0.37 | 0.96 | 0.57 | 0.24 | 1.78 |
| Ni1Mg1Al-R | 0.13 | 0.19 | 0.14 | 0.46 | 0.62 | 0.87 | 0.66 | 2.15 |
| Ni2Mg1Al-R | 0.04 | 0.14 | 0.20 | 0.39 | 0.20 | 0.72 | 1.01 | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldureid, A.; Montané, D.; Llorca, J.; Medina, F. Ni-Mg/Al Mixed Oxides Prepared from Layered Double Hydroxides as Catalysts for the Conversion of Furfural to Tetrahydrofurfuryl Alcohol. Chemistry 2023, 5, 571-588. https://doi.org/10.3390/chemistry5010041
Aldureid A, Montané D, Llorca J, Medina F. Ni-Mg/Al Mixed Oxides Prepared from Layered Double Hydroxides as Catalysts for the Conversion of Furfural to Tetrahydrofurfuryl Alcohol. Chemistry. 2023; 5(1):571-588. https://doi.org/10.3390/chemistry5010041
Chicago/Turabian StyleAldureid, Abdulaziz, Daniel Montané, Jordi Llorca, and Francesc Medina. 2023. "Ni-Mg/Al Mixed Oxides Prepared from Layered Double Hydroxides as Catalysts for the Conversion of Furfural to Tetrahydrofurfuryl Alcohol" Chemistry 5, no. 1: 571-588. https://doi.org/10.3390/chemistry5010041
APA StyleAldureid, A., Montané, D., Llorca, J., & Medina, F. (2023). Ni-Mg/Al Mixed Oxides Prepared from Layered Double Hydroxides as Catalysts for the Conversion of Furfural to Tetrahydrofurfuryl Alcohol. Chemistry, 5(1), 571-588. https://doi.org/10.3390/chemistry5010041










