Kinetic and Spectroscopic Studies of Methyl Ester Promoted Methanol Dehydration to Dimethyl Ether on ZSM-5 Zeolite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
2.3. Kinetic Tests
2.4. FT-IR Spectroscopic Analysis
2.5. NMR Spectroscopic Analysis
3. Results and Discussion
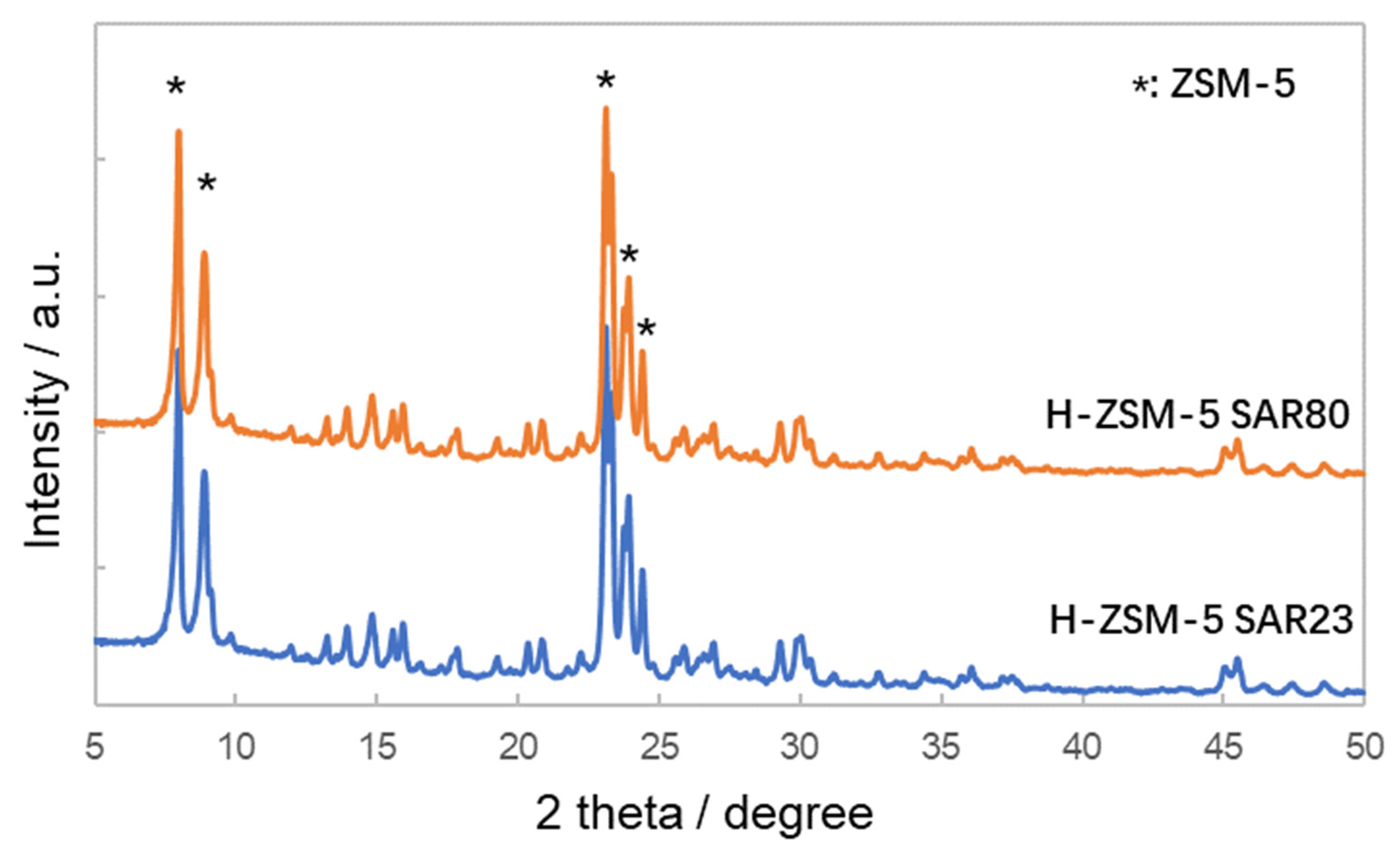
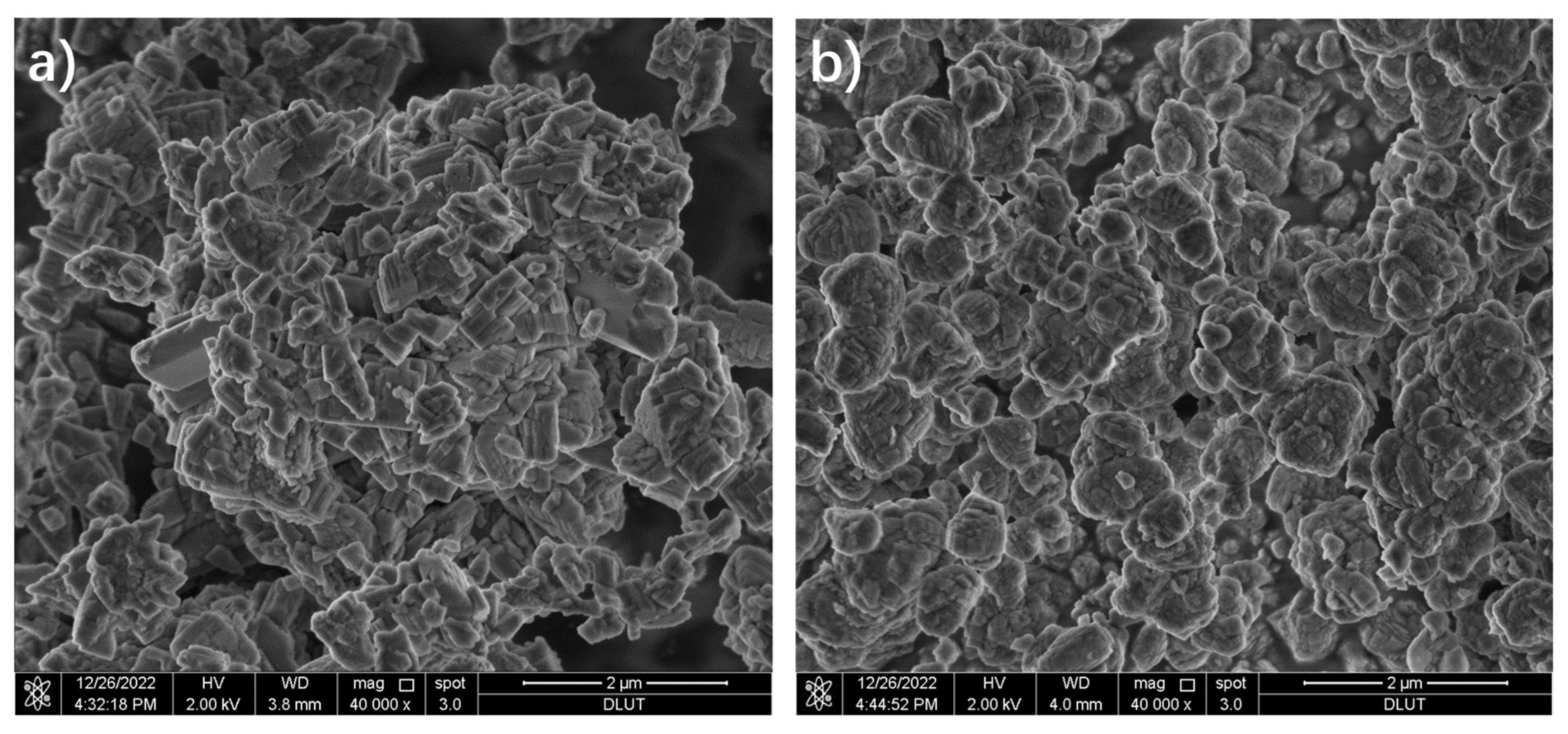
3.1. Characterization
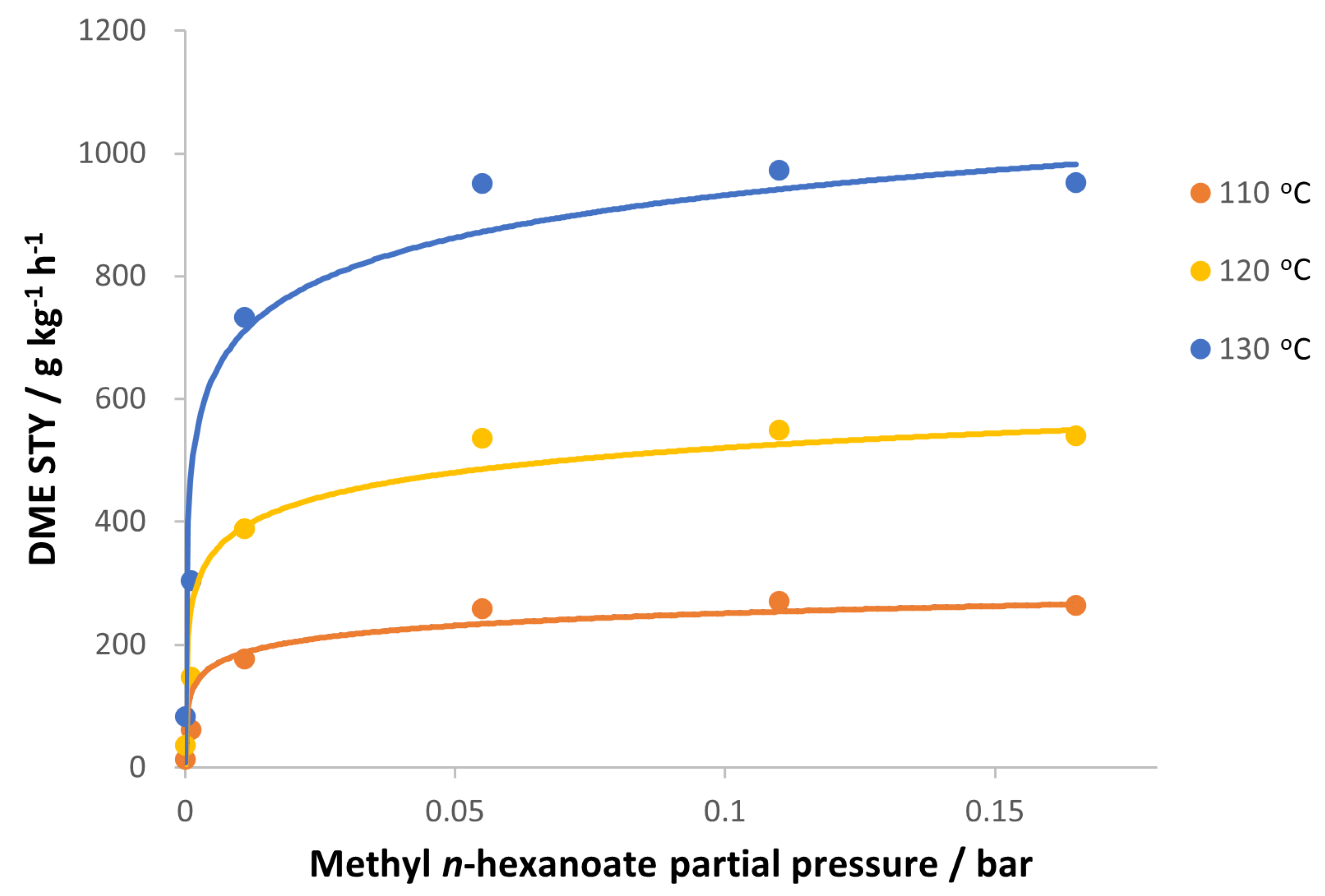
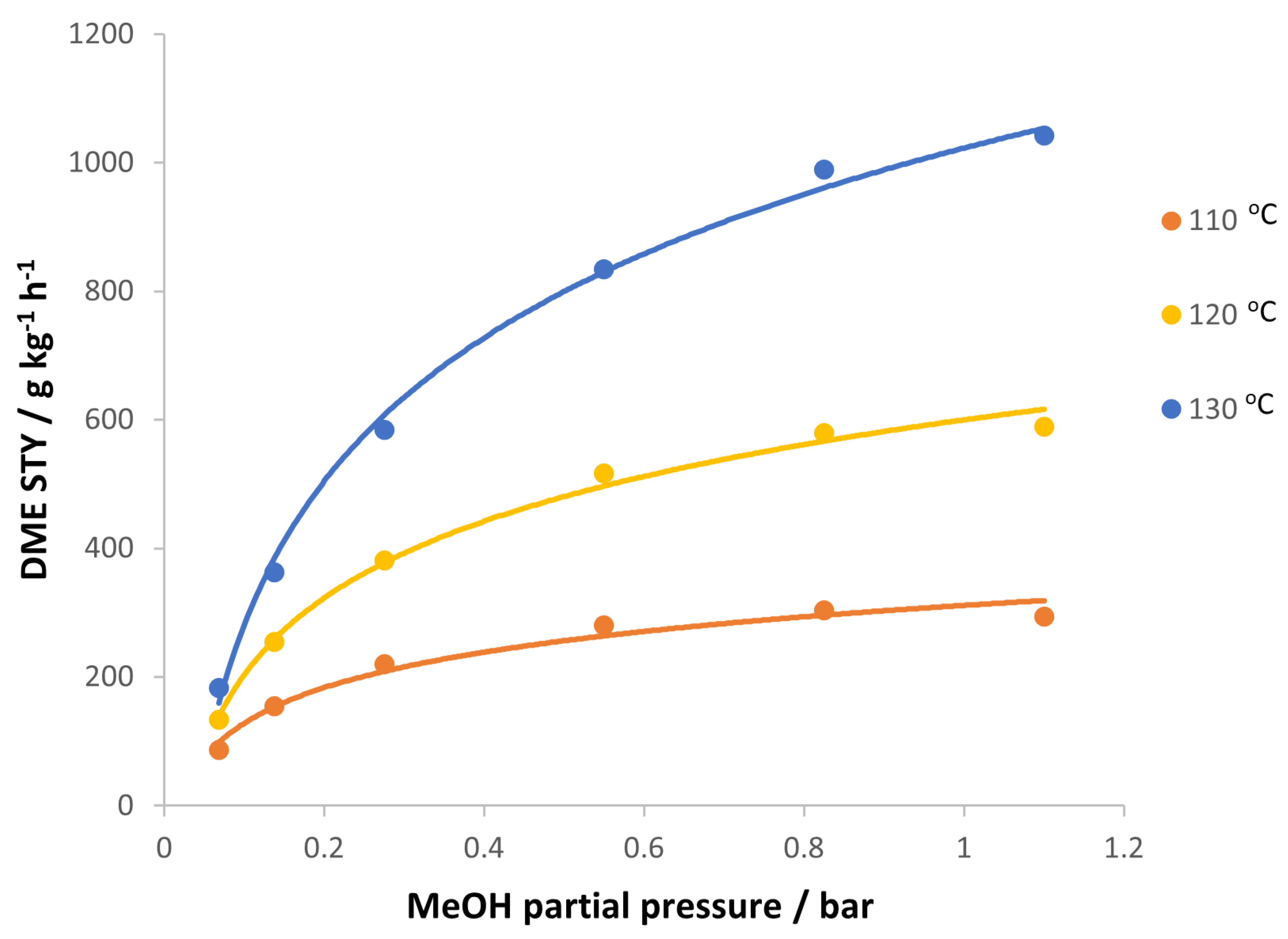
3.2. Reaction Kinetic of Promoted Methanol Dehydration to DME
3.3. FT-IR Study
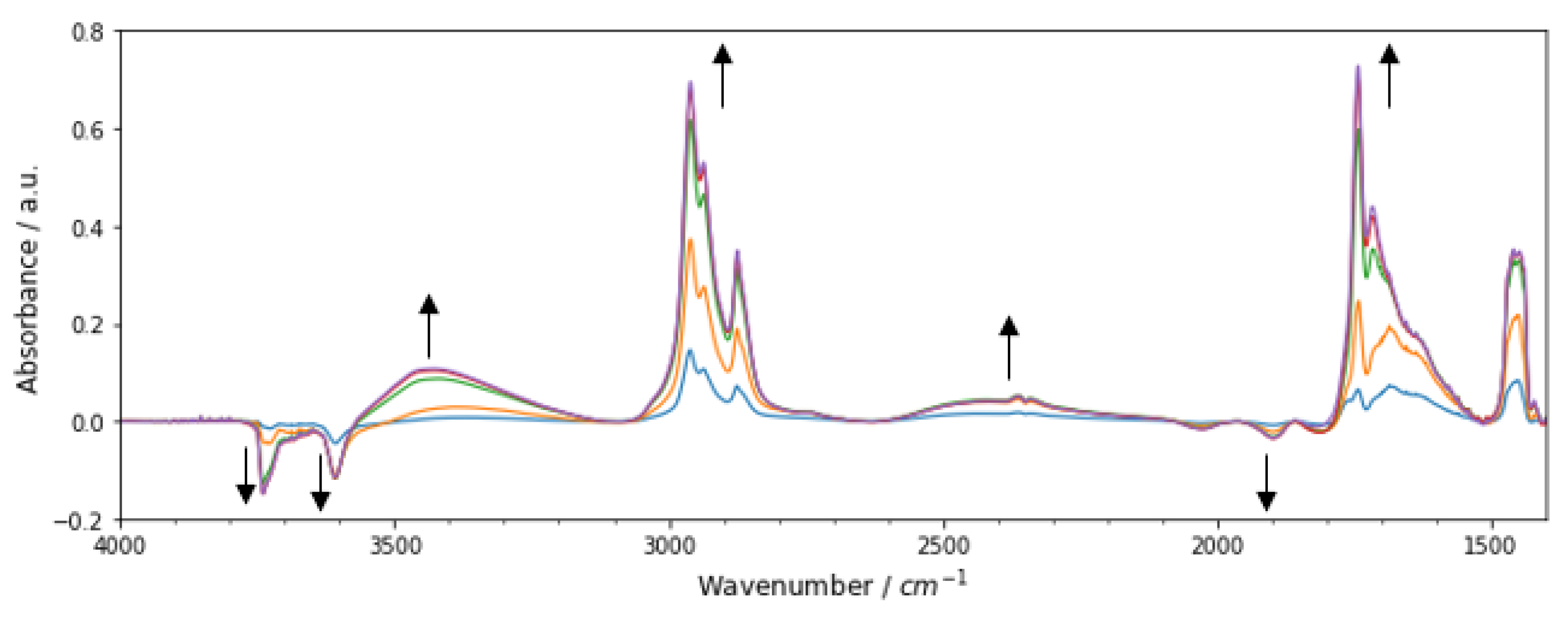
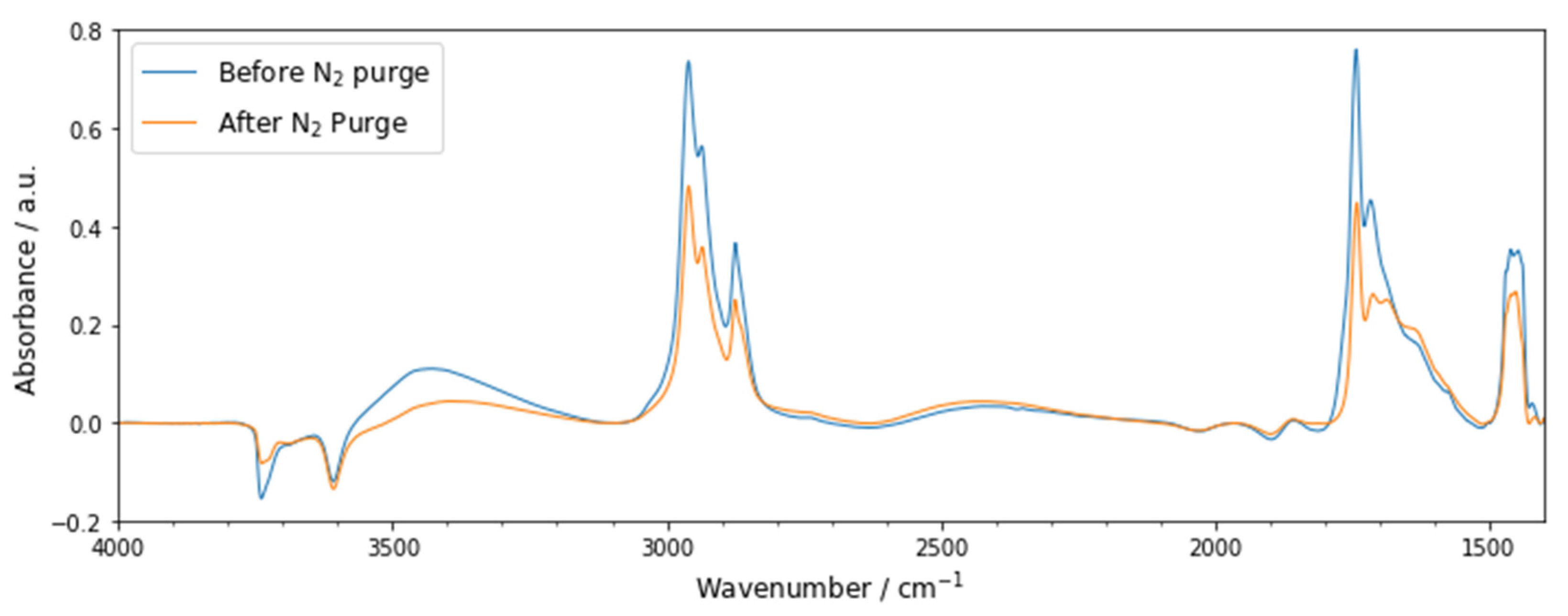
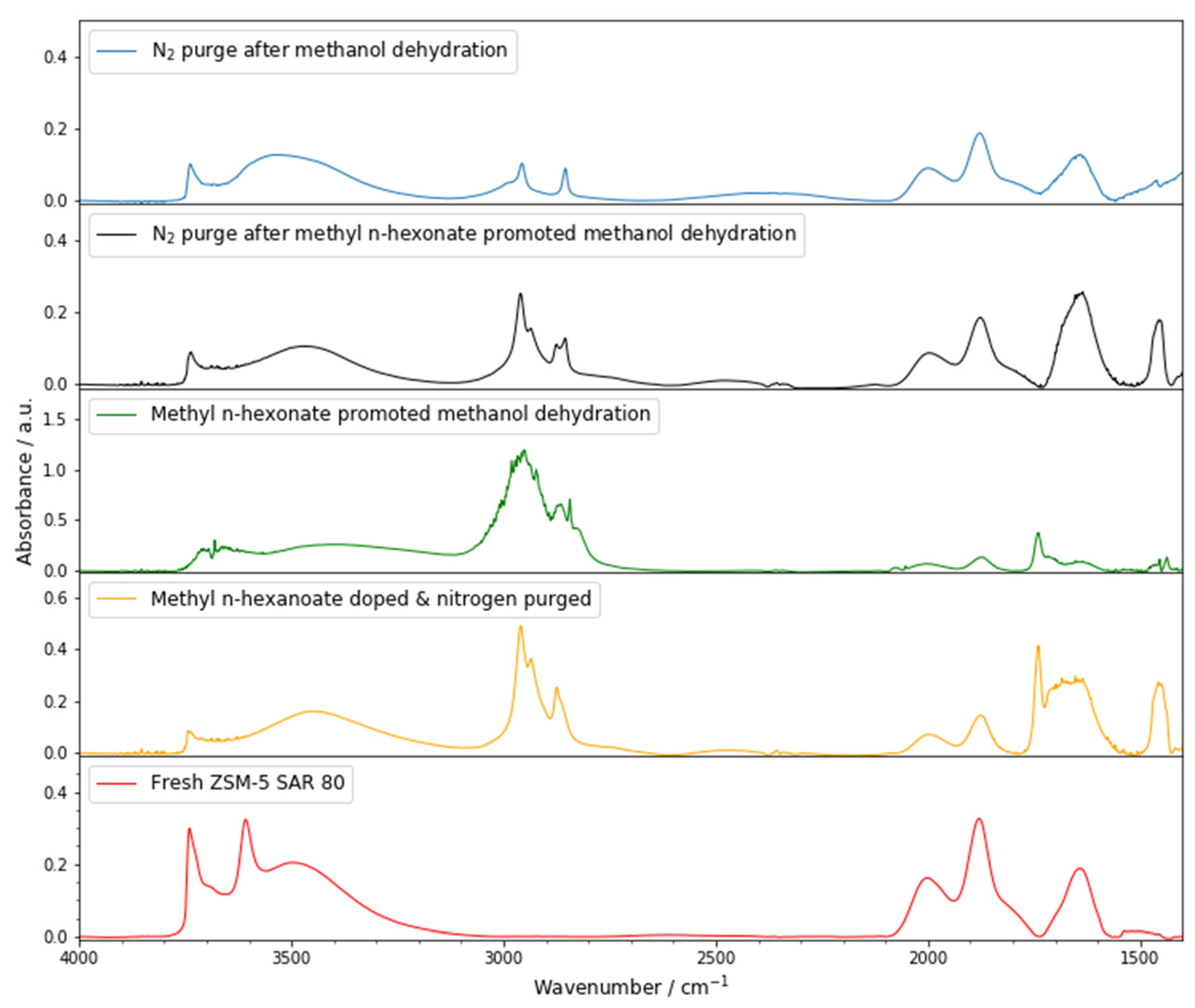
3.3.1. Methyl n-Hexanoate Adsorption
3.3.2. In-Situ FT-IR of Methanol Dehydration to DME over Methyl n-Hexanoate-Doped Zeolite
3.4. NMR Study
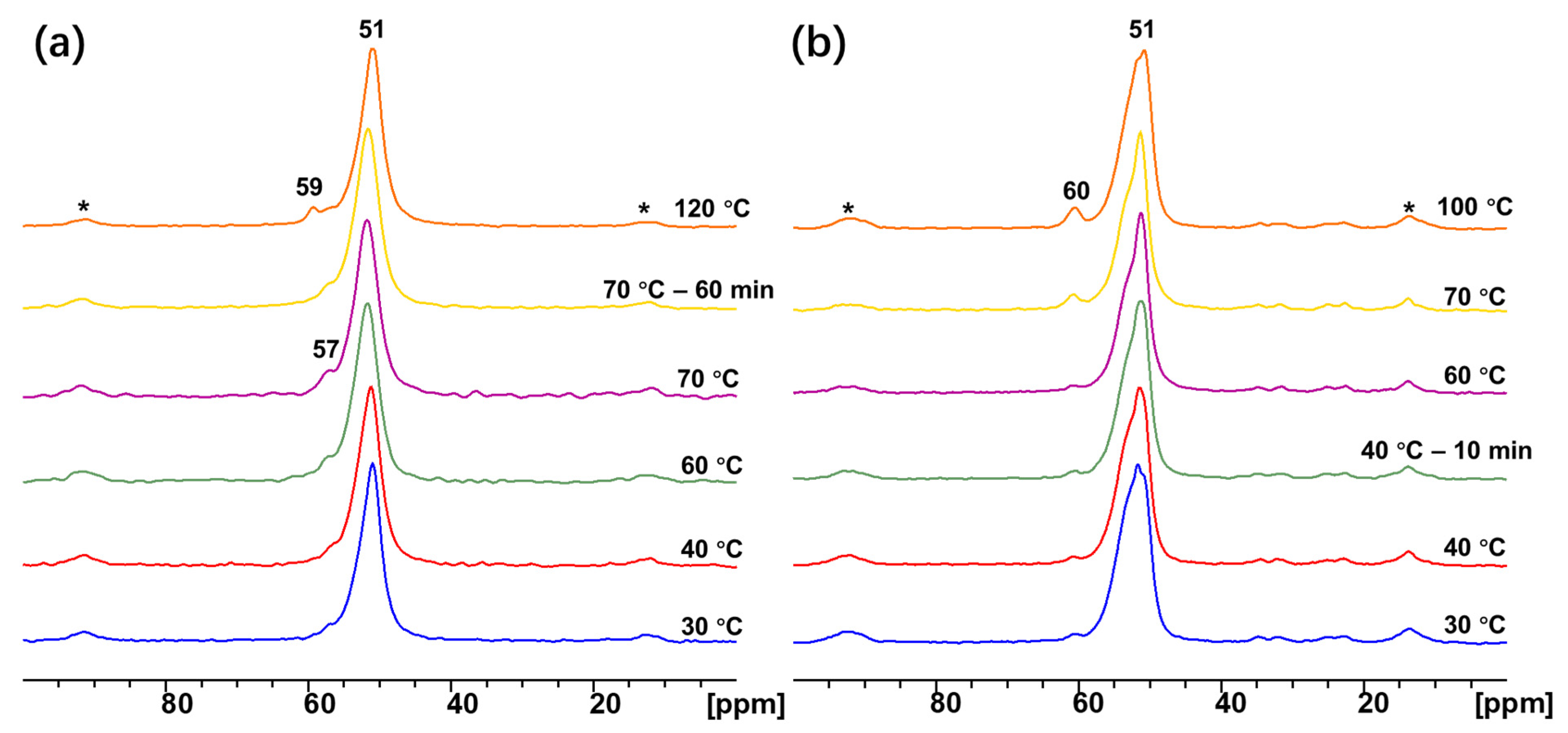
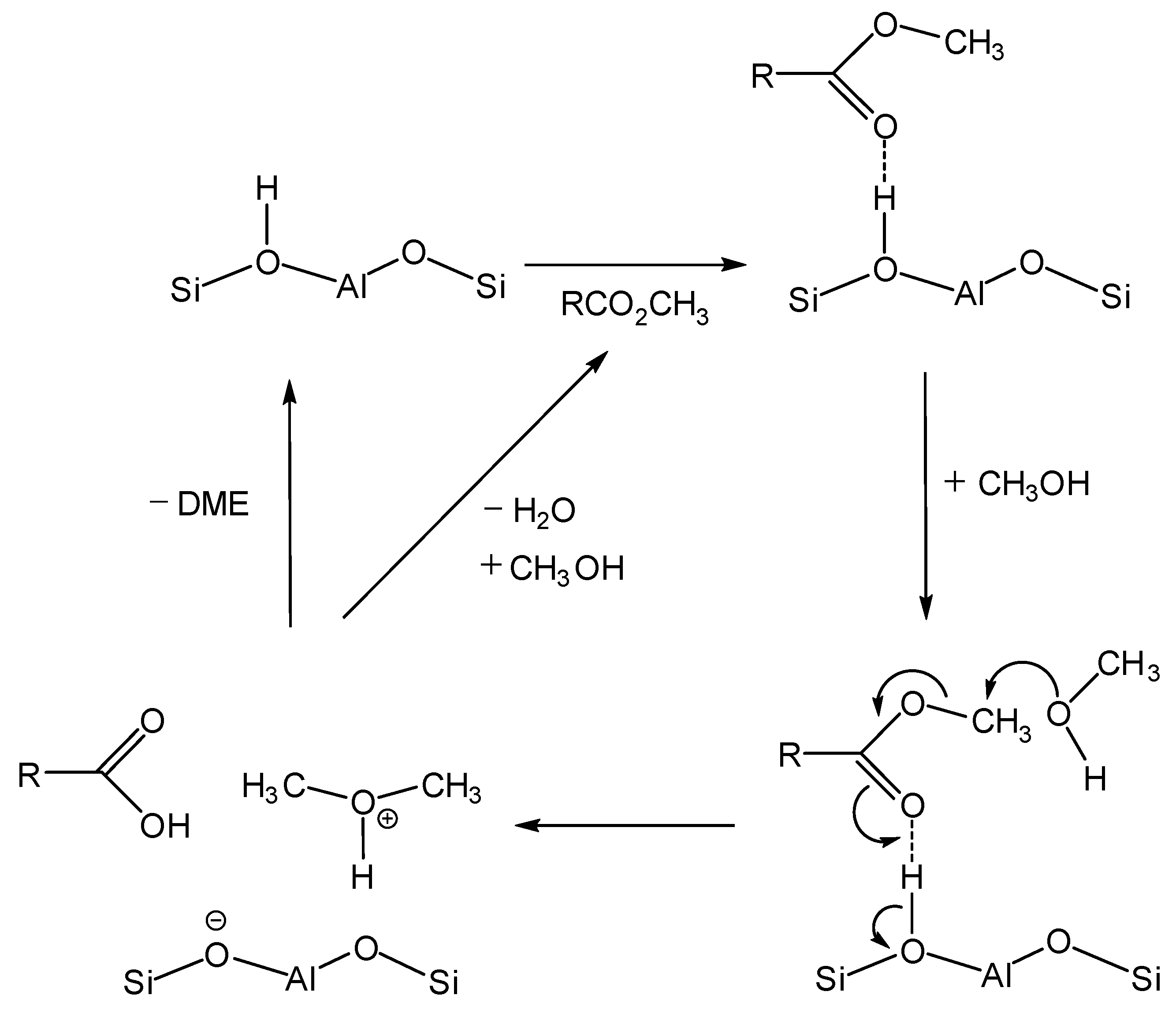
3.4.1. In-Situ Solid-State NMR
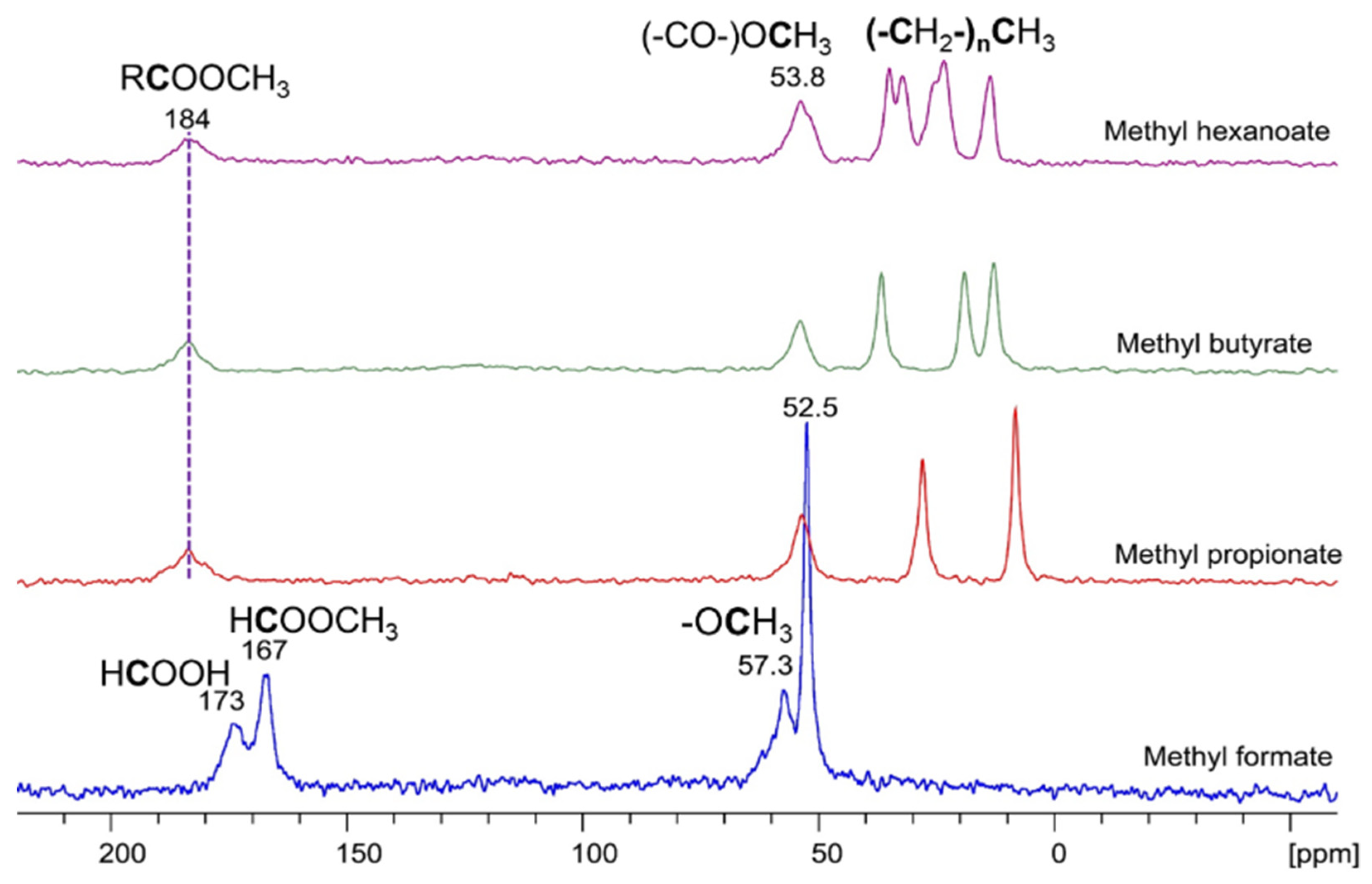
3.4.2. Adsorption of Methyl Esters with Different Alkyl Chain Lengths
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Corma, A.; Zones, S.; Cejka, J. Zeolites and Catalysis; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Vermeiren, W.; Gilson, J.-P. Impact of Zeolites on the Petroleum and Petrochemical Industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Yilmaz, B.; Trukhan, N.; Müller, U. Industrial Outlook on Zeolites and Metal Organic Frameworks. Chin. J. Catal. 2012, 33, 3. [Google Scholar] [CrossRef]
- Abate, S.; Barbera, K.; Centi, G.; Lanzafame, P.; Perathoner, S. Disruptive catalysis by zeolites. Catal. Sci. Technol. 2016, 6, 2485–2501. [Google Scholar] [CrossRef]
- Gounder, R.; Iglesia, E. The Roles of Entropy and Enthalpy in Stabilizing Ion-Pairs at Transition States in Zeolite Acid Catalysis. Accounts Chem. Res. 2012, 45, 229–238. [Google Scholar] [CrossRef]
- Bhan, A.; Iglesia, E. A Link between Reactivity and Local Structure in Acid Catalysis on Zeolites. Acc. Chem. Res. 2008, 41, 559–567. [Google Scholar] [CrossRef]
- Jones, A.J.; Zones, S.I.; Iglesia, E. Implications of Transition State Confinement within Small Voids for Acid Catalysis. J. Phys. Chem. C 2014, 118, 17787–17800. [Google Scholar] [CrossRef]
- Bhan, A.; Allian, A.D.; Sunley, G.J.; Law, D.J.; Iglesia, E. Specificity of Sites within Eight-Membered Ring Zeolite Channels for Carbonylation of Methyls to Acetyls. J. Am. Chem. Soc. 2007, 129, 4919–4924. [Google Scholar] [CrossRef]
- Blaszkowski, S.R.; Van Santen, R.A. The mechanism of dimethyl ether formation from methanol catalyzed by zeolitic protons. J. Am. Chem. Soc. 1996, 118, 5152–5153. [Google Scholar] [CrossRef]
- Blaszkowski, S.R.; Van Santen, R.A. Theoretical study of the mechanism of surface methoxy and dimethyl ether formation from methanol catalyzed by zeolitic protons. J. Phys. Chem. B 1997, 101, 2292–2305. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. Kinetic, Spectroscopic, and Theoretical Assessment of Associative and Dissociative Methanol Dehydration Routes in Zeolites. Angew. Chem. Int. Ed. 2014, 53, 12177–12181. [Google Scholar] [CrossRef]
- Moses, P.G.; Nørskov, J.K. Methanol to Dimethyl Ether over ZSM-22: A Periodic Density Functional Theory Study. ACS Catal. 2013, 3, 735–745. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Park, M.-J.; Lee, W.B. Microkinetic modeling of DME synthesis from methanol over H-zeolite catalyst: Associative vs. dissociative pathways. Catal. Today 2021, 375, 314–323. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Paioni, A.L.; Houben, K.; Whiting, G.T.; Baldus, M.; Weckhuysen, B.M. Bridging the Gap between the Direct and Hydrocarbon Pool Mechanisms of the Methanol-to-Hydrocarbons Process. Angew. Chem. Int. Ed. 2018, 57, 8095–8099. [Google Scholar] [CrossRef] [PubMed]
- Haw, J.F.; Song, W.; Marcus, D.M.; Nicholas, J.B. The Mechanism of Methanol to Hydrocarbon Catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Ilias, S.; Khare, R.; Malek, A.; Bhan, A. A descriptor for the relative propagation of the aromatic- and olefin-based cycles in methanol-to-hydrocarbons conversion on H-ZSM-5. J. Catal. 2013, 303, 135–140. [Google Scholar] [CrossRef]
- McCann, D.M.; Lesthaeghe, D.; Kletnieks, P.W.; Guenther, D.R.; Hayman, M.J.; Van Speybroeck, V.; Waroquier, M.; Haw, J.F. A Complete Catalytic Cycle for Supramolecular Methanol-to-Olefins Conversion by Linking Theory with Experiment. Angew. Chem. Int. Ed. 2008, 47, 5179–5182. [Google Scholar] [CrossRef]
- Dennis-Smither, B.J.; Yang, Z.; Buda, C.; Liu, X.; Sainty, N.; Tan, X.; Sunley, G.J. Getting zeolite catalysts to play your tune: Methyl carboxylate esters as switchable promoters for methanol dehydration to DME. Chem. Commun. 2019, 55, 13804–13807. [Google Scholar] [CrossRef]
- Zeolyst International. ZSM-5. Available online: https://www.zeolyst.com/our-products/standard-zeolite-powders/zsm-5.html (accessed on 10 January 2023).
- Phung, T.K.; Carnasciali, M.M.; Finocchio, E.; Busca, G. Catalytic conversion of ethyl acetate over faujasite zeolites. Appl. Catal. A Gen. 2014, 470, 72–80. [Google Scholar] [CrossRef]
- Fujino, T.; Kashitani, M.; Kondo, J.; Domen, K.; Hirose, C.; Ishida, M.; Goto, F.; Wakabayashi, F. FT-IR and Quantum Chemical Studies of the Interaction between Dimethyl Ether and HZSM-5 Zeolite. J. Phys. Chem. 1996, 100, 11649–11653. [Google Scholar] [CrossRef]
- Wakabayashi, F.; Kondo, J.N.; Domen, K.; Hirose, C. FT-IR Study of H218O Adsorption on H-ZSM-5: Direct Evidence for the Hydrogen-Bonded Adsorption of Water. J. Phys. Chem. 1996, 100, 1442–1444. [Google Scholar] [CrossRef]
- Nolin, B.; Jones, R.N. The Infrared Absorption Spectra of Deuterated Esters: I. Methyl Acetate. Can. J. Chem. 1956, 34, 1382–1391. [Google Scholar] [CrossRef]
- Docquir, F.; Toufar, H.; Su, B. Infrared Evidence of Three Types of Interaction between Methylamine and a Series of Alkali Cation Exchanged Faujasite Zeolites. Langmuir 2001, 17, 6282–6288. [Google Scholar] [CrossRef]
- Phung, T.K.; Casazza, A.A.; Aliakbarian, B.; Finocchio, E.; Perego, P.; Busca, G. Catalytic conversion of ethyl acetate and acetic acid on alumina as models of vegetable oils conversion to biofuels. Chem. Eng. J. 2013, 215, 838–848. [Google Scholar] [CrossRef]
- Mowla, O.; Kennedy, E.; Stockenhuber, M. In-situ FTIR study on the mechanism of both steps of zeolite-catalysed hydroesterification reaction in the context of biodiesel manufacturing. Fuel 2018, 232, 12–26. [Google Scholar] [CrossRef]
- Forester, T.R.; Howe, R.F. In situ FTIR studies of methanol and dimethyl ether in ZSM-5. J. Am. Chem. Soc. 1987, 109, 5076–5082. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Dennis-Smither, B.J.; Xu, Z.; Zhao, Z.; Guo, M.; Sainty, N.; Hou, G.; Liu, X.; Sunley, G.J. Kinetic and Spectroscopic Studies of Methyl Ester Promoted Methanol Dehydration to Dimethyl Ether on ZSM-5 Zeolite. Chemistry 2023, 5, 511-525. https://doi.org/10.3390/chemistry5010037
Yang Z, Dennis-Smither BJ, Xu Z, Zhao Z, Guo M, Sainty N, Hou G, Liu X, Sunley GJ. Kinetic and Spectroscopic Studies of Methyl Ester Promoted Methanol Dehydration to Dimethyl Ether on ZSM-5 Zeolite. Chemistry. 2023; 5(1):511-525. https://doi.org/10.3390/chemistry5010037
Chicago/Turabian StyleYang, Zhiqiang, Benjamin J. Dennis-Smither, Zhuoran Xu, Zhenchao Zhao, Meiling Guo, Neil Sainty, Guangjin Hou, Xuebin Liu, and Glenn J. Sunley. 2023. "Kinetic and Spectroscopic Studies of Methyl Ester Promoted Methanol Dehydration to Dimethyl Ether on ZSM-5 Zeolite" Chemistry 5, no. 1: 511-525. https://doi.org/10.3390/chemistry5010037
APA StyleYang, Z., Dennis-Smither, B. J., Xu, Z., Zhao, Z., Guo, M., Sainty, N., Hou, G., Liu, X., & Sunley, G. J. (2023). Kinetic and Spectroscopic Studies of Methyl Ester Promoted Methanol Dehydration to Dimethyl Ether on ZSM-5 Zeolite. Chemistry, 5(1), 511-525. https://doi.org/10.3390/chemistry5010037






