Abstract
The excessive utilization of petroleum diesel has led to the depletion of fossil resources and severe environmental pollution. Biodiesel produced from renewable triglycerides (TGs) or waste lipids is a low-emission fuel substitute for diesel. Biodiesel is mainly produced by transesterification reactions over homogeneous base catalysts with excellent activity and low cost. In comparison, solid base catalysts are more attractive due to their lower environmental impact and simpler production and purification processes. It remains a challenge to further improve the stability and activity of solid base catalysts. Because of the high surface area, superior stability, and tunable basicity, basic zeolites, especially two-dimensional zeolites, have emerged as promising solid basic catalysts for the transesterification of TGs. In this review, we present recent advancements in the synthesis, characterization, and catalytic performance of basic zeolites for the transesterification of TGs. Challenges and development prospects of basic zeolites for biodiesel production via transesterification are also pointed out. We expect that this review will inspire the more efficient and rational design of zeolites for sustainable fuel production.
1. Introduction
The limited reserves of resources, the rapid depletion and unsustainability of fossil energy, and the worsening of the greenhouse effect caused by burning fossil fuels are the main energy problems that need to be solved at present [1]. Therefore, the development of renewable energy alternatives is essential to maintain environmental sustainability. Biodiesel, a mixture of fatty acid esters, produced by esterification or transesterification reactions of renewable plant oil or animal lipids, has become one of the most promising renewable energy sources to date [2,3,4,5,6]. Compared with conventional diesel fuel, biodiesel has the same performance and engine durability, but has a higher flash point and is non-flammable and non-toxic, and thus safe to transport and store. Furthermore, biodiesel is free of sulfur and aromatics, so its pollutant emissions are not dangerous [7]. Since biodiesel is an excellent lubricant when blended with diesel, it can improve the lubricity of the diesel fuel blend and reduce the long-term wear of diesel engines [5]. Due to its attractive advantages, such as safety, biodegradability, renewability, and engine compatibility, biodiesel has become a blending component or direct substitute for fossil diesel. Currently, the worldwide production of biodiesel is increasing and has exceeded 30 million tons per year due to favorable government policies and incentives, such as fiscal subsidies and tax preferences [8]. Despite its recent success, biodiesel still holds a relatively small share of the current global diesel market, and economic feasibility remains a significant factor affecting its commercial production. The cost of producing biodiesel is generally higher than that of conventional diesel due to the high cost of feedstock and the relatively low yields of biodiesel per unit of feedstock. To tackle this challenge, industry and academic researchers are striving to develop high-performing catalysts that can enhance the efficiency of the biodiesel production process.
Nowadays, ionic liquids catalysis [9], enzyme bio-catalysis [10,11], and acid–base catalysis [7,12,13] are the main processes in research on biodiesel production. However, the preparation of ionic liquid catalysts often requires complicated steps and is more expensive than other types of catalysts. Similarly, the enzymatic catalysis process is effective and environmentally benign, but is also costly, which impedes its widespread utilization in the industry. Nowadays, the main industrial process for the production of biodiesel is utilizing strong bases as homogeneous catalysts in the transesterification reaction of vegetable oils. Although the homogenous base-catalyzed approach can be carried out industrially under mild conditions and give high catalytic performance, the liquid–base catalytic system generally suffers from the corrosion of catalytic instruments and yields stable emulsions and soaps. Moreover, the homogeneous base catalyst is difficult to remove from biofuel products, which leads to high costs and waste pollution [5]. Therefore, it is necessary to develop efficient and reusable heterogeneous basic catalysts for green and sustainable processes. The use of the solid base for the transesterification reaction not only can provide higher activity and selectivity as well as longer catalyst lifetime, but also can facilitate production efficiency, owing to the advantages of solid bases, such as non-corrosiveness, environmental friendliness, and fewer problems in their disposal.
Therefore, various solid basic catalysts, such as alkaline earth oxides, supported alkali metals/oxides, and hydrotalcites, have been extensively applied in the transesterification reaction [14,15,16,17]. However, the catalytic activity and efficiency of conventional solid base catalysts in the transesterification reactions are generally subject to their poor porosity, low specific surface area, and limited concentration of active sites [18]. Furthermore, the air tolerance of solid base catalysts is a critical criterion for accessing the application potential of catalysts. Nevertheless, most basic solid catalysts tend to be inactivated in the air with the chemical adsorption of carbon dioxide and water on the surface. Therefore, the design and development of porous basic catalysts with an ideal porous structure, adjustable surface hydrophobicity/hydrophilicity, and high stability have attracted wide research interests in the past decade [7,19].
Zeolites are a significant class of open-framework aluminosilicate materials with cages and channels, consisting of orderly distributed micropores in a molecular dimension [20,21,22,23,24,25]. The incorporation of the Al3+ ions into the zeolite framework generates a negative charge, which can be compensated by metal cations or protons to create the acid–basic character of the zeolites. Recently, zeolites have emerged as promising catalysts for the conversion of biomass into biofuels and other value-added chemicals [26,27]. Thanks to the adjustable acidity/basicity, large specific surface, tuneable hydrophobicity/hydrophilicity, and high stability, zeolites have become significant catalysts and support materials for biodiesel production via transesterification reactions [28,29,30,31].
Various types of zeolite catalysts have been used in transesterification reactions for biodiesel production. Acid zeolites are stable and will not be affected by the presence of water and free fatty acids in the catalytic system [7,32,33]. However, the reaction rates of acid zeolites for transesterification are so slow that it limits their commercial application [7]. Zeolite-supported enzymes have attracted attention due to their high selectivity and activity at relatively low temperatures [34], while the higher cost and longer reaction time of zeolite-supported enzymes have become resistant to being industrialized [11]. The basic zeolites show high activity and stability at relatively mild reaction conditions with low alcohol consumption, thus considered promising heterogeneous catalysts for transesterification reactions [35,36,37,38,39,40].
This review summarizes recent progress in basic zeolites for biodiesel production via transesterification, with a focus on preparation strategies of basic zeolites in the past decades. State-of-the-art characterization techniques are also presented to unravel how the basicity of zeolites impacts the basicity and porosity of zeolites and the catalytic performance for transesterification reactions of triglycerides. Furthermore, current challenges and future perspectives regarding the preparation, characterization, and catalysis of basic zeolites are also addressed. The review aims to advance the development of synthetic strategies for basic zeolites with high catalytic performance and expand the practical application of basic zeolites in the production of biodiesel and other chemicals.
2. Synthesis of Basic Zeolite Catalysts
2.1. Origin of Basicity in Zeolites
Zeolites are a class of crystalline inorganic materials that are constructed from TO4 (T = Si, Al, P, etc.) tetrahedra with each apical oxygen atom shared with an adjacent tetrahedron [20]. Replacing atoms in the zeolite framework with another with a lower valence bears negative charges on the framework, which need to be neutralized by protons or alkali metal cations. The appearance of the positive and negative charges creates the acidity or basicity of zeolites.
To date, the basic properties of zeolites have been mainly identified as two kinds, i.e., intrinsic basicity and additional basicity. The intrinsic basic character of zeolites relies on the negatively charged oxygen in the zeolite framework, which depends on both structure and chemical composition. Furthermore, additional basicity in zeolites may also originate from other sites, which are not framework oxygens, such as basic hydroxyls, oxide clusters, supported metals, etc.
The intrinsic basicity of a zeolite is generated by the compensated alkaline cations in the zeolite that can enhance the electron density of the framework oxygen, hence yielding basic character [41]. The strength of the basic sites depends on the chemical composition and framework structure of the zeolites, as well as the nature, content, location, and valency of the cations [42,43]. Furthermore, the interactive nature between the cations and zeolite framework affects the basicity of zeolites [42].
The additional basicity of zeolite materials may originate from other basic sites, such as basic hydroxyls, oxide clusters, supported metals, etc. [44,45]. Some basic metal oxides (Na2O, MgO, or CaO) can be dispersed on the zeolite via the decomposition of impregnated alkali metal precursors to form small clusters with basic properties. The basic metal oxides in zeolites not only perform as basic sites, but also can improve basic sites in the parent zeolites by enhancing the negative charge of the framework oxygen atoms [46].
2.2. Synthetic Strategies of Basic Zeolites
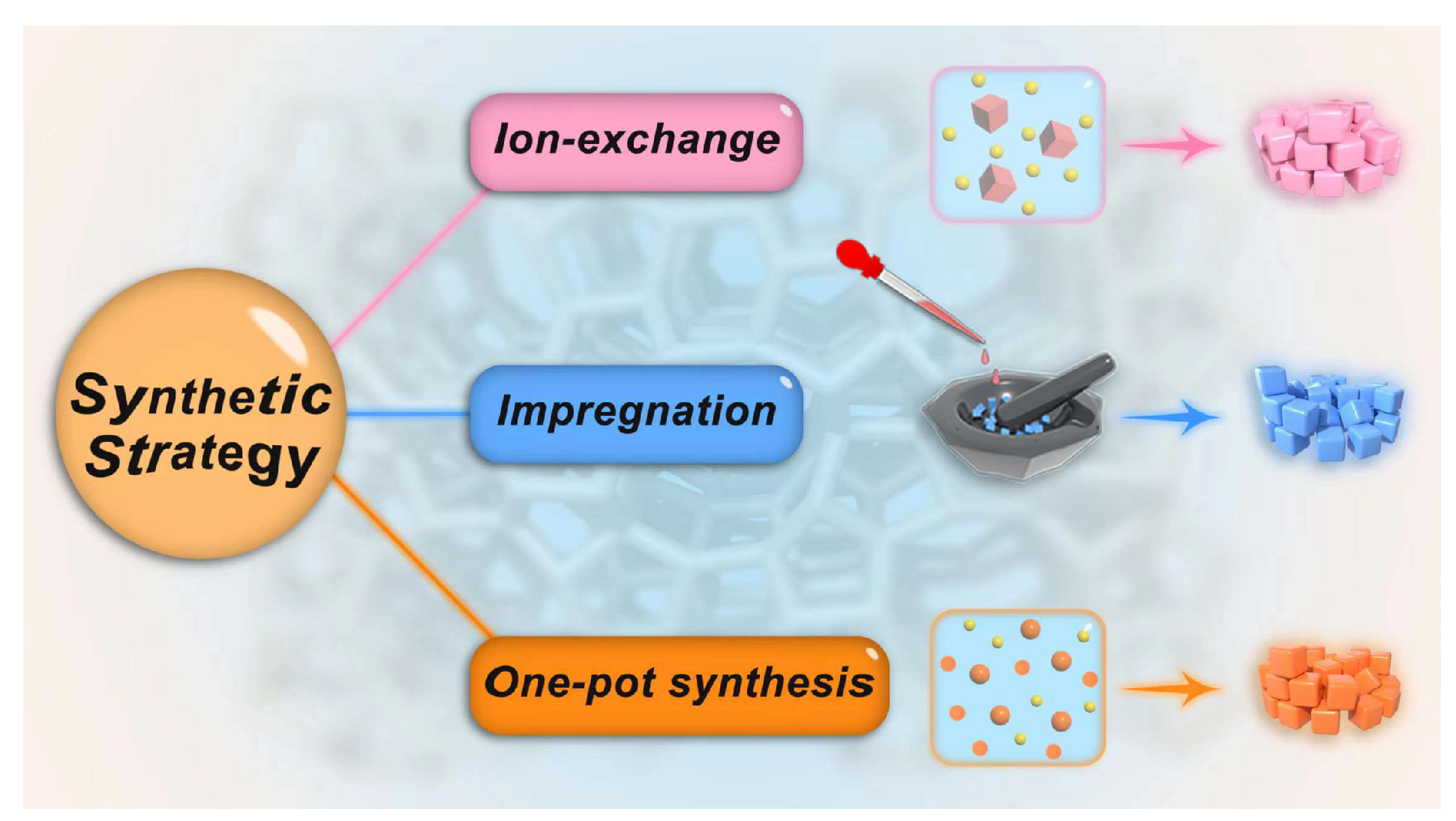
The ion exchange method and impregnation approach are widely used to prepare basic zeolites. The ion exchange approach relies on the introduction of alkaline cations into the zeolite framework, thereafter generating structural basicity by lowering the electronegativity of the zeolite framework. In the impregnation method, the solvated alkaline metal precursors are introduced into the zeolite followed by the calcination that can decompose the doped alkaline compound and yield basic sites in zeolites [21,28,29]. These alkaline species may possess their basic character, or they may modify the basicity of the zeolite framework by an interaction. Herein, some representative synthesis methods (Figure 1) of basic zeolites for transesterification reactions are presented and discussed.
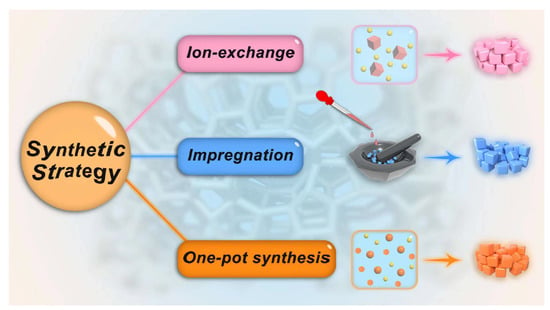
Figure 1.
Representative synthesis strategies of basic zeolites.
2.2.1. Ion Exchange Synthesis
Traditional aluminosilicate zeolites possess negative charges on their frameworks, which can be neutralized by metal cations with low electronegativities, such as Na+, K+, and Cs+, etc. Ion exchange approaches are mostly applied to introduce alkali cations into the pores of zeolites by exchanging with H+ in the framework of zeolites. The presence of alkali cations in the zeolites enhances the electron density of the framework oxygen, hence functioning as basic sites.
In general, more framework aluminum in the aluminosilicate zeolites can provide more ion exchange sites for alkali cations; thus, zeolite A (LTA), X (FAU), and Y (FAU) that possess a high content of aluminum were widely utilized in the ion exchange process. Chen et al. prepared various Na-Y (FAU) zeolites via the ion exchange method using different precursors of Na+ cations (NaOH, NaCl, and Na2SO4) [35,47]. They found that the NaOH could dope more Na+ in the Y zeolites than the other two sodium precursors. In addition, both high pH and high concentration of NaOH in the ion exchange solutions were beneficial to increasing sodium loading.
Since the highly electropositive cations in the zeolites can boost the basic character of zeolites, Cs+ and K+ cations were exchanged with zeolites to further increase the basicity of zeolites. Babajide et al. introduced the K+ into Na-X zeolite with the ion exchange approach to further increase the basicity of the Na-X [48]. Al-Ani et al. prepared hierarchical X and Y zeolites using a post-treatment strategy that reserved a high ion exchange capacity [49]. Afterward, Cs+ and K+ cations were introduced into the hierarchical zeolites via ion exchange to generate strong basic sites for transesterification reactions.
In the abovementioned reports, the conventional liquid ion exchange (LIE) method was applied to introduce alkali cations into zeolites. The wetness-state LIE method requires multiple exchange experiments in solution to increase the exchange degree of cations. Furthermore, repeated washing and drying processes of exchanged zeolites tend to result in the leaching of alkali cations. To avoid the above drawbacks, a solid-state ion exchange (SSIE) strategy involving mechanical mixing of zeolite and cationic salts easily endows zeolite hosts with a high exchange degree and homogeneous distribution of cations in one-step processing. Recently, Yu et al. prepared Na/ITQ-2 zeolites by both LIE and SSIE approaches for transesterification reactions [50]. Compared with the conventional LIE approach, the SSIE method was favorable for the generation of more strong basic sites, which were highly immobilized in the ITQ-2 zeolite.
2.2.2. Impregnation Method
Zeolites with a neutral framework are infeasible to apply to the ion exchange method, since the ion exchange capacity of zeolite hosts resulting from negative charges on their frameworks is the key for the ion exchange method. For the neutral zeolites, the impregnation approach, i.e., mixing the dehydrated zeolite with a metal-containing solution, is mostly applied to dope basic cation precursors in the zeolite matrix. In addition, the impregnation strategy can render high loading of basic species, regardless of the ion exchange capacity of zeolites. Thus, the metal precursors can be doped on zeolite crystals in accordance with the required loading. The excess cations after calcination can be transformed into basic metal oxides on/in zeolite cavities. These cation-added zeolites containing both cations and metal oxides possess higher basicity than the cation-exchanged zeolites for base-catalyzed reactions.
When the solvated alkali metal (Li, Na, K, Cs) precursors were doped on the zeolites by the impregnation method, the alkali metal salts can be decomposed by calcination to form alkali cations in the framework or/and to generate the alkali metal oxides on the zeolites. Both of them render zeolite hosts with basicity.
Wet impregnation is a typical method to introduce alkali cations or alkali oxides into zeolites by immersing zeolites in the solution of solvated alkali precursors. Chen et al. [40] prepared high-silica Beta zeolite in a fluoride-containing system, and subsequently impregnated them with diluted NaOH solutions. The impregnated Na+ cations were present in the cages and the defect sites of prepared Na/Beta zeolite, performing necessary basic sites for the transesterification reaction of triolein.
Manadee et al. [51] introduced potassium species into zeolite Na/X zeolite using the impregnation method with a potassium acetate buffer solution. The carbonate was generated on the zeolite after the calcination of the impregnated zeolite, which endowed prepared K/NaX materials with strong basicity for the transesterification reaction.
Likewise, Li et al. [38] prepared lithium-doped Li/NaY zeolite materials with the microemulsion-assisted impregnation strategy using various molar ratios of Li2CO3 to NaY. A large number of alkali species were introduced into the zeolites, thus improving the catalytic activity and stability of Li/NaY catalysts in the transesterification reactions of castor oil with ethanol.
Besides alkali cations and alkali metal oxides, alkaline earth metal oxides supported on zeolite hosts possess strong basic properties, due to their low electronegativity. For instance, four nitrates (K, Na, Ca, and Mg) were doped on the clinoptilolite zeolite using the impregnation method under magnetic stirring and ultrasonic irradiation. CaO and MgO were generated on the clinoptilolite zeolite after calcination and showed higher activity than K- and Na-doped samples in the transesterification of commercial waste cooking oil [52]. Among basic metal oxides, the cost-effective CaO possesses high basicity and is insoluble in methanol; hence, it is a promising basic specie supported on the zeolite host for transesterification reactions. Wu et al. loaded CaO on KL, NaY, and Na/ZSM-5 zeolites using the impregnation approach under microwave irradiation [53]. They reported that the utilization of microwave radiation was beneficial to scattering CaO on zeolites, hence enhancing the catalyst basicity. The prepared CaO/zeolites showed excellent activity in the transesterification reaction of soybean oil with methanol. Yuan et al. determined the optimum concentration of calcium precursor used in the impregnation solution [46]. The resultant CaO/ZSM-5 zeolite, which was prepared using a 35% (wt./vol.) aqueous solution of calcium acetate for impregnation, gave a superior yield of biodiesel in the microwave reactor.
2.2.3. One-Pot Synthesis
The one-pot synthesis system of introducing metal precursors into zeolites is an effective strategy to modify the acid–base properties of zeolites [31,54]. Peng and coworkers prepared metallosilicate MFI-type zeolites with a one-pot synthesis method, in which alkaline earth cations (Mg2+, Ca2+, Sr2+, or Ba2+) were incorporated into the silicalite-1 zeolite [55]. The one-pot hydrothermal synthesis of alkaline zeolites involved an important pretreatment process for co-hydrolysis/condensation of silica sources with hydrochloric acid solutions. The intracrystalline strong basic centers were constructed and stabilized in well-preserved frameworks of zeolite. CO2 sorption measurements and 1H→13C and 1H→29Si cross-polarization magic-angle spinning NMR spectra comprehensively confirmed the presence of alkaline earth cation-derived basic sites in the MFI framework.
To summarize, the direct one-pot synthesis approach is a promising strategy for developing excellent basic zeolites for solid base catalysis, as it is both time- and energy-saving, and helps to preserve the zeolitic framework. Another alternative method is the facile SSIE approach, which can anchor more basic sites and stabilize them in a simple ion exchange step. These methods show great potential for preparing high-performing basic zeolites for transesterification reactions.
3. Characterization of Basic Zeolites
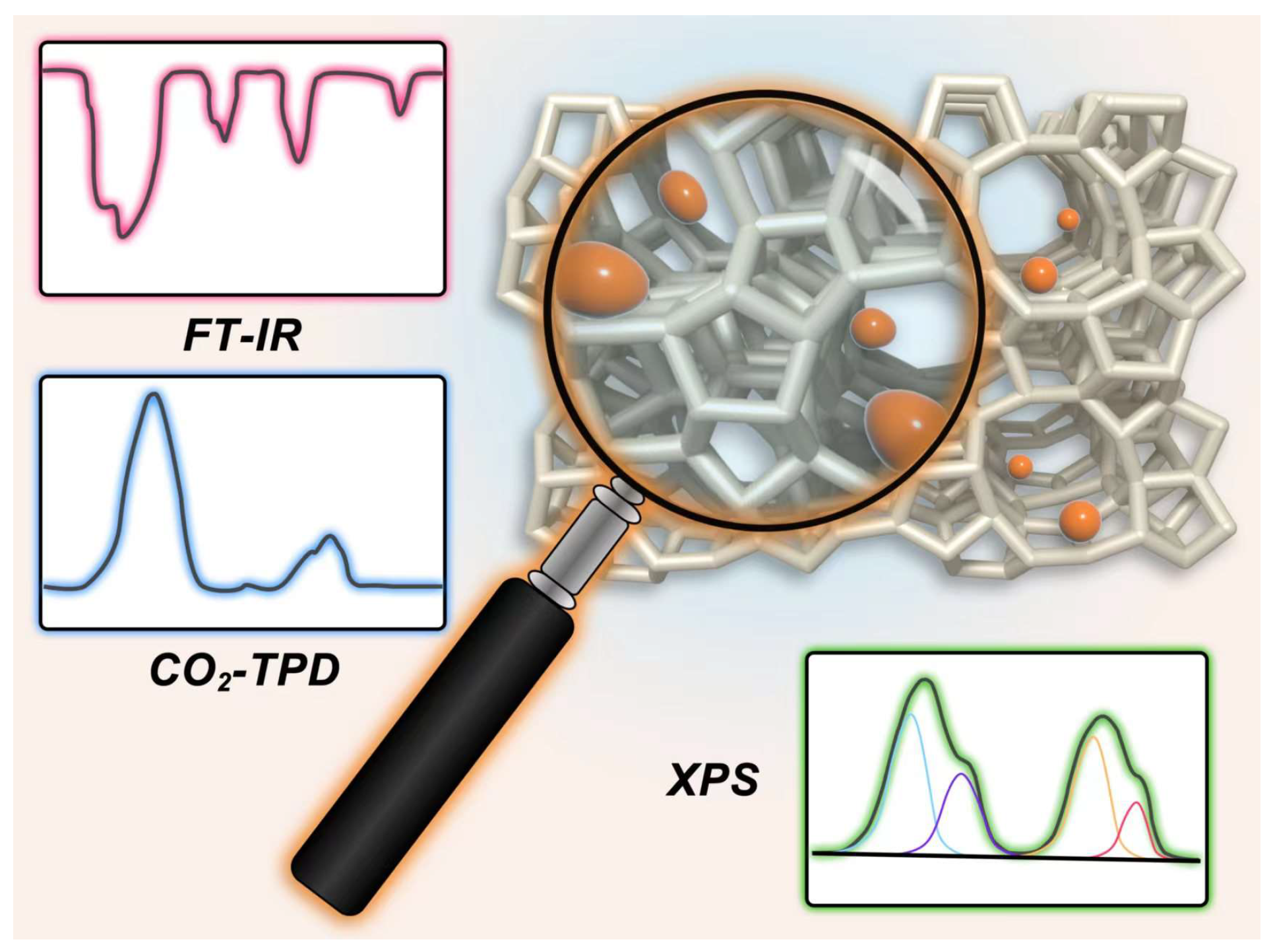
Basic sites in zeolites are the active centers for transesterification reactions. An in-depth understanding of the chemical states and the locations of basic sites in zeolites is essential to design more efficient basic zeolite catalysts for transesterification reactions. Various characterization techniques, such as temperature-programmed desorption (TPD), infrared spectroscopy (IR), nuclear magnetic resonance (NMR), and X-ray photoelectron spectroscopy (XPS) have been extensively employed to gain deeper insights into the characteristics of basic sites in zeolites [56]. Herein, some representative characterization techniques that can monitor the zeolite basicity will be briefly introduced in the following content (Figure 2).
Figure 2.
Representative characterization techniques of basic sites in zeolites.
3.1. TPD of Carbon Dioxide (CO2-TPD)
Probe molecules coupled with the appropriate characterization techniques are widely used to measure zeolite basicity by monitoring the interaction of probe molecules with zeolite basic sites. CO2 is a common probe molecule and is frequently used to determine the basic properties of alkali cation-doped zeolites. CO2-TPD is a facile approach for estimating the concentration and strength of basic sites according to the desorption temperature and peak area in TPD desorption profiles. For example, Hattori et al. prepared a series of alkali cation-exchanged zeolite X and alkali cation-added X zeolites that contained an excess of the alkali cations (K+, Cs+, Rb+) [57]. The basicity of the alkali/zeolites was monitored by TPD-CO2. The alkali cation-added zeolites showed larger desorption peak areas than the alkali cation ion exchanged zeolites, indicating that the over-introduced cations generated abundant additional basic sites in X zeolites. TPD-CO2 can evaluate the relative strength and proportion of basic sites in different zeolites under the same conditions, whereas it is difficult to identify the state of adsorbed CO2 and precisely quantify the concentration of basic sites in zeolites.
3.2. Infrared Spectroscopy
Infrared spectroscopy using probe molecules can qualitatively/quantitatively study zeolite basicity by analyzing the infrared adsorption band of probe molecules [58]. For instance, infrared spectroscopy of CO2 adsorption (IR-CO2) can determine the state of CO2 adsorbed on the zeolites, according to the strong interaction of CO2 with basic sites, thereby providing structural information on basic sites [59,60]. H. Bekhti et al. used in situ Fourier transform infrared (FTIR) spectroscopy of CO2 adsorption to study the state of adsorbed CO2 on the MgO impregnated on NaY. They reported that the chemically adsorbed CO2 was present in various forms, including carbonates, carboxylate species, chelated and bridged bidentate carbonate, bicarbonate and unidentate, etc. The carboxylate species and chelated bidentate carbonate corresponded to the porosity and basicity of MgO-doped NaY zeolites, indicating the formation of various adsorption basic sites on the studied basic zeolites.
Among various probe molecules, such as pyrrole [61], chloroform [55,62,63], N2O4 [64,65], and methanol [66,67], pyrrole has attracted special attention due to its sensitive spectroscopic characteristics that correlate closely with zeolite basicity [58]. The IR detection of pyrrole adsorbed on zeolite basic sites can characterize the basic strength and local environment of basic zeolites. Furthermore, pyrrole is non-reactive on basic sites of zeolite and remains intact under IR experimental conditions. Barthomeuf et al. used IR of pyrrole adsorption (IR-pyrrole) to monitor the basic properties of different zeolites with varying structures and chemical compositions [41,43]. They reported that as the zeolite basicity increased, the IR band corresponding to the N-H vibration shifted to lower wavenumbers, due to the interaction of pyrrole with the oxygen of the zeolite framework. This resulted in the bridge formation and polarizing of the N–H bond [41]. Similarly, the basic strength of alkali cations exchanged FAU-type zeolites was evaluated according to the shift of the infrared band corresponding to the N–H vibration of pyrrole adsorbed on basic zeolites. The obtained results signified that NaX possessed higher basicity than NaY, and the basic strengths of alkali ion exchanged zeolites increased in the following order: NaY< KY < NaX < CsX [43]. Huang and Kaliaguine determined the basicity of alkali metal cation-exchanged ZSM-5, mordenite, X, and Y zeolites according to the NH stretching vibration of infrared pyrrole adsorption [61]. The basic sites in these cation-exchanged zeolites were Lewis bases originating from framework oxygens adjacent to the cations. Furthermore, two NH-stretching bands were observed in the infrared spectrum of pyrrole adsorbed on a zeolite containing two cations, implying that the basic strength varied from site to site and the alkalinity of the zeolite mainly depended on the local environment rather than the composition of the zeolite. Compared with FAU-type zeolites, high Si zeolites showed higher NH-stretching frequencies and weaker band intensities in the IR-pyrrole spectra, indicating weaker basicity and lower concentrations of basic sites.
3.3. NMR
Besides its use in infrared adsorption spectroscopy, pyrrole has also been employed as a sensitive probe in NMR analysis to measure the basicity of zeolites. Blasco et al. used 1H NMR to investigate the intrinsic basicity of alkali cation-exchanged zeolites (FAU-type) by monitoring the chemical shift of the N–H group of chemisorbed pyrrole [68]. Their findings revealed that the 1H chemical shift corresponding to the N–H proton resonance shifted up to 3 ppm from LiY to KX zeolites, indicating an increase in the zeolite basicity. Notably, the 1H NMR chemical shifts were closely related to the average frequency of N–H calculated from the infrared N–H band [58], highlighting the effectiveness of 1H NMR analysis of pyrrole in determining the average zeolite basicity. The solid-state NMR analysis of alkali metal nuclei in Blasco’s work evidenced the interaction between pyrrole and alkali metal cations in FAU-type zeolites [63,68]. In addition, the chemical shift of the methoxide group in the 13C NMR spectra of methanol adsorbed on basic zeolites decreased with increasing basicity. Thus, 13C NMR analysis of chemisorbed methoxide species is also an effective approach for determining the basicity of zeolite [69].
3.4. XPS
XPS is a useful technique for characterizing the basicity of zeolites by measuring the binding energy (BE) for framework oxygen. The influence of the type of alkali cations and zeolite compositions on the BE of the related elements was investigated by Okamoto and his coworkers. They found that the basicity of framework oxygen increased with the decreasing electronegativity of the exchanged cation [70]. Moreover, XPS analysis of probe molecules chemisorbed on zeolite basic sites can effectively reflect the basic strength of zeolites. The BE of N 1s of the pyrrole chemisorbed on LiY and NaY zeolites was determined by Huang et al. [71]. Three different deconvoluted N 1s peaks were observed, suggesting an inhomogeneous basic strength of the framework oxygen in the zeolite. The XPS study evidenced that the alkali cations only affected the adjacent framework atoms.
Zeolite basicity can be described by conceptual density functional theory (DFT) models. According to reports, the Fukui function of the framework oxygens, local hardness/softness, and the minimum of the molecular electrostatic potential at the oxygen atoms are crucial descriptors of zeolite framework basicity [64,69].
These typical techniques described above provide in-depth information on the basicity of studied zeolites, including basic strength, proportion, and concentration of basic sites. Such information is crucial for evaluating the catalytic behavior of basic zeolites. However, common probe molecules used in these techniques may decompose or react upon interacting with basic sites or adjacent cations. Thus, it is a challenge to develop an ideal probe molecule that can selectively adsorb on basic sites without causing additional alterations in the zeolite. The integration of several characterization techniques using specific probe molecules is necessary to efficiently interrogate the zeolite basicity.
4. Transesterification Reactions Utilizing Basic Zeolites
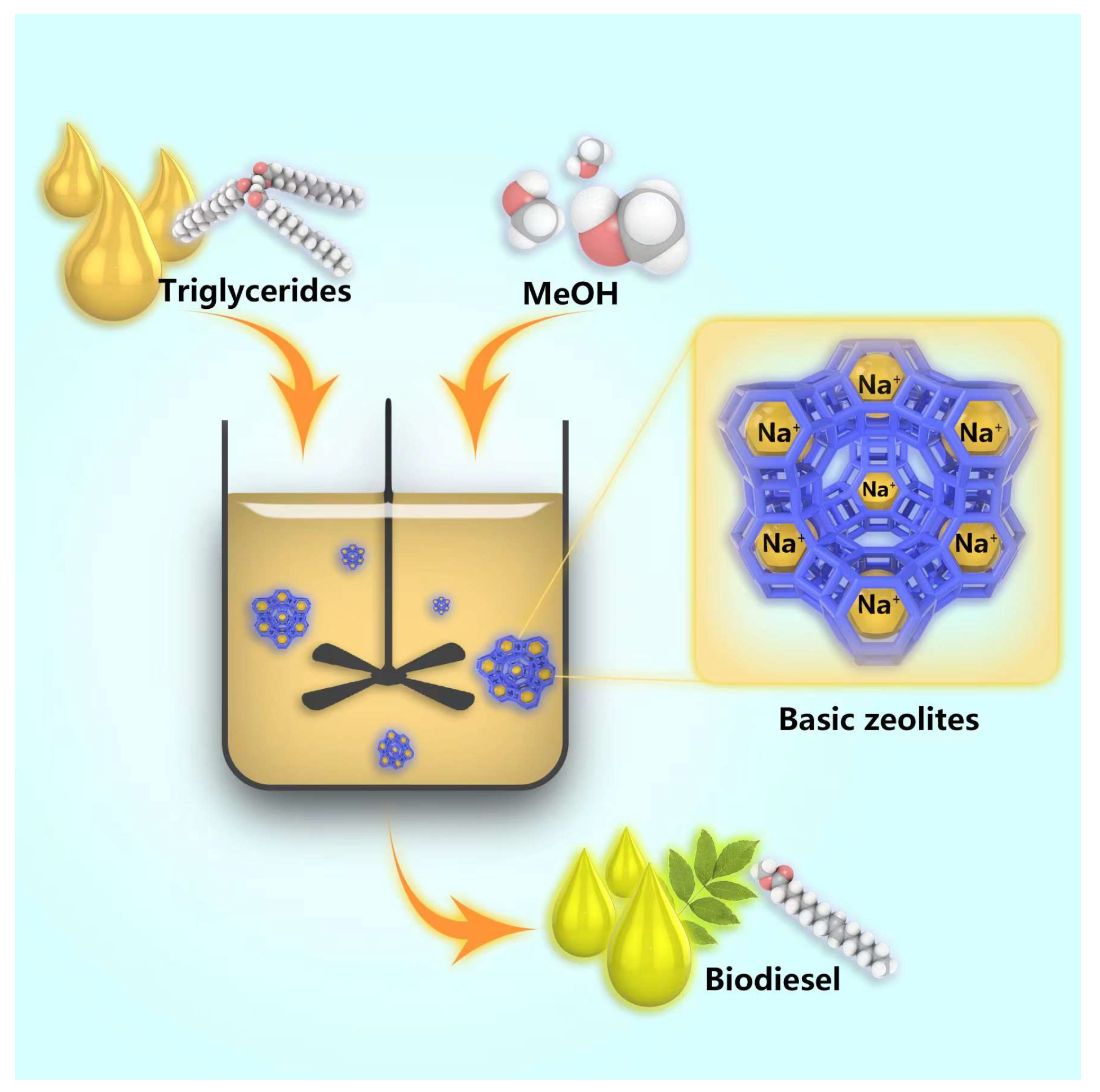
Transesterification of various lipid sources with small alcohols by the base catalysis is a promising process for biodiesel production, because it can achieve high conversions in short reaction times at relatively low costs, as depicted in Figure 3. Alkyl esters (mono and diglycerides) and glycerin are produced in the transesterification reactions. Basic zeolites, with their high surface area, adjustable basicity, and superior hydrothermal stability, have been widely utilized in transesterification reactions to produce biodiesel [35,36,37,38,39,40].
Figure 3.
Biodiesel production through transesterification reaction using basic zeolites.
4.1. Conventional Microporous Basic Zeolites
Wang and co-workers prepared a variety of Na/Y [35], Na/Beta [40], and Na/MCM-22 [36] zeolites using the impregnation and ion exchange methods. These Na-doped zeolite catalysts were effective in converting triglycerides to biodiesel in the transesterification reactions. For instance, Na/Y and Na/MCM-22 converted 98% and 99% of triolein into biodiesel, respectively, in 5.5 h. The Na/Beta also showed good stability in nine cycles of the transesterification reaction. To further increase the basicity of Na-form zeolites, additional alkali cations were introduced. Manadee et al. impregnated NaX zeolites with 4, 8, 12, and 16 wt.% of potassium [47]. The catalytic activity of the prepared K-doped NaX increased with the rising loading of potassium; 16K/NaX (16 wt.% of K) gave 95.2 ± 0.96% yield of biodiesel in the transesterification reaction of Jatropha seed oil. Likewise, Li-doped NaY zeolite (Li/NaY) was synthesized for the transesterification of castor oil with ethanol [30]. The authors reported that plentiful basic sites in the complex channel structure of Li/NaY catalyst afforded enhanced stability and recyclability.
In addition, alkaline-earth oxides with strong basicity are frequently used in solid base catalysis. For instance, the prepared CaO/NaY material yielded 95% biodiesel and showed outstanding water and acid resistance in the transesterification of soybean oil with methanol [53]. Yuan et al. loaded CaO on ZSM-5 zeolites (CaO/ZSM-5) and tested their catalytic activity under microwave heating conditions for the transesterification reaction [46]. Microwave heating is highly efficient and requires less energy, which can remarkably reduce the reaction time to produce a high yield of bio-diesel over prepared CaO/ZSM-5. Rare earth oxides, e.g., La2O3, are widely used as promoters/supports for catalysis. Their surface characteristics are basic rather than acidic, thus being promising materials for transesterification reactions. Du et al. prepared La2O3/NaY materials using the physical mixing method for the production of biodiesel from castor oil and ethanol [31]. The prepared La2O3/NaY materials gave a biodiesel yield of 84.6% in 50 min, and exhibited excellent reusability as well as crushing strength in the transesterification reaction.
Notably, the basicity of zeolites plays a crucial role in transesterification reactions. By introducing more electropositive metal species into zeolites, their basicity can be enhanced. For example, the ion exchanging Na-X zeolite with potassium increased its basicity strength for transesterification reactions [48]. The resulting K-X zeolite exhibited higher catalytic activity due to the inclusion of potassium species in the zeolite pore cavities. Furthermore, the decomposition of impregnated alkali metal salts resulted in the occlusion of alkali metal oxide clusters in zeolite cages, which further increased the basicity of these materials. Suppes et al. [37] occluded NaOx species in an X zeolite, significantly increasing the basic strength and concentration of the X zeolite, thereby enhancing the catalytic activity in the transesterification of soybean oil. Therefore, it can be concluded that stronger basicity and more basic centers in zeolites are beneficial for increasing conversion and selectivity under the same reaction conditions.
4.2. Nanoscale and Hierarchical Basic Zeolites
The aforementioned studies signify that increasing the ion exchange capacity of zeolites and incorporating more electropositive cation species can enhance their basicity, thereby boosting the catalytic activity in transesterification reactions. However, the intracrystalline micropores of zeolites pose a diffusion limitation for bulky molecules, which can be mitigated by utilizing nanoscale zeolites with larger surface areas and shorter diffusion lengths, facilitating the diffusion of reactants. Furthermore, the preparation of hierarchical zeolites via mesoporogen, mesoporogen-free, and demetallization strategies [30] has introduced an alternative and promising avenue for improving mass transfer and molecular accessibility of bulky TGs molecules in basic zeolites [72,73,74,75,76,77,78]. Al-Ani et al. prepared nanoscale zeolite P and hierarchical FAU-type zeolites (X and Y), and introduced K+ or/and Cs+ cations using ion exchange to catalyze the transesterification reaction of rapeseed oil [47,49]. Their characterization studies and reaction results indicated that both the accessibility to the basic sites and the basic strength of zeolites should be finely tuned to improve their catalytic performance in transesterification reactions.
4.3. Two-Dimensional (2D) Basic Zeolites
Besides nanoscale and hierarchical zeolites, 2D zeolites with an open framework structure provide an alternative route to improve diffusion limitations [79,80,81]. Two-dimensional zeolites possess 1–2 unit cells in one specific crystallographic direction, corresponding to a width of 2–3 nm. Thus, the adsorption and catalysis of reactants mainly occur on the external surface of 2D zeolites, thereby improving the access of bulky molecules to the active sites in zeolites. Dr. Avelino Corma, an eminent researcher and mentor in the field of zeolites and catalysis [82,83,84,85,86,87,88,89], made significant contributions to the preparation and catalysis of 2D zeolites. For instance, Corma et al. reported on the preparation of 2D ITQ-2 zeolites with high surface area; the zeolite layers are randomly arranged in the form of bends and coils [90]. ITQ-2 was prepared by swelling the MCM-22P (MWW) precursor with an aqueous solution of surfactant and TPAOH followed by ultra-sonication to delaminate the zeolite layers. The catalytic behavior of ITQ-2 was tested by the catalytic cracking of alkanes, in which ITQ-2 showed higher activity and selectivity than the 3D MWW-type counterpart [91]. Similarly, Corma’s group prepared 2D FER-type monolayer zeolites (ITQ-6) by performing similar swelling and delamination post-treatment of the FER precursor [92]. Compared to the 3D FER zeolite, the 2D ITQ-6 zeolite possessed open access to acid sites in the layered structure, hence exhibiting superior catalytic activity in the cracking of bulky 1,3,5-triisopropylbenzene. Besides the post-synthetic approach, Corma et al. reported a one-pot synthetic strategy to prepare a delaminated MWW zeolite by using hexamethyleneimine coupled with a designed bifunctional surfactant agent. The obtained delaminated materials not only retained structural integrity but also showed excellent catalytic activity in the alkylation reaction of benzene with propylene [93].
Inspired by Corma’s pioneering work, our group prepared 2D basic zeolites, which showed great potential to be superior catalysts for the transesterification reaction of TGs [50]. Na-formed 2D zeolites were prepared using the SSIE method, which is more advantageous for stabilizing and dispersing strong basic sites than the conventional LIE method. The abundant accessible basic sites on the large external surface of 2D basic zeolites promoted mass transportation and increased the distribution and accessibility of basic sites. Consequentially, 2D basic zeolites prepared using the SSIE approach showed prominent catalytic activity and stability in the transesterification reactions. This is a crucial implication that the synthesis strategies and different types of zeolites can be regulated to improve the catalytic performance of basic zeolites. In addition, Macario et al. reported that K doped on the 2D ITQ-6 zeolite showed higher activity than 3D zeolites and mesoporous silica in biodiesel production by transesterification of waste oil owing to its open structure [94].
5. Challenges and Outlooks
Traditional solid basic catalysts often suffer from poisoning or leaching of basic sites due to impurities or moisture in the reaction medium. This can lead to the deactivation of the solid basic catalysts and they are hardly regenerated after several consecutive runs. Basic zeolites can overcome the aforementioned problems and improve the stability and distribution of basic sites due to their unique characteristics in terms of the uniform porous structure, large surface area, tunable hydrophobic/hydrophilic properties, etc. To expand the practical application of basic zeolites in the transesterification of TGs, there are still important challenges to overcome. For example, the narrow pore size of zeolites limits the transport of bulky and viscous C12-C22 TGs or FFAs molecules into the active sites located in the micropores. The long diffusion path results in low catalyst utilization and reduces the catalytic rates. In addition, the basic sites in zeolites tend to leak after several reaction runs because of the weak interaction of the basic site with the zeolite framework in the reaction system. Preferential adsorption of glycerol (or water) on the basic sites can lead to the deactivation of the zeolites. Notably, basic zeolites are more competent for oils with a small number of FFAs, whereas feedstocks for biodiesel production generally are low-quality oil (tallow or used cooking oil) that contains numerous FFAs.
Therefore, the following important strategies should be considered to confront the challenges. Firstly, the basic zeolites with excellent interconnected porosity and two or more levels of pore size are highly desirable to improve mass transportation and retard the deactivation and poisoning of catalysts. Likewise, prominent porosity (large pore diameter, large surface area, as well as high pore volume) is also advantageous for increasing the distribution and accessibility of basic sites on the zeolite surface. Secondly, hydrophobic modification of the zeolite surface can enhance the adsorption of hydrophobic species (TGs and FFAs) on the zeolite and restrain the strong adsorption of polar compounds such as glycerol and water that generally leads to the deactivation of the basic catalysts. Furthermore, the interaction between the basic sites and zeolite framework structure should be strengthened, by which, leaching of the basic sites can be inhibited. In addition, bifunctional acid-base zeolites may be developed for the industrial production of biodiesel, since hybrid processes involving acid-catalyzed esterification of FFAs and base-catalyzed transesterification are suitable for raw feedstocks containing vast FFA.
Future research for the development of basic zeolite catalysts for biodiesel production by transesterification could focus on the following areas: (1) fabrication of hierarchical basic zeolites that integrate intrinsic micropores with mesopores and/or macropores to improve mass transfer and molecular accessibility; (2) preparation of nanosized or 2D basic zeolites, where the content and distribution of basic sites can be well regulated; (3) developing one-pot synthesis strategies of basic zeolites to promote the stability and intensity of basic sites; (4) designing hydrophobic zeolites with grafting methods using silane reagents; (5) synthesizing a proto-zeolite with a partially formed structure, which possesses a large external surface area and short-range order, but more open pores; (6) carefully designing bifunctional catalysts endowed with acid–base functions, which are capable of esterifying the free fatty acids on acid sites and transesterifying the triglycerides on the basic sites for raw oil feedstocks.
6. Conclusions
In recent years, the development of highly efficient catalysts for the production of sustainable biodiesel has sparked worldwide research interest. From an economic and eco-friendly perspective, basic zeolites have demonstrated the critical potential as efficient and reusable catalysts in the transesterification process. This review provides a comprehensive summary of the synthesis approaches, characterization techniques, and catalytic performance of basic zeolite materials for the production of biodiesel by transesterification. Furthermore, the current challenges and the perspectives of basic zeolites used in transesterification reactions are outlined in this work. This review aims to advance the development of synthetic strategies for basic zeolites with high catalytic performance and expand the practical application of basic zeolites in the production of biodiesel and other chemicals.
Author Contributions
Writing—review and editing, G.Y. and J.Y.; supervision, J.Y.; project administration, J.Y.; funding acquisition, J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 22288101, 22001090, 21835002, and 21920102005, and Jilin Province Science and Technology Development Plan, grant number 20200201096JC.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
This article is dedicated to the 70th birthday of Avelino Corma. This work acknowledges the 111 Project (B17020).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Izadyar, N.; Ong, H.C.; Chong, W.T.; Leong, K.Y. Resource assessment of the renewable energy potential for a remote area: A review. Renew. Sustain. Energy Rev. 2016, 62, 908–923. [Google Scholar] [CrossRef]
- Luque, R.; Lovett, J.C.; Datta, B.; Clancy, J.; Campelo, J.M.; Romero, A.A. Biodiesel as feasible petrol fuel replacement: A multidisciplinary overview. Energy Environ. Sci. 2010, 3, 1706–1721. [Google Scholar] [CrossRef]
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, S108–S117. [Google Scholar] [CrossRef]
- Yusuf, N.N.A.N.; Kamarudin, S.K.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Pang, H.; Yang, G.; Li, L.; Yu, J. Esterification of Oleic Acid to Produce Biodiesel over 12-Tungstophosphoric Acid Anchored Two-dimensional Zeolite. Chem. Res. Chin. Univ. 2021, 37, 1072–1078. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y.H. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Y. Chapter 21—Biodiesel Production: Status and Perspectives. In Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels (Second Edition); Pandey, A., Larroche, C., Dussap, C.-G., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 503–522. [Google Scholar]
- Ishak, Z.I.; Sairi, N.A.; Alias, Y.; Aroua, M.K.T.; Yusoff, R. A review of ionic liquids as catalysts for transesterification reactions of biodiesel and glycerol carbonate production. Catal. Rev. 2017, 59, 44–93. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Tosa, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification - Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Melero, J.A.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Girish, N.; Niju, S.P.; Meera Sheriffa Begum, K.M.; Anantharaman, N. Utilization of a cost effective solid catalyst derived from natural white bivalve clam shell for transesterification of waste frying oil. Fuel 2013, 111, 653–658. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Lu, D.; Hu, S.; Han, H. Preparation of KF/CaO nanocatalyst and its application in biodiesel production from Chinese tallow seed oil. Fuel 2010, 89, 2267–2271. [Google Scholar] [CrossRef]
- Alonso, D.M.; Mariscal, R.; Granados, M.L.; Maireles-Torres, P. Biodiesel preparation using Li/CaO catalysts: Activation process and homogeneous contribution. Catal. Today 2009, 143, 167–171. [Google Scholar] [CrossRef]
- Baroutian, S.; Aroua, M.K.; Raman, A.A.A.; Sulaiman, N.M.N. Potassium hydroxide catalyst supported on palm shell activated carbon for transesterification of palm oil. Fuel Process. Technol. 2010, 91, 1378–1385. [Google Scholar] [CrossRef]
- Hattori, H. Heterogeneous Basic Catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]
- Sun, L.-B.; Liu, X.-Q.; Zhou, H.-C. Design and fabrication of mesoporous heterogeneous basic catalysts. Chem. Soc. Rev. 2015, 44, 5092–5147. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, M.; Tian, P.; Liu, Z. Progress in Seed-assisted Synthesis of (Silico)Aluminophosphate Molecular Sieves. Chem. Res. Chin. Univ. 2022, 38, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Rong, H.; Chen, C.; Li, Z.; Zuo, J.; Wang, W.; Liu, X.; Guan, Y.; Yang, X.; Liu, Y.; et al. Synthesis Optimization of SSZ-13 Zeolite Membranes by Dual Templates for N2/NO2 Separation. Chem. Res. Chin. Univ. 2022, 38, 250–256. [Google Scholar] [CrossRef]
- Lin, S.; Zhi, Y.; Liu, Z.; Yuan, J.; Liu, W.; Zhang, W.; Xu, Z.; Zheng, A.; Wei, Y.; Liu, Z. Multiscale dynamical cross-talk in zeolite-catalyzed methanol and dimethyl ether conversions. Natl. Sci. Rev. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, P. New progress in zeolite synthesis and catalysis. Natl. Sci. Rev. 2022, 9. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef]
- Luo, W.; Cao, W.; Bruijnincx, P.C.A.; Lin, L.; Wang, A.; Zhang, T. Zeolite-supported metal catalysts for selective hydrodeoxygenation of biomass-derived platform molecules. Green Chem. 2019, 21, 3744–3768. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J. New stories of zeolite structures: Their descriptions, determinations, predictions, and evaluations. Chem. Rev. 2014, 114, 7268–7316. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Yu, J. Applications of zeolites in sustainable chemistry. Chem 2017, 3, 928–949. [Google Scholar] [CrossRef]
- Bai, R.; Song, Y.; Li, Y.; Yu, J. Creating hierarchical pores in zeolite catalysts. Trends Chem. 2019, 1, 601–611. [Google Scholar] [CrossRef]
- Wang, N.; Sun, Q.; Yu, J. Ultrasmall metal nanoparticles confined within crystalline nanoporous materials: A fascinating class of nanocatalysts. Adv. Mater. 2019, 31, 1803966. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Alaba, P.A.; Sani, Y.M.; Mohammed, I.Y.; Abakr, Y.A.; Daud, W.M.A.W. Synthesis and characterization of sulfated hierarchical nanoporous faujasite zeolite for efficient transesterification of shea butter. J. Clean. Prod. 2017, 142, 1987–1993. [Google Scholar] [CrossRef]
- MacArio, A.; Giordano, G.; Setti, L.; Parise, A.; Campelo, J.M.; Marinas, J.M.; Luna, D. Study of lipase immobilization on zeolitic support and transesterification reaction in a solvent free-system. Biocatal. Biotransform. 2007, 25, 328–335. [Google Scholar] [CrossRef]
- Wang, Y.; Chou, H.; Chen, B.; Lee, D. Optimization of sodium loading on zeolite support for catalyzed transesterification of triolein with methanol. Bioresour. Technol. 2013, 145, 248–253. [Google Scholar] [CrossRef]
- Wang, Y.; Dang, T.; Chen, B.; Lee, D. Transesterification of Triolein to Biodiesel Using Sodium-Loaded Catalysts Prepared from Zeolites. Ind. Eng. Chem. Res. 2012, 51, 9959–9965. [Google Scholar] [CrossRef]
- Suppes, G.J.; Dasari, M.A.; Doskocil, E.J.; Mankidy, P.J.; Goff, M.J. Transesterification of soybean oil with zeolite and metal catalysts. Appl. Catal. A 2004, 257, 213–223. [Google Scholar] [CrossRef]
- Li, Z.; Ding, S.; Chen, C.; Qu, S.; Du, L.; Lu, J.; Ding, J. Recyclable Li/NaY zeolite as a heterogeneous alkaline catalyst for biodiesel production: Process optimization and kinetics study. Energy Convers. Manag. 2019, 192, 335–345. [Google Scholar] [CrossRef]
- Du, L.; Ding, S.; Li, Z.; Lv, E.; Lu, J.; Ding, J. Transesterification of castor oil to biodiesel using NaY zeolite-supported La2O3 catalysts. Energy Convers. Manag. 2018, 173, 728–734. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, B. High-silica zeolite beta as a heterogeneous catalyst in transesterification of triolein for biodiesel production. Catal. Today 2016, 278, 335–343. [Google Scholar] [CrossRef]
- Barthomeuf, D. Basic Zeolites: Characterization and Uses in Adsorption and Catalysis. Catal. Rev. 1996, 38, 521–612. [Google Scholar] [CrossRef]
- Hathaway, P.E.; Davis, M.E. Base catalysis by alkali modified zeolites: III. Alkylation with methanol. J. Catal. 1989, 119, 497–507. [Google Scholar] [CrossRef]
- Barthomeuf, D. Acidity and Basicity in Zeolites. In Studies in Surface Science and Catalysis; Öhlmann, G., Pfeifer, H., Fricke, R., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 65, pp. 157–169. [Google Scholar]
- Davis, R.J.; Doskocil, E.J.; Bordawekar, S. Structure/function relationships for basic zeolite catalysts containing occluded alkali species. Catal. Today 2000, 62, 241–247. [Google Scholar] [CrossRef]
- Sun, H.; Wu, D.; Liu, K.; Guo, X.; Navrotsky, A. Energetics of Alkali and Alkaline Earth Ion-Exchanged Zeolite A. J. Phys. Chem. C 2016, 120, 15251–15256. [Google Scholar] [CrossRef]
- Lawan, I.; Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. Synergies between the microwave reactor and CaO/zeolite catalyst in waste lard biodiesel production. Renew. Energy 2020, 145, 2550–2560. [Google Scholar] [CrossRef]
- Al-Ani, A.; Darton, R.J.; Sneddon, S.; Zholobenko, V. Nanostructured Zeolites: The Introduction of Intracrystalline Mesoporosity in Basic Faujasite-type Catalysts. ACS Appl. Nano Mater. 2018, 1, 310–318. [Google Scholar] [CrossRef]
- Babajide, O.; Musyoka, N.; Petrik, L.; Ameer, F. Novel zeolite Na-X synthesized from fly ash as a heterogeneous catalyst in biodiesel production. Catal. Today 2012, 190, 54–60. [Google Scholar] [CrossRef]
- Al-Ani, A.; Mordvinova, N.E.; Lebedev, O.I.; Khodakov, A.Y.; Zholobenko, V. Ion-exchanged zeolite P as a nanostructured catalyst for biodiesel production. Energy Rep. 2019, 5, 357–363. [Google Scholar] [CrossRef]
- Pang, H.; Yang, G.; Li, L.; Yu, J. Efficient transesterification over two-dimensional zeolites for sustainable biodiesel production. Green Energy Environ. 2020, 5, 405–413. [Google Scholar] [CrossRef]
- Manadee, S.; Sophiphun, O.; Osakoo, N.; Supamathanon, N.; Kidkhunthod, P.; Chanlek, N.; Wittayakun, J.; Prayoonpokarach, S. Identification of potassium phase in catalysts supported on zeolite NaX and performance in transesterification of Jatropha seed oil. Fuel Process. Technol. 2017, 156, 62–67. [Google Scholar] [CrossRef]
- AbuKhadra, M.R.; Basyouny, M.G.; El-Sherbeeny, A.M.; El-Meligy, M.A.; Abd Elgawad, A.E.E. Transesterification of commercial waste cooking oil into biodiesel over innovative alkali trapped zeolite nanocomposite as green and environmental catalysts. Sustain. Chem. Pharm. 2020, 17, 100289. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Wei, Q.; Zheng, J.; Zhang, J. Transesterification of soybean oil to biodiesel using zeolite supported CaO as strong base catalysts. Fuel Process. Technol. 2013, 109, 13–18. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef]
- Zhou, Y.; Jin, Y.; Wang, M.; Zhang, W.; Xie, J.; Gu, J.; Wen, H.; Wang, J.; Peng, L. One-Pot Synthesis of Zeolitic Strong Solid Bases: A Family of Alkaline-Earth Metal-Containing Silicalite-1. Chem. Eur. J. 2015, 21, 15412–15420. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, S.; Lamberti, C.; Bonino, F.; Travert, A.; Thibault-Starzyk, F. Probing zeolites by vibrational spectroscopies. Chem. Soc. Rev. 2015, 44, 7262–7341. [Google Scholar] [CrossRef] [PubMed]
- Yagi, F.; Tsuji, H.; Hattori, H. IR and TPD (temperature-programmed desorption) studies of carbon dioxide on basic site active for 1-butene isomerization on alkali-added zeolite X. Microporous Mater. 1997, 9, 237–245. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Blasco, T. Characterization of zeolite basicity using probe molecules by means of infrared and solid state NMR spectroscopies. Catal. Today 2009, 143, 293–301. [Google Scholar] [CrossRef]
- Bekhti, H.; Boucheffa, Y.; Blal, A.H.A.; Travert, A. In situ FTIR investigation of CO2 adsorption over MgO–Impregnated NaY zeolites. Vib. Spectrosc. 2021, 117, 103313. [Google Scholar] [CrossRef]
- Stevens, R.W.; Siriwardane, R.V.; Logan, J. In Situ Fourier Transform Infrared (FTIR) Investigation of CO2 Adsorption onto Zeolite Materials. Energy Fuels 2008, 22, 3070–3079. [Google Scholar] [CrossRef]
- Huang, M.; Kaliaguine, S. Zeolite basicity characterized by pyrrole chemisorption: An infrared study. J. Chem. Soc. Faraday Trans. 1992, 88, 751–758. [Google Scholar] [CrossRef]
- Wilson, J.N.; Idriss, H. Structure Sensitivity and Photocatalytic Reactions of Semiconductors. Effect of the Last Layer Atomic Arrangement. J. Am. Chem. Soc. 2002, 124, 11284–11285. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Blasco, T. Investigation on the Nature of the Adsorption Sites of Pyrrole in Alkali-Exchanged Zeolite Y by Nuclear Magnetic Resonance in Combination with Infrared Spectroscopy. J. Am. Chem. Soc. 2002, 124, 3443–3456. [Google Scholar] [CrossRef] [PubMed]
- Vos, A.M.; Mignon, P.; Geerlings, P.; Thibault-Starzyk, F.; Schoonheydt, R.A. Probing the basicity of zeolite frameworks with N2O4: A DFT approach. Microporous Mesoporous Mater. 2006, 90, 370–376. [Google Scholar] [CrossRef]
- Mignon, P.; Pidko, E.A.; Van Santen, R.A.; Geerlings, P.; Schoonheydt, R.A. Understanding the Reactivity and Basicity of Zeolites: A Periodic DFT Study of the Disproportionation of N2O4 on Alkali-Cation-Exchanged Zeolite Y. Chem. Eur. J. 2008, 14, 5168–5177. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.F.; Simperler, A.; Bell, R.G. Adsorption of Methanol on Zeolites X and Y. An Atomistic and Quantum Chemical Study. J. Phys. Chem. B 2006, 110, 6170–6178. [Google Scholar] [CrossRef]
- Schenkel, R.; Jentys, A.; Parker, S.F.; Lercher, J.A. INS and IR and NMR Spectroscopic Study of C1−C4 Alcohols Adsorbed on Alkali Metal-Exchanged Zeolite X. J. Phys. Chem. B 2004, 108, 15013–15026. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.; Blasco, T. Pyrrole as an NMR probe molecule to characterise zeolite basicity. Chem. Commun. 2000, 6, 491–492. [Google Scholar] [CrossRef]
- Schoonheydt, R.A.; Geerlings, P.; Pidko, E.A.; van Santen, R.A. The framework basicity of zeolites. J. Mater. Chem. 2012, 22, 18705–18717. [Google Scholar] [CrossRef]
- Okamoto, Y.; Ogawa, M.; Maezawa, A.; Imanaka, T. Electronic structure of zeolites studied by X-Ray photoelectron spectroscopy. J. Catal. 1988, 112, 427–436. [Google Scholar] [CrossRef]
- Huang, M.; Adnot, A.; Kaliaguine, S. Characterization of basicity in alkaline cation faujasite zeolites—An XPS study using pyrrole as a probe molecule. J. Catal. 1992, 137, 322–332. [Google Scholar] [CrossRef]
- Han, J.; Yang, G.; Zou, Y.; Chen, X.; Valtchev, V. Hierarchical SAPO-34 Preparation Based on the Crystal Metastability in Mother Liquor Solution. Adv. Mater. Interfaces 2021, 8, 2002029. [Google Scholar] [CrossRef]
- Han, J.; Yang, G.; Ding, H.; Chen, X. Revealing inherent factors of SAPO-34 zeolites etching towards the fabrication of hierarchical structure. Microporous Mesoporous Mater. 2021, 319, 111067. [Google Scholar] [CrossRef]
- Yang, G.; Qiu, Z.; Han, J.; Chen, X.; Yu, J. Fluoride etching opens the access for bulky molecules to active sites in microporous Ti-Beta zeolite. Mater. Chem. Front. 2020, 4, 2982–2989. [Google Scholar] [CrossRef]
- Yang, G.; Han, J.; Qiu, Z.; Chen, X.; Feng, Z.; Yu, J. An amino acid-assisted approach to fabricate nanosized hierarchical TS-1 zeolites for efficient oxidative desulfurization. Inorg. Chem. Front. 2020, 7, 1975–1980. [Google Scholar] [CrossRef]
- Yang, G.; Han, J.; Liu, Y.; Qiu, Z.; Chen, X. The synthetic strategies of hierarchical TS-1 zeolites for the oxidative desulfurization reactions. Chin. J. Chem. Eng. 2020, 28, 2227–2234. [Google Scholar] [CrossRef]
- Yang, G.; Han, J.; Huang, Y.; Chen, X.; Valtchev, V. Busting the efficiency of SAPO-34 catalysts for the methanol-to-olefin conversion by post-synthesis methods. Chin. J. Chem. Eng. 2020, 28, 2022–2027. [Google Scholar] [CrossRef]
- Chen, X.; Yang, G.; Valtchev, V. Environmentally benign synthesis of crystalline nanosized molecular sieves. Green Energy Environ. 2020, 5, 394–404. [Google Scholar] [CrossRef]
- Opanasenko, M.V.; Roth, W.J.; Čejka, J. Two-dimensional zeolites in catalysis: Current status and perspectives. Catal. Sci. Technol. 2016, 6, 2467–2484. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J. Recent Advances in the Synthesis and Application of Two-Dimensional Zeolites. Adv. Energy Mater. 2016, 6, 1600441. [Google Scholar] [CrossRef]
- Corma, A. State of the art and future challenges of zeolites as catalysts. J. Catal. 2003, 216, 298–312. [Google Scholar] [CrossRef]
- Grirrane, A.; Corma, A.; García, H. Gold-Catalyzed Synthesis of Aromatic Azo Compounds from Anilines and Nitroaromatics. Science 2008, 322, 1661–1664. [Google Scholar] [CrossRef] [PubMed]
- Simancas, R.; Dari, D.; Velamazan, N.; Navarro, M.T.; Cantin, A.; Jorda, J.L.; Sastre, G.; Corma, A.; Rey, F. Modular Organic Structure-Directing Agents for the Synthesis of Zeolites. Science 2010, 330, 1219–1222. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Jorda, J.L.; Yu, J.; Baumes, L.A.; Mugnaioli, E.; Diaz-Cabanas, M.J.; Kolb, U.; Corma, A. Synthesis and Structure Determination of the Hierarchical Meso-Microporous Zeolite ITQ-43. Science 2011, 333, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Bereciartua, P.J.; Cantín, Á.; Corma, A.; Jordá, J.L.; Palomino, M.; Rey, F.; Valencia, S.; Corcoran, E.W.; Kortunov, P.; Ravikovitch, P.I.; et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 2017, 358, 1068–1071. [Google Scholar] [CrossRef]
- Li, C.; Paris, C.; Martínez-Triguero, J.; Boronat, M.; Moliner, M.; Corma, A. Synthesis of reaction-adapted zeolites as methanol-to-olefins catalysts with mimics of reaction intermediates as organic structure-directing agents. Nat. Catal. 2018, 1, 547–554. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Gallego, E.M.; Portilla, M.T.; Paris, C.; Leon-Escamilla, A.; Boronat, M.; Moliner, M.; Corma, A. “Ab initio” synthesis of zeolites for preestablished catalytic reactions. Science 2017, 355, 1051–1054. [Google Scholar] [CrossRef]
- Corma, A.; Fornes, V.; Pergher, S.B.; Maesen, T.L.M.; Buglass, J.G. Delaminated zeolite precursors as selective acidic catalysts. Nature 1998, 396, 353. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Guil, J.M.; Pergher, S.; Maesen, T.L.M.; Buglass, J.G. Preparation, characterisation and catalytic activity of ITQ-2, a delaminated zeolite. Microporous Mesoporous Mater. 2000, 38, 301–309. [Google Scholar] [CrossRef]
- Corma, A.; Diaz, U.; Domine, M.E.; Fornés, V. New Aluminosilicate and Titanosilicate Delaminated Materials Active for Acid Catalysis, and Oxidation Reactions Using H2O2. J. Am. Chem. Soc. 2000, 122, 2804–2809. [Google Scholar] [CrossRef]
- Margarit, V.J.; Martínez-Armero, M.E.; Navarro, M.T.; Martínez, C.; Corma, A. Direct Dual-Template Synthesis of MWW Zeolite Monolayers. Angew. Chem. Int. Ed. 2015, 54, 13724–13728. [Google Scholar] [CrossRef] [PubMed]
- Macario, A.; Giordano, G.; Onida, B.; Cocina, D.; Tagarelli, A.; Giuffrè, A.M. Biodiesel production process by homogeneous/heterogeneous catalytic system using an acid–base catalyst. Appl. Catal. A 2010, 378, 160–168. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).