Complementarity and Preorganisation in the Assembly of Heterometallic–Organic Cages via the Metalloligand Approach—Recent Advances
Abstract
:1. Introduction
2. Metalloligand Strategies for the Assembly of MOCs
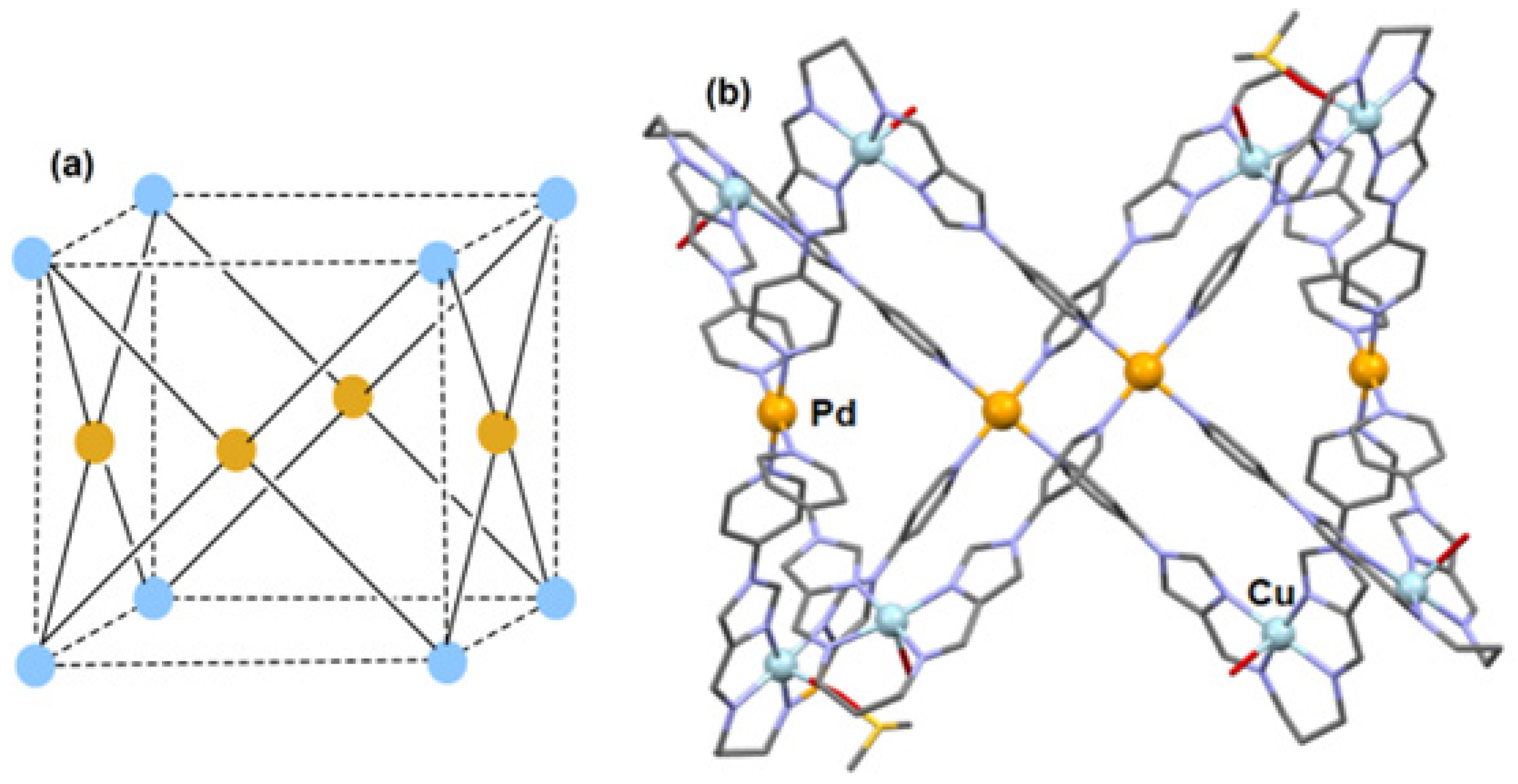
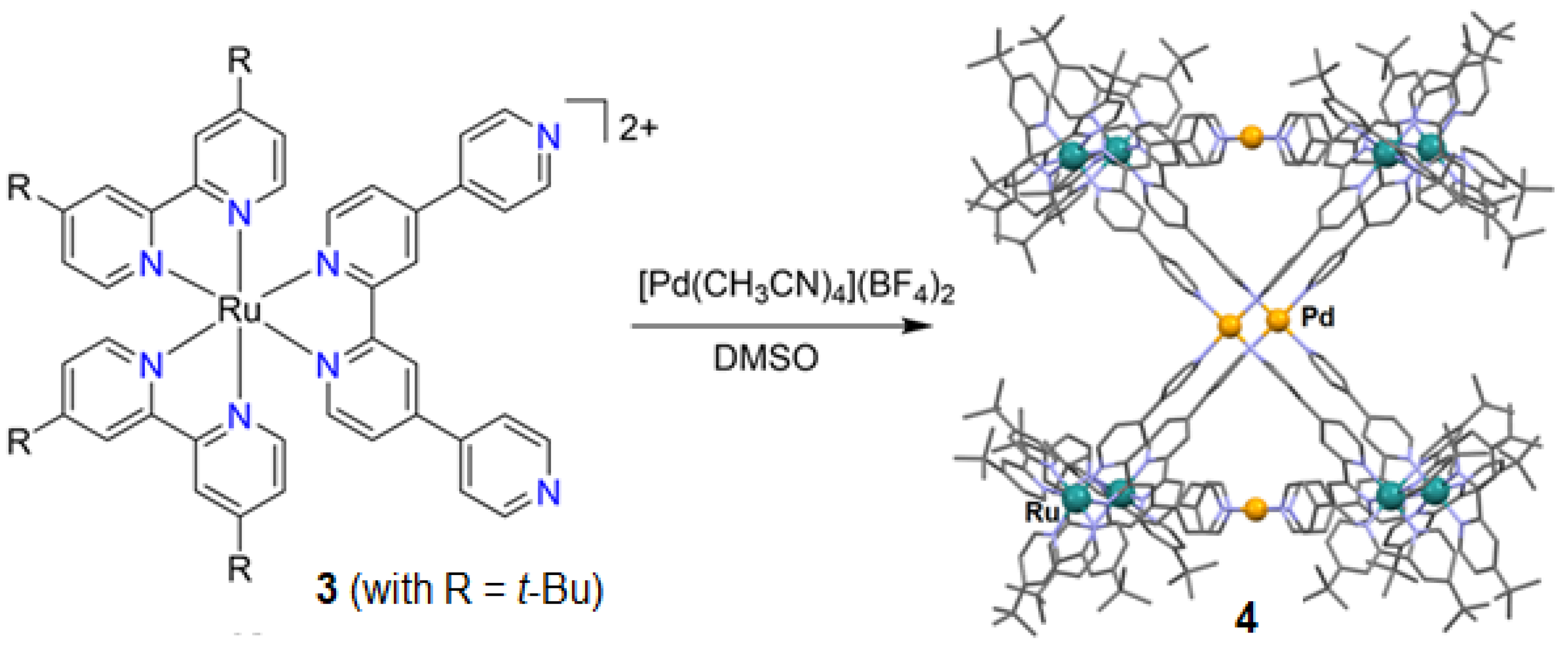
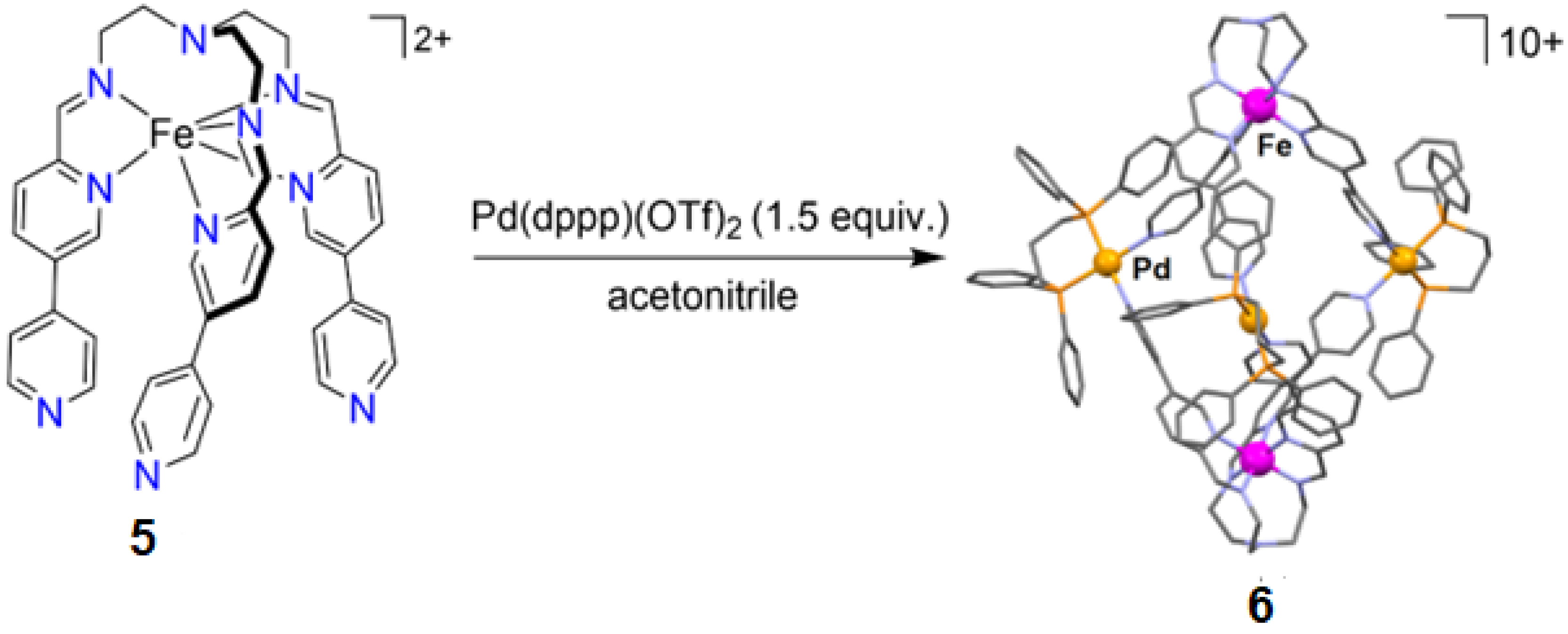
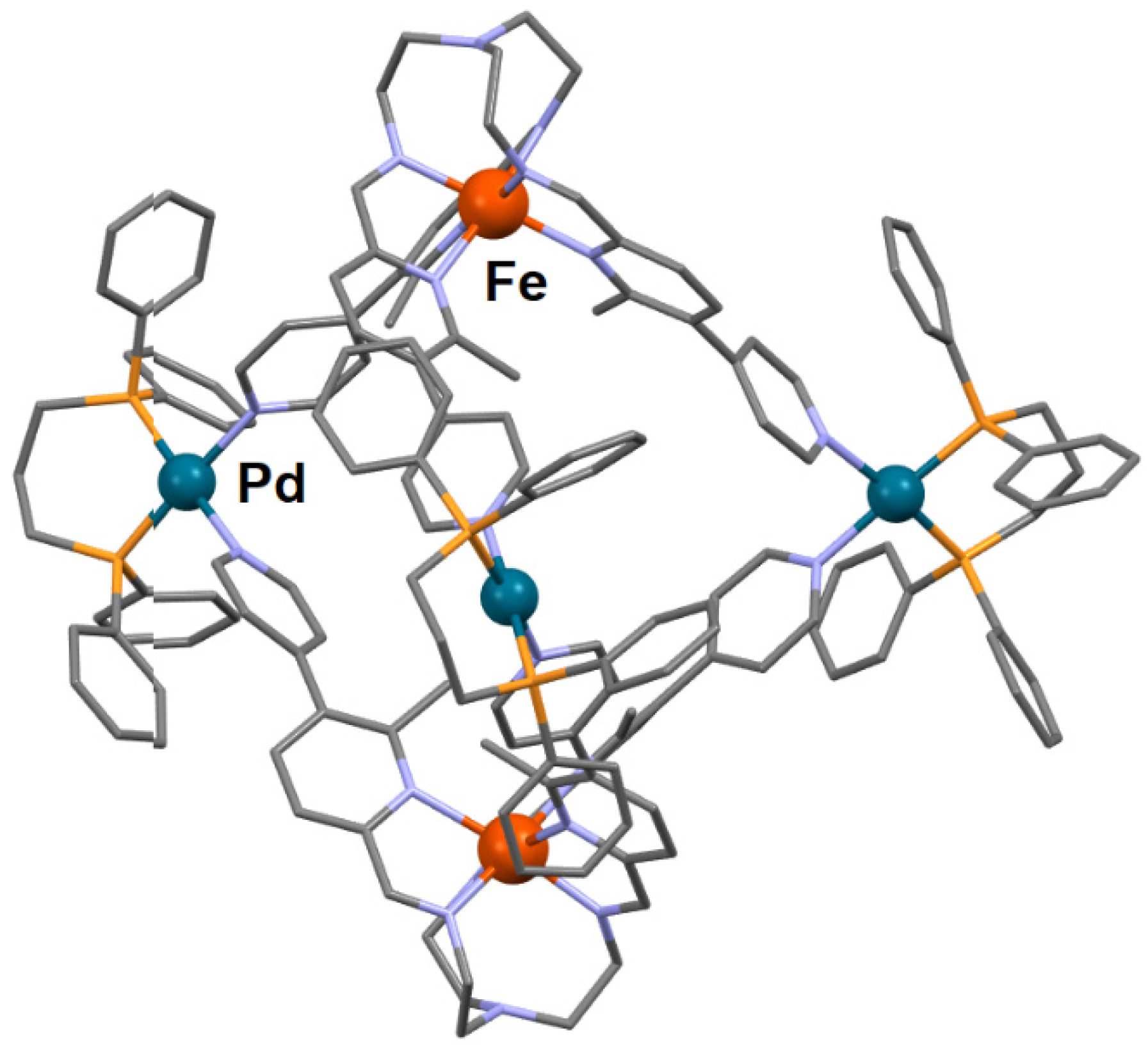
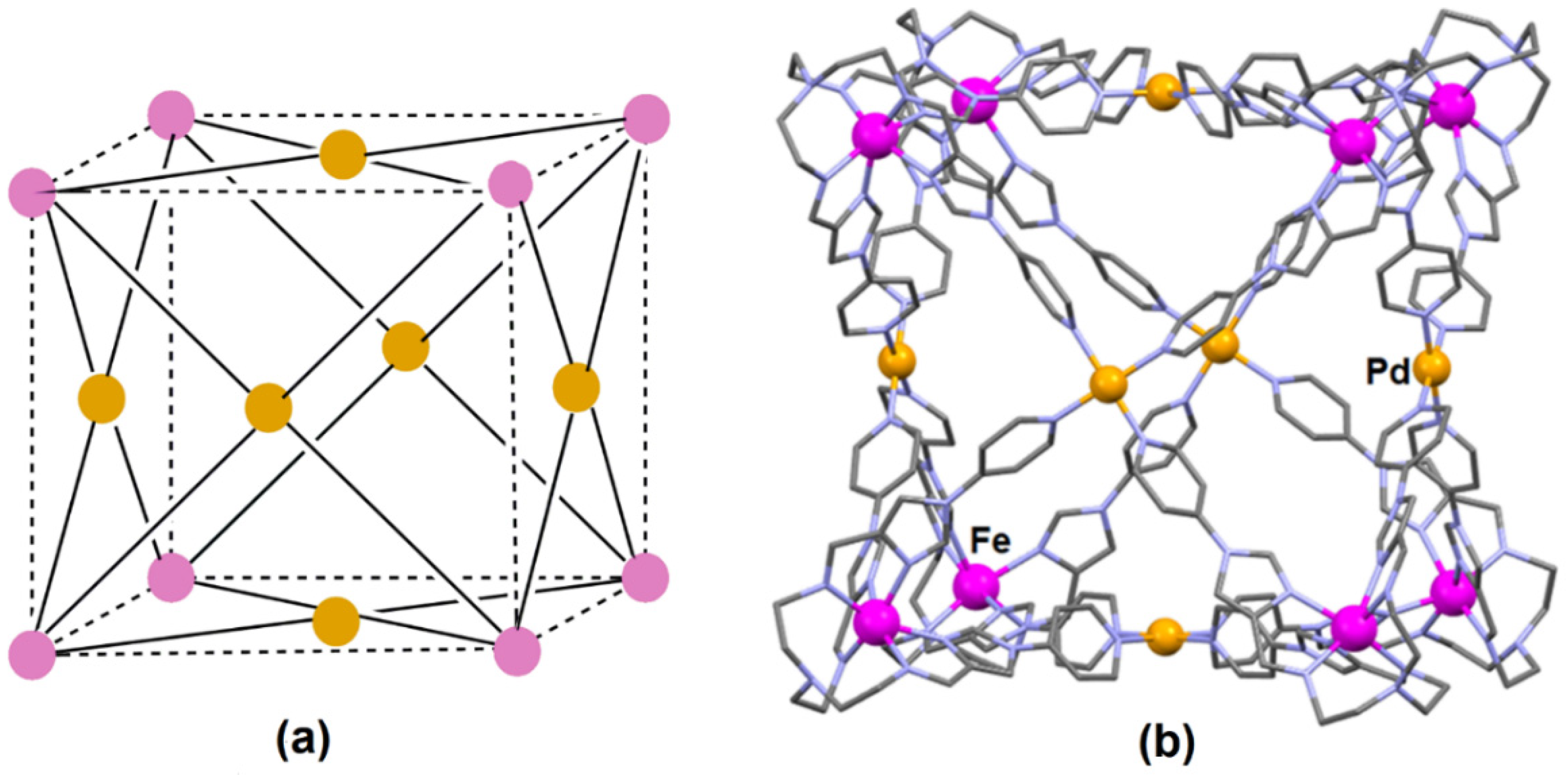
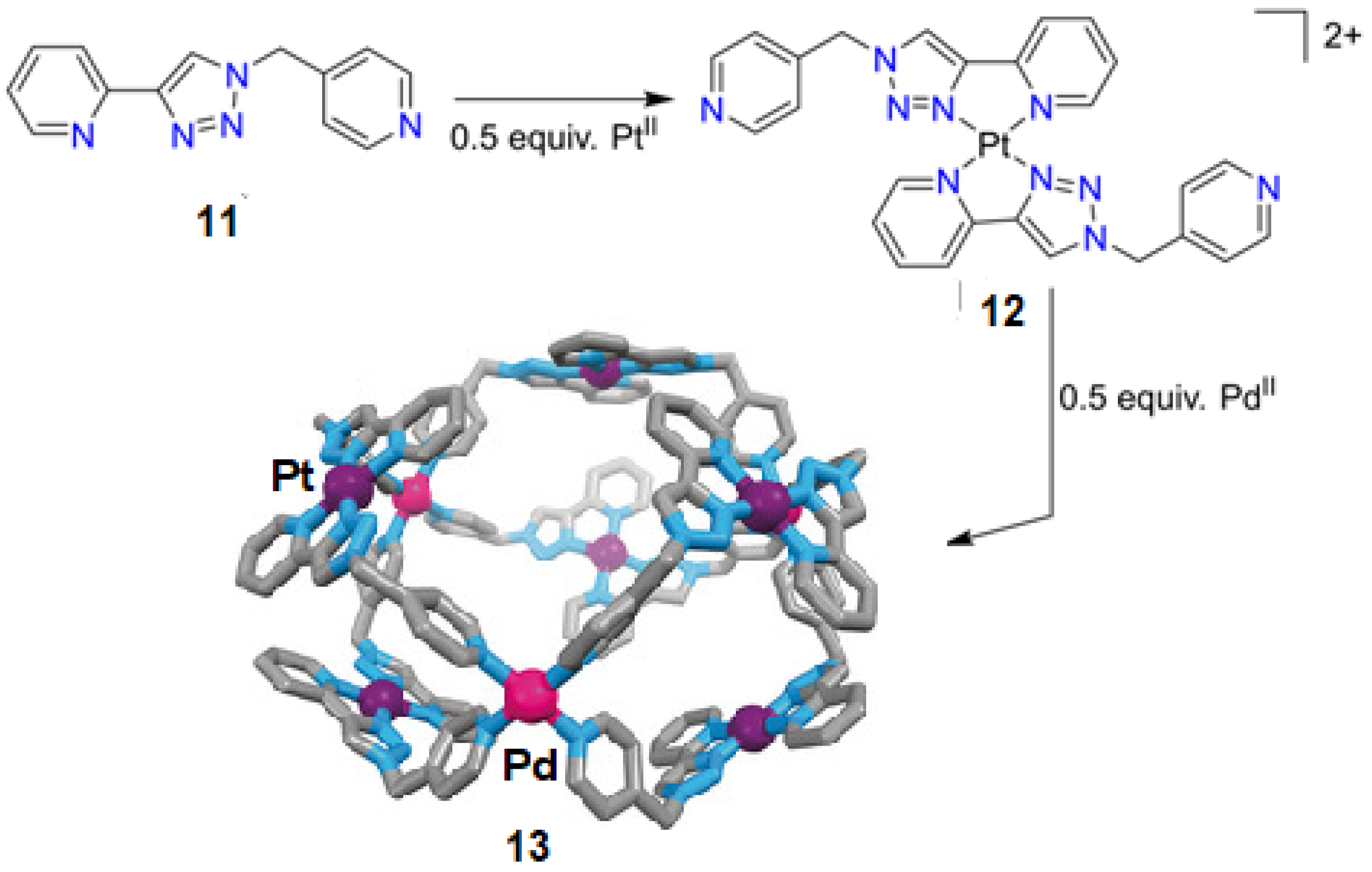
2.1. Metalloligand Systems Incorporating a Bound Primary Metal Ion That Sterically Organises Initially Unbound Secondary Donor Atoms for Cage Formation

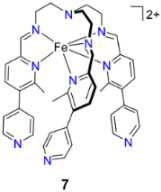
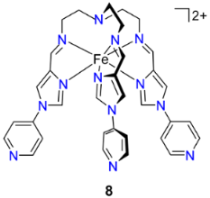
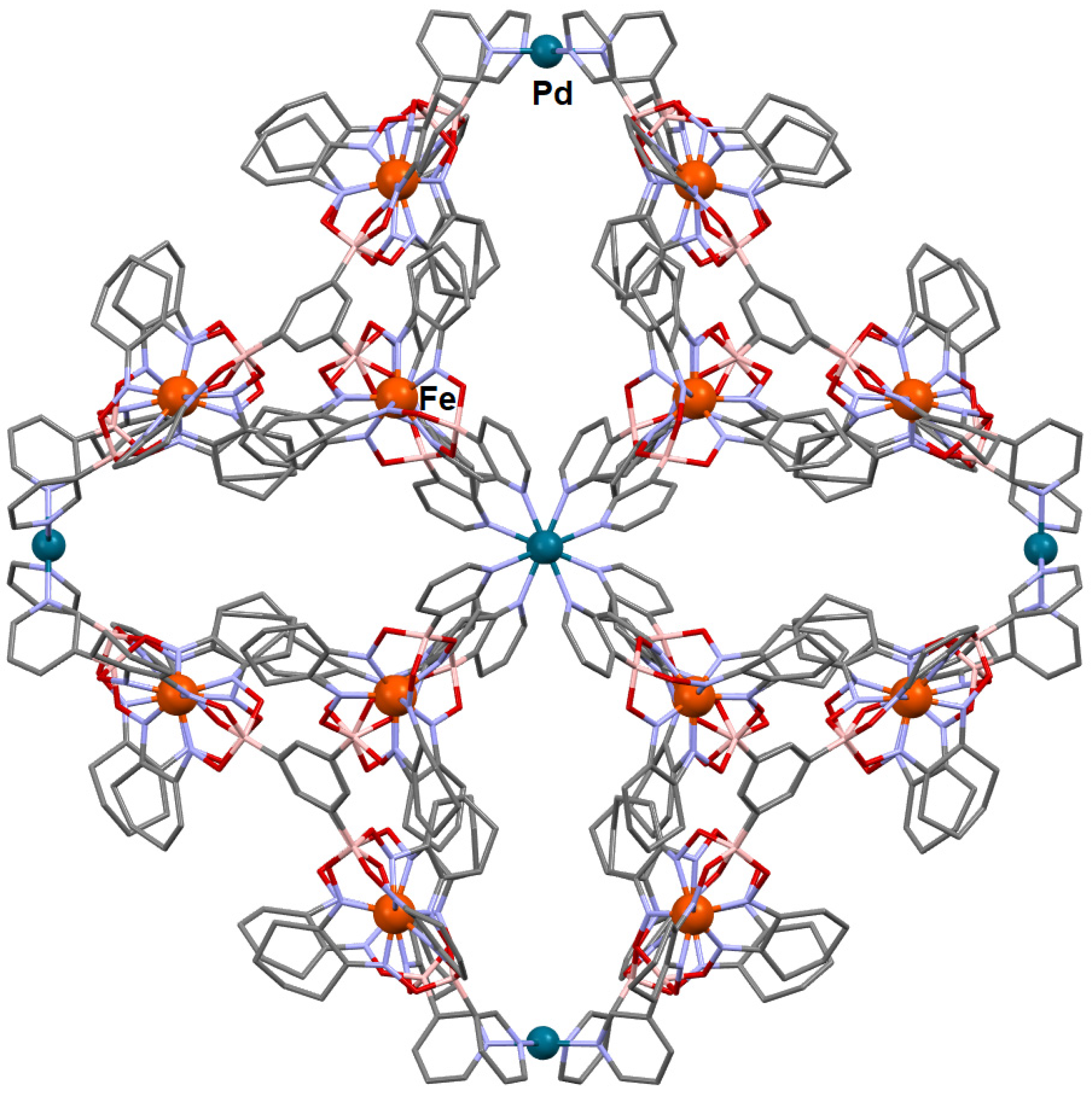
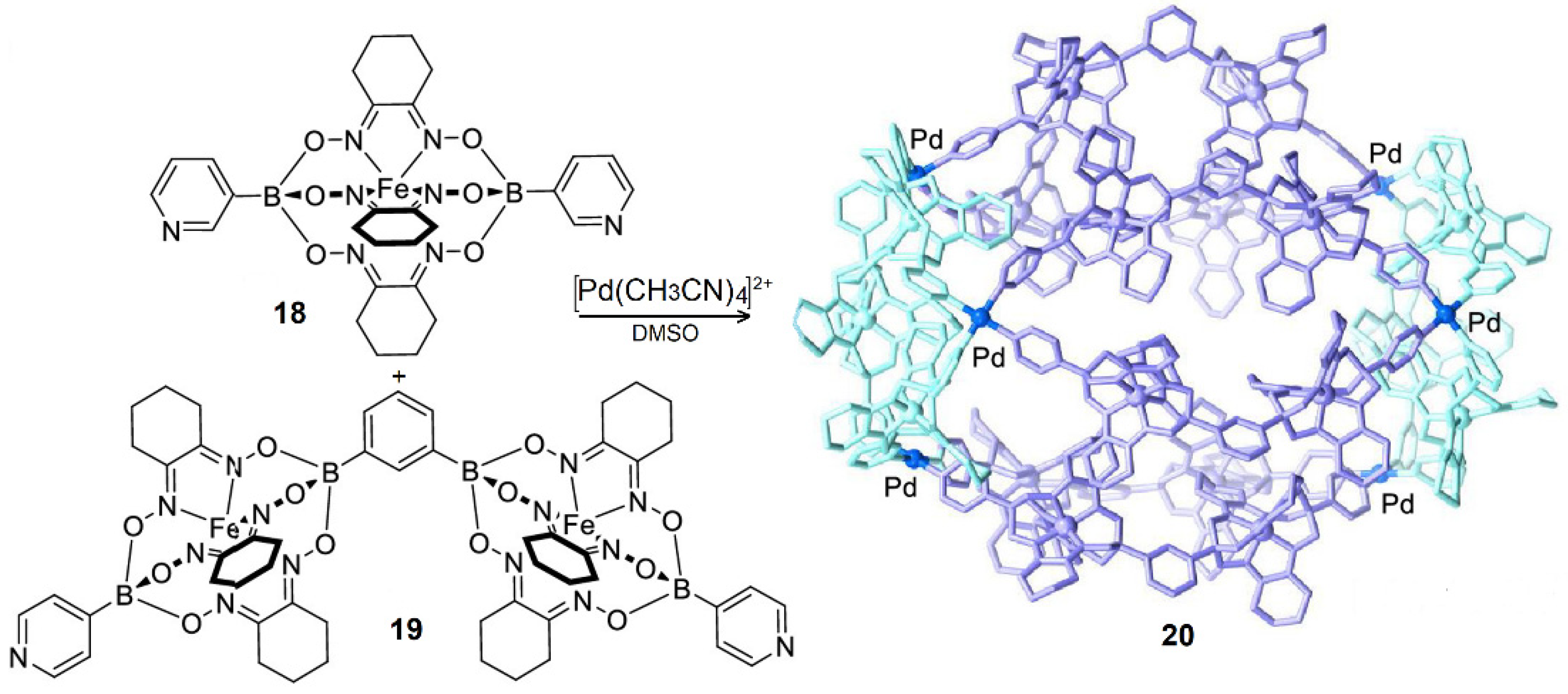
2.2. Cage Assembly Employing Clathrochelate Metalloligand Systems

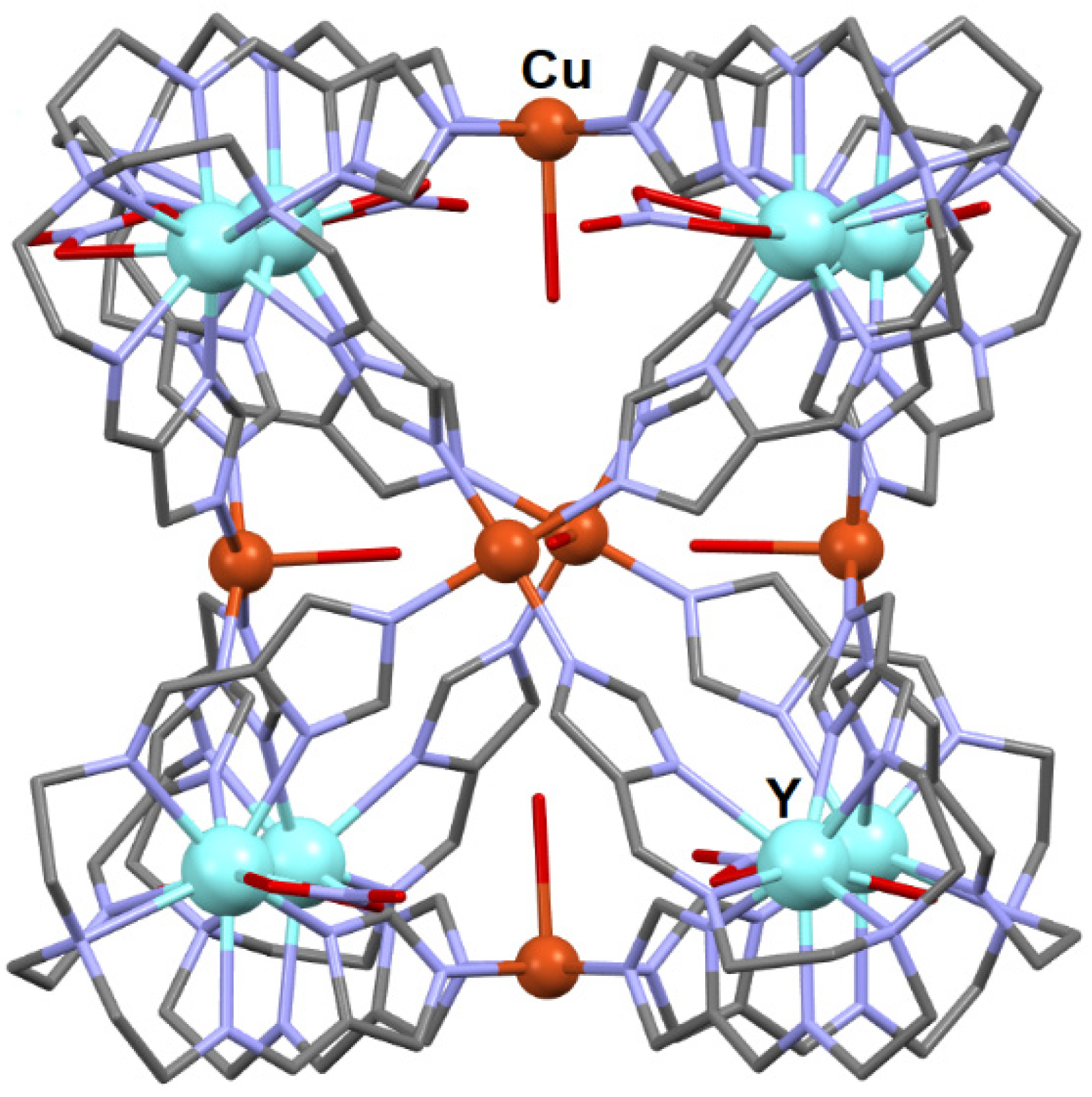
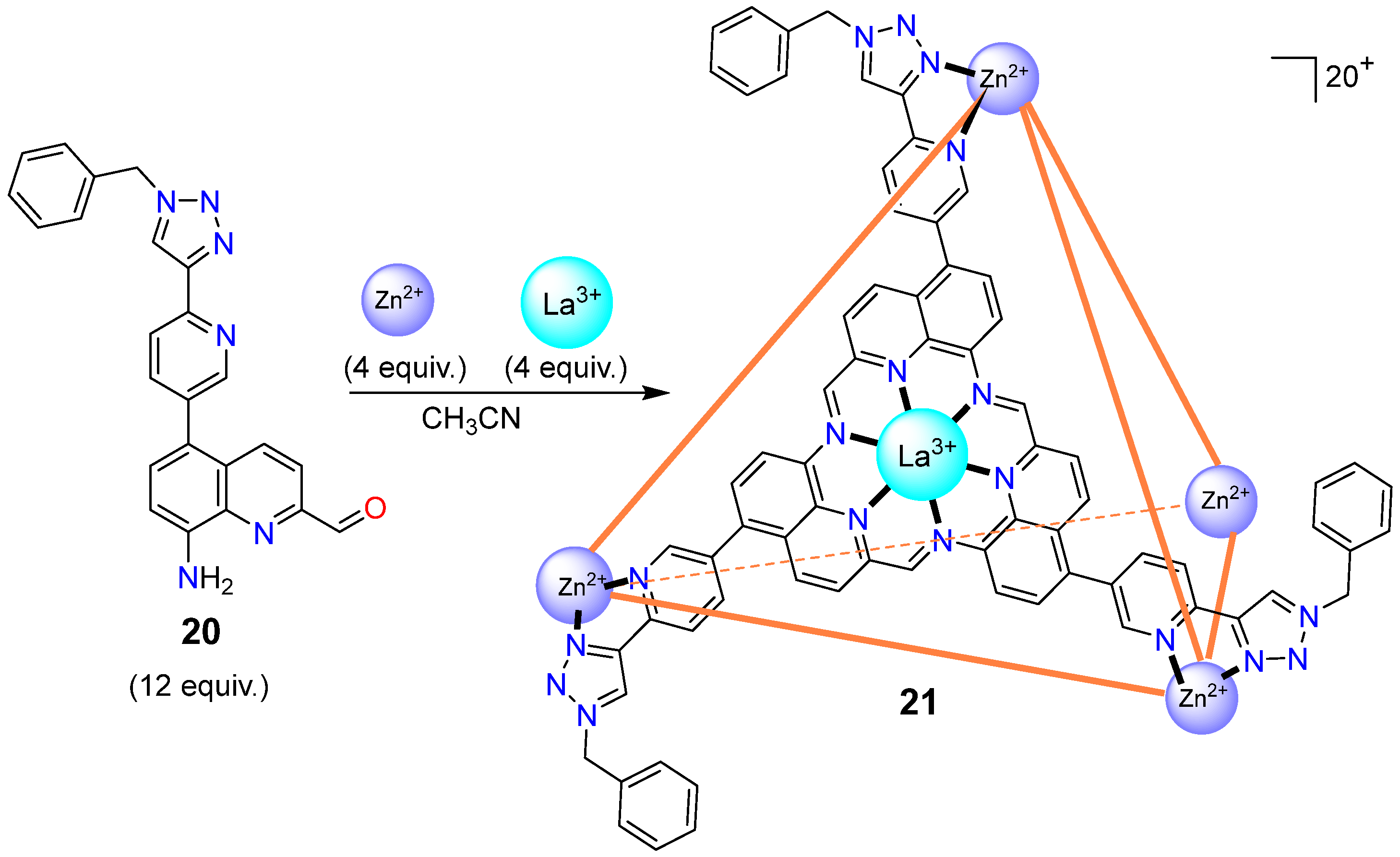
2.3. Cage Assembly Associated with In Situ Metalloligand Formation Involving a Metal-Ion Template Process
3. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saalfrank, R.W.; Stark, A.; Peters, K.; Vonschnering, H.G. Adamantoid chelate complexes. 1. The first adamantoid alkaline-earth metal chelate complex—Synthesis, structure, and reactivity. Angew. Chem. Int. Ed. 1988, 27, 851–853. [Google Scholar] [CrossRef]
- Zhu, X.-W.; Luo, D.; Zhou, X.-P.; Li, D. Imidazole-based metal-organic cages: Synthesis, structures, and functions. Coord. Chem. Rev. 2022, 455, 214354. [Google Scholar] [CrossRef]
- Virovets, A.V.; Peresypkina, E.; Scheer, M. Structural chemistry of giant metal based supramolecules. Chem. Rev. 2021, 121, 14485–14554. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, A.J.; Rowland, C.A.; Bloch, E.D. Permanently microporous metal—Organic polyhedra. Chem. Rev. 2020, 120, 8987–9014. [Google Scholar] [CrossRef]
- Lindoy, L.F. Tetrahedral metallocages assembled from oligopyridine ligands and transition metal ions. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 121–131. [Google Scholar] [CrossRef]
- Chakraborty, S.; Newkome, G.R. Terpyridine-based metallosupramolecular constructs: Tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef]
- Vasdev, R.A.S.; Preston, D.; Crowley, J.D. Multicavity metallosupramolecular architectures. Chem. Asian J. 2017, 12, 2513–2523. [Google Scholar] [CrossRef] [PubMed]
- Amouri, H.; Desmarets, C.; Moussa, J. Confined nanospaces in metallocages: Guest molecules, weakly encapsulated anions, and catalyst sequestration. Chem. Rev. 2012, 112, 2015–2041. [Google Scholar] [CrossRef]
- Davis, A.V.; Fiedler, D.; Seeber, G.; Zahl, A.; van Eldik, R.; Raymond, K.N. Guest exchange dynamics in an M4L6 tetrahedral host. J. Am. Chem. Soc. 2006, 128, 1324–1333. [Google Scholar] [CrossRef]
- Kedia, M.; Shankar, B.; Sathiyendiran, M. Rhenium (I)-based neutral coordination cages with a spherical cavity for selective recognition of fluoride. Inorg. Chem. 2022, 61, 14506–14510. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, H.M.; Tipping, W.J.; Vallejo, J.; Nichol, G.S.; Faulds, K.; Graham, D.; Brechin, E.K.; Lusby, P.J. Utilizing Raman spectroscopy as a tool for solid- and solution-phase analysis of metalloorganic cage host-guest complexes. Inorg. Chem. 2022, accepted. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, K.; Wang, H.; Fang, Y.; Feng, L.; Zhang, J.; Xiao, Z.; Wu, X.; Li, Y.; Fu, Y.; et al. Enantioseparation in hierarchically porous assemblies of homochiral cages. ACS Central Sci. 2022, 8, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.D.; Tipping, M.B.; Taylor, C.G.P.; Piper, J.R.; Pritchard, C.; Mozaceanu, C.; Ward, M.D. A family of externally-functionalised coordination cages. Chemistry 2021, 3, 88. [Google Scholar] [CrossRef]
- Kieffer, M.; Bilbeisi, R.A.; Thoburn, J.D.; Clegg, J.K.; Nitschke, J.R. Guest binding drives host redistribution in libraries of CoII4L4 cages. Angew. Chem. Int. Ed. 2020, 59, 11369–11373. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.G.P.; Argent, S.P.; Ludden, M.D.; Piper, J.R.; Mozaceanu, C.; Barnett, S.A.; Ward, M.D. One Guest or Two? A crystallographic and solution study of guest binding in a cubic coordination cage. Chem. Eur. J. 2020, 26, 3054–3064. [Google Scholar] [CrossRef]
- Kim, T.Y.; Vasdev, R.A.S.; Preston, D.; Crowley, J.D. Strategies for reversible guest uptake and release from metallosupramolecular architectures. Chem. Eur. J. 2018, 24, 14878–14890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-N.; Lu, Y.; Gao, W.-X.; Lin, Y.-J.; Jin, G.-X. Selective Encapsulation and separation of dihalobenzene isomers with discrete heterometallic macrocages. Chem. Eur. J. 2018, 24, 18913–18921. [Google Scholar] [CrossRef]
- Roukala, J.; Zhu, J.F.; Giri, C.; Rissanen, K.; Lantto, P.; Telkki, V.V. Encapsulation of xenon by a self-assembled Fe4L6 metallosupramolecular cage. J. Am. Chem. Soc. 2015, 137, 2464–2467. [Google Scholar] [CrossRef]
- Schmidt, A.; Casini, A.; Kuehn, F.E. Self-assembled M2L4 coordination cages: Synthesis and potential applications. Coord. Chem. Rev. 2014, 275, 19–36. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, J.; Wu, P.; Zhao, L.; He, C.; Zhang, J.; Duan, C. Cerium-based M4L4 tetrahedra as molecular flasks for selective reaction prompting and luminescent reaction tracing. Chem. Eur. J. 2014, 20, 2224–2231. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, H.; Zhang, C.; He, C.; Duan, C. Cerium-based M4L4 tetrahedrons containing hydrogen bond groups as functional molecular flasks for selective reaction prompting. New J. Chem. 2014, 38, 3137–3145. [Google Scholar] [CrossRef]
- Clegg, J.K.; Li, F.; Jolliffe, K.A.; Meehan, G.V.; Lindoy, L.F. An expanded neutral M4L6 cage that encapsulates four tetrahydrofuran molecules. Chem. Commun. 2011, 47, 6042–6044. [Google Scholar] [CrossRef]
- Brzechwa-Chodzyńska, A.; Drożdż, W.; Harrowfield, J.; Stefankiewicz, A.R. Fluorescent sensors: A bright future for cages. Coord. Chem. Rev. 2021, 434, 213820. [Google Scholar] [CrossRef]
- Hu, X.; Han, M.; Shao, L.; Zhang, C.; Zhang, L.; Kelley, S.P.; Zhang, C.; Lin, J.; Dalgarno, S.J.; Atwood, D.A.; et al. Self-assembly of a semiconductive and photoactive heterobimetallic metal-organic capsule. Angew. Chem. Int. Ed. 2021, 60, 10516–10520. [Google Scholar] [CrossRef]
- Wezenberg, S.J. Light-switchable metal-organic cages. Chem. Lett. 2020, 49, 609–615. [Google Scholar] [CrossRef]
- Jing, X.; He, C.; Yang, Y.; Duan, C. A Metal–organic tetrahedron as a redox vehicle to encapsulate organic dyes for photocatalytic proton reduction. J. Am. Chem. Soc. 2015, 137, 3967–3974. [Google Scholar] [CrossRef]
- Scott, A.J.; Vallejo, J.; Sarkar, A.; Smythe, L.; Regincós Martí, E.; Nichol, G.S.; Klooster, W.T.; Coles, S.J.; Murrie, M.; Rajaraman, G.; et al. Exploiting host-guest chemistry to manipulate magnetic interactions in metallosupramolecular M4L6 tetrahedral cages. Chem. Sci. 2021, 12, 5134–5142. [Google Scholar] [CrossRef] [PubMed]
- Capel Berdiell, I.; Hochdörffer, T.; Desplanches, C.; Kulmaczewski, R.; Shahid, N.; Wolny, J.A.; Warriner, S.L.; Cespedes, O.; Schünemann, V.; Chastanet, G.; et al. Supramolecular iron metallocubanes exhibiting site-selective thermal and light-induced spin-crossover. J. Am. Chem. Soc. 2019, 141, 18759–18770. [Google Scholar] [CrossRef] [PubMed]
- Campanella, A.J.; Ozvat, T.M.; Zadrozny, J.M. Ligand design of zero-field splitting in trigonal prismatic Ni (ii) cage complexes. Dalton Trans. 2022, 51, 3341–3348. [Google Scholar] [CrossRef]
- Li, L.; Craze, A.R.; Mustonen, O.; Zenno, H.; Whittaker, J.J.; Hayami, S.; Lindoy, L.F.; Marjo, C.E.; Clegg, J.K.; Aldrich-Wright, J.R.; et al. A mixed-spin spin-crossover thiozolylimine Fe4L68+ cage. Dalton Trans. 2019, 48, 9935–9938. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Jia, J.H.; Zheng, X.Y.; Zhang, Q.; Xiong, K.C.; Zhang, Z.M.; Wang, Q.M. Enantiopure magnetic heterometallic coordination cubic cages (M8Cu6II)-CuII (M = Ni, Co). Cryst. Growth Des. 2018, 18, 4555–4561. [Google Scholar] [CrossRef]
- McConnell, A.J. Spin-state switching in Fe (II) helicates and cages. Supramol. Chem. 2018, 30, 858–868. [Google Scholar] [CrossRef]
- Struch, N.; Bannwarth, C.; Ronson, T.K.; Lorenz, Y.; Mienert, B.; Wagner, N.; Engeser, M.; Bill, E.; Puttreddy, R.; Rissanen, K.; et al. An octanuclear metallosupramolecular cage designed to exhibit spin-crossover behavior. Angew. Chem. Int. Ed. 2017, 56, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, E.; Tsang, M.Y.; Troyano, J.; Craig, G.A.; Furukawa, S. Assembling metal-organic cages as porous materials. Chem. Soc. Rev. 2022, 51, 4876–4889. [Google Scholar] [CrossRef]
- Bell, D.J.; Natrajan, L.S.; Riddell, I.A. Design of lanthanide based metal-organic polyhedral cages for application in catalysis, sensing, separation and magnetism. Coord. Chem. Rev. 2022, 472, 214786. [Google Scholar] [CrossRef]
- Pan, M.; Wu, K.; Zhang, J.H.; Su, C.Y. Chiral metal-organic cages/containers (MOCs): From structural and stereochemical design to applications. Coord. Chem. Rev. 2019, 378, 333–349. [Google Scholar] [CrossRef]
- Ward, M.D.; Hunter, C.A.; Williams, N.H. Coordination cages based on bis (pyrazolylpyridine) ligands: Structures, dynamic behavior, guest binding, and catalysis. Acc. Chem. Res. 2018, 51, 2073–2082. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Ronson, T.K.; Zou, Y.-Q.; Nitschke, J.R. Metal-organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. [Google Scholar] [CrossRef]
- Zhu, J.; Yan, Z.; Bošković, F.; Haynes, C.J.E.; Kieffer, M.; Greenfield, J.L.; Wang, J.; Nitschke, J.R.; Keyser, U.F. FeII4L4 tetrahedron binds and aggregates DNA G-quadruplexes. Chem. Sci. 2021, 12, 14564–14569. [Google Scholar] [CrossRef]
- Percástegui, E.G.; Ronson, T.K.; Nitschke, J.R. Design and applications of water-soluble coordination cages. Chem. Rev. 2020, 120, 13480–13544. [Google Scholar] [CrossRef]
- Rizzuto, F.J.; von Krbek, L.K.S.; Nitschke, J.R. Strategies for binding multiple guests in metal-organic cages. Nat. Rev. Chem. 2019, 3, 204–222. [Google Scholar] [CrossRef]
- Xu, N.; Tan, Y.X.; El-Sayed, E.S.M.; Yuan, D. Two zirconium metal-organic cages with S4 and D2d Symmetry: Construction and detection of antibiotics. Cryst. Grow. Des. 2022, 22, 2768–2773. [Google Scholar] [CrossRef]
- Wei, L.; Ding, J.; Wu, J.; Li, L.; Li, Q.; Shao, L.-X.; Lu, J.; Qian, J. An efficient glucose sensor thermally calcined from copper-organic coordination cages. Talanta 2022, 241, 123263. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, X.; Leng, X.; Liu, X.; Schipper, D. A high-nuclearity CdII–NdIII nanocage for the rapid ratiometric fluorescent detection of quercetin. CrystEngComm 2022, 24, 4534–4539. [Google Scholar] [CrossRef]
- Ren, H.; Liu, C.; Yang, W.; Jiang, J. Sensitive and selective sensor based on porphyrin porous organic cage fluorescence towards copper ion. Dye. Pigment. 2022, 200, 110117. [Google Scholar] [CrossRef]
- McTernan, C.T.; Davies, J.A.; Nitschke, J.R. Beyond platonic: How to build metal-organic polyhedra capable of binding low-symmetry, information-rich molecular cargoes. Chem. Rev. 2022, 122, 10393–10437. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Sun, L.; Liu, C.L.; Rotaru, A.; Robeyns, K.; Singleton, M.L.; Garcia, Y. Supramolecular FeII4L4 cage for fast ammonia sensing. J. Mater. Chem. C 2022, 10, 9216–9221. [Google Scholar] [CrossRef]
- Yao, Y.; Li, J.Y.; Zhou, Y.Y.; Gao, T.; Li, H.F.; Yan, P.F. Turn-on luminescence detection of biogenic amine with an Eu (III) tetrahedron cage. Dye. Pigment. 2021, 192, 109441. [Google Scholar] [CrossRef]
- Li, Y.-W.; Li, J.; Wan, X.-Y.; Sheng, D.-F.; Yan, H.; Zhang, S.-S.; Ma, H.-Y.; Wang, S.-N.; Li, D.-C.; Gao, Z.-Y.; et al. Nanocage-based N-rich metal-organic framework for luminescence sensing toward Fe3+ and Cu2+ ions. Inorg. Chem. 2021, 60, 671–681. [Google Scholar] [CrossRef]
- Fang, Y.; Dehaen, W. Small-molecule-based fluorescent probes for f-block metal ions: A new frontier in chemosensors. Coord. Chem. Rev. 2021, 427, 213524. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Z.; Wu, L.; Lu, S.; Ling, S.; Li, G.; Xu, L.; Ma, L.; Hou, Y.; Wang, X.; et al. Emissive platinum (II) cages with reverse fluorescence resonance energy transfer for multiple sensing. J. Am. Chem. Soc. 2020, 142, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, B.S.; Champness, N.R. Metal-organic frameworks and metal-organic cages—A perspective. ChemPlusChem 2020, 85, 1842–1856. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.Y.; Zhu, T.Y.; Gao, T.; Li, H.F.; Yan, P.F. Eu (III) tetrahedron cage as a luminescent chemosensor for rapidly reversible and turn-on detection of volatile amine/NH3. Acs Appl. Mat. Interfaces 2020, 12, 15338–15347. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.X.; Liu, J.P.; Huang, J.J.; Rees, T.W.; Wang, Y.L.; Wang, H.; Li, X.P.; Chao, H.; Stang, P.J. A self-assembled Ru-Pt metallacage as a lysosome-targeting photosensitizer for 2-photon photodynamic therapy. Proc. Natl. Acad. Sci. USA 2019, 116, 20296–20302. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhou, L.-P.; Zhao, T.-H.; Cai, L.-X.; Guo, X.-Q.; Duan, P.-F.; Sun, Q.-F. Hierarchical self-assembly and chiroptical studies of luminescent 4d–4f cages. Inorg. Chem. 2018, 57, 7982–7992. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jordan, J.H.; Hu, X.-Y.; Wang, L. Supramolecular strategies for controlling reactivity within confined nanospaces. Angew. Chem. Int. Ed. 2020, 59, 13712–13721. [Google Scholar] [CrossRef]
- Galan, A.; Ballester, P. Stabilization of reactive species by supramolecular encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. [Google Scholar] [CrossRef]
- Vardhan, H.; Verpoort, F. Metal-organic polyhedra: Catalysis and reactive intermediates. Adv. Synth. Catal. 2015, 357, 1351–1368. [Google Scholar] [CrossRef]
- Mal, P.; Breiner, B.; Rissanen, K.; Nitschke, J.R. White phosphorus is air-stable within a self-assembled tetrahedral capsule. Science 2009, 324, 1697–1699. [Google Scholar] [CrossRef]
- Fang, Y.; Powell, J.A.; Li, E.; Wang, Q.; Perry, Z.; Kirchon, A.; Yang, X.; Xiao, Z.; Zhu, C.; Zhang, L.; et al. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. [Google Scholar] [CrossRef]
- Saha, R.; Mondal, B.; Mukherjee, P.S. Molecular cavity for catalysis and formation of metal nanoparticles for use in catalysis. Chem. Rev. 2022, 122, 12244–12307. [Google Scholar] [CrossRef]
- Chen, S.; Chen, L.-J. Metal-organic cages: Applications in organic reactions. Chemistry 2022, 4, 36. [Google Scholar] [CrossRef]
- Yu-Lin, L. A redox-active supramolecular Fe4L6 cage based on organic vertices with acid-base-dependent charge tunability for dehydrogenation catalysis. J. Am. Chem. Soc. 2022, 144, 8778–8788. [Google Scholar]
- Taylor, C.G.P.; Ward, M.D. Supramolecular catalysis with a cubic coordination cage: Contributions from cavity and external-surface binding. Supramol. Catal. 2022, 241–254. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, Z.; Bai, D.; Li, J.; Cui, X.; Xu, Z.J.; Tang, Y.; Tang, X.; Liu, W. A Discrete 3d–4f metallacage as an efficient catalytic nanoreactor for a three-component aza-Darzens reaction. Inorg. Chem. 2022, 61, 4009–4017. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hang, X.; Ding, J.; Li, B.; Zhu, R.; Pang, H.; Xu, Q. Catalysis within coordination cages. Coord. Chem. Rev. 2021, 430, 213656. [Google Scholar] [CrossRef]
- Yadav, S.; Kannan, P.; Qiu, G. Cavity-based applications of metallo-supramolecular coordination cages (MSCCs). Org. Chem. Front. 2020, 7, 2842–2872. [Google Scholar] [CrossRef]
- Tan, C.; Chu, D.; Tang, X.; Liu, Y.; Xuan, W.; Cui, Y. Supramolecular coordination cages for asymmetric catalysis. Chem. Euro. J. 2019, 25, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, R.J.; Rowlands, G.J.; Plieger, P.G. Coordination cages in catalysis. J. Incl. Phenom. Macrocycl. Chem. 2019, 96, 29–42. [Google Scholar] [CrossRef]
- Gao, W.-X.; Zhang, H.-N.; Jin, G.-X. Supramolecular catalysis based on discrete heterometallic coordination-driven metallacycles and metallacages. Coord. Chem. Rev. 2019, 386, 69–84. [Google Scholar] [CrossRef]
- Ward, M.D.; Raithby, P.R. Functional behaviour from controlled self-assembly: Challenges and prospects. Chem. Soc. Rev. 2013, 42, 1619–1636. [Google Scholar] [CrossRef] [PubMed]
- Sinha, I.; Mukherjee, P.S. Chemical transformations in confined space of coordination architectures. Inorg. Chem. 2018, 57, 4205–4221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, C.J.; Toste, F.D.; Bergman, R.G.; Raymond, K.N. Supramolecular catalysis in metal-ligand cluster hosts. Chem. Rev. 2015, 115, 3012–3035. [Google Scholar] [CrossRef] [PubMed]
- Hastings, C.J.; Pluth, M.D.; Bergman, R.G.; Raymond, K.N. Enzyme-like catalysis of the Nazarov cyclization by supramolecular encapsulation. J. Am. Chem. Soc. 2010, 132, 6938–6940. [Google Scholar] [CrossRef] [Green Version]
- Brown, C.J.; Bergman, R.G.; Raymond, K.N. Enantioselective catalysis of the aza-Cope rearrangement by a chiral supramolecular assembly. J. Am. Chem. Soc. 2009, 131, 17530–17531. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.K.; Isaacs, L. Biomedical applications of metal organic polygons and polyhedra (MOPs). Coord. Chem. Rev. 2020, 410, 213181. [Google Scholar] [CrossRef]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Verpoort, F. Metal-organic molecular cages: Applications of biochemical implications. Chem. Soc. Rev. 2015, 44, 9–25. [Google Scholar] [CrossRef]
- Sokolow, G.E.; Crawley, M.R.; Morphet, D.R.; Asik, D.; Spernyak, J.A.; McGray, A.J.R.; Cook, T.R.; Morrow, J.R. Metal-organic polyhedron with four Fe (III) centers producing enhanced T1 magnetic resonance imaging contrast in tumors. Inorg. Chem. 2022, 61, 2603–2611. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Yu, H.-J.; Wang, Y.-P.; Li, C.-J.; Wang, X.-F.; Ye, C.-G.; Yao, H.-L.; Pan, M.; Su, C.-Y. A photoactive Ir-Pd bimetallic cage with high singlet oxygen yield for efficient one/two-photon activated photodynamic therapy. Mater. Chem. Front. 2022, 6, 948–955. [Google Scholar] [CrossRef]
- Lisboa, L.S.; Preston, D.; McAdam, C.J.; Wright, L.J.; Hartinger, C.G.; Crowley, J.D. Heterotrimetallic double cavity cages: Syntheses and selective guest binding. Angew. Chem. Int. Ed. 2022, 61, e202201700. [Google Scholar] [CrossRef]
- Xu, W.Q.; Fan, Y.Z.; Wang, H.P.; Teng, J.; Li, Y.H.; Chen, C.X.; Fenske, D.; Jiang, J.J.; Su, C.Y. Investigation of binding behavior between drug molecule 5-fluoracil and M4L4-type tetrahedral cages: Selectivity, capture, and release. Chem. Eur. J. 2017, 23, 3542–3547. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; Lewis, J.E.M.; Crowley, J.D. Multicavity PdnL4 (2n+) cages with controlled segregated binding of different guests. J. Am. Chem. Soc. 2017, 139, 2379–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.R.; Suntharalingam, K.; Johnstone, T.C.; Lippard, S.J. Encapsulation of Pt (IV) prodrugs within a Pt (II) cage for drug delivery. Chem. Sci. 2015, 6, 1189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, J.E.M.; Gavey, E.L.; Cameron, S.A.; Crowley, J.D. Stimuli-responsive Pd2L4 metallosupramolecular cages: Towards targeted cisplatin drug delivery. Chem. Sci. 2012, 3, 778–784. [Google Scholar] [CrossRef]
- McConnell, A.J. Metallosupramolecular cages: From design principles and characterisation techniques to applications. Chem. Soc. Rev. 2022, 51, 2957–2971. [Google Scholar] [CrossRef]
- Hardy, M.; Lutzen, A. Better together: Functional heterobimetallic macrocyclic and cage-like assemblies. Chem. Eur. J. 2020, 26, 13332–13346. [Google Scholar] [CrossRef] [PubMed]
- Pullen, S.; Tessarolo, J.; Clever, G.H. Increasing structural and functional complexity in self-assembled coordination cages. Chem. Sci. 2021, 12, 7269–7293. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, C.; Liu, J.; Stang, P.J. Recent developments in the construction and applications of platinum-based metallacycles and metallacages via coordination. Chem. Soc. Rev. 2020, 49, 3889–3919. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.M.; Crowley, J.D. Metallo-supramolecular self-assembly with reduced-symmetry ligands. ChemPlusChem 2020, 85, 815–827. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.J.; Liu, D.; Jin, G.X. Multi-component coordination-driven self-assembly toward heterometallic macrocycles and cages. Coord. Chem. Rev. 2015, 293, 139–157. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef] [PubMed]
- Caulder, D.L.; Raymond, K.N. The rational design of high symmetry coordination clusters. J. Chem. Soc. Dalton Trans. 1999, 8, 1185–1200. [Google Scholar] [CrossRef]
- Fujita, M.; Umemoto, K.; Yoshizawa, M.; Fujita, N.; Kusukawa, T.; Biradha, K. Molecular paneling via coordination. Chem. Commun. 2001, 6, 509–518. [Google Scholar] [CrossRef]
- Zhou, M.-Y.; Tong, J.; Lu, H.-L.; Wang, X.-Y.; Yu, S.-Y. Hierarchical self-assembly and packing models of dipalladium (II,II)-based metallacapsules and metallacages based on amide-functionalized multi-pyrazoles. Inorg. Chem. Commun. 2022, 136, 109145. [Google Scholar] [CrossRef]
- Fujita, M.; Ogura, K. Transition-metal-directed assembly of well-defined organic architectures possessing large voids: From macrocycles to 2-catenanes. Coord. Chem. Rev. 1996, 148, 249–264. [Google Scholar] [CrossRef]
- Zhang, D.; Ronson, T.K.; Nitschke, J.R. Functional capsules via subcomponent self-assembly. Acc. Chem. Res. 2018, 51, 2423–2436. [Google Scholar] [CrossRef]
- Li, F.; Lindoy, L.F. Metalloligand strategies for assembling heteronuclear nanocages—Recent developments. Aust. J. Chem. 2019, 72, 731–741. [Google Scholar] [CrossRef]
- Rota Martir, D.; Zysman-Colman, E. Photoactive supramolecular cages incorporating Ru (II) and Ir (III) metal complexes. Chem. Commun. 2019, 55, 139–158. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Y.; Gao, W.-X.; Lin, L.; Jin, G.-X. Recent advances in the construction and applications of heterometallic macrocycles and cages. Coord. Chem. Rev. 2017, 344, 323–344. [Google Scholar] [CrossRef]
- Pachisia, S.; Gupta, R. Tailored inorganic-organic architectures via metalloligands. Chem. Rec. 2022, e202200121. [Google Scholar] [CrossRef]
- Zhou, X.-C.; Wu, L.-X.; Wang, X.-Z.; Lai, Y.-L.; Ge, Y.-Y.; Su, J.; Zhou, X.-P.; Li, D. Self-assembly of a Pd4Cu8L8 cage for epoxidation of styrene and its derivatives. Inorg. Chem. 2022, 61, 5196–5200. [Google Scholar] [CrossRef] [PubMed]
- Martir, D.R.; Cordes, D.B.; Slawin, A.M.Z.; Escudero, D.; Jacquemin, D.; Warriner, S.L.; Zysman-Colman, E. A luminescent Pd4Ru8 (24+) supramolecular cage. Chem. Commun. 2018, 54, 6016–6019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Liu, Y.; Wang, Y.; Guo, J.; Pan, M. Assembly and properties of Pd4Ru8 metal-organic cages based on polypyridine Ru (II)-metalloligand. Sci. Sin. Chim. 2020, 50, 687–694. [Google Scholar]
- Li, X.; Gorle, A.K.; Sundaraneedi, M.K.; Keene, F.R.; Collins, J.G. Kinetically-inert polypyridylruthenium(II) complexes as therapeutic agents. Coord. Chem. Rev. 2018, 375, 134–147. [Google Scholar] [CrossRef]
- O’Connor, H.M.; Sanz, S.; Scott, A.J.; Pitak, M.B.; Klooster, W.T.; Coles, S.J.; Chilton, N.F.; McInnes, E.J.L.; Lusby, P.J.; Weihe, H.; et al. (CrIII8NiII6)n+ Heterometallic coordination cubes. Molecules 2021, 26, 757. [Google Scholar] [CrossRef]
- Sanz, S.; O’Connor, H.M.; Comar, P.; Baldansuren, A.; Pitak, M.B.; Coles, S.J.; Weihe, H.; Chilton, N.F.; McInnes, E.J.L.; Lusby, P.J.; et al. Modular [FeIII8MII6]n+ (MII = Pd, Co, Ni, Cu) coordination cages. Inorg. Chem. 2018, 57, 3500–3506. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, H.M.; Sanz, S.; Pitak, M.B.; Coles, S.J.; Nichol, G.S.; Piligkos, S.; Lusby, P.J.; Brechin, E.K. [CrIII8MII6]n+ (MII = Cu, Co) face-centred, metallosupramolecular cubes. CrystEngComm 2016, 18, 4914–4920. [Google Scholar] [CrossRef] [Green Version]
- Sanz, S.; O’Connor, H.M.; Pineda, E.M.; Pedersen, K.S.; Nichol, G.S.; Mønsted, O.; Weihe, H.; Piligkos, S.; McInnes, E.J.L.; Lusby, P.J.; et al. [CrIII8MII6]12+ coordination cubes (MII = Cu, Co). Angew. Chem. Int. Ed. 2015, 54, 6761–6764. [Google Scholar] [CrossRef] [Green Version]
- Hardy, M.; Struch, N.; Topić, F.; Schnakenburg, G.; Rissanen, K.; Lützen, A. Stepwise construction of heterobimetallic cages by an extended molecular library approach. Inorg. Chem. 2018, 57, 3507–3515. [Google Scholar] [CrossRef]
- Hardy, M.; Struch, N.; Holstein, J.J.; Schnakenburg, G.; Wagner, N.; Engeser, M.; Beck, J.; Clever, G.H.; Lützen, A. Dynamic complex-to-complex transformations of heterobimetallic systems influence the cage structure or spin state of iron (II) ions. Angew. Chem. Int. Ed. 2020, 59, 3195–3200. [Google Scholar] [CrossRef] [Green Version]
- Min, H.; Craze, A.R.; Taira, T.; Wallis, M.J.; Bhadbhade, M.M.; Tian, R.; Fanna, D.J.; Wuhrer, R.; Hayami, S.; Clegg, J.K.; et al. Self-assembly of a rare high spin FeII/PdII tetradecanuclear cubic cage constructed via the metalloligand approach. Chemistry 2022, 4, 38. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Harman, D.G.; Avdeev, M.; Karatchevtseva, I. Cu (II) ion directed self-assembly of a Y8/Cu6 heterometallic coordination cage via an Y (III) metalloligand. Inorg. Chim. Acta 2019, 484, 521–526. [Google Scholar] [CrossRef]
- Preston, D.; Tucker, R.A.J.; Garden, A.L.; Crowley, J.D. Heterometallic [MnPtn(L)2n]x+ macrocycles from dichloromethane-derived bis-2-pyridyl-1,2,3-triazole ligands. Inorg. Chem. 2016, 55, 8928–8934. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; Sutton, J.J.; Gordon, K.C.; Crowley, J.D. A nona-nuclear heterometallic Pd3Pt6 “donut”-shaped cage: Molecular recognition and photocatalysis. Angew. Chem. Int. Ed. 2018, 57, 8659–8663. [Google Scholar] [CrossRef] [PubMed]
- Planes, O.M.; Schouwink, P.A.; Bila, J.L.; Fadaei-Tirani, F.; Scopelliti, R.; Severin, K. Incorporation of clathrochelate-based metalloligands in metal-organic frameworks by solvent-assisted ligand exchange. Cryst. Growth Des. 2020, 20, 1394–1399. [Google Scholar] [CrossRef]
- Giraldi, E.; Depallens, A.B.; Ortiz, D.; Fadaei-Tirani, F.; Scopelliti, R.; Severin, K. Boronate ester-capped helicates. Chem. Eur. J. 2020, 26, 7578–7582. [Google Scholar] [CrossRef]
- Bila, J.L.; Pijeat, J.; Ramorini, A.; Fadaei-Tirani, F.; Scopelliti, R.; Baudat, E.; Severin, K. Porous networks based on iron (II) clathrochelate complexes. Dalton Trans. 2019, 48, 4582–4588. [Google Scholar] [CrossRef]
- Jansze, S.M.; Severin, K. Clathrochelate metalloligands in supramolecular chemistry and materials science. Acc. Chem. Res. 2018, 51, 2139–2147. [Google Scholar] [CrossRef]
- Jansze, S.M.; Ortiz, D.; Fadaei Tirani, F.; Scopelliti, R.; Menin, L.; Severin, K. Inflating face-capped Pd6L8 coordination cages. Chem. Commun. 2018, 54, 9529–9532. [Google Scholar] [CrossRef]
- Ronson, T.K.; Carruthers, C.; Fisher, J.; Brotin, T.; Harding, L.P.; Rizkallah, P.J.; Hardie, M.J. Tripodal 4-pyridyl-derived host ligands and their metallo-supramolecular chemistry: Stella octangula and bowl-shaped assemblies. Inorg. Chem. 2010, 49, 675–685. [Google Scholar] [CrossRef]
- Sudan, S.; Li, R.-J.; Jansze, S.M.; Platzek, A.; Rudolf, R.; Clever, G.H.; Fadaei-Tirani, F.; Scopelliti, R.; Severin, K. Identification of a heteroleptic Pd6L6L’6 coordination cage by screening of a virtual combinatorial library. J. Am. Chem. Soc. 2021, 143, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
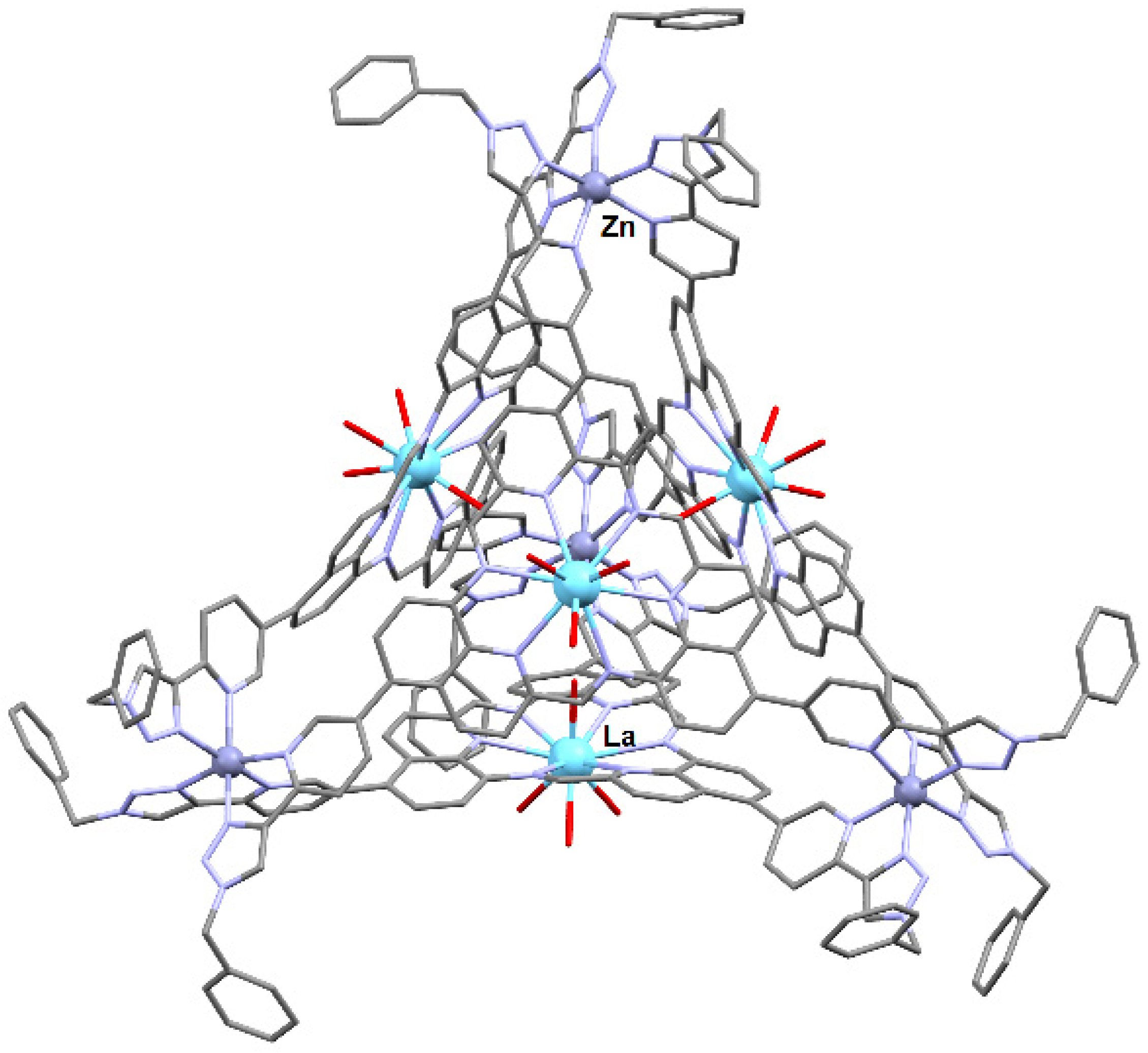
- Yang, D.; Greenfield, J.L.; Ronson, T.K.; von Krbek, L.K.S.; Yu, L.; Nitschke, J.R. La-III and Zn-II cooperatively template a metal-organic capsule. J. Am. Chem. Soc. 2020, 142, 19856–19861. [Google Scholar] [CrossRef] [PubMed]
- Cabbiness, D.K.; Margerum, D.W. Macrocyclic effect on the stability of copper (II) tetramine complexes. J. Am. Chem. Soc. 1969, 91, 6540–6542. [Google Scholar] [CrossRef]
- Cabbiness, D.K.; Margerum, D.W. Effect of macrocyclic structures on the rate of formation and dissociation of copper (II) complexes. J. Am. Chem. Soc. 1970, 92, 2151–2153. [Google Scholar] [CrossRef]
- Lindoy, L.F. The Chemistry of Macrocyclic Ligand Complexes; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Lindoy, L.F. Complementarity and Preorganisation in the Assembly of Heterometallic–Organic Cages via the Metalloligand Approach—Recent Advances. Chemistry 2022, 4, 1439-1456. https://doi.org/10.3390/chemistry4040095
Li F, Lindoy LF. Complementarity and Preorganisation in the Assembly of Heterometallic–Organic Cages via the Metalloligand Approach—Recent Advances. Chemistry. 2022; 4(4):1439-1456. https://doi.org/10.3390/chemistry4040095
Chicago/Turabian StyleLi, Feng, and Leonard F. Lindoy. 2022. "Complementarity and Preorganisation in the Assembly of Heterometallic–Organic Cages via the Metalloligand Approach—Recent Advances" Chemistry 4, no. 4: 1439-1456. https://doi.org/10.3390/chemistry4040095
APA StyleLi, F., & Lindoy, L. F. (2022). Complementarity and Preorganisation in the Assembly of Heterometallic–Organic Cages via the Metalloligand Approach—Recent Advances. Chemistry, 4(4), 1439-1456. https://doi.org/10.3390/chemistry4040095





