Cytotoxicity and Lipase Inhibition of Essential Oils from Amazon Annonaceae Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Collection
2.3. Essential Oil Extraction
2.4. Cell Viability Assay
2.5. Lipase Inhibition Assay
2.6. Chromatographic Analysis
2.7. Statistical Analysis
2.8. Molecular Docking Analysis
3. Results and Discussion
3.1. Cytotoxic Evaluation of Essential Oils
3.2. Lipase Enzyme Inhibition Assessment
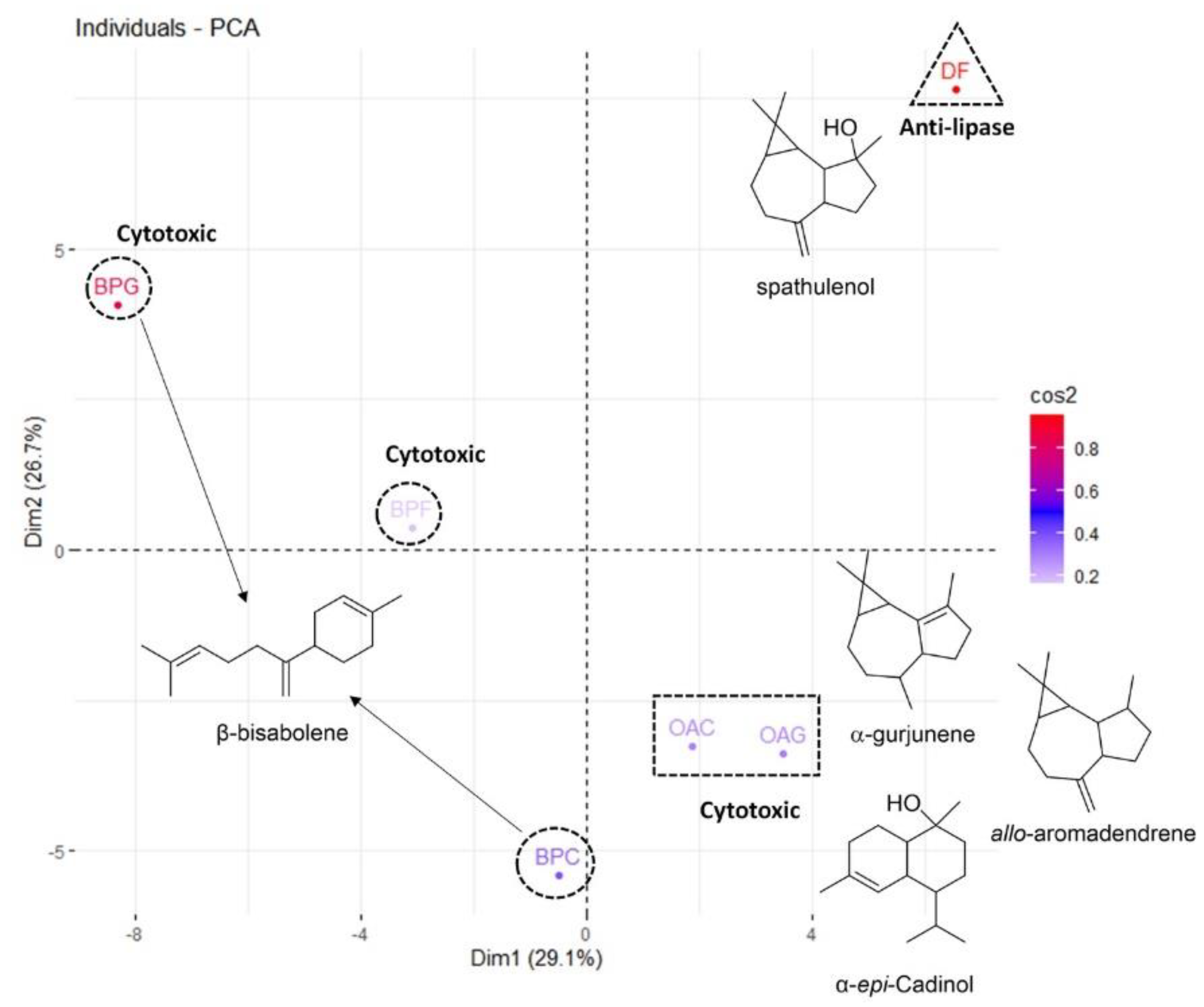
Principal Component Analysis (PCA)
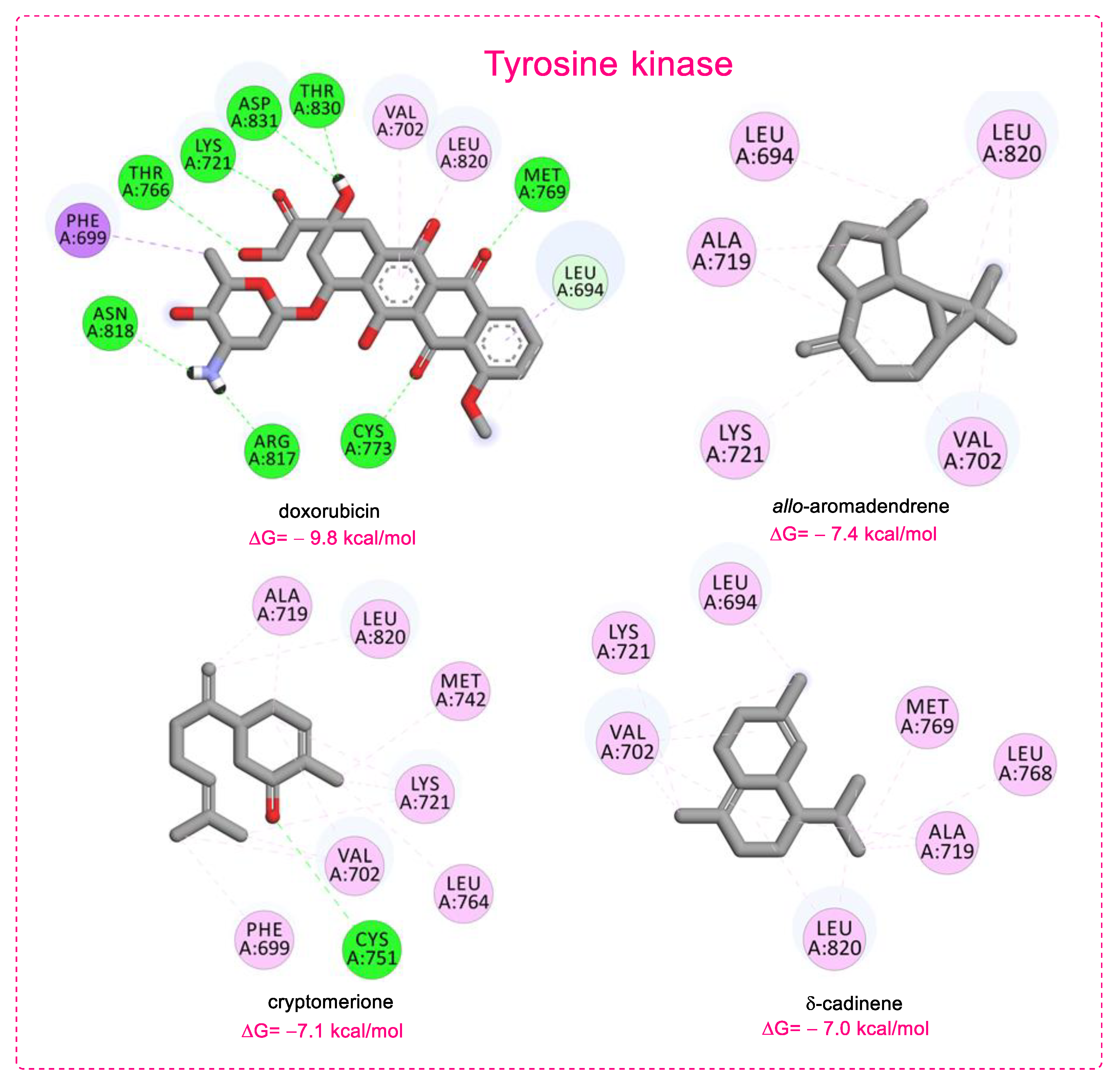
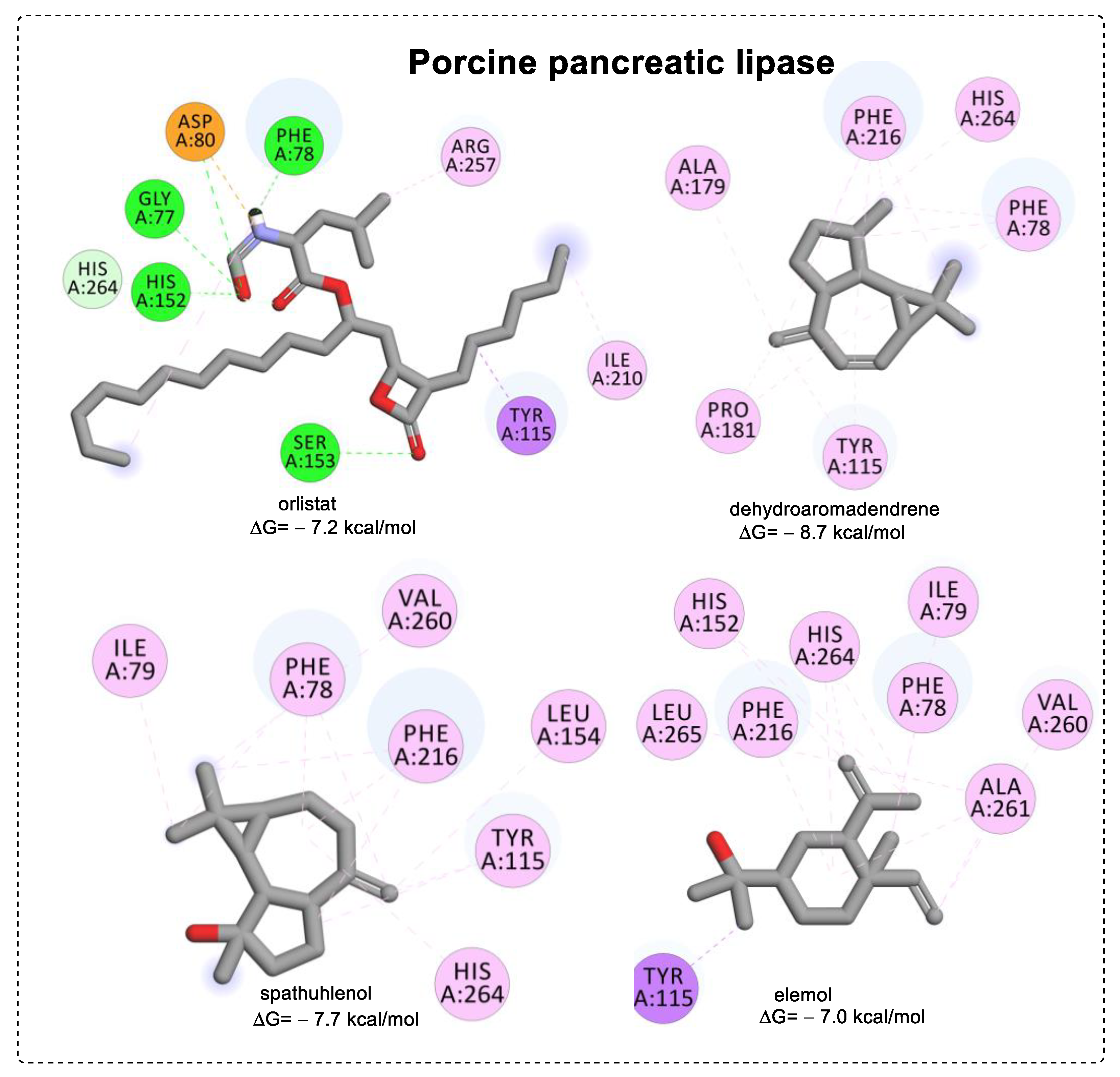
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- ter Steege, H.; Vaessen, R.W.; Cárdenas-López, D.; Sabatier, D.; Antonelli, A.; Oliveira, S.M.; Pitman, N.C.; Jørgensen, P.M.; Salomão, R.P. The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Sci. Rep. 2016, 6, 29549. [Google Scholar] [CrossRef]
- Ibiapina, A.; Gualberto, L.S.; Dias, B.B.; Freitas, B.C.B.; Martins, G.A.S.; Melo-Filho, A.A. Essential and fixed oils from Amazonian fruits: Proprieties and applications. Crit. Rev. Food Sci. Nutr. 2021, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.G.S.; Mourão, R.H.V. Amazon rosewood (Aniba rosaeodora Ducke) oils. In Essential Oils in Food Preservation, Flavor and Safety, 1st ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 20; pp. 193–201. [Google Scholar]
- Santos, R.C.V.; Alves, C.F.S.; Schneider, T.; Quintana, L.; Aurich, C.; Giogo, J.L.; Brandelli, A.; Vaucher, R.A. Antimicrobial activity of Amazonian oils against Paenibacillus species. J. Invertebr. Pathol. 2012, 109, 265–268. [Google Scholar] [CrossRef]
- Silva, B.J.M.; Hage, A.A.P.; Silva, E.O.; Rodrigues, A.P.D. Medicinal plants from the Brazilian Amazonian region and their antileishmanial activity: A review. J. Integr. Med. 2018, 16, 211–222. [Google Scholar] [CrossRef]
- Okhale, S.E.; Ugbabe, G.E.; Nwanosike, E.M.; Okoro, I. Comparative study on the essential oils of Curcuma longa L., Zingiber officinale Roscoe and Xylopia aethiopica (Dunal) A. Rich. J. Pharmacogn. Phytother. 2021, 13, 1–6. [Google Scholar]
- Fournier, G.; Leboeuf, M.; Cavé, A. Annonaceae essential oils: A review. J. Essent. Oil Res. 1999, 11, 131–142. [Google Scholar] [CrossRef]
- Bomfim, D.S.; Ferraz, R.P.C.; Carvalho, N.C.; Soares, M.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P. Eudesmol isomers induce caspase-mediated apoptosis in human hepatocellular carcinoma HepG2 cells. Basic Clin. Pharmacol. Toxicol. 2013, 113, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.P.C.; Cardoso, G.M.B.; da Silva, T.B.; Fontes, J.E.N.; Prata, A.P.N.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumor properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013, 141, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.C.B.C.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.A.; Dias, R.B.; Soares, M.B.P.; Prata, A.P.N.; Rocha, C.A.G.; Costa, E.V.; Bezerra, D.P. Antitumor properties of the essential oil from the leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Koorbanally, N.A.; Islam, M.S. Anti-diabetic effect of Xylopia aethiopica (Dunal) A. rich. (Annonaceae) fruit acetone fraction in a type 2 diabetes model of rats. J. Ethnopharmacol. 2016, 180, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Majoulia, K.; Hlilab, M.B.; Hamdic, A.; Flaminid, G.; Jannete, H.B.; Kenani, A. Antioxidant activity and α-glucosidase inhibition by essential oils from Hertia cheirifolia (L.). Ind. Crops Prod. 2015, 82, 223–228. [Google Scholar] [CrossRef]
- Quiroga, P.R.; Grosso, N.R.; Lante, A.; Lomolino, G.; Zygadlo, J.A.; Nepote, V. Chemical composition, antioxidant activity and anti-lipase activity of Origanum vulgare and Lippia turbinata essential oils. Int. J. Food Sci. Technol. 2013, 48, 642–649. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, R.Z.; Sun, S.L.; Zhao, C.; Fan, T.Y.; Chen, M.; Li, N.G.; Shi, Z.H. Small-molecule fms-like tyrosine kinase 3 inhibitors: An attractive and efficient method for the treatment of acute myeloid leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef]
- Dimić, D.S.; Kaluđerović, G.N.; Avdović, E.H.; Milenković, D.A.; Živanović, M.N.; Potocnak, I.; Samol’ova, E.; Dimitrijević, M.S.; Saso, L.; Marković, Z.S.; et al. Synthesis, crystallographic, quantum chemical, antitumor, and molecular docking/dynamic sudies of 4-hydroxycoumarin-neurotransmitter derivatives. Int. J. Mol. Sci. 2022, 23, 1001. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-obesity attributes; UHPLC-QTOF-MS/MS-based metabolite profiling and molecular docking insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.M.A.; Koolen, H.F.; Pereira, J.L.S.; Flach, A.; da Costa, L.A.M.A.; Souza, A.D.L.; Pinheiro, M.L.B. Chemotaxonomy of the Amazonian Unonopsis species based on GC-MS and chemometric analysis of the leaf essential oils. Rec. Nat. Prod. 2016, 9, 530–537. [Google Scholar]
- Soares, E.R.; da Silva, F.M.A.; de Almeida, R.A.; de Lima, B.R.; Koolen, H.F.; Lourenço, C.C. Chemical composition and antimicrobial evaluation of the essential oils of Bocageopsis pleiosperma Maas. Nat. Prod. Res. 2015, 29, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- de Lima, B.R.; da Silva, F.M.A.; Soares, E.R.; de Almeida, R.A.; da Silva-Filho, F.A.; Junior, R.C.P. Chemical composition and antimicrobial activity of the essential oils of Onychopetalum amazonicum R.E.Fr. Nat. Prod. Res. 2016, 30, 2356–2359. [Google Scholar] [CrossRef]
- Slanc, P.; Doljak, B.; Kreft, S.; Lunder, M.; Janeš, D.; Štrukelj, B. Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition. Phyther. Res. 2009, 23, 874–877. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Silva, F.M.A.; Silva, K.P.A.; Oliveira, L.P.M.; Costa, E.V.; Koolen, H.H.; Pinheiro, M.L.B.; Souza, A.Q.L.; Souza, A.D.L. Flavonoid glycosides and their putative human metabolites as potential inhibitors of the SARS-CoV-2 main protease (Mpro) and RNA-dependent RNA polymerase (RdRp). Mem. Inst. Oswaldo Cruz 2020, 115, e200207. [Google Scholar] [CrossRef]
- Stewart, J.J. Stewart Computational Chemistry, Colorado Springs, CO, USA. 2016. Available online: http://OpenMOPAC.net (accessed on 11 January 2022).
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [PubMed]
- Fechine, I.M.; Navarro, V.R.; da Cunha, E.V.L.; Silva, M.S.; Maia, J.G.S.; Barbosa-Filho, J.M. Alkaloids and volatile constituents from Duguetia flagellaris. Biochem. Syst. Ecol. 2002, 30, 267–269. [Google Scholar] [CrossRef]
- Jürgens, A.; Webber, A.C.; Gottsberger, G. Floral scent compounds of Amazonian Annonaceae species pollinated by small beetles and thrips. Phytochemistry 2000, 55, 551–558. [Google Scholar] [CrossRef]
- Mulyaningsiha, S.; Younsb, M.; El-Readia, M.Z.; Ashoura, M.L.; Nibreta, E.; Sporera, F.; Herrmanna, F.; Reichlinga, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major componentes. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef]
- Babahmada, R.A.; Aghrazb, A.; Boutafdaa, A.; Papazoglouc, E.G.; Tarantilisd, P.A.; Kanakisd, C.; Hafidia, M.; Ouhdouche, Y.; Outzourhitf, A.; Ouhammou, A. Chemical composition of essential oil of Jatropha curcas L. leaves and its antioxidant and antimicrobial activities. Ind. Crops Prod. 2018, 121, 405–410. [Google Scholar] [CrossRef]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.C.; Ho, C.L. Composition, in vitro cytotoxic, and antimicrobial activities of the flower essential oil of Diospyros discolor from Taiwan. Nat. Prod. Commun. 2015, 10, 1311–1314. [Google Scholar] [CrossRef]
- Costa, R.G.A.; da Anunciação, T.A.; Araujo, M.S.; Souza, C.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.P.B.; da Silva, F.M.A.; Koolen, H.F.; et al. In vitro and in vivo growth inhibition of human acute promyelocytic leucemia HL-60 cells by Guatteria megalophylla Diels (Annonaceae) leaf essential oil. Biomed. Pharmacother. 2020, 122, 109713. [Google Scholar] [CrossRef] [PubMed]
- Monk, N.R.; Bordignon, S.A.L.; Ferraz, A.; Machado, K.R.; Faria, D.H.; Lopes, R.M.; Mondin, C.A.; Souza, I.C.C.; Lima, M.F.S.; Rocha, A.B.; et al. Anti-tumour screening of Brazilians plants. Pharm. Biol. 2002, 40, 603–616. [Google Scholar] [CrossRef]
- Ibrahim, A.; Umar, I.A.; Aimola, I.A.; Mohammed, A. Inhibition of key enzymes linked to diabetes by Annona senegalensis Pers (Annonaceae) leaf in vitro. J. Herb. Med. 2018, 16, 100248. [Google Scholar] [CrossRef]
- Faria, J.V.; Valido, I.H.; Paz, W.H.P.; da Silva, F.M.A.; de Souza, A.D.L.; Acho, L.R.D.; Lima, E.S.; Boleti, A.P.A.; Marinho, J.V.N.; Salvador, M.J.; et al. Comparative evaluation of chemical composition and biological activities of tropical fruits consumed in Manaus, central Amazonia, Brazil. Food Res. Int. 2021, 139, 109836. [Google Scholar] [CrossRef]
- Bahadoria, M.B.; Maggib, F.; Zenginc, G.; Asgharid, B.; Eskandanie, M. Essential oils of hedgenettles (Stachys inflata, S. lavandulifolia, and S. byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: A comparative study. Ind. Crops Prod. 2020, 145, 112089. [Google Scholar] [CrossRef]
- de Freitas, R.A.; Lima, V.V.; Giachini, F.R.; Alves, J.D.; Lopes, A.S.; David, F.L.; Ndiaye, E.A. Chronic treatment with Duguetia furfuracea improves hyperglycemia and metabolic parameters in diabetic rats. Rev. Panorâmica 2018, 1, 56–68. [Google Scholar]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a sesquiterpene from the essential oil extract of Opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.M.A.; Brandão, M.G.L.; Oliveira, G.B.; Fortes, I.C.P.; Chartone-Souza, E. Synergistic bactericidal activity of Eremanthus erythropappus oil or β-bisabolene with ampicillin against Staphylococcus aureus. Antonie van Leeuwenhoek. 2007, 92, 95–100. [Google Scholar] [CrossRef]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem. Toxicol. 2013, 56, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Dutra, L.M.; Jesus, H.C.R.; Nogueira, P.C.L.; Moraes, V.R.S.; Salvador, M.J.; Cavalcanti, S.C.H.; Santos, R.L.C.; Prata, A.P.N. Chemical composition and antioxidant, antimicrobial, and larvicidal activities of the essential oils of Annona salzmannii and A. pickelii (Annonaceae). Nat. Prod. Commun. 2011, 6, 907–912. [Google Scholar] [CrossRef] [PubMed]
- An, Z.; Aksoy, O.; Zheng, T.; Fan, Q.W.; Weiss, W.A. Epidermal growth factor receptor and EGFRvIII in glioblastoma: Signaling pathways and targeted therapies. Oncogene 2018, 37, 1561–1575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, J.; Cheng, J.; Yang, W.; Zhu, Y.; Li, H.; Lu, T.; Chen, Y.; Lu, S. FLT3 inhibitors in acute myeloid leukemia: Challenges and recent developments in overcoming resistance. J. Med. Chem. 2021, 64, 2878–2900. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Shi, Y.; Cao, W.; Shi, J. The inhibition mechanisms of pancreatic lipase by apigenin and its anti-obesity mechanisms revealed by using network pharmacology. Food Biosci. 2022, 45, 101515. [Google Scholar] [CrossRef]
- Hegazy, M.E.F.; Elshamy, A.I.; Mohamed, T.A.; Hamed, A.R.; Ibrahim, M.A.; Ohta, S.; Paré, P.W. Cembrene diterpenoids with ether linkages from Sarcophyton ehrenbergi: An anti-proliferation and molecular-docking assessment. Mar. Drug 2017, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Yen, S.C.; Chen, L.C.; Huang, H.L.; Ngo, S.T.; Wu, Y.W.; Lin, T.E.; Sung, T.; Lein, S.; Tseng, H.; Pan, S.; et al. Investigation of selected flavonoid derivatives as potent FLT3 inhibitors for the potential treatment of acute myeloid leukemia. J. Nat. Prod. 2021, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mermer, A.; Demirci, S.; Tatar, G. Synthesis of novel pancreatic lipase inhibitors: Biological investigation and in silico studies. J. Biomol. Struct. Dyn. 2022, 40, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Anigboro, A.A.; Avwioroko, O.J.; Akeghware, O.; Tonukari, N.J. Anti-obesity, antioxidant and in silico evaluation of Justicia carnea bioactive compounds as potential inhibitors of an enzyme linked with obesity: Insights from kinetics, semi-empirical quantum mechanics and molecular docking analysis. Biophys. Chem. 2021, 274, 106607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ma, Y.; Gao, F.; Zhao, Y.; Cai, S.; Pang, M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods 2018, 40, 729–735. [Google Scholar] [CrossRef]
- Zhou, J.F.; Wang, W.J.; Yin, Z.P.; Zheng, G.D.; Chen, J.G.; Li, J.E.; Chen, L.L.; Zhang, Q.F. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021, 43, 101248. [Google Scholar] [CrossRef]

| Scheme | UGL | USL | UFL | URL | UDL | BPL | BPB | BPS | OAL | OAB | OAS | XBB | DFB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Artemisia triene | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 |
| α-Pinene | - | - | - | - | - | - | - | - | - | - | - | 3.3 | - |
| β-Pinene | - | - | - | - | - | - | - | - | - | - | - | 2.2 | - |
| α-Phellandrene | - | - | - | - | - | - | - | - | - | - | - | 14.1 | - |
| Limonene | - | - | - | - | - | - | - | - | - | - | - | 1.4 | - |
| Linalool | - | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| Verbenol | - | - | - | - | - | - | - | - | - | - | - | - | 1.7 |
| Citronellol | - | - | - | - | - | - | - | - | - | - | - | - | - |
| δ-Elemene | 2.51 | 1 | 0.27 | - | 0.48 | - | - | - | 0.7 | - | - | - | - |
| α-Cubebene | 1.19 | - | 0.83 | 0.24 | 0.34 | - | 1.62 | - | 0.7 | 1.3 | - | - | - |
| Citronellyl acetate | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cyclosativene | 0.71 | 0.11 | 0.27 | - | 0.4 | - | - | - | - | - | - | - | - |
| α-Ylangene | 0.34 | - | 0.17 | 0.31 | - | - | - | - | - | - | - | - | - |
| Isoledene | - | - | - | - | - | - | - | - | - | - | - | - | 1.8 |
| α-Copaene | 11.26 | 0.4 | 6.26 | 3.71 | 1.99 | 1.16 | 3.28 | - | 8.4 | 3.4 | 0.2 | - | - |
| β-Bourbonene | 0.9 | - | 1.24 | 1.44 | 0.46 | - | - | - | 0.6 | - | - | - | - |
| β-Cubebene | 0.84 | - | 0.84 | 0.26 | 0.49 | - | 1.10 | - | - | - | - | - | - |
| β-Elemene | 2.03 | 1.31 | 0.89 | 0.42 | 2.65 | - | 0.88 | 0.22 | 2.7 | 2.9 | 4.2 | - | 1.6 |
| 7-epi-Sesquithujene | - | - | - | - | - | 0.56 | - | - | - | - | - | - | - |
| β-Longipinene | - | - | - | - | - | - | 0.47 | - | - | - | - | - | - |
| α-Gurjunene | - | - | 0.19 | - | 1.26 | - | - | - | 3.6 | 14.9 | 10.6 | - | 4.1 |
| (E)-Caryophyllene | 3.91 | 18.76 | 4.06 | 3.97 | 1.22 | - | 3.61 | - | 17.0 | 3.8 | - | 46.9 | - |
| β-Gurjunene | 0.76 | - | 0.15 | 0.32 | 0.8 | - | - | - | - | - | - | - | - |
| (E)-α-Bergamotene | - | - | - | - | - | 6.9 | 1.54 | 2.49 | - | - | - | - | 0.3 |
| γ-Elemene | 0.44 | - | 0.12 | - | - | - | - | - | - | - | - | - | - |
| α-Guaiene | - | - | 2.48 | 2.58 | - | - | - | - | - | - | - | - | - |
| Aromadendrene | 1.13 | 2.38 | - | - | 0.44 | - | - | 0.63 | 1.6 | - | - | - | - |
| (Z)-β-Farnesene | - | - | - | - | - | 2.09 | - | - | - | - | - | - | - |
| Sinularene | 0.18 | 0.21 | - | - | - | 0.98 | - | - | - | - | - | - | |
| α-Neoclevene | - | - | - | - | - | - | - | - | - | - | - | - | 2.6 |
| α-Humulene | 1.74 | 5.18 | 0.94 | 0.84 | 0.96 | - | - | - | 3.1 | 0.9 | - | 4.5 | - |
| (E)-β-Farnesene | - | - | - | - | - | 6.05 | 1.24 | 1.04 | - | - | - | - | - |
| allo-Aromadendrene | 3.55 | - | - | - | 0.4 | - | 3.66 | - | - | 21.2 | 2.4 | - | - |
| Dehydroaromadendrene | - | - | - | - | - | - | - | - | - | - | - | - | 6.1 |
| γ-Gurjunene | - | - | - | - | - | - | - | - | - | 1.3 | - | - | 0.7 |
| γ-Muurolene | 2.79 | 0.45 | 4.18 | 6.4 | 1.11 | - | 1.70 | - | 2.9 | - | - | - | - |
| ar-Curcumene | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Germacrene D | 1.62 | 4.56 | 1.54 | 2.59 | 1.24 | - | - | - | 1.7 | - | - | - | 1.4 |
| β-Selinene | 0.31 | 0.3 | 0.8 | 0.5 | 0.54 | 0.29 | 6.46 | 0.39 | 0.7 | 0.7 | 1.4 | - | - |
| δ-Selinene | - | 0.22 | 0.53 | - | - | - | - | - | - | - | - | - | - |
| (E)-Muurola-4(14)-5-diene | - | - | - | - | - | - | 0.35 | - | - | - | - | - | - |
| Valencene | 1.94 | - | 2.1 | - | 0.4 | - | - | - | - | - | - | - | - |
| α-Selinene | - | - | - | - | - | - | 5.2 | - | - | - | - | - | - |
| Bicyclogermacrene | - | 20 | 1.55 | 3.7 | - | - | - | - | 5.4 | - | - | 12.5 | 0.6 |
| α-Muurolene | 1.26 | 0.23 | 1.93 | 2.38 | 0.57 | 0.25 | 0.96 | - | 1.2 | 0.7 | - | - | 0.9 |
| β-Bisabolene | - | - | - | - | - | 55.71 | 38.53 | 34.37 | - | - | - | - | - |
| (E,E)-α-Farnesene | - | - | - | - | - | - | - | - | - | - | 0.4 | - | - |
| α-Bulnesene | - | 0.4 | 1.69 | 1.24 | - | - | - | - | - | - | - | - | 1.3 |
| γ-Cadinene | 1.91 | 0.26 | 4.96 | 4.26 | 0.92 | 1.60 | - | - | 1.9 | 1.8 | 1.4 | 0.4 | |
| (E)-Calamene | - | - | - | - | - | - | - | - | - | - | 1.9 | - | - |
| δ-Cadinene | 2.24 | 0.7 | 5.37 | 4.02 | 0.83 | 1.42 | 7.55 | - | 5.9 | 3.9 | 2.4 | - | - |
| (E)-γ-Bisabolene | - | - | - | - | - | - | - | 0.62 | - | - | - | - | - |
| (E)-Cadina-1,4-diene | - | - | 0.43 | - | - | - | - | - | - | - | - | - | - |
| α-Cadinene | 0.25 | - | 0.39 | 0.55 | - | - | - | - | - | - | - | - | - |
| α-Calacorene | - | - | 2.16 | 2.56 | - | 1.00 | 0.52 | - | 2.7 | - | 1.3 | - | 3.3 |
| Elemol | 1.6 | 1.29 | 1.3 | 0.68 | 7.86 | - | - | - | - | - | 2.5 | - | 6.5 |
| β-Vetivenene | 0.63 | 0.53 | 1.14 | 1.09 | 1.29 | - | - | - | - | - | - | - | - |
| Germacrene B | 1.84 | 0.56 | 0.52 | 0.29 | - | - | 0.32 | - | 1.0 | - | - | - | 1.6 |
| (E)-Nerolidol | 0.52 | - | - | - | 0.63 | - | - | - | - | - | - | - | - |
| β-Calacorene | - | - | - | - | - | - | - | - | 0.8 | - | - | - | - |
| 3-(Z)-Hexenyl benzoate | - | - | - | - | 0.53 | - | - | - | - | - | - | - | - |
| 1.5-Epoxysalvial-4(14)-ene | 0.46 | 0.88 | 0.3 | 0.26 | 3.65 | - | - | - | - | - | - | - | - |
| Dendrolasin | - | - | - | - | - | - | - | 0.26 | - | - | - | - | - |
| (E)-Sesquisabinene hydrate | - | - | - | - | - | - | - | - | - | - | 0.5 | - | - |
| Spathulenol | 4.8 | 20.5 | 15.7 | 17.13 | 19.1 | - | - | - | 10.4 | - | - | - | 11.9 |
| Caryophyllene oxide | 4.8 | 8.41 | 9.77 | 15.9 | 9.7 | 2.8 | 3.00 | - | 11.9 | 3.7 | 4.9 | 0.8 | 2.5 |
| ar-Turmerol | - | - | - | - | - | - | - | 0.72 | - | - | - | - | - |
| Gleenol | - | - | - | - | - | - | 0.34 | - | - | - | - | - | - |
| β-Copaen-4-α-ol | 0.32 | 0.27 | 0.75 | 0.56 | - | - | - | - | - | - | - | - | - |
| Viiridiflorol | 1.61 | 1.6 | 0.99 | 0.84 | - | - | - | - | 0.7 | 0.9 | 1.4 | 1.5 | 2.0 |
| Salvial-4(14)-en-1-one | - | 0.47 | - | - | 0.25 | - | - | 0.31 | - | - | - | - | - |
| Widdrol | - | - | 1.14 | 0.63 | - | - | - | - | - | - | - | - | 4.1 |
| Guaiol | 5.14 | 0.85 | 1.43 | 1.57 | 6.41 | - | - | - | 1.1 | - | - | - | - |
| Rosifoliol | - | 0.8 | - | - | - | - | - | - | - | - | - | - | - |
| Ledol | - | - | - | - | 1.83 | - | - | - | - | - | - | 4.6 | 3.6 |
| Sesquithuriferol | - | - | - | - | - | - | - | - | - | - | 0.7 | - | - |
| Humulene epoxide II | 0.95 | 0.98 | 2.26 | 2.89 | 3.21 | - | 0.89 | - | 1.1 | - | 3.4 | - | - |
| β-atlanol | - | - | - | - | - | - | - | 4.09 | - | - | - | - | - |
| 1,10-di-epi-Cubenol | - | - | - | - | - | - | - | - | - | - | 3.2 | - | - |
| Isolongifolan-7-α-ol | - | - | - | - | - | - | - | - | - | - | 2.4 | - | - |
| 1-epi-Cubenol | - | - | - | - | - | 0.41 | 3.55 | - | - | 3.2 | 2.8 | - | - |
| Isospathulenol | 5.51 | 1.6 | 3.14 | 2.21 | 2.57 | - | - | - | - | - | - | - | - |
| α-epi-Cadinol | - | - | - | - | - | - | - | - | - | 24.1 | 14.0 | 2.9 | - |
| Epoxy-allo-aromadendrene | - | - | - | - | - | 0.60 | - | - | - | - | - | - | - |
| Cubenol | - | - | - | - | - | - | 3.2 | - | 1.4 | 0.8 | - | - | - |
| α-Muurolol | 2.45 | - | 1.35 | 0.78 | 1.37 | 0.22 | 0.97 | - | - | - | - | - | - |
| Torreyol | - | - | - | - | - | - | - | - | - | - | - | - | 1.8 |
| β-Eudesmol | 1.13 | - | 1.1 | 1.16 | 1.49 | - | - | - | - | - | - | - | 2.3 |
| α-Cadinol | 3.64 | 0.59 | 1.62 | 2.09 | 3.05 | 0.47 | 0.37 | - | 0.5 | 1.3 | 0.7 | - | - |
| α-Bisabolol oxide B | - | - | - | - | - | - | - | 1.43 | - | - | - | - | - |
| 14-Hydroxy-(Z)-caryophyllene | - | - | - | - | - | 0.36 | - | - | - | - | - | - | - |
| β-Atlantone | - | - | - | - | - | - | - | 0.74 | - | - | - | - | - |
| E-calamen-10-ol | - | - | - | - | - | 0.23 | - | - | - | - | - | - | - |
| Bulnesol | 2.65 | - | 0.7 | 0.8 | 2.4 | - | - | - | - | - | - | - | - |
| β-Bisabolol | - | - | - | - | - | 0.47 | - | 0.94 | - | - | - | - | - |
| Cadalene | - | - | 0.48 | 0.62 | 0.52 | 0.22 | - | - | - | - | 0.9 | - | - |
| Mustakone | - | - | - | - | - | - | - | - | - | - | 1.1 | - | - |
| α-Bisabolol | - | - | - | - | - | 1.45 | 2.21 | 1.9 | - | - | - | - | - |
| Eudesma-4(15),7-dien-1-β-ol | - | - | - | - | - | - | - | - | - | - | 1.8 | - | - |
| Cyperotundone | - | - | - | - | - | - | - | - | - | 1.3 | 8.1 | - | - |
| (2Z.6Z)-Farnesol | - | - | - | - | - | - | - | 7.2 | - | - | - | - | - |
| β-Sinensal | - | - | - | - | - | - | - | 0.35 | - | - | - | - | - |
| (2E.6Z)-Farnesol | - | - | - | - | - | 0.98 | - | 3.54 | - | - | - | - | - |
| Cryptomerione | - | - | - | - | - | 2.58 | 1.03 | 9.6 | - | - | - | - | - |
| (2E.6E)-Farnesol | - | - | - | - | - | - | - | 0.5 | - | - | - | - | - |
| β-Bisabolenal | - | - | - | - | - | - | - | 0.62 | - | - | - | - | - |
| β-Bisabolenol | - | - | - | - | - | 0.64 | - | 0.2 | - | - | - | - | - |
| (2E.6E)-Farnesyl acetate | - | - | - | - | 0.24 | - | - | - | - | - | - | - | - |
| (5E.9E)-Farnesyl acetone | 1.52 | - | - | - | - | - | - | - | - | - | - | - | - |
| Phytol | - | - | - | - | - | - | - | - | - | - | - | - | 3.4 |
| Cembrene A | - | - | - | - | - | - | - | - | - | - | - | - | 0.4 |
| Samples | Cell Lines IC50 (in μg/mL) and 95% CI (in µg/mL) | |||
|---|---|---|---|---|
| HepG2 | HL-60 | K562 | PBMC | |
| U. duckei (leaves) (UDL) | >50 | >50 | >50 | >50 |
| U. floribunda (leaves) (UFL) | >50 | >50 | >50 | >50 |
| U. guatterioides (leaves) (UGL) | >50 | >50 | >50 | >50 |
| U. rufescens (leaves) (URL) | >50 | >50 | >50 | >50 |
| U. stipitata (leaves) (USL) | >50 | >50 | >50 | >50 |
| O. amazonicum (leaves) (OAL) | >50 | >50 | >50 | >50 |
| O. amazonicum (barks) (OAB) | 40.37 34.38–47.39 | 10.32 7.13–14.93 | 17.70 13.01–24.08 | 46.02 38.70–54.73 |
| O. amazonicum (stems) (OAS) | 28.58 25.00–32.67 | 5.36 4.23–6.79 | 29.73 23.53–37.55 | 33.47 28.20–39.71 |
| B. pleiosperma (barks) (BPB) | 35.93 30.96–41.69 | 15.22 10.63–21.80 | 20.33 12.12–34.12 | >50 |
| B. pleiosperma (leaves) (BPL) | >50 | 11.74 9.65–14.29 | 37.92 31.72–45.33 | 43.65 37.02–51.48 |
| B. pleiosperma (stems) (BPS) | 43.41 35.08–53.72 | 8.70 5.19–14.57 | 28.93 20.62–40.59 | 33.32 28.34–39.18 |
| D. flagellaris (barks) (DFB) | >50 | >50 | - | - |
| X. benthamii (barks) (XBB) | >50 | >50 | - | - |
| Doxorubicin | 0.46 0.33–0.62 | 0.06 0.05–0.08 | 0.33 0.19–0.59 | 0.67 0.49–0.91 |
| Samples | Dimension 1 | Dimension 2 |
|---|---|---|
| BPL | 6.90 | 0.10 |
| BPB | 0.17 | 23.10 |
| BPS | 50.38 | 13.01 |
| OAB | 2.53 | 8.44 |
| OAS | 8.80 | 9.07 |
| DFB | 31.19 | 46.25 |
| Compounds | Protein (PDB ID) | Binding Energy (kcal/mol) | Main Interactions |
|---|---|---|---|
| Doxorubicin a | Tyrosine kinase (1M17) | −9.8 | HB (Lys721, Thr766, Met769, Cys773, Arg817, Asn818, Thr830, Asp831), PSi (Phe699), PAl (Val702, Leu820) |
| allo-aromadendrene | −7.4 | Al (Leu694, Val702, Ala719, Lys721, Leu820) | |
| cryptomerione | −7.1 | HB (Cys751), PAl (Ala719, Leu820), Al (Phe699, Val702, Ala719, Lys721, Met742, Leu764,) | |
| δ-cadinene | −7.0 | Al (Leu694, Val702, Ala719, Lys721, Leu768, Met769, Leu820) | |
| (−)-β-bisabolene | −6.8 | Al (Val702, Ala719, Lys721, Met742, Leu764, Leu768, Met769, Leu820) | |
| (+)-β-bisabolene | −6.8 | Al (Val702, Ala719, Lys721, Met742, Leu764, Leu768, Met769, Leu820) | |
| epi-α-cadinol | −6.8 | HB (Asp831), Al (Leu694, Val702, Ala719, Leu768, Leu820) | |
| α-gurjunene | −6.8 | Al (Val702, Ala719, Lys721, Cys773, Leu820) | |
| doxorubicin a | FMS-like tyrosine kinase-3 (4RT7) | −8.1 | HB (Val808, Asp829), PAl (Ile836), PAm (Gly831) |
| (-)-β-bisabolene | −9.0 | PSi (Phe691), PAl (Val624, Phe830), Al (Leu616, Val624, Ala642, Lys644, Val675, Phe691, Tyr693, Leu818, Cys828, Phe830) | |
| (+)-β-bisabolene | −8.9 | PSi (Phe691), PAl (Val624, Phe830), Al (Leu616, Val624, Ala642, Lys644, Val675, Phe691, Tyr693, Leu818, Cys828, Phe830) | |
| cryptomerione | −8.6 | PAl (Leu616, Ala642, Leu818), Al (Leu616, Val624, Ala642,Val675, Phe691, Tyr693, Leu818, Cys828) | |
| δ-cadinene | −6.9 | Al (Met664, Met665, Leu668, Val675, Leu802, Cys807, Cys828) | |
| allo-aromadendrene | −6.7 | PAl (Met664), Al (Met665, Leu668, Ile674, Val675, Phe691, Leu802, Ile827, Cys828) | |
| epi-α-cadinol | −6.2 | PAl (Met665), Al (Ile674, Val675, His809, Cys828) | |
| α-gurjunene | −6.2 | Al (Met664, Met665, Val675, His809, Ile827, Cys828) | |
| orlistat a | Porcine pancreatic lipase (1ETH) | −7.2 | HB (Gly77, Phr78, Asp80, His152, Ser153), PSi (Tyr115), Al (Arg257, Ile210), AC (Asp80) |
| dehydroaromadendrene | −8.7 | PAl (Phe78, Tyr115, Phe216, His264), Al (Ala179, Pro181) | |
| spathulenol | −7.7 | PAl (Phe78, Tyr115, Leu154), Al (Phe78, Ile79, Tyr115, Phe216, Val260, His264) | |
| elemol | −7.0 | PSi (Tyr115), PAl (Phe78, His152, Phe216, His264), Al (Ile79, Val260, Ala261, Leu265) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima Barros, A.; de Lima, E.J.S.P.; Faria, J.V.; Acho, L.R.D.; Lima, E.S.; Bezerra, D.P.; Soares, E.R.; de Lima, B.R.; Costa, E.V.; Pinheiro, M.L.B.; et al. Cytotoxicity and Lipase Inhibition of Essential Oils from Amazon Annonaceae Species. Chemistry 2022, 4, 1208-1225. https://doi.org/10.3390/chemistry4040081
de Lima Barros A, de Lima EJSP, Faria JV, Acho LRD, Lima ES, Bezerra DP, Soares ER, de Lima BR, Costa EV, Pinheiro MLB, et al. Cytotoxicity and Lipase Inhibition of Essential Oils from Amazon Annonaceae Species. Chemistry. 2022; 4(4):1208-1225. https://doi.org/10.3390/chemistry4040081
Chicago/Turabian Stylede Lima Barros, André, Emilly J. S. P. de Lima, Jéssica V. Faria, Leonard R. D. Acho, Emerson S. Lima, Daniel P. Bezerra, Elzalina R. Soares, Bruna R. de Lima, Emmanoel V. Costa, Maria Lúcia B. Pinheiro, and et al. 2022. "Cytotoxicity and Lipase Inhibition of Essential Oils from Amazon Annonaceae Species" Chemistry 4, no. 4: 1208-1225. https://doi.org/10.3390/chemistry4040081
APA Stylede Lima Barros, A., de Lima, E. J. S. P., Faria, J. V., Acho, L. R. D., Lima, E. S., Bezerra, D. P., Soares, E. R., de Lima, B. R., Costa, E. V., Pinheiro, M. L. B., Bataglion, G. A., da Silva, F. M. A., Cardozo, N. M. D., Gonçalves, J. F. C., & Koolen, H. H. F. (2022). Cytotoxicity and Lipase Inhibition of Essential Oils from Amazon Annonaceae Species. Chemistry, 4(4), 1208-1225. https://doi.org/10.3390/chemistry4040081










