Recent Advances in Synthesis and Properties of Pyrazoles
Abstract
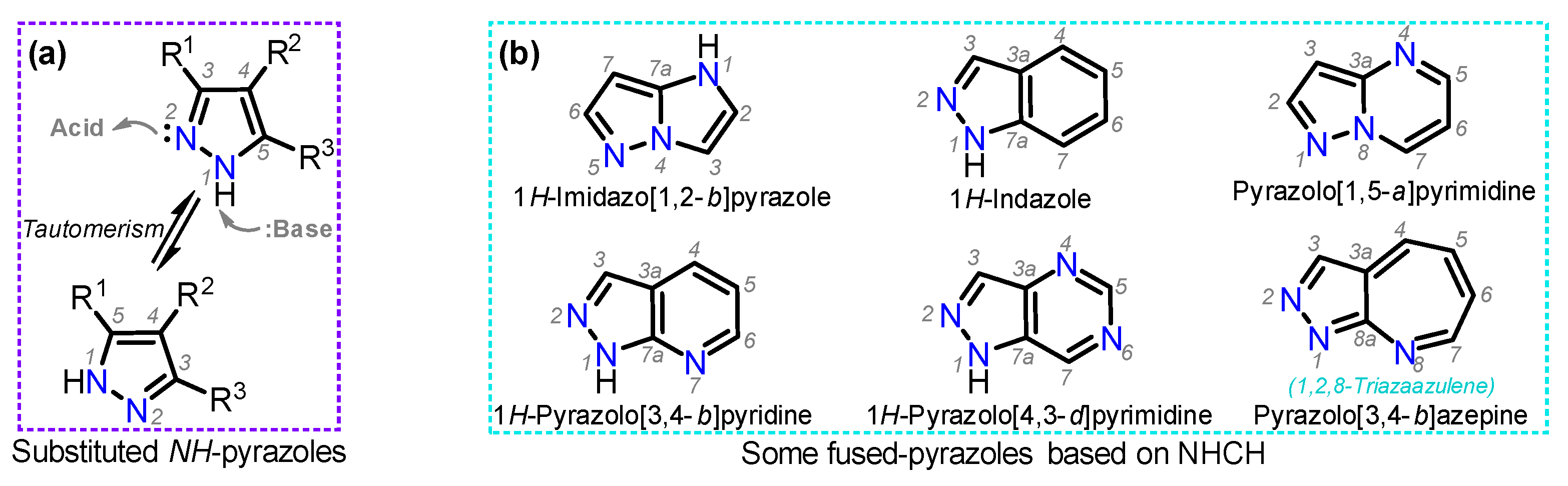
1. Introduction
2. Synthesis and Functionalization of Pyrazoles
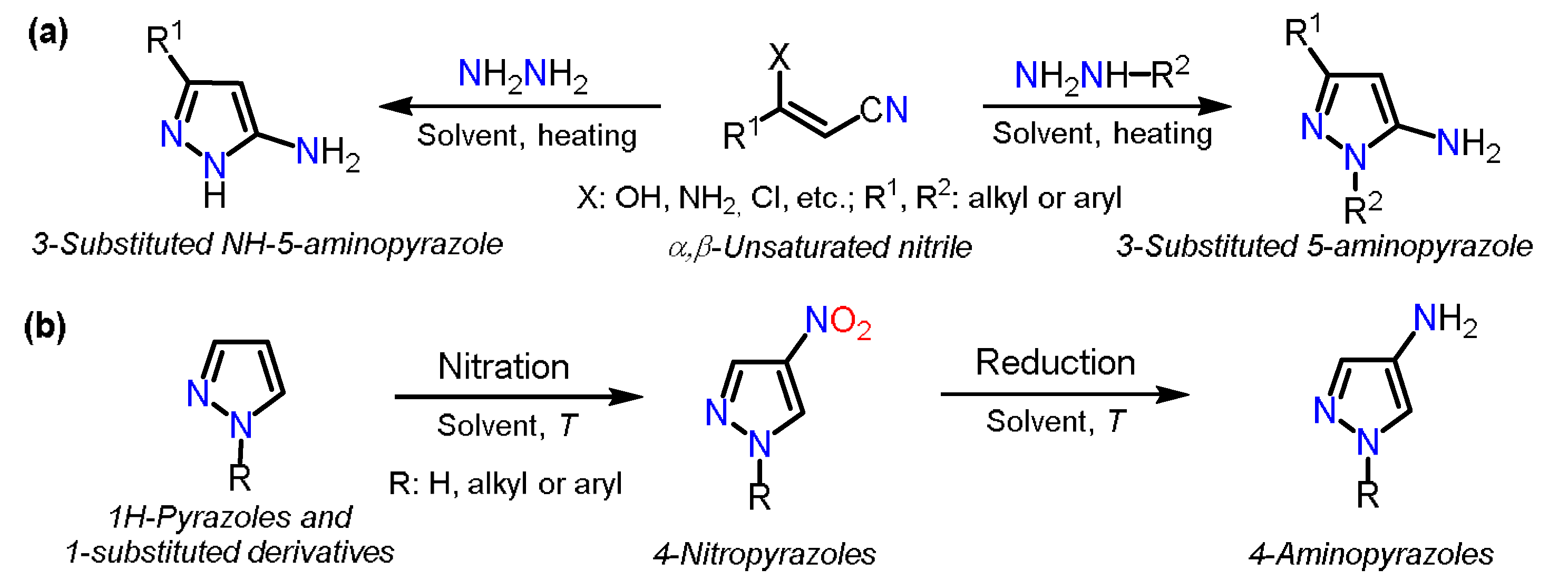
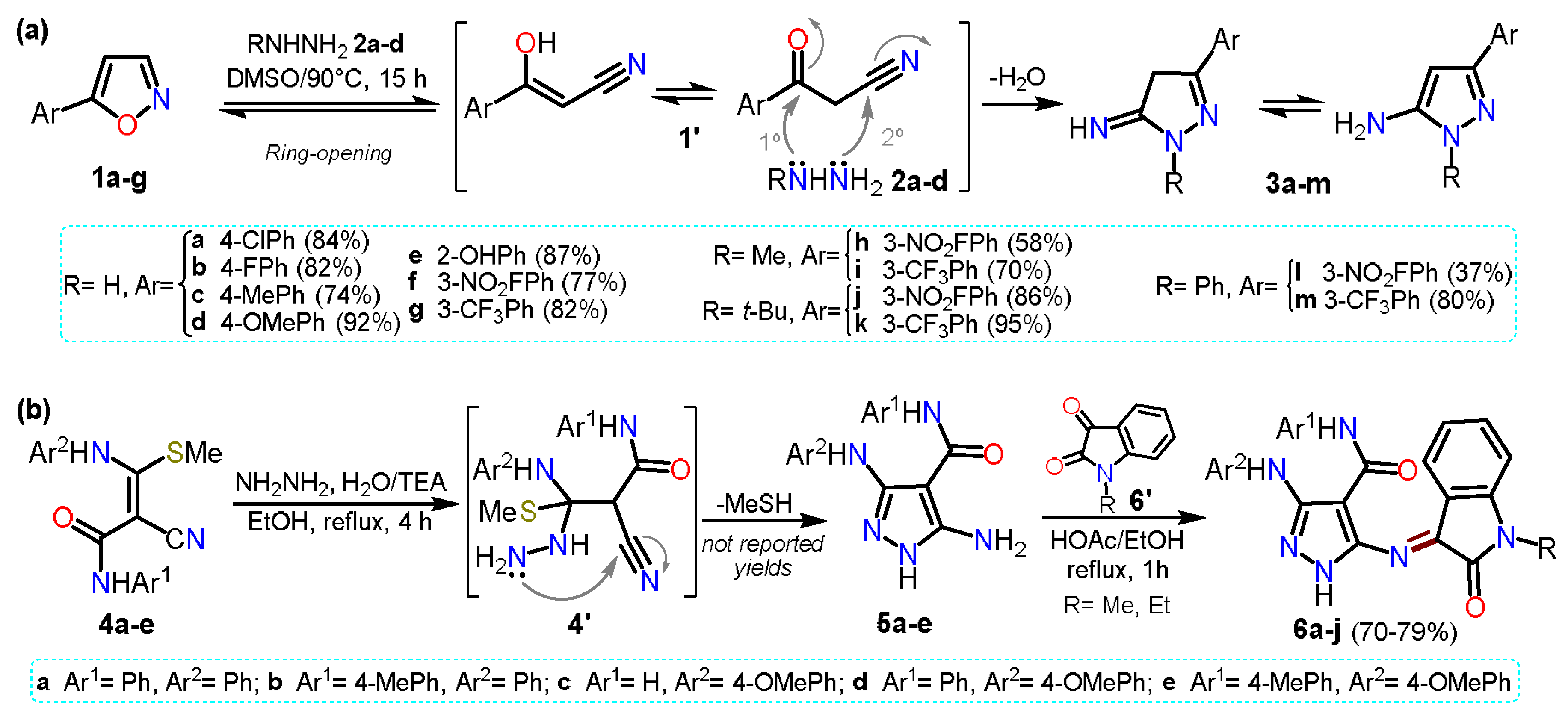
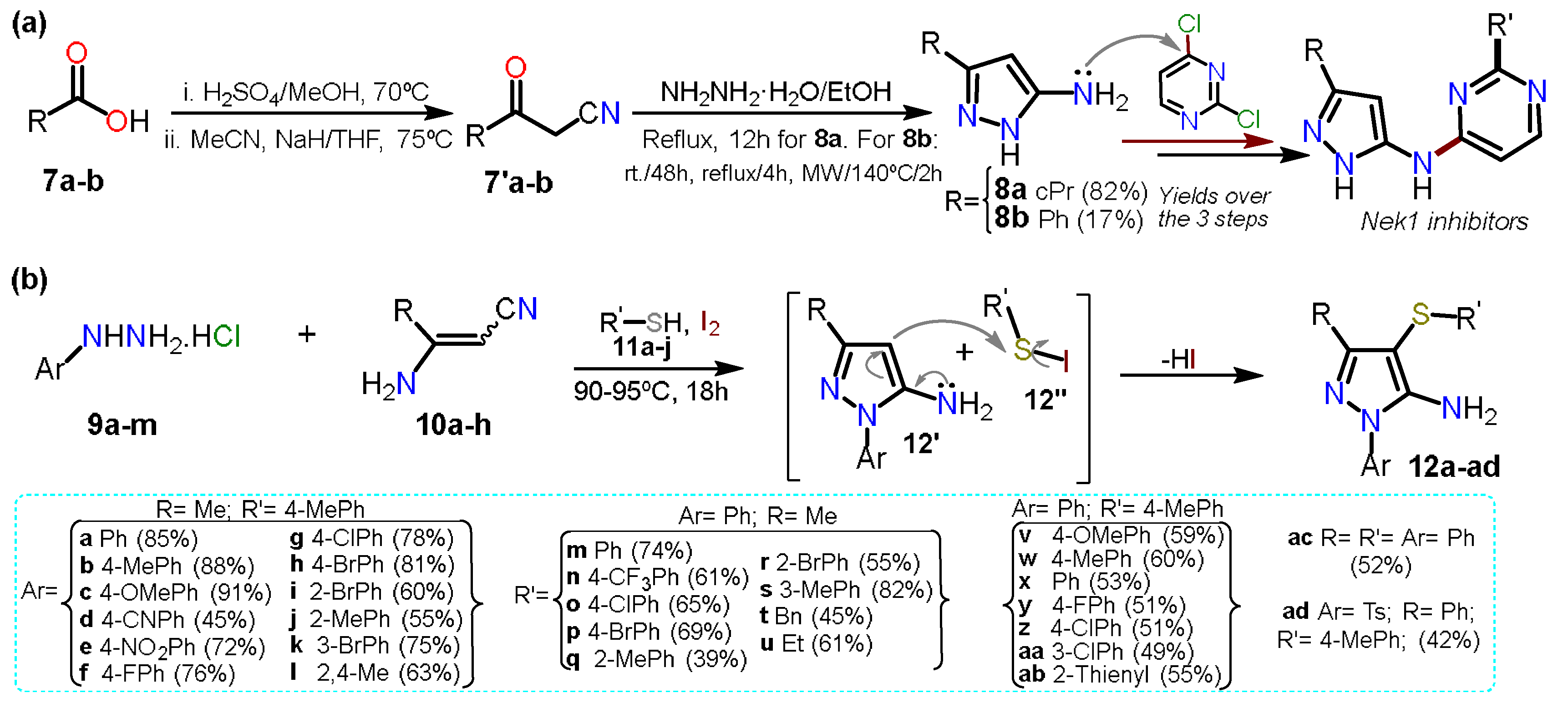
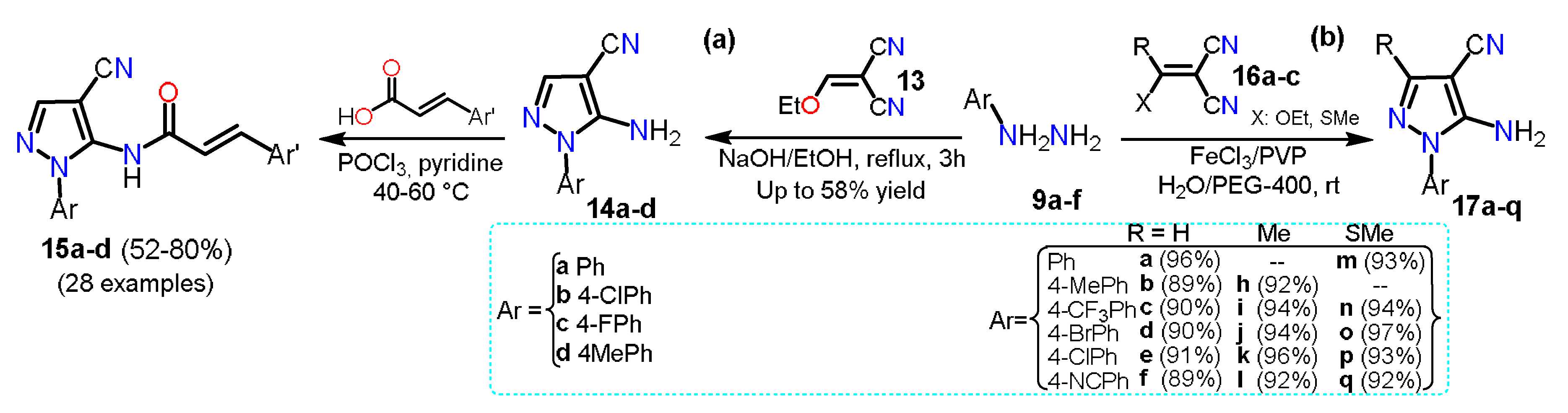
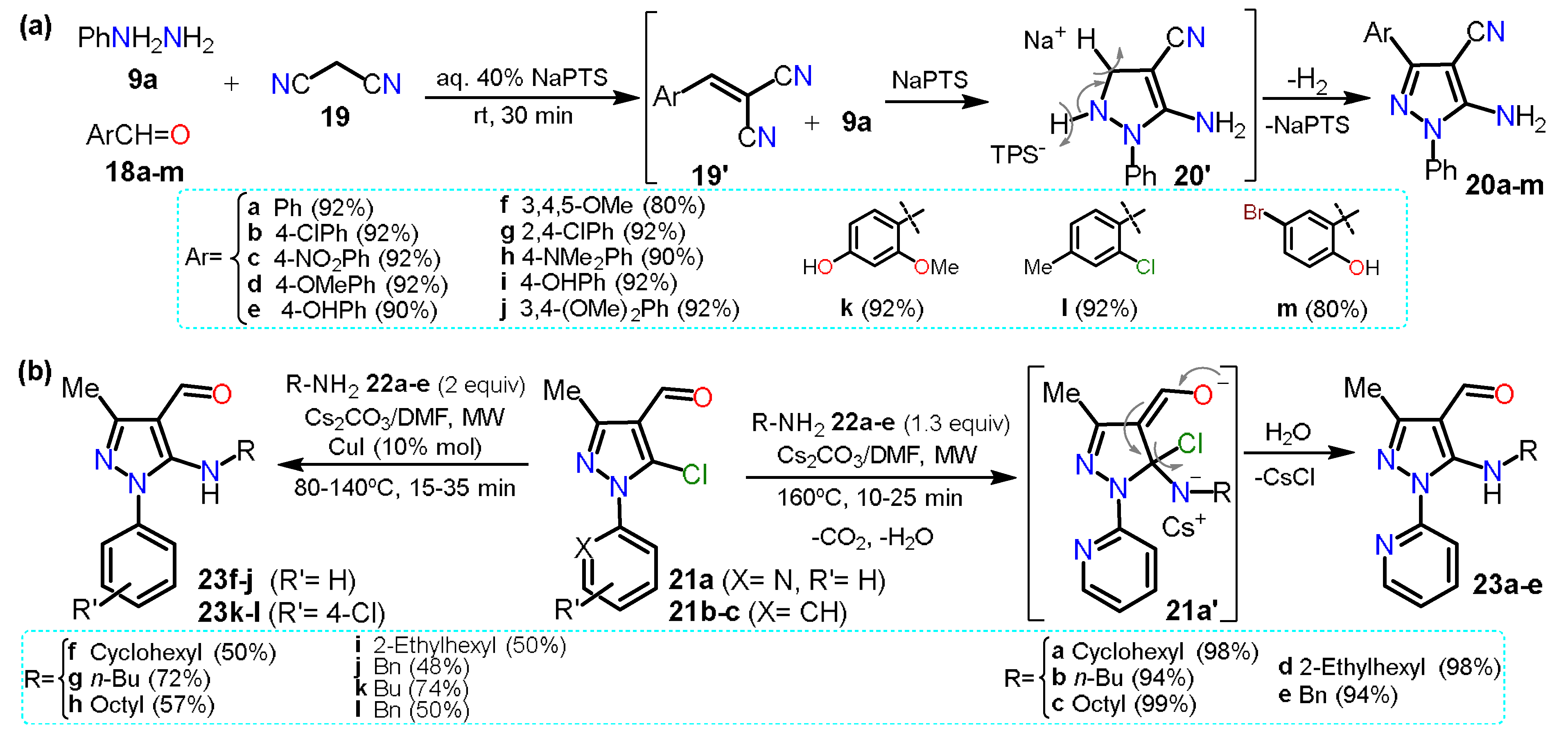
2.1. Aminopyrazoles
2.2. Acylpyrazoles
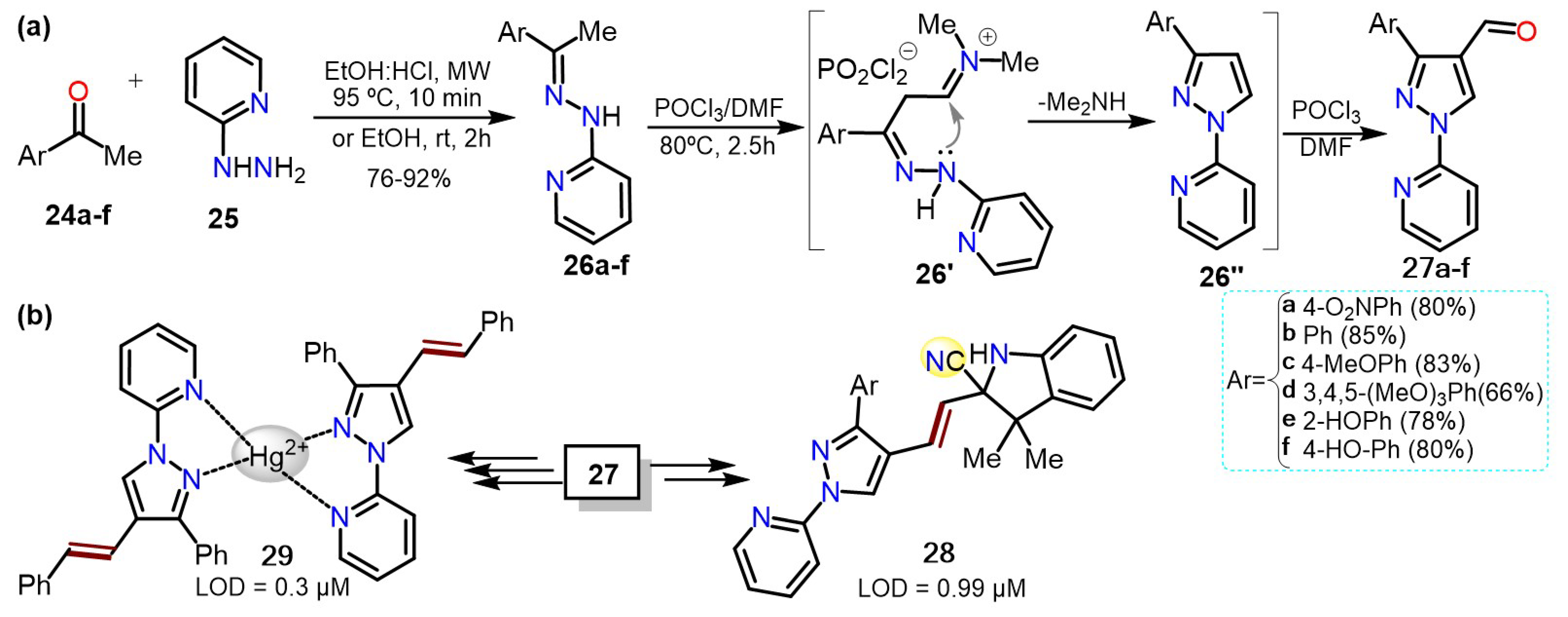
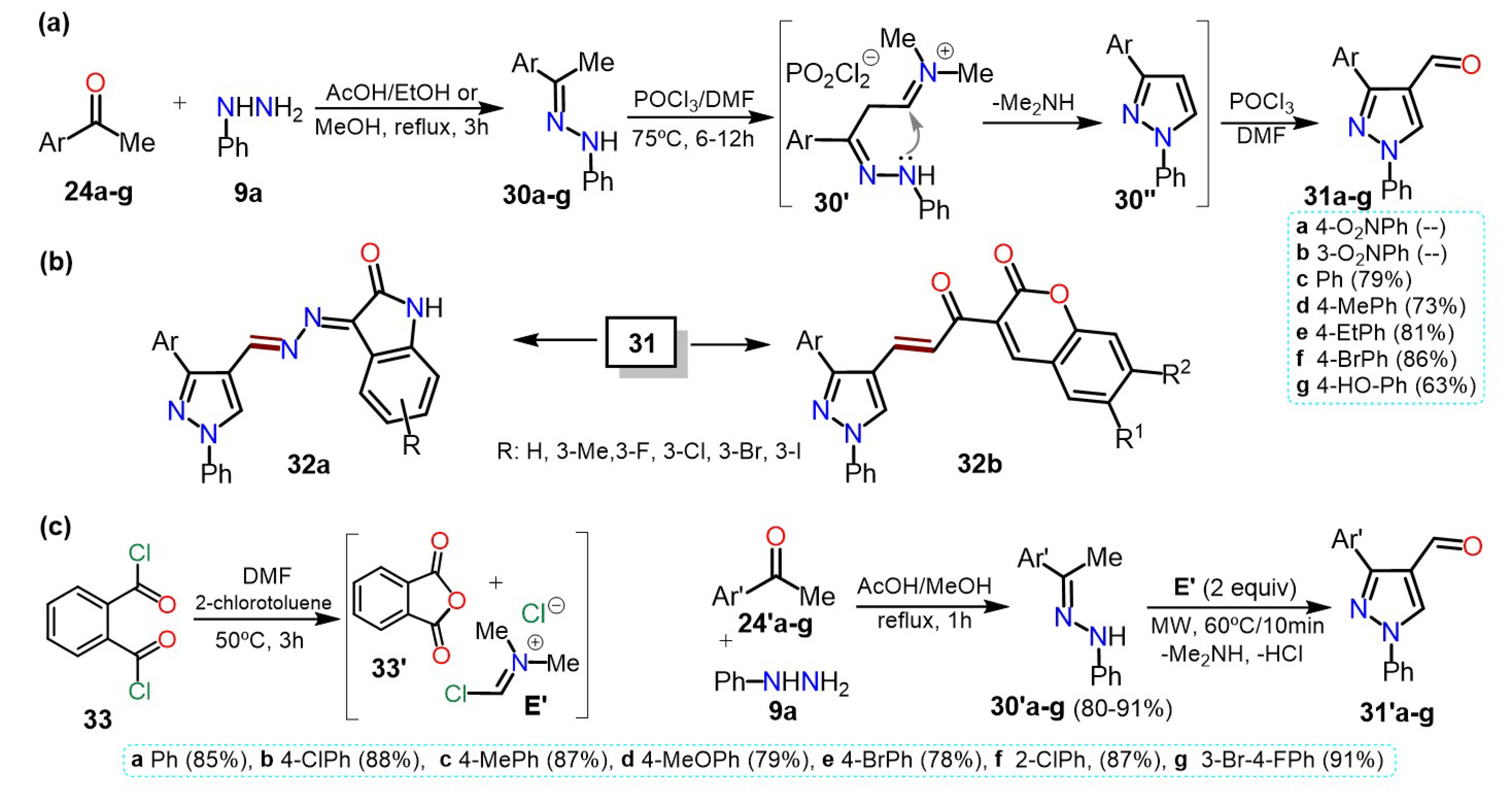
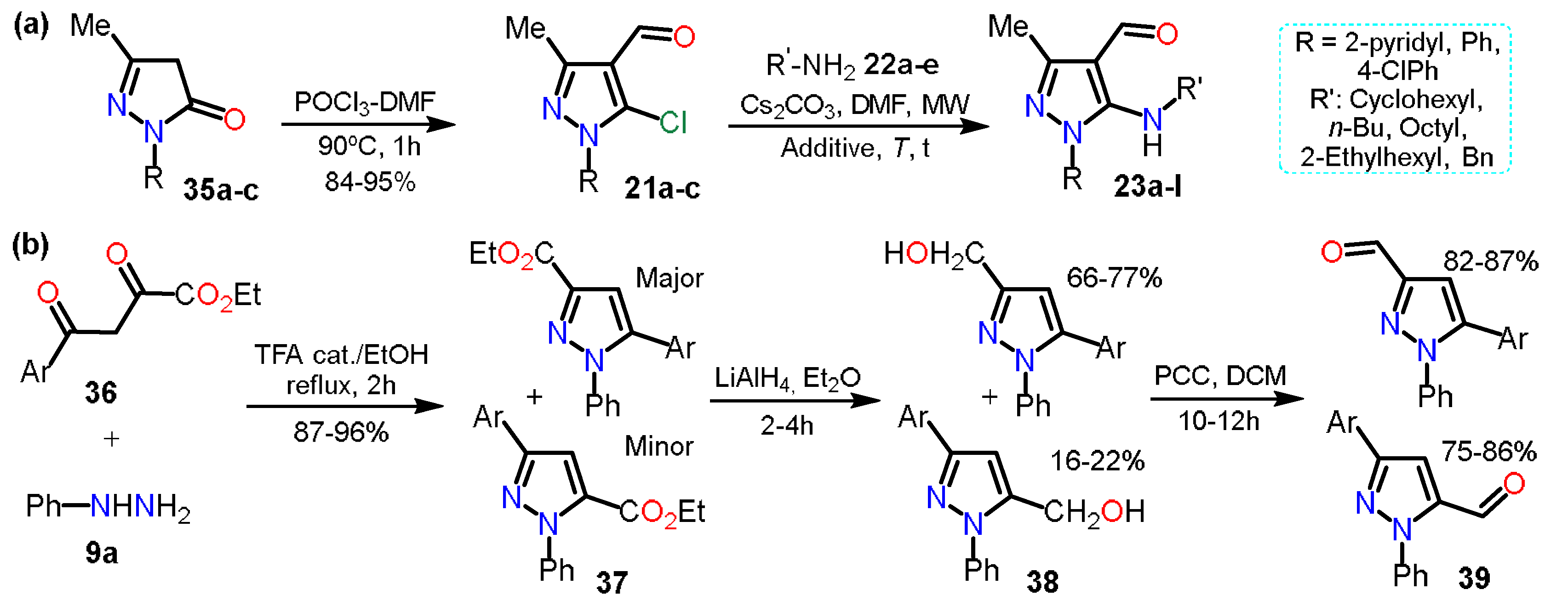
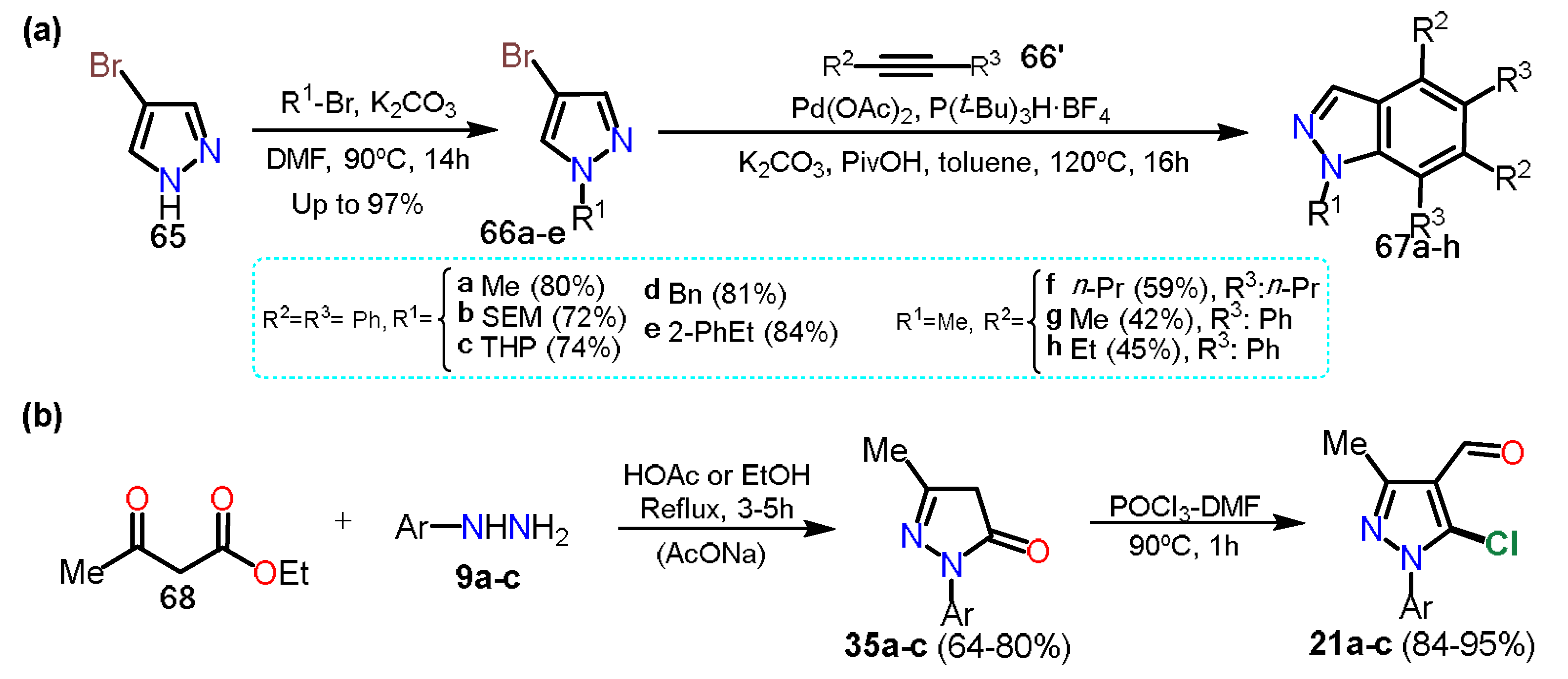
2.2.1. Formylpyrazoles
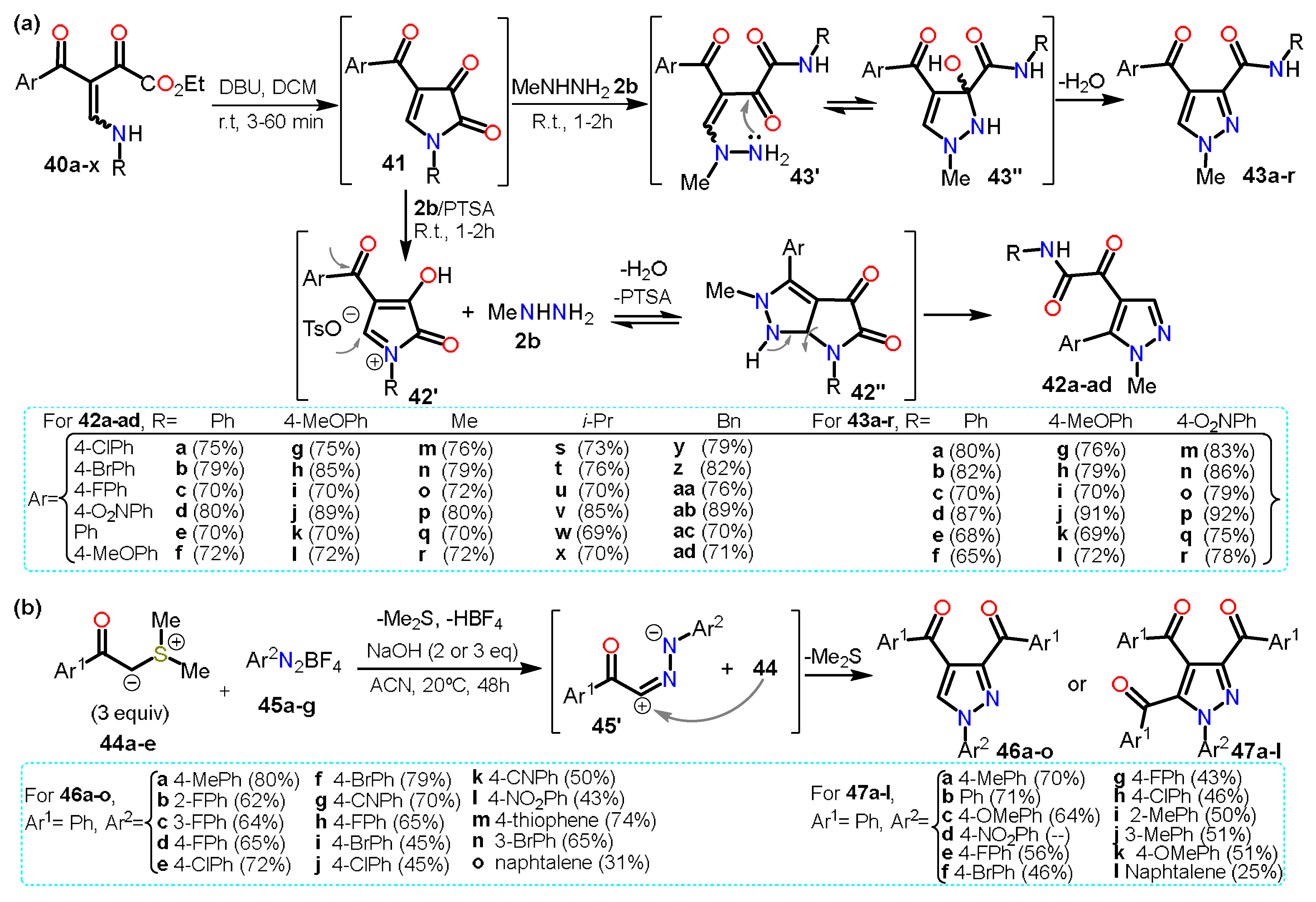
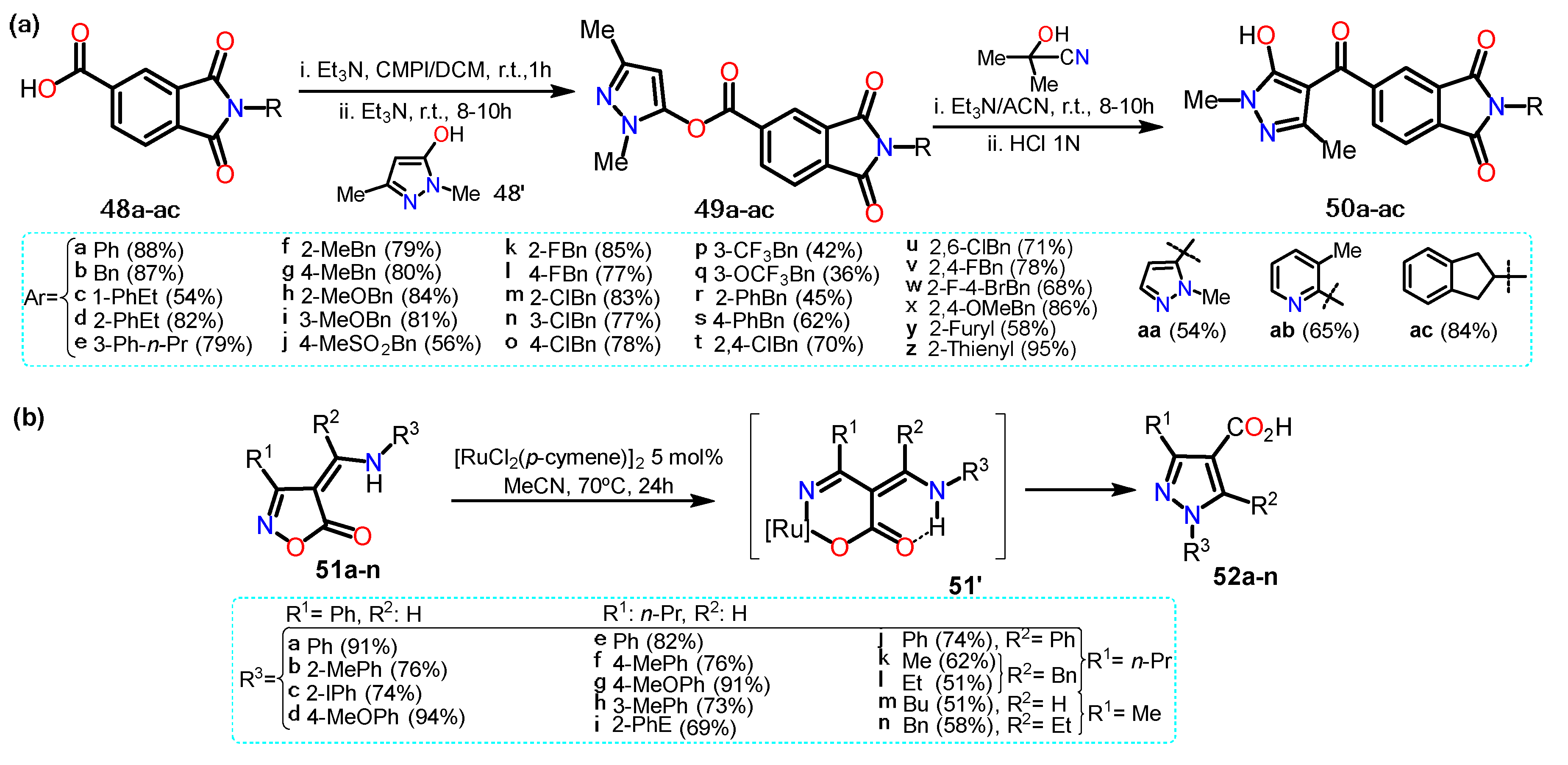
2.2.2. Other Acylated Derivatives
2.3. Further Functional Pyrazoles
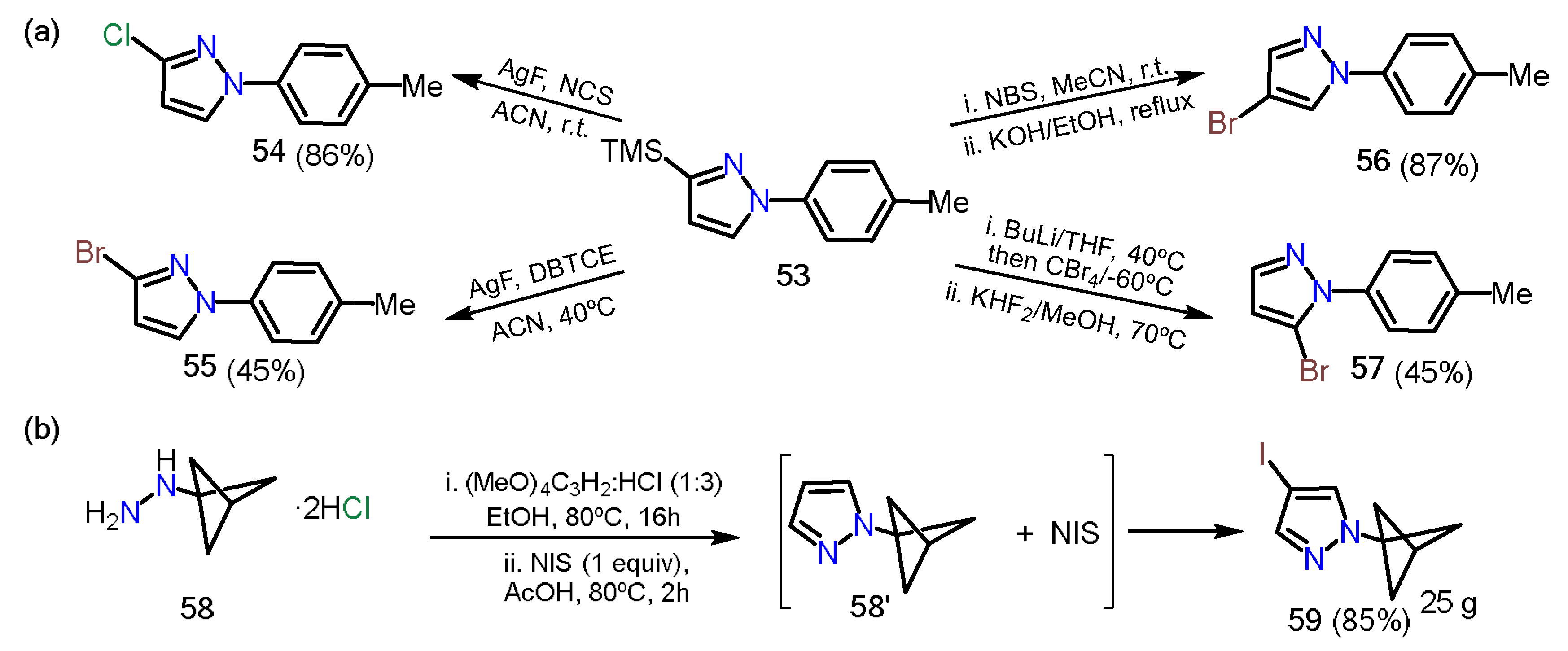
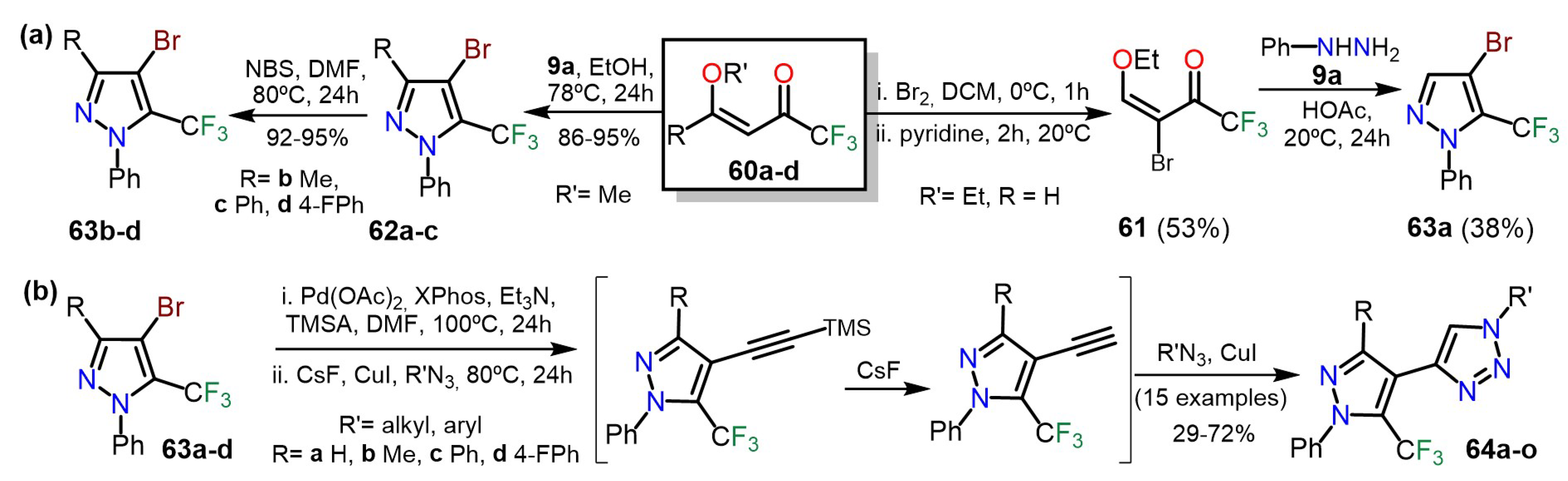
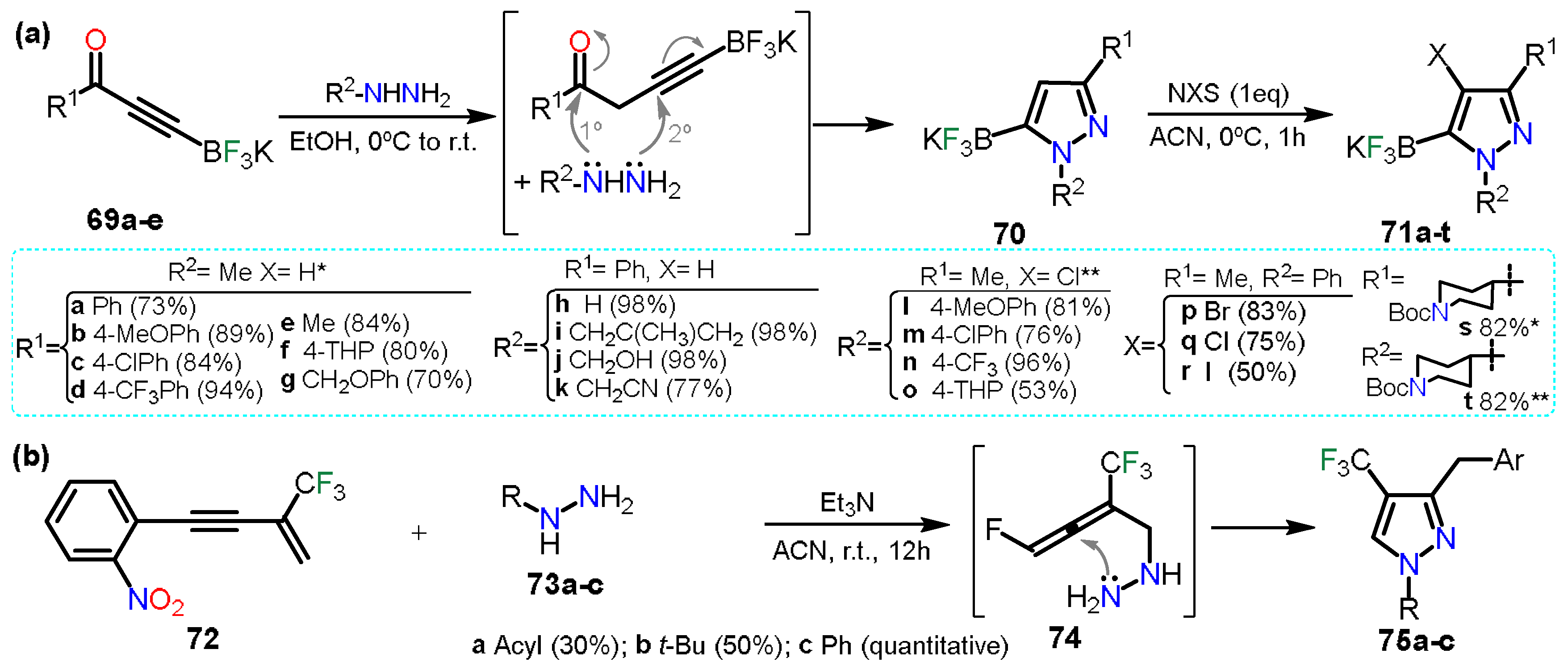
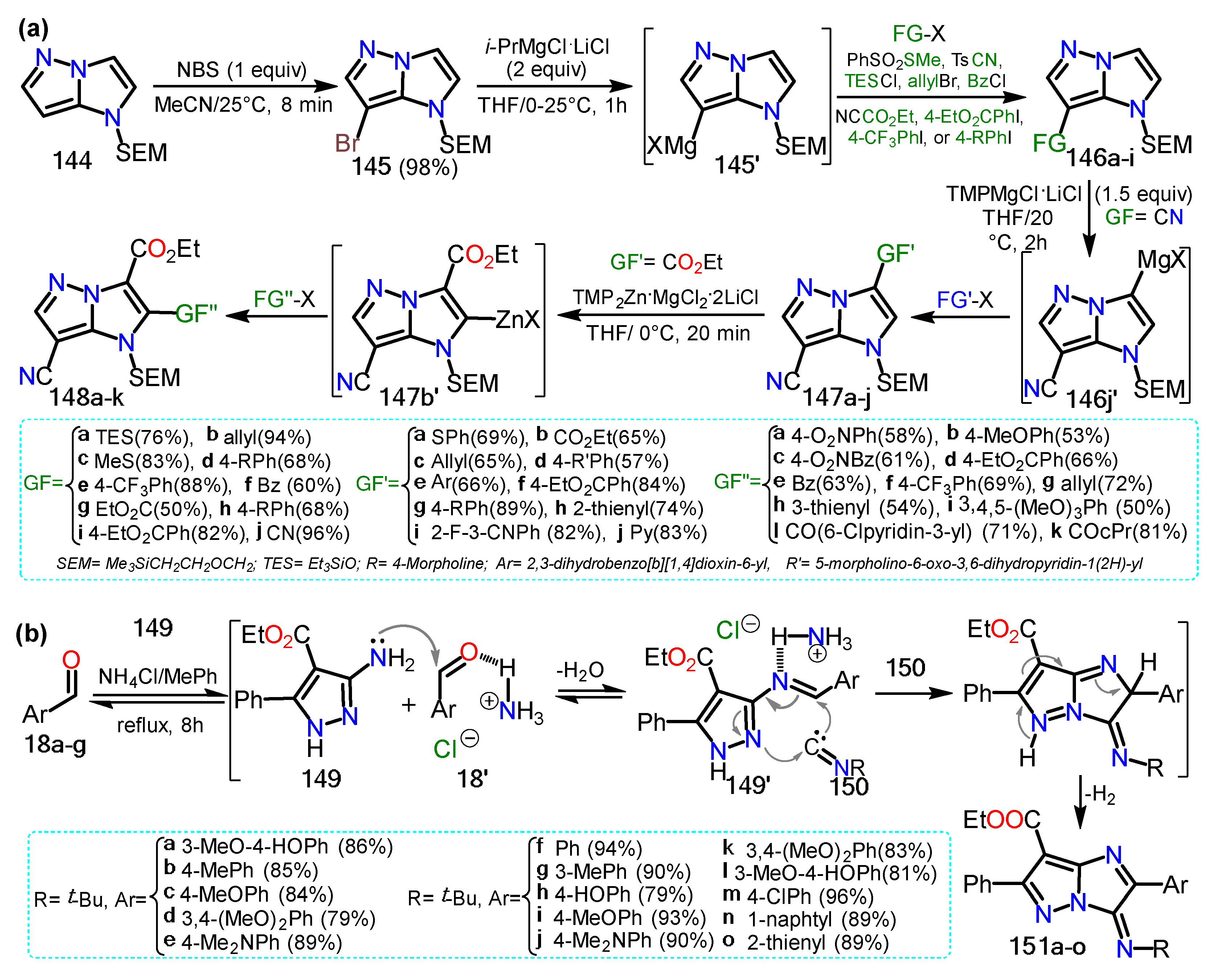
2.3.1. Halopyrazoles
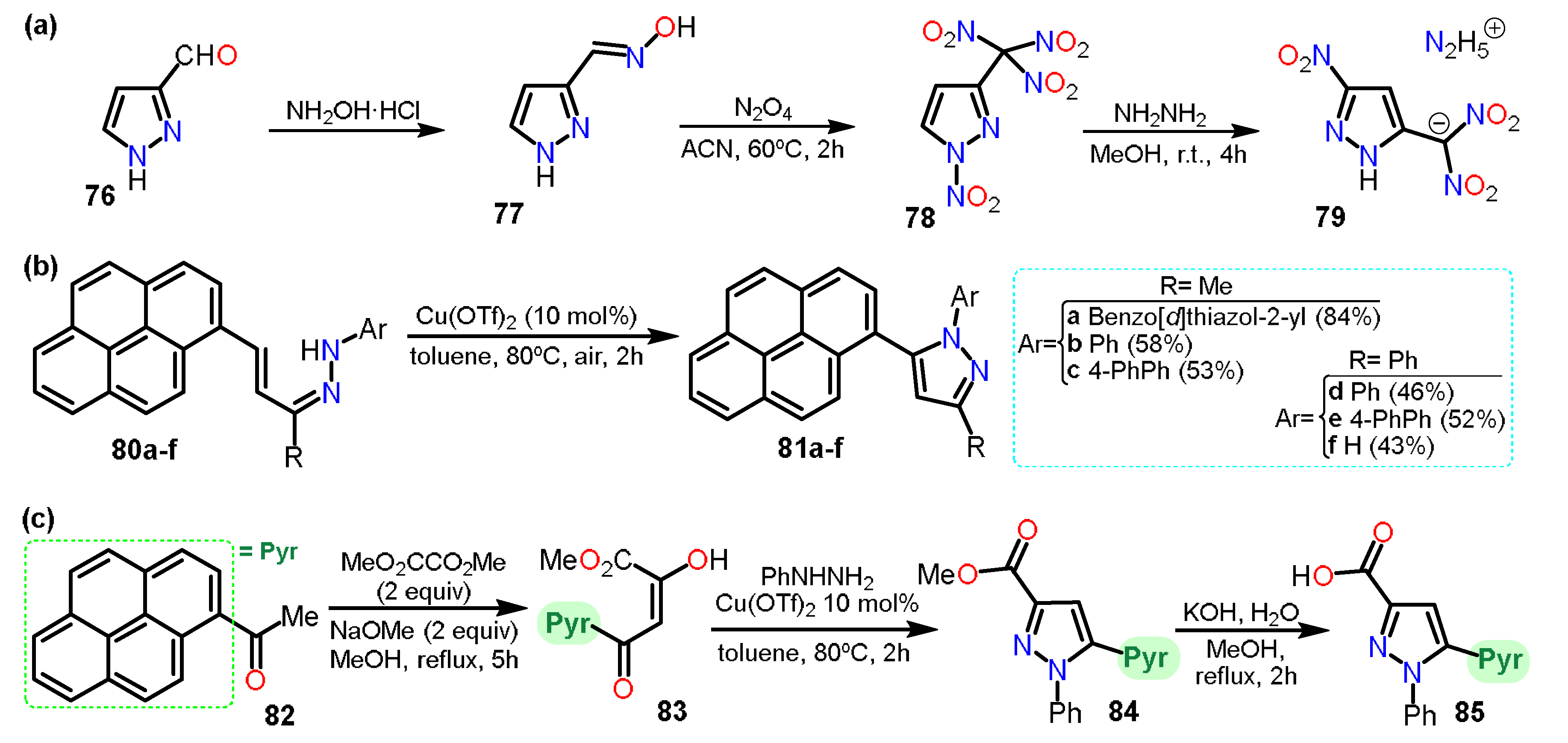
2.3.2. Additional Systems
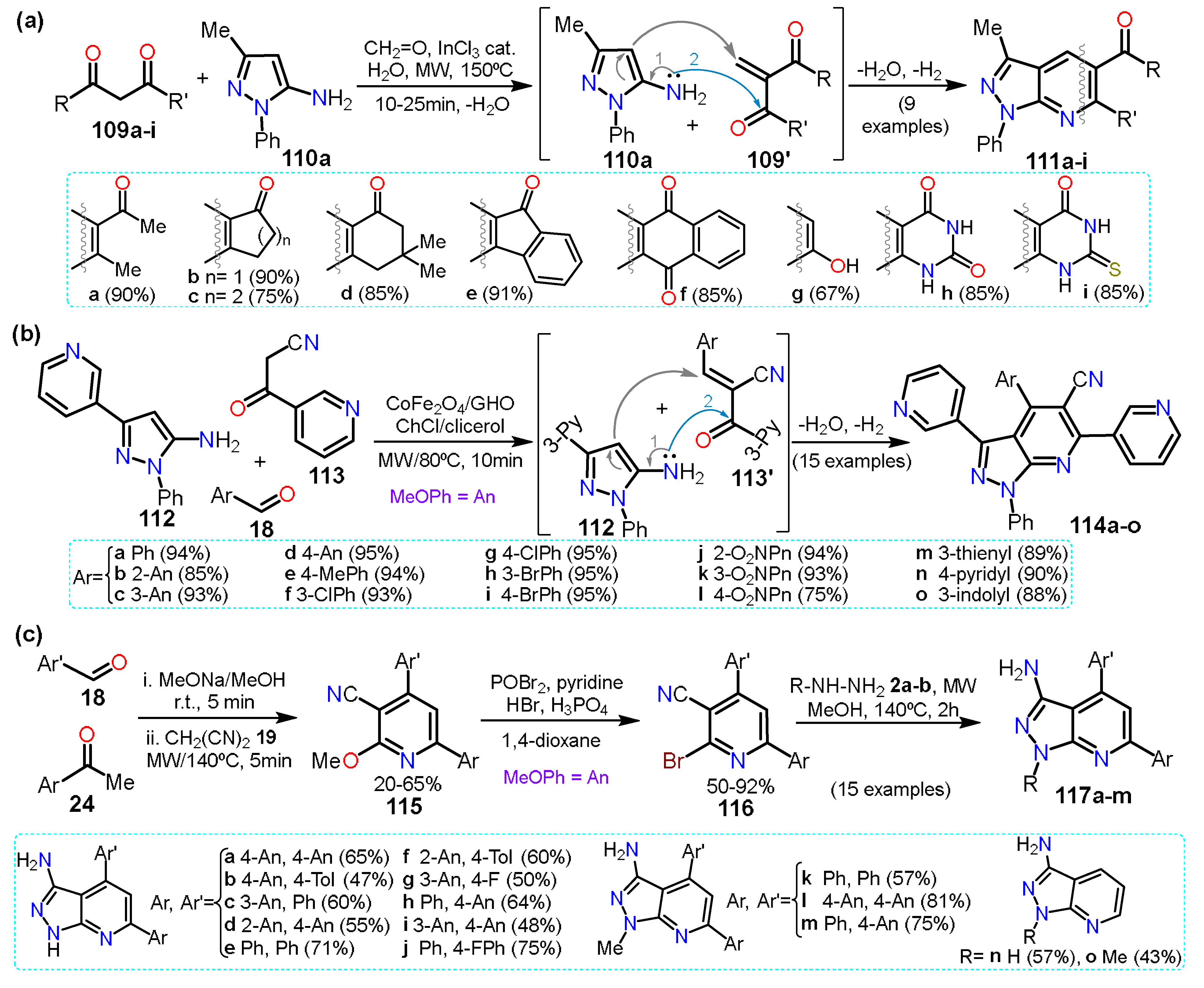
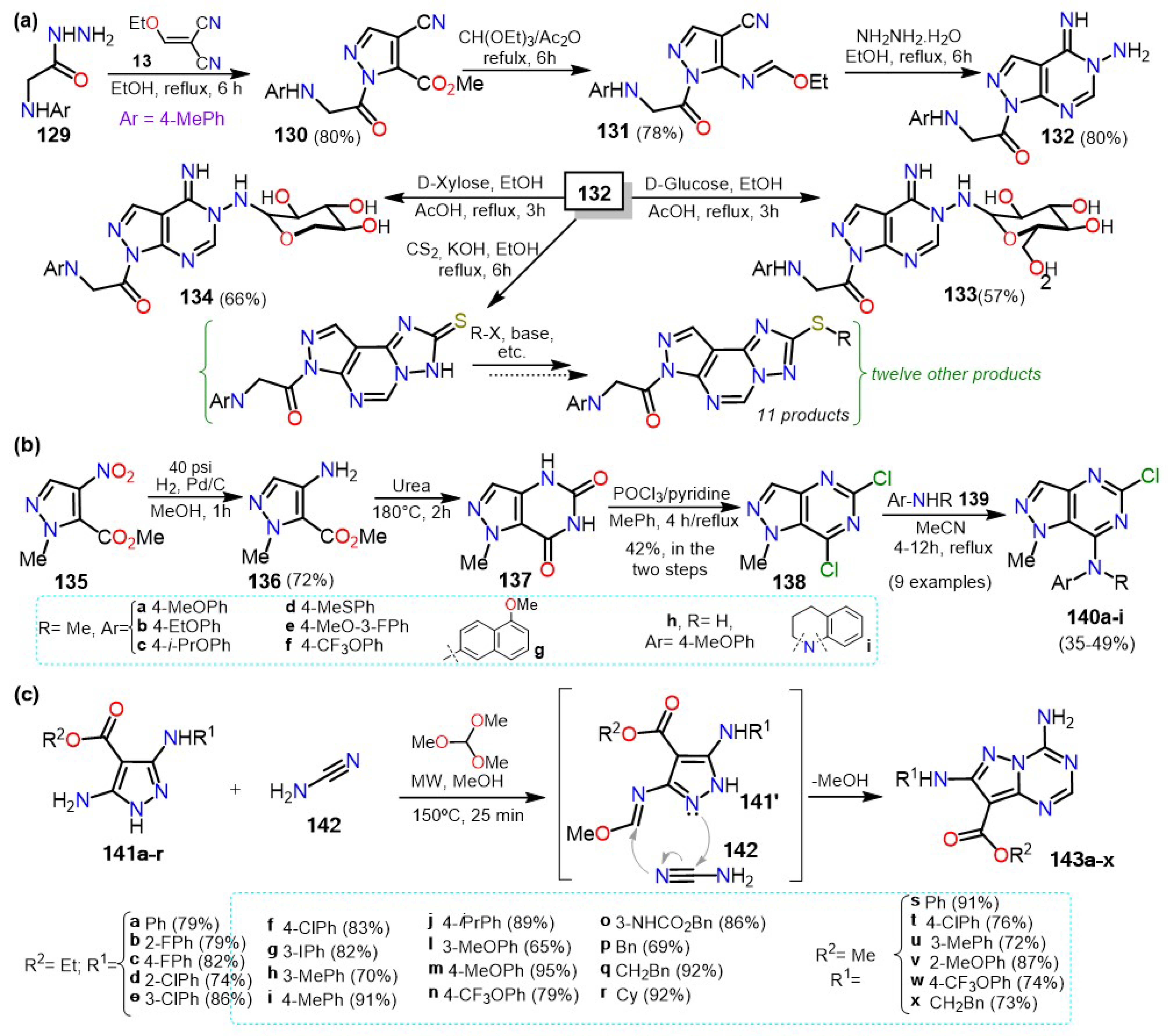
3. Applications in Fused Systems
3.1. Pyrazolo[1,5-a]pyrimidines
3.2. Pyrazolo[3,4-b]pyridines
3.3. Indazoles and Other Bicyclic Pyrazole-Based
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rostami, H.; Shiri, L.; Khani, Z. Recent advances in the synthesis of pyrazole scaffolds via nanoparticles: A review. Tetrahedron 2022, 110, 132688. [Google Scholar] [CrossRef]
- Fustero, S.; Antonio, S.F.; Sanz-Cervera, J.F. Recent advances in the synthesis of pyrazoles. A review. Org. Prep. Proced. Int. 2009, 41, 253–290. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.-X.; Xi, Z. Carbodiimide-based synthesis of N-heterocycles: Moving from two classical reactive sites to chemical bond breaking/forming reaction. Chem. Soc. Rev. 2020, 49, 5810–5849. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J. Pyrazoles. In Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Elsevier: Oxford, UK, 1996; Volume 3, pp. 1–75. [Google Scholar]
- Regan, A.C. Bicyclic 5-6 Systems with One Bridgehead (Ring Junction) Nitrogen Atom: Two Extra Heteroatoms 1:1. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 11, pp. 551–587. [Google Scholar]
- Arias-Gómez, A.; Godoy, A.; Portilla, J. Functional Pyrazolo[1,5-a]pyrimidines: Current Approaches in Synthetic Transformations and Uses As an Antitumor Scaffold. Molecules. 2021, 26, 2708. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento, J.T.; Portilla, J. Current advances in chemosensors diazoles-based for CN– and F–detection. Curr. Org. Synth. 2022, 19. [Google Scholar] [CrossRef]
- Camargo, A.F.; Marangoni, M.A.; De Moraes, P.A.; Nogara, P.A.; Afolabi, B.A.; Bencke, C.E.; Rocha, J.B.T.; Bonacorso, H.G.; Martins, M.A.P.; Zanatta, N. Regioselective synthesis of pyrazolyl-pyrimidine hybrids of pharmacological interest. Synthesis 2020, 52, 2347–2356. [Google Scholar] [CrossRef]
- Donaire-Arias, A.; Montagut, A.M.; de la Bellacasa, R.P.; Estrada-Tejedor, R.; Teixidó, J.; Borrell, J.I. 1H-Pyrazolo[3,4-b]pyridines: Biomedical Applications Synthesis and Biomedical Applications. Molecules 2022, 27, 2237. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Pati, A.; Patra, M.; Behera, R.K.; Behera, A.K. Acid hydrazides, potent reagents for synthesis of oxygen-, nitrogen-, and/or sulfur-containing heterocyclic rings. Chem. Rev. 2014, 114, 2942–2977. [Google Scholar] [CrossRef]
- Surendra Kumar, R.; Arif, I.A.; Ahamed, A.; Idhayadhulla, A. Anti-inflammatory and antimicrobial activities of novel pyrazole analogues. Saudi J. Biol. Sci. 2016, 23, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Gingell, C.; Collins, M.; Wicker, P.A.; Osterloh, I.H. Clinical safety of oral sildenafil citrate (VIAGRA®) in the treatment of erectile dysfunction. Int. J. Impot. Res. 1998, 10, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.A.; Wingrove, P.B.; Connelly, L.; Whiting, P.J.; Wafford, K.A. Tracazolate reveals a novel type of allosteric interaction with recombinant γ-aminobutyric acidA receptors. Mol. Pharmacol. 2002, 61, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Gross-Goupil, M.; François, L.; Quivy, A.; Ravaud, A. Axitinib: A review of its safety and efficacy in the treatment of adults with advanced renal cell carcinoma. Clin. Med. Insights Oncol. 2013, 7, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Rao Saketi, J.M.; Boddapati, S.N.M.; Raghuram, M.; Adil, S.F.; Shaik, M.R.; Alduhaish, O.; Siddiqui, M.R.H.; Bollikolla, H.B. Pd(PPh3)4 catalyzed synthesis of indazole derivatives as potent anticancer drug. Appl. Sci. 2020, 10, 3792. [Google Scholar] [CrossRef]
- Tigreros, A.; Macías, M.; Portilla, J. Expeditious ethanol quantification present in hydrocarbons and distilled spirits: Extending photophysical usages of the pyrazolo[1,5-a]pyrimidines. Dye. Pigment. 2022, 202, 110299. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Vieira, J.C.B.; Araújo, J.N.; Souza, V.B.; Frizzo, C.P.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. Solution and Solid-State Optical Properties of Trifluoromethylated 5-(Alkyl/aryl/heteroaryl)-2-methyl-pyrazolo[1,5-a]pyrimidine System. Photochem 2022, 2, 345–357. [Google Scholar] [CrossRef]
- Yang, Z.L.; Wang, Z.; Cao, W.L.; Li, T.; Yang, J.Q.; Zhang, J.G. New green energetic materials based on unsymmetrically substituted pyrazole-tetrazines and their hydroperchlorates. New J. Chem. 2019, 43, 18637–18646. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Ecological and Economic Efforts in the Development of Molecular Sensors for the Optical Detection of Cyanide Ions. Eur. J. Org. Chem. 2022, 2022, e202200249. [Google Scholar] [CrossRef]
- Molina, P.; Zapata, F.; Caballero, A. Anion Recognition Strategies Based on Combined Noncovalent Interactions. Chem. Rev. 2017, 117, 9907–9972. [Google Scholar] [CrossRef] [PubMed]
- Ríos, M.-C.; Bravo, N.-F.; Sánchez, C.-C.; Portilla, J. Chemosensors based on N-heterocyclic dyes: Advances in sensing highly toxic ions such as CN− and Hg2+. RSC Adv. 2021, 11, 34206–34234. [Google Scholar] [CrossRef] [PubMed]
- Abu Elmaati, T.M.; El-Taweel, F.M. New Trends in the Chemistry of 5-Aminopyrazoles. J. Heterocycl. Chem. 2004, 41, 109–134. [Google Scholar] [CrossRef]
- Alvarez-Builla, J.; Vaquero, J.J.; Barluenga, J. Modern Heterocyclic Chemistry; Wiley-VCH Verlag and Co. KGaA: Weinheim, Germany, 2011; Volume 4. [Google Scholar]
- Ortiz, M.-C.; Portilla, J. Access to five-membered N-heteroaromatic compounds: Current approach based on microwave-assisted synthesis. Targets Heterocycl. Syst. 2022, 25, 436. [Google Scholar] [CrossRef]
- Kallman, N.J.; Cole, K.P.; Koenig, T.M.; Buser, J.Y.; McFarland, A.D.; McNulty, L.A.M.; Mitchell, D. Synthesis of Aminopyrazoles from Isoxazoles: Comparison of Preparative Methods by in situ NMR Analysis. Synthesis 2016, 48, 3537–3543. [Google Scholar] [CrossRef][Green Version]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M.; Nossier, E.S.; Mady, M.F. Design, synthesis, anticancer evaluation, enzymatic assays, and a molecular modeling study of novel pyrazole-indole hybrids. ACS Omega 2021, 6, 12361–12374. [Google Scholar] [CrossRef]
- Pilakowski, J.; Baumann, G.; Shih, Y.H.; Meckel, T.; Schmidt, B. Design, synthesis and biological evaluation of novel aminopyrazole- and 7-azaindole-based Nek1 inhibitors and their effects on zebrafish kidney development. Bioorg. Med. Chem. Lett. 2021, 53, 128418. [Google Scholar] [CrossRef]
- Annes, S.B.; Saritha, R.; Chandru, K.; Mandali, P.K.; Ramesh, S. Metal- A nd Solvent-Free Cascade Reaction for the Synthesis of Amino Pyrazole Thioether Derivatives. J. Org. Chem. 2021, 86, 16473–16484. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Liu, H.; Jiao, D.; Hu, H.T.; Wang, W.; Gong, J.X.; Wang, A.L.; Cao, H.Q.; Lv, X.H. Design, synthesis, and antifungal activity of novel cinnamon–pyrazole carboxamide derivatives. Drug Dev. Res. 2018, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Elnagdy, H.M.F.; Sarma, D. FeCl3/PVP as Green Homogeneous Catalyst to Synthesize 5-Amino-1H-Pyrazole-4-Carbonitriles from Malononitrile Derivatives. ChemistrySelect 2019, 4, 783–787. [Google Scholar] [CrossRef]
- Sapkal, A.; Kamble, S. Sodium toluene-4-sulfonate as a reusable and ecofriendly catalyst for greener synthesis of 5-aminopyrazole-4-carbonitrile in aqueous medium. J. Heterocycl. Chem. 2020, 57, 3597–3604. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Cobo, J.; Portilla, J. Chemoselective Synthesis of 5-Alkylamino-1H-pyrazole-4-carbaldehydes by Cesium- and Copper-Mediated Amination. Eur. J. Org. Chem. 2015, 2015, 5064–5069. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Portilla, J. Synthesis of Dicyanovinyl-Substituted 1-(2-Pyridyl)pyrazoles: Design of a Fluorescent Chemosensor for Selective Recognition of Cyanide. J. Org. Chem. 2017, 82, 13376–13385. [Google Scholar] [CrossRef]
- Garzón, L.-M.; Portilla, J. Synthesis of Novel D-π-A Dyes for Colorimetric Cyanide Sensing Based on Hemicyanine-Functionalized N -(2-Pyridyl)pyrazoles: Synthesis of Novel D-π-A Dyes for Colorimetric Cyanide Sensing Based on Hemicyanine-Functionalized N -(2-Pyridyl)pyra. Eur. J. Org. Chem. 2019, 2019, 7079–7088. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Cobo, J.; Portilla, J. Synthesis, Photophysical Properties, and Metal-Ion Recognition Studies of Fluoroionophores Based on 1-(2-Pyridyl)-4-Styrylpyrazoles. ACS Omega 2019, 4, 16689–16700. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Palta, K.; Kumar, M. Hybrids of Isatin-Pyrazole as Potential α-Glucosidase Inhibitors: Synthesis, Biological Evaluations and Molecular Docking Studies. ChemistrySelect 2019, 4, 13219–13227. [Google Scholar] [CrossRef]
- Kumar, G.; Siva Krishna, V.; Sriram, D.; Jachak, S.M. Pyrazole–coumarin and pyrazole–quinoline chalcones as potential antitubercular agents. Arch. Pharm. 2020, 353, 2000077. [Google Scholar] [CrossRef]
- Kumari, P.; Sood, S.; Kumar, A.; Singh, K. Microwave-assisted Vilsmeier-Haack synthesis of Pyrazole-4-carbaldehydes. J. Heterocycl. Chem. 2020, 57, 796–804. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, V. Exploration of pyrazole based ALDO-X bifunctional building blocks for the synthesis of pyrazole annulated molecular architectures. J. Heterocycl. Chem. 2020, 57, 3735–3762. [Google Scholar] [CrossRef]
- Nag, S.; Singh, V.; Batra, S. Studies on the Baylis-Hillman reaction of pyrazolecarbaldehydes under the influence of DABCO: Positional effect on the reactivity of the formyl group. Arkivoc 2007, 2007, 185–203. [Google Scholar] [CrossRef]
- Poletto, J.; da Silva, M.J.V.; Pianoski, K.E.; Willig, J.C.M.; Rosa, F.A. Regiodivergent Synthesis of 3,4- and 4,5-Disubstituted N -Methylpyrazoles from 4-Acyl-1 H -pyrrole-2,3-dione and Methylhydrazine. J. Org. Chem. 2022, 87, 8544–8550. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Cao, Y.; Zeng, L.; Zhang, C.; Fu, L.; Zhang, J.; Zhu, H.; Shao, J. (2 + 1 + 1 + 1)-Annulation Reactions of Aryldiazonium Tetrafluoroborates with Sulfur Ylides to Polysubstituted Pyrazoles. J. Org. Chem. 2021, 86, 8997–9006. [Google Scholar] [CrossRef]
- He, B.; Dong, J.; Lin, H.Y.; Wang, M.Y.; Li, X.K.; Zheng, B.F.; Chen, Q.; Hao, G.F.; Yang, W.C.; Yang, G.F. Pyrazole-isoindoline-1,3-dione hybrid: A promising scaffold for 4-hydroxyphenylpyruvate dioxygenase inhibitors. J. Agric. Food Chem. 2019, 67, 10844–10852. [Google Scholar] [CrossRef]
- Loro, C.; Molteni, L.; Papis, M.; Lo Presti, L.; Foschi, F.; Beccalli, E.M.; Broggini, G. Non-Decarboxylative Ruthenium-Catalyzed Rearrangement of 4-Alkylidene-isoxazol-5-ones to Pyrazole- and Isoxazole-4-carboxylic Acids. Org. Lett. 2022, 24, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Onodera, S.; Kochi, T.; Kakiuchi, F. Synthesis of N-Arylpyrazoles by Palladium-Catalyzed Coupling of Aryl Triflates with Pyrazole Derivatives. J. Org. Chem. 2019, 84, 6508–6515. [Google Scholar] [CrossRef] [PubMed]
- Zarate, C.; Ardolino, M.; Morriello, G.J.; Logan, K.M.; Kaplan, W.P.; Torres, L.; Li, D.; Chen, M.; Li, H.; Su, J.; et al. Development of Scalable Routes to 1-Bicyclo[1.1.1]pentylpyrazoles. Org. Process Res. Dev. 2021, 25, 642–647. [Google Scholar] [CrossRef]
- Bonacorso, H.G.; Dal Forno, G.M.; Wiethan, C.; Ketzer, A.; Zanatta, N.; Frizzo, C.P.; Martins, M.A.P.; Stradiotto, M. Sequential one-pot three-step synthesis of polysubstituted 4-(5-(trifluoromethyl)-1: H -pyrazol-4-yl)-1H-1,2,3-triazole systems. RSC Adv. 2017, 7, 43957–43964. [Google Scholar] [CrossRef]
- Kim, O.S.; Jang, J.H.; Kim, H.T.; Han, S.J.; Tsui, G.C.; Joo, J.M. Synthesis of Fluorescent Indazoles by Palladium-Catalyzed Benzannulation of Pyrazoles with Alkynes. Org. Lett. 2017, 19, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Fricero, P.; Bialy, L.; Brown, A.W.; Czechtizky, W.; Méndez, M.; Harrity, J.P.A. Synthesis and Modular Reactivity of Pyrazole 5-Trifluoroborates: Intermediates for the Preparation of Fully Functionalized Pyrazoles. J. Org. Chem. 2017, 82, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ding, S.; Liu, M.; Yu, Z.; Xiao, Y. Synthesis of Trifluoromethylated Pyrazolidines, Pyrazolines and Pyrazoles via Divergent Reaction of β-CF3-1,3-Enynes with Hydrazines. Org. Lett. 2021, 23, 7718–7723. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yu, T.; Liu, Y.; Chen, S.; Ge, Z.; Sun, C.; Pang, S. Synthesis and Properties of Energetic Hydrazinium 5-Nitro-3-dinitromethyl-2H-pyrazole by Unexpected Isomerization of N-Nitropyrazole. ACS Omega 2019, 4, 19011–19017. [Google Scholar] [CrossRef]
- Sar, D.; Srivastava, I.; Misra, S.K.; Ostadhossein, F.; Fathi, P.; Pan, D. Copper-Catalyzed Syntheses of Pyrene-Pyrazole Pharmacophores and Structure Activity Studies for Tubulin Polymerization. ACS Omega 2018, 3, 6378–6387. [Google Scholar] [CrossRef] [PubMed]
- Galenko, E.E.; Ivanov, V.K.; Novikov, M.S.; Zolotarev, A.A.; Khlebnikov, A.F. Synthesis of N-aminopyrazoles by Fe(II)-catalyzed rearrangement of 4-hydrazonomethyl-substituted isoxazoles. Tetrahedron 2018, 74, 6288–6298. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, P.; Liu, Y.; He, J.; Li, X.; Li, S.; Zhao, J. NIS-promoted three-component reaction of 3-oxo-3-arylpropanenitriles with arylsulfonyl hydrazides. Org. Biomol. Chem. 2021, 19, 3932–3939. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, O.; Tamaru, M.; Nakasato, H.; Shimai, S.; Panthai, S.; Kuroda, Y.; Yamaguchi, K.; Fujisawa, K.; Hisano, K. Highly efficient aggregation-induced room-temperature phosphorescence with extremely large Stokes shift emitted from trinuclear gold(I) complex crystals. Molecules 2019, 24, 4606. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.G.; Liang, C.G.; Zhang, W.H. Recent advances in indazole-containing derivatives: Synthesis and biological perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef]
- Cherukupalli, S.; Hampannavar, G.A.; Chinnam, S.; Chandrasekaran, B.; Sayyad, N.; Kayamba, F.; Reddy Aleti, R.; Karpoormath, R. An appraisal on synthetic and pharmaceutical perspectives of pyrazolo[4,3-d]pyrimidine scaffold. Bioorg. Med. Chem. 2018, 26, 309–339. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.H.; Mohamed, M.S.; Ragb, E.A. Design, synthesis, anticancer evaluation, molecular docking and cell cycle analysis of 3-methyl-4,7-dihydropyrazolo[1,5-a]pyrimidine derivatives as potent histone lysine demethylases (KDM) inhibitors and apoptosis inducers. Bioorg. Chem. 2019, 88, 102929. [Google Scholar] [CrossRef]
- Stefanello, F.S.; Kappenberg, Y.G.; Araújo, J.N.; Franceschini, S.Z.; Martins, M.A.P.; Zanatta, N.; Iglesias, B.A.; Bonacorso, H.G. Trifluoromethyl-substituted aryldiazenyl-pyrazolo[1,5-a]pyrimidin-2-amines: Regioselective synthesis, structure, and optical properties. J. Fluor. Chem. 2022, 255–256, 109967. [Google Scholar] [CrossRef]
- Castillo, J.C.; Rosero, H.A.; Portilla, J. Simple access toward 3-halo- and 3-nitro-pyrazolo[1,5-a] pyrimidines through a one-pot sequence. RSC Adv. 2017, 7, 28483–28488. [Google Scholar] [CrossRef]
- Tigreros, A.; Aranzazu, S.-L.; Bravo, N.-F.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]pyrimidines based fluorophores: A comprehensive theoretical-experimental study. RSC Adv. 2020, 10, 39542–39552. [Google Scholar] [CrossRef]
- Tigreros, A.; Zapata-Rivera, J.; Portilla, J. Pyrazolo[1,5-a]pyrimidinium Salts for Cyanide Sensing: A Performance and Sustainability Study of the Probes. ACS Sustain. Chem. Eng. 2021, 9, 12058–12069. [Google Scholar] [CrossRef]
- Tigreros, A.; Macías, M.; Portilla, J. Photophysical and crystallographic study of three integrated pyrazolo[1,5-a]pyrimidine–triphenylamine systems. Dye. Pigment. 2021, 184, 108730. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Lizarazo, C.; Cobo, J.; Portilla, J. Pyrazolo-fused 4-azafluorenones as key reagents for the synthesis of fluorescent dicyanovinylidene-substituted derivatives. RSC Adv. 2019, 9, 27318–27323. [Google Scholar] [CrossRef] [PubMed]
- Charris-Molina, A.; Castillo, J.C.; Macías, M.; Portilla, J. One-Step Synthesis of Fully Functionalized Pyrazolo[3,4-b]pyridines via Isobenzofuranone Ring Opening. J. Org. Chem. 2017, 82, 12674–12681. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Romero, I.; Portilla, J. Synthesis of Fluorescent 1,7-Dipyridyl-bis-pyrazolo[3,4-b:4′,3′-e]pyridines: Design of Reversible Chemosensors for Nanomolar Detection of Cu2+. ACS Omega 2019, 4, 6757–6768. [Google Scholar] [CrossRef] [PubMed]
- Polo, E.; Ferrer-Pertuz, K.; Trilleras, J.; Quiroga, J.; Gutiérrez, M. Microwave-assisted one-pot synthesis in water of carbonylpyrazolo[3,4-b]pyridine derivatives catalyzed by InCl3and sonochemical assisted condensation with aldehydes to obtain new chalcone derivatives containing the pyrazolopyridinic moiety. RSC Adv. 2017, 7, 50044–50055. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, P.; Liu, Y.H.; Shang, Z.R.; Hu, H.C.; Zhang, Z.H. Magnetically separable graphene oxide anchored sulfonic acid: A novel, highly efficient and recyclable catalyst for one-pot synthesis of 3,6-di(pyridin-3-yl)-1H-pyrazolo[3,4-b]pyridine-5-carbonitriles in deep eutectic solvent under microwave irradiation. RSC Adv. 2016, 6, 106160–106170. [Google Scholar] [CrossRef]
- Bou-Petit, E.; Hümmer, S.; Alarcon, H.; Slobodnyuk, K.; Cano-Galietero, M.; Fuentes, P.; Guijarro, P.J.; Muñoz, M.J.; Suarez-Cabrera, L.; Santamaria, A.; et al. Overcoming Paradoxical Kinase Priming by a Novel MNK1 Inhibitor. J. Med. Chem. 2022, 65, 6070–6087. [Google Scholar] [CrossRef]
- Sawant, A.S.; Kamble, S.S.; Pisal, P.M.; Meshram, R.J.; Sawant, S.S.; Kamble, V.A.; Kamble, V.T.; Gacche, R.N. Synthesis and evaluation of a novel series of 6-bromo-1-cyclopentyl-1H-indazole-4-carboxylic acid-substituted amide derivatives as anticancer, antiangiogenic, and antioxidant agents. Med. Chem. Res. 2020, 29, 17–32. [Google Scholar] [CrossRef]
- Zhang, M.; Fang, X.; Wang, C.; Hua, Y.; Huang, C.; Wang, M.; Zhu, L.; Wang, Z.; Gao, Y.; Zhang, T.; et al. Design and synthesis of 1H-indazole-3-carboxamide derivatives as potent and selective PAK1 inhibitors with anti-tumour migration and invasion activities. Eur. J. Med. Chem. 2020, 203, 112517. [Google Scholar] [CrossRef]
- Nassar, I.F.; Abdel Aal, M.T.; El-Sayed, W.A.; Shahin, A.E.M.; Elsakka, E.G.E.; Mokhtar, M.M.; Hegazy, M.; Hagras, M.; Mandour, A.A.; Ismail, N.S.M. Discovery of pyrazolo[3,4-d]pyrimidine and pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives as novel CDK2 inhibitors: Synthesis, biological and molecular modeling investigations. RSC Adv. 2022, 12, 14865–14882. [Google Scholar] [CrossRef]
- Islam, F.; Quadery, T.M.; Bai, R.; Luckett-Chastain, L.R.; Hamel, E.; Ihnat, M.A.; Gangjee, A. Novel pyrazolo[4,3-d]pyrimidine microtubule targeting agents (MTAs): Synthesis, structure–activity relationship, in vitro and in vivo evaluation as antitumor agents. Bioorg. Med. Chem. Lett. 2021, 41, 127923. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Halcovitch, N.R.; Tiekink, E.R.T.; Dolzhenko, A.V. A new microwave-assisted, three-component reaction of 5-aminopyrazole-4-carboxylates: Selective synthesis of substituted 5-aza-9-deaza-adenines. Tetrahedron 2018, 74, 1868–1879. [Google Scholar] [CrossRef]
- Schwärzer, K.; Rout, S.K.; Bessinger, D.; Lima, F.; Brocklehurst, C.E.; Karaghiosoff, K.; Bein, T.; Knochel, P. Selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold. A new potential non-classical isostere of indole and a precursor of push-pull dyes. Chem. Sci. 2021, 12, 12993–13000. [Google Scholar] [CrossRef] [PubMed]
- Peytam, F.; Adib, M.; Shourgeshty, R.; Mohammadi-Khanaposhtani, M.; Jahani, M.; Imanparast, S.; Faramarzi, M.A.; Mahdavi, M.; Moghadamnia, A.A.; Rastegar, H.; et al. Design and synthesis of new imidazo[1,2-b]pyrazole derivatives, in vitro α-glucosidase inhibition, kinetic and docking studies. Mol. Divers. 2020, 24, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Romo, P.E.; Isaza, J.H.; Insuasty, B.; Abonia, R.; del Crespo, M.P.; Quiroga, J. Synthesis of pyrazolo[3,4-b]azepines and their antioxidant and antibacterial studies. Monatshefte Chem.-Chem. Mon. 2019, 150, 1503–1511. [Google Scholar] [CrossRef]
- Bortňák, D.; Milata, V.; Šofranko, J.; Végh, D.; Fronc, M.; Herich, P.; Kožíšek, J.; Hrivnáková, V.; Šoral, M. On the formation of uncommon pyrazoloazepines from 5-aminopyrazoles as by-products in the Clauson-Kaas reaction. J. Mol. Struct. 2018, 1166, 243–251. [Google Scholar] [CrossRef]


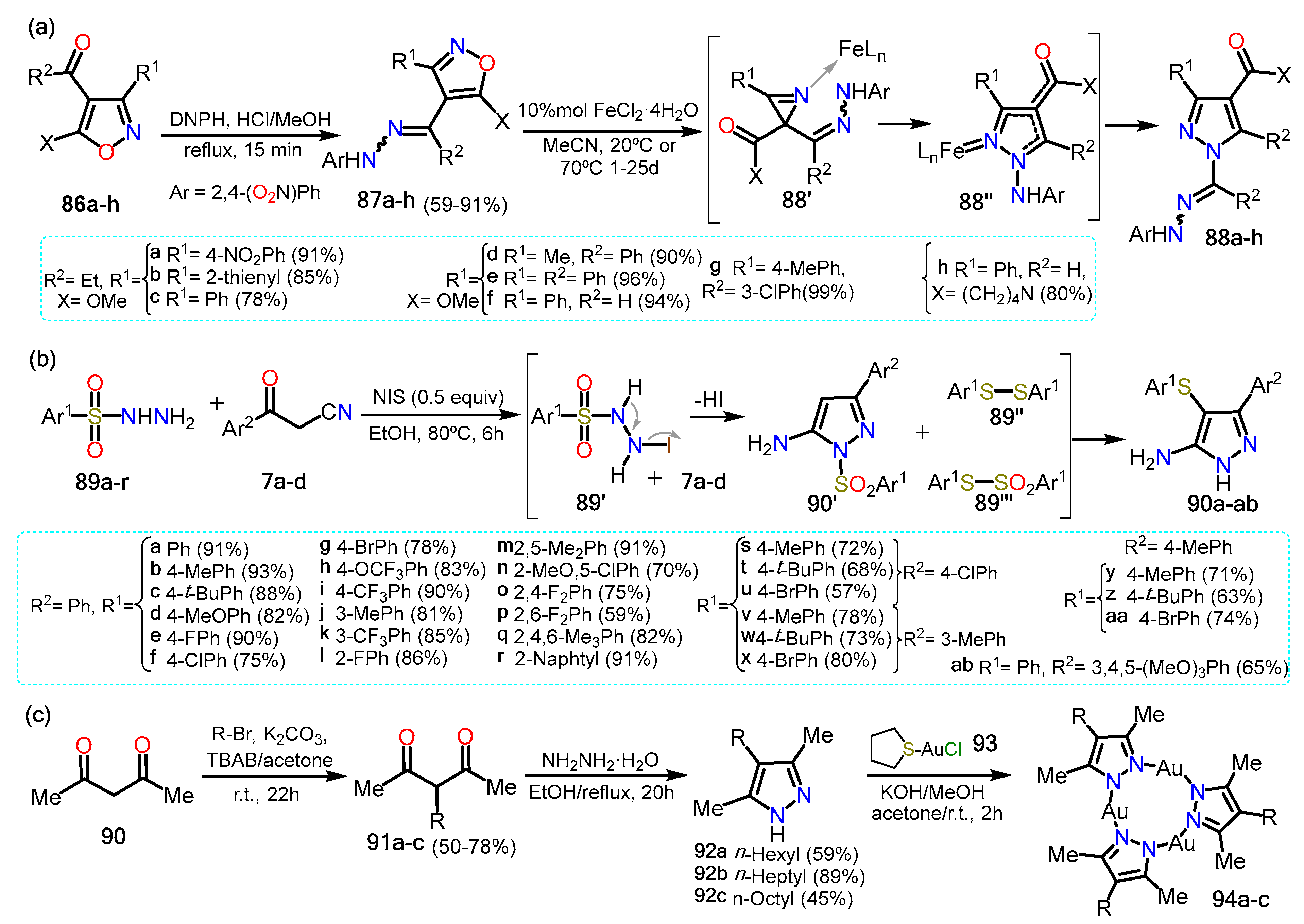
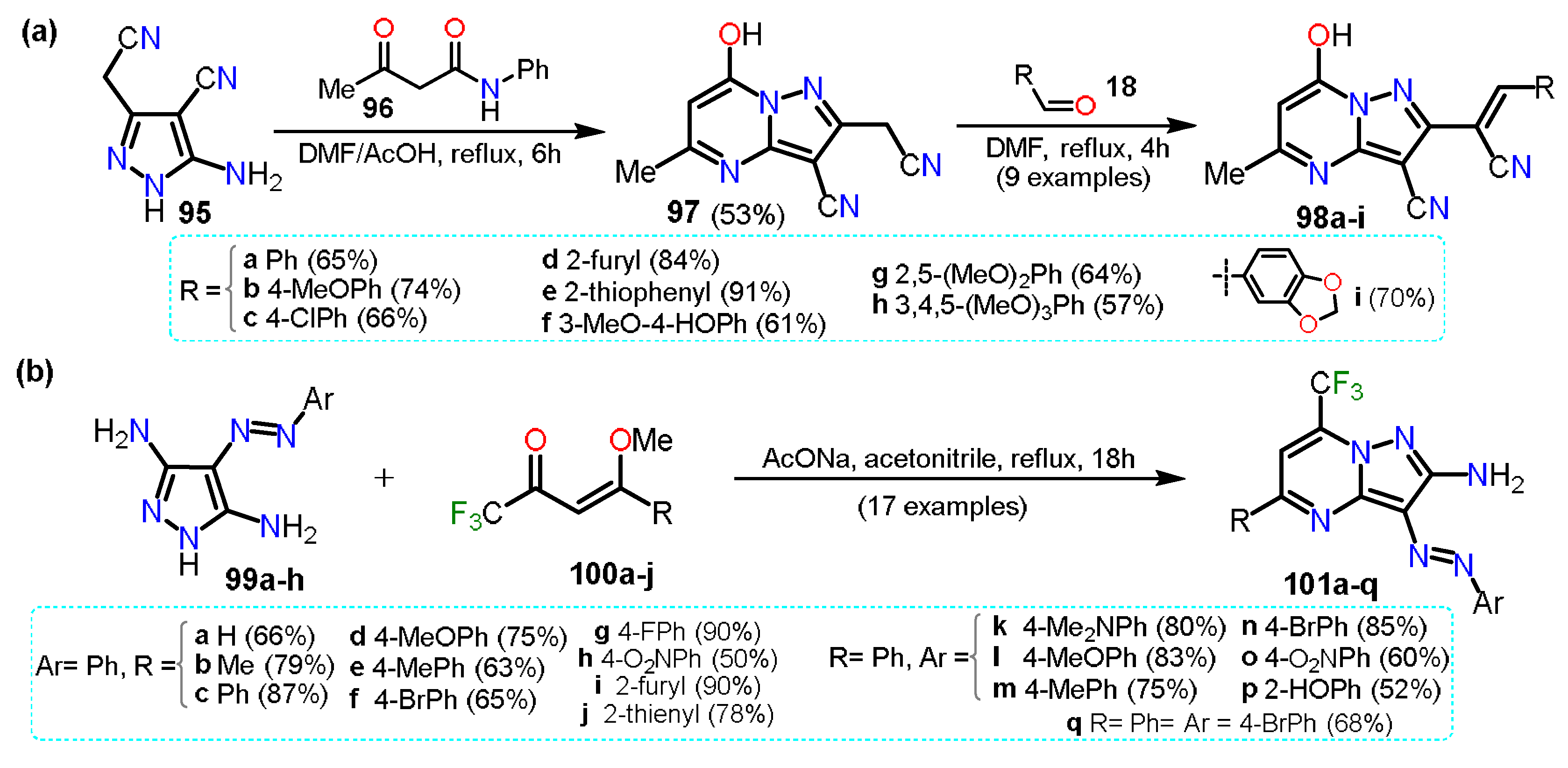
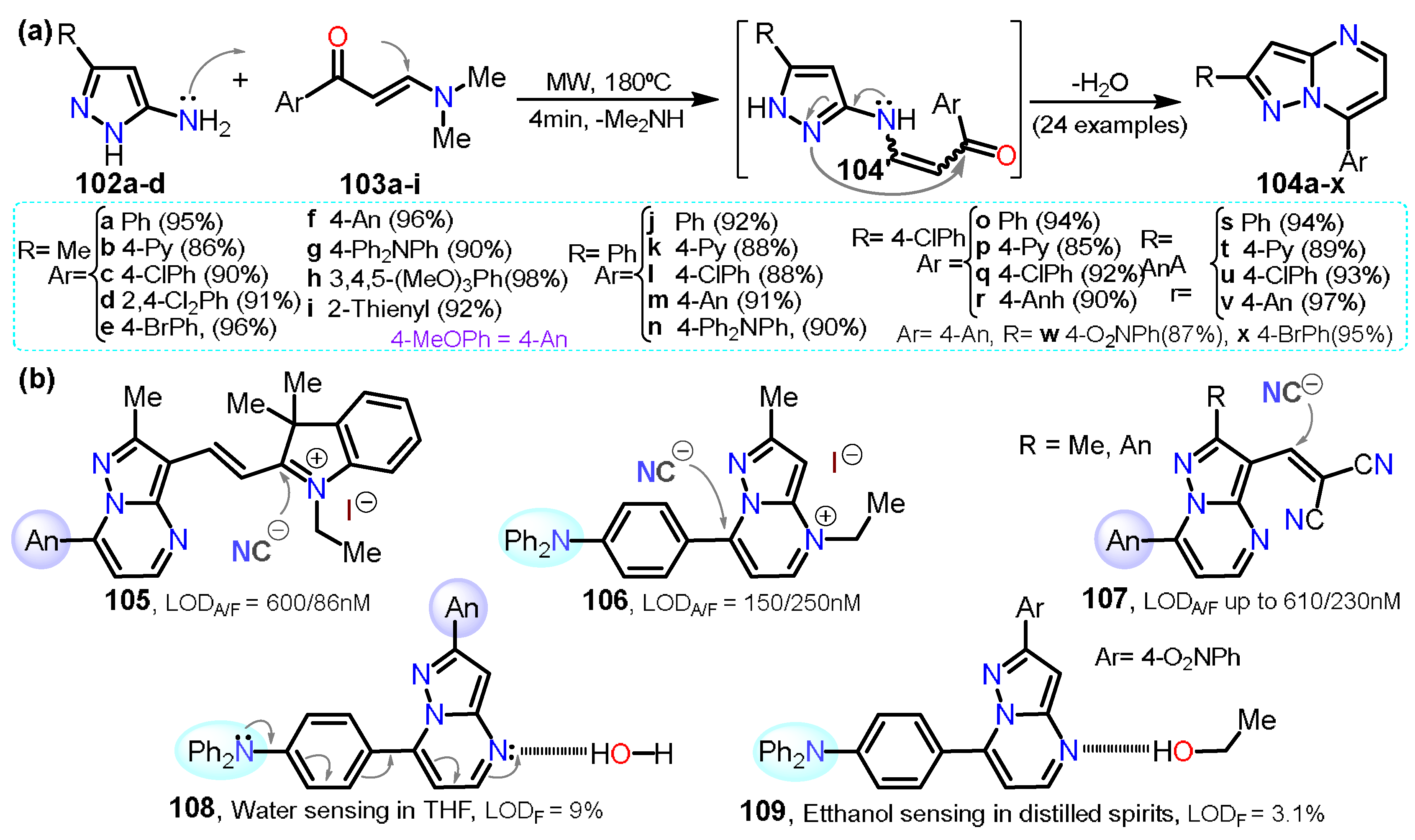
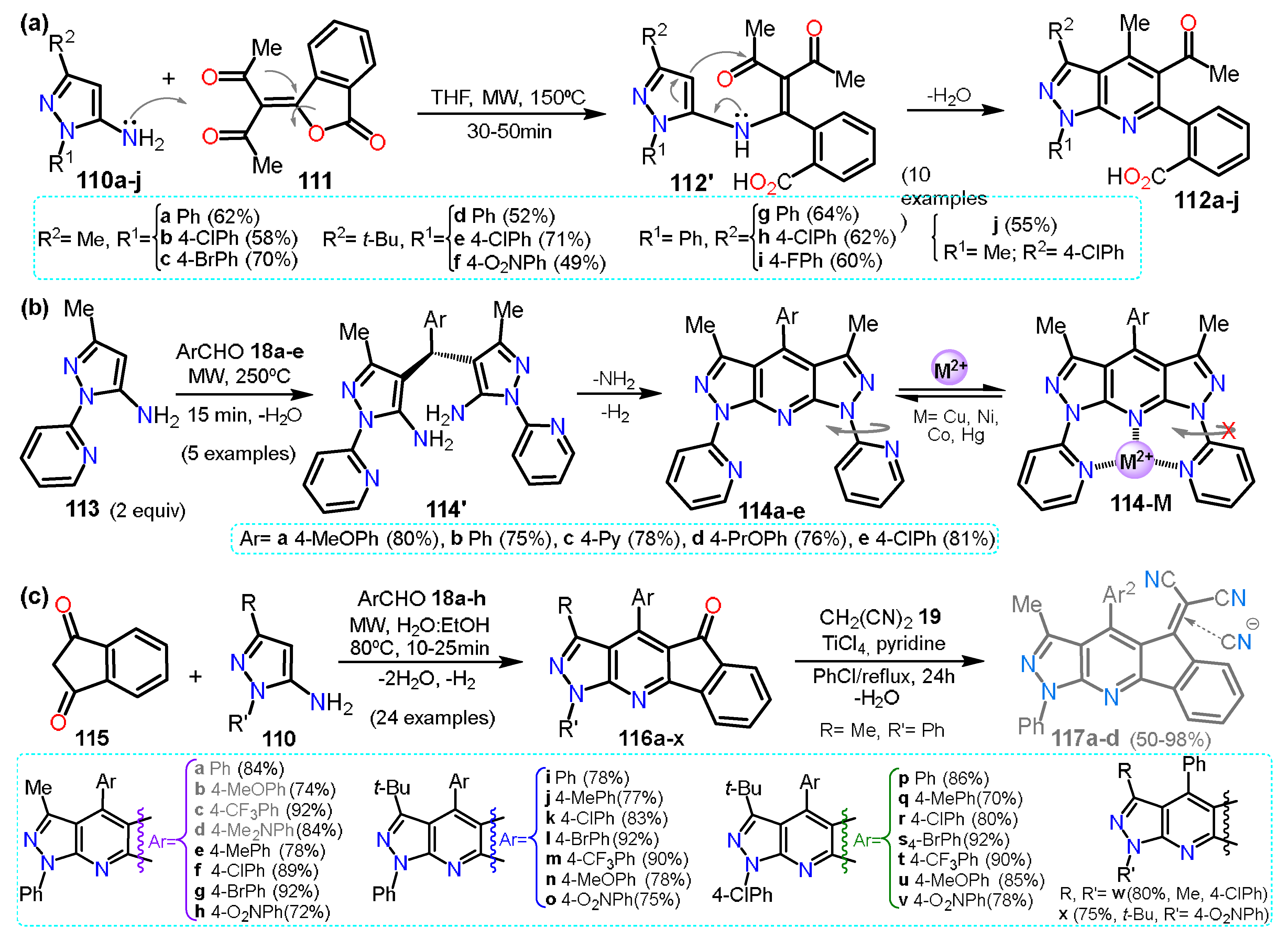


| Synthesis and Functionalization | Pyrazole Derivatives | Synthesis of Fused Pyrazoles | ||
|---|---|---|---|---|
| Section 2.1 | Aminopyrazoles: β-ketonitriles, β-enaminonitriles, acrylonitriles, etc. |  | Pyrazolo[1,5-a]pyrimidines: NH-aminopyrazoles and 1,3-biselectrophiles. | Section 3.1 |
| Section 2.2 | Acylpyrazoles: Vilsmeier-Haack conditions and using already acylated precursors.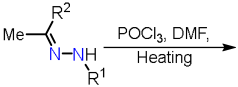 | Pyrazolo[3,4-b]pyridines: NR-aminopyrazoles and 1,3-biselectrophiles. | Section 3.2 | |
| Section 2.3 | Further functional Pyrazoles: halogenations, nitrations, etc.  | Other fused rings: imidazopyrazoles, pyrazoloazepines, indazoles, etc. | Section 3.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos, M.-C.; Portilla, J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry 2022, 4, 940-968. https://doi.org/10.3390/chemistry4030065
Ríos M-C, Portilla J. Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry. 2022; 4(3):940-968. https://doi.org/10.3390/chemistry4030065
Chicago/Turabian StyleRíos, María-Camila, and Jaime Portilla. 2022. "Recent Advances in Synthesis and Properties of Pyrazoles" Chemistry 4, no. 3: 940-968. https://doi.org/10.3390/chemistry4030065
APA StyleRíos, M.-C., & Portilla, J. (2022). Recent Advances in Synthesis and Properties of Pyrazoles. Chemistry, 4(3), 940-968. https://doi.org/10.3390/chemistry4030065







