Abstract
In the present study, the conversion performance of hydrogen sulfide (H2S) to elemental sulfur in ionic-liquid-incorporated transition metals (ILTMs) is predicted using a conductor-like screening model for realistic solvents (COSMO-RS). The predictions were made via the establishment of a correlation between the conversion performance and solubility of H2S in ionic liquids (ILs). All molecules involved were optimized at the DFT/TZVP/M06 computational level and imported on the COSMOtherm program at equimolar conditions. For validation purposes, the solubility of ILs was predicted at 1 bar pressure. Simple regression analysis was used to establish a relationship between the solubility and conversion performance of H2S. The results indicate that the solubility prediction of ILs is accurate (R2 = 93.40%) with a p-value of 0.0000000777. Additionally, the conversion performance is generally found to be dependent on the solubility value. Furthermore, 1-butyl-3-methylimidazolium chloride [bmim][Cl] was chosen as the base IL for incorporating the transition metal, owing to its solubility and selectivity to H2S. The solubility trend of ILTMs is found to follow the following order: [bmim][NiCl3] > [bmim][FeCl4] > [bmim][CoCl3] > [bmim][CuCl3]. According to the viscosity measurements of ILTMs, [bmim][NiCl3] and [bmim][FeCl4] exhibited the highest and lowest viscosity values, respectively. Therefore, [bmim][FeCl4] is a promising ILTM owing to its higher solubility and low viscosity for the application studied.
1. Introduction
Hydrogen sulfide (H2S) is a contaminant well known for its hazardous and catastrophic effects on human health, the environment, and materials. It is commonly generated from a variety of industrial processes, such as natural gas processing, petroleum refining, and biogas processing. Moreover, it is extremely corrosive in nature, highly toxic, flammable, and explosive; thus, it is stringently regulated by authorities [1,2,3]. Hence, its presence often imposes difficulties in operation, transportation, and storage. Therefore, it is very important to capture and convert H2S to a safer product, such as elemental sulfur. Several existing conventional sulfur technologies are available and have been applied in the industries. The Claus process, redox process, amine absorption, activated carbon adsorption, and biological processes are among the existing commercial technologies [4,5,6,7,8,9,10,11]. However, there are several drawbacks with the existing technology, such as the Claus process, for example, which is very energy-intensive since it requires two stages with a high operating temperature: 1000–1200 °C at the H2S oxidation stage and 200–350 °C at the catalytic conversion stage [5,12,13,14,15]. Additionally, the process suffers from catalyst deactivation issues due to the condensation of sulfur on the surface of the inorganic catalyst in addition to harsh operating conditions, which in turn leads to high operating costs and imposes operational difficulties [4,16]. The conventional redox process also suffers a set of drawbacks, including the requirements to maintain the solution’s pH between 8.5–9.0 and gas streams containing more than 1–2 g of H2S/m3 of gas [6]. Moreover, due to this limitation, the solution must be circulated through the absorber at a higher rate, resulting in a pumping energy consumption penalty. The other technologies display operational difficulty, incomplete H2S absorption, stringent conditions, and the production of low-quality products [17,18].
Therefore, to overcome the shortcoming of the conventional process, ionic-liquid-incorporated transition metals (ILTMs) are introduced. This type of IL design consists of transition metals coupled either to the anion, cation, or both [16], thus, in our case, forming chlorocomplexes that are anions (CuCl3, FeCl4, CoCl3, NiCl3). The presence of the transition metal presents a considerable advantage to the ILs as it can exist in many oxidation states owing to its incomplete d-orbital; thus, it can facilitate oxidation and reduction processes [19]. Furthermore, as a new type of IL, it is also known for its unique properties, including negligible vapor pressure, low flammability, high thermal stability, and the ability to maintain it in a liquid state over a wide range of temperatures. These unique properties of ILs have an economic contribution, which made their recovery and reuse feasible for repeated cycles. Several recovery methods, such as distillation, extraction, adsorption, induced phase separation, membrane-based separation, magnetic separation, and centrifugation, are commonly employed to recover the used ILs [20]. The magnetic separation method is also one of the appropriate methods used to recover [bmim]FeCl4] [21]. Moreover, ILTMs are task-specific ILs, often known as designer solvents, implying that they are tailored to meet a specific requirement with desirable physicochemical properties [22,23,24,25,26,27,28,29,30,31]. Due to its advantages, the use of an ILTM is believed to provide economical, effective, and sustainable approaches for the direct conversion of H2S to elemental sulfur.
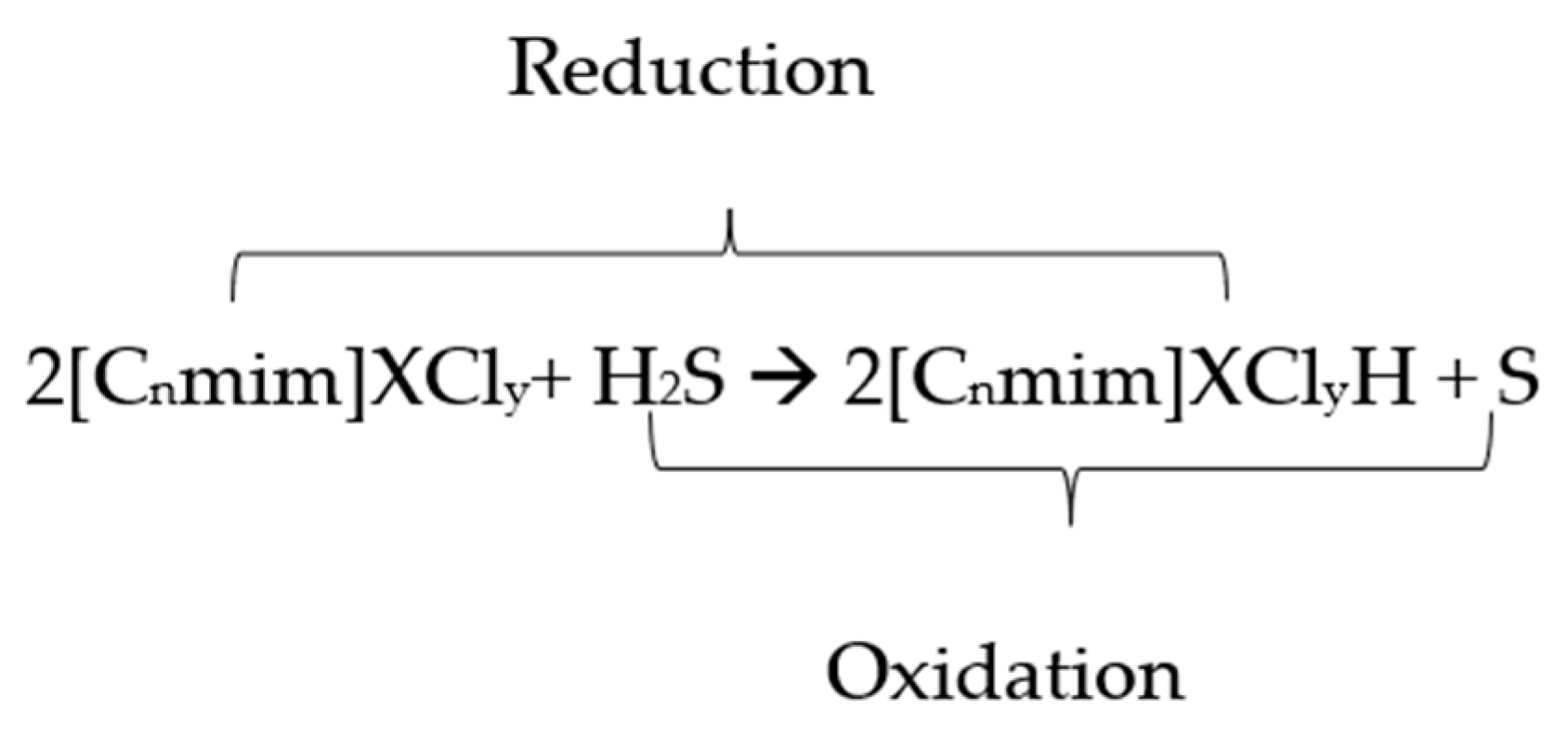
The conversion of H2S to elemental sulfur can be described by the mechanism hypothesized below (Scheme 1) [32]:
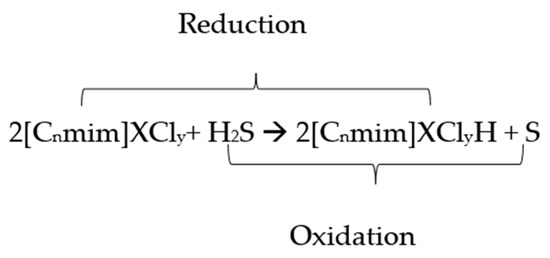
Scheme 1.
Hypothesized mechanism for the conversion of H2S to elemental sulfur.
From the above reaction, it is believed that the metal species in the ionic liquid accommodates the oxidation of H2S to elemental sulfur by reducing itself to a lower oxidation state owing to its ability to exist in many oxidation states due to the incompleteness of the d-orbital. Hence, ILs can possibly be used as a good solvent to assist the conversion of H2S to elemental sulfur [32,33,34,35]. In addition, since the reaction occurs in the IL-incorporated transition metal (liquid phase), it is a prerequisite for the solute gas to be soluble in the base ILs for the conversion reaction to occur.
There are numerous IL-incorporated transition metals that are, theoretically, possible to form via various combinations of cations, anions, and metals. However, from a practical point of view, the performance evaluations of the numerous ILs and their properties, experimentally, are costly and time-consuming [13,36]. Therefore, to search for potential IL candidates for the conversion of H2S, it is necessary to have a reliable predictive computational approach to screen the ILs. For this context of study, COSMO-RS serves as the best screening tool that allows the quantum chemical calculation of individual molecules and the prediction of various thermodynamic properties [37]. Furthermore, the conductor-like screening model for realistic models (COSMO-RS) provides rapid IL screening, as many common cations and anions are readily available in the database [38]. Hence, this computational tool serves as a robust platform enabling the screening of numerous ILs at a reduced time, cost, and using resources.
To date, only one literature report is available that explores the COSMO-RS screening of metal chloride-based ILs reported by Aminuddin et al. [39]. However, the study does not correlate the role of H2S solubility in different metal chloride-based ILs for the conversion to elemental sulfur. Hence, the science behind the correlation between conversion and solubility is not well understood and is yet to be explored. Therefore, this study aims to use COSMO-RS to predict the conversion performance of IL-incorporated transition metals for the conversion of H2S to elemental sulfur. Initially, the H2S solubility in various ILs is predicted using COSMO-RS. The experimental solubility data are collected from the literature for different ILs to validate the predicted solubility values predicted by COSMO-RS during identical experimental conditions. Based on the validation results, a correlation between the solubility of H2S and the conversion of H2S to elemental sulfur is established and further used to predict the H2S conversion performance of ILTMs to elemental sulfur.
2. Materials and Methods
2.1. Materials
Ionic liquid, i.e., 1-butyl-3-methylimidazolium chloride, 98% purity, was purchased from Acros Organics. The metal chlorides, such as copper(II) chloride (≥98% purity), nickel(II) chloride anhydrous (≥98% purity), ferric(III) chloride anhydrous (≥98% purity), and cobalt(II) chloride (≥98% purity), were all purchased from Merck. All the chemicals were used as received, without any further purification.
2.2. The Selection of Cations and Anions of Ionic Liquids Related to H2S Capture for COSMO-RS Screening
In the present work, it was first assumed that the conversion performance of H2S to elemental sulfur was in correlation with the solubility of H2S in the ILs. This assumption was based on the fundamentals of reactions in liquid media, where the reaction between the solute and solvent occurs effectively. It is first required that the solute be adequately soluble in the solvent. Therefore, it is necessary to determine or calculate the solubility of H2S in the ILs. However, obtaining the solubility values for a wide range of ILs via experimentation is time- and resource-consuming. Hence, a computational platform COSMO-RS was opted to predict the solubility value of H2S in the ILs.
Subsequently, the experimental data of ILs for H2S capture were collected from the available literature. Then, the anions and cations were selected. The cations were selected from the 1-alkyl-3-methylimidazolium group, such as 1-(2-hydroxyethyl)-3-methylimidazolium [HOemim], 1-octyl-3-methylimidazolium [omim], 1-hexyl-3-methylimidazolium [hmim], 1-butyl-3-methylimidazolium [bmim], and 1-ethyl-3-methylimidazolium [emim]. This was due to the simplicity of its synthesis route, commercially available at a more reasonable price than other functional ILs, and proven ability in capturing and converting H2S to sulfur [32,34,35,40,41,42], whereas the anions were chosen from halide groups. The halide-based anions chosen were tetrafluoroborate [BF4], trifluoroacetate [TfA], bis(trifluoromethylsulfonyl)imide [Tf2N], trifluoromethanesulfonate [TfO], hexafluorophosphate [PF6], and chloride [Cl]. The anions in the halide group are known for their physical adsorption isotherms [43,44,45,46,47]. From the selected anions and cations, the following combinations forming ILs were selected due to the availability of H2S capture values in the literature: 1-octyl-3-methylimidazolium tetrafluoroborate [omim][BF4], 1-octyl-3-methylimidazolium hexafluorophosphate [omim][PF6], 1-hexyl-3-methylimidazolium tetrafluoroborate [hmim][BF4], 1-hexyl-3-methylimidazolium trifluoroacetate [hmim][TfA], 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [hmim][Tf2N], 1-butyl-3-methylimidazolium trifluoromethanesulfonate [bmim][TfO], 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4], 1-butyl-3-methylimidazolium chloride [bmim][Cl], 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [bmim][Tf2N], 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [emim][Tf2N], 1-ethyl-3-methylimidazolium tetrafluoroborate [emim][BF4], hydroxyethyl)-3-methylimidazolium trifluoromethanesulfonate [HOemim][TfO], and 1-(2-hydroxyethyl)-3-methylimidazolium hexafluorophosphate [HOemim][PF6].
2.3. The Prediction of H2S Solubility in ILs and the Validation of the Accuracy of COSMO-RS
The anions and cations studied in the present paper are within the available COSMOtherm database. These anions and cations were selected at a single conformer with the least ground-state energy and TZVP basis set for the computation. The solubility of these ILs was then predicted using COSMO-RS at 1 bar pressure. The solubility was calculated by COSMO-RS, according to the following expression (Equation (1)) [48]:
where , are the partial pressure and vapor pressure of the pure H2S, respectively; is the mole fraction; and is the activity coefficient of H2S [48]. The solubilities are presented in the form of a mole fraction of H2S in the liquid phase. The obtained result was then divided with the molecular weight of the respective ILs. Hence, solubility in mol/kg was acquired. This was to ease the comparison to the experimental data collected, which were described in mol/kg.
The obtained solubility results were then normalized and compared to the normalized solubility data collected from the literature. The data were then plotted and their correlation coefficient (R2) values were determined to evaluate the accuracy of the COSMO-RS prediction.
2.4. The Formation of New ILs for H2S Conversion to Elemental Sulfur for COSMO-RS Screening
Then, new IL formations were formed in accordance to the available conversion data in the literature: 1-hexyl-3-methylimidazolium tetrafluoroborate [hmim][BF4], 1-hexyl-3-methylimidazolium trifluoroacetate [hmim][TfA], 1-hexyl-3-methylimidazolium chloride [hmim][Cl], 1-butyl-3-methylimidazolium trifluoromethanesulfonate [bmim][TfO], 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [emim][Tf2N], and 1-ethyl-3-methylimidazolium acetate [emim][OAc]. The solubility of these ILs was then predicted using COSMO-RS. The prediction method is similar to the method described in the previous section.
2.5. The Establishment of the Correlation between H2S Solubility in ILs and Conversion Performance
Furthermore, the normalized experimental H2S solubility and normalized COSMO-RS-predicted H2S solubility values in ILs were plotted against the conversion ratio of H2S to elemental sulfur in order to establish a correlation between H2S capture and H2S conversion by ILs. The establishment of the correlation allows the utilization of COSMO-RS for the preliminary prediction of the conversion performance of H2S to elemental sulfur in ILs. The conversion ratio value was taken from the literature [35].
2.6. The Selection of the Best ILs to Be Paired and Incorporated with Transition Metals
The IL with the highest solubility and conversion performance in the previous step was selected for further optimization. This was achieved by varying the alkyl chain length of the selected IL from n = 2 to n = 8, since the alkyl chain length impacts the solubility performance. Their solubility and selectivity values for H2S were predicted using COSMO-RS. The selectivity was calculated from the activity coefficient at infinite dilution, as described in the following expression (Equation (2)) [49]:
where and are the activity coefficients for CO2 and H2S at infinite dilution, respectively. The selectivity value was one of the crucial parameters to be predicted in this work because most systems appear in mixtures of gases. From a practical perspective, acid gases are mainly composed of CO2 in higher concentrations than H2S. Thus, the selectivity value of ILs towards H2S against CO2 is a crucial parameter.
Additionally, to further understand the behavior of the molecules under study, the sigma profile and sigma potential were studied. The sigma profile and sigma potential are divided into three segments: the hydrogen-bond donor region belongs to a range of polarities of α < −0.0082 e/Å2, the hydrogen-bond acceptor region belongs to a range of polarities of α > +0.0082 e/Å2, and lastly, −0.0082 e/Å2 < α < 0.0082 e/Å2 is known as the non-polar region [50]. Nevertheless, according to Bavoh et al. [51], it is comparatively easier to interpret the sigma potential compared to the sigma profile, since it presents detailed results with simplicity. Understanding the behavior and interaction of the molecules allows for the effective designation of ILs for specific applications and helps in selecting the right ILs to perform the work. The expressions used for the calculation of both the sigma profile and sigma potential are presented in Equations (3) and (4), respectively [48]:
Sigma profile of the molecule
where is the sigma profile of any molecule X, is the number of distributed segments that have a surface charge density σ, is the total number of distributed segments, is the segment surface area that has surface charge density σ, is the area of the whole surface cavity rooted in the medium, and is the polarity of the surface.
Sigma potential
where is the chemical potential of a surface segment, is the universal gas constant, T is the temperature at which the vapor pressure is estimated, is the chemical potential of an effective surface segment of area, is the sigma profile of the whole system, and is the chemical potential of a surface segment. Therefore, the best IL is selected based on the solubility and selectivity values. The sigma profile and sigma potential are used to analyze and describe the behavior of the selected ILs.
2.7. The Prediction of H2S Solubility and Viscosity for Direct H2S Conversion in ILTMs, and the Selection of the Best ILTM
Different metal chlorides xCln (x = Fe, Ni, Co and Cu, n = 2 or 3) are added to the structure of the selected IL, forming a list of IL-incorporated transition metals. The new structures are optimized using Turbomole software at the DFT/M06/TZVP computational level. For organo- and inorgano-metallic chemistry and noncovalent interactions, the use of an M06 functional is recommended [52]. The optimized structures are then imported to COSMO-RS for the predictions of solubility and viscosity. As a crucial physicochemical property for the current application, the viscosity value is predicted to assist the selection at ambient conditions.
The transition metals chosen in this study are commonly used catalysts in the industry, inexpensive, and abundantly available [53]. These transition metals possess an incomplete d-orbital; hence, they exist in many oxidation states, promoting the oxidization of H2S to elemental sulfur. The imidazolium-based IL is chosen according to its overall performance and interaction with the solute gas.
2.8. Synthesis of Ionic-Liquid-Incorporated Transition Metals
To validate the COSMO-RS viscosity predictions, the ILTMs were synthesized. The synthesis protocol for the incorporation of transition metals in the ionic liquids is according to the procedure reported by Wang et al. [32], where 1-butyl-3-methylimidazolium chloride [bmim][Cl] is used as a base IL for the incorporation of the transition metals. [bmim][Cl] and the metal chlorides, such as Fe(III)Cl3, Ni(II)Cl2, Cu(II)Cl2, and Co(II)Cl2, were mixed at a 1:1 molar ratio and stirred for 24 h at a speed of 120 rpm using a hot-plate magnetic stirrer at room temperature. The proposed reaction mechanism for the synthesis of ILTMs can be described as:
[bmim][Cl] + xCln → [bmim][xCln+1]
The viscosity of the ILTM samples was then tested using a rheometer (Model DHR1, TA instruments, New Castle, DE, USA) at a shear rate of 10.01/s, temperature 298.15 K, and pressure 1 bar.
3. Results and Discussions
3.1. The Accuracy of COSMO-RS in Predicting H2S Solubility in ILs
The validation of the COSMO-RS-predicted H2S solubility using experimental data is crucial for the reliability and accuracy of the model. Therefore, to cross-check the accuracy of the COSMO-RS model, the available experimental solubility data in the literature were collected and compared against the COSMO-RS-predicted solubility values. The experimental solubility data collected from the literature in mol/kg, along with the references and the COSMO-RS-predicted solubility in mol/mol and mol/kg, are presented in Table 1.

Table 1.
List of ILs with experimental and COSMO-RS-predicted solubility values at 1 bar.
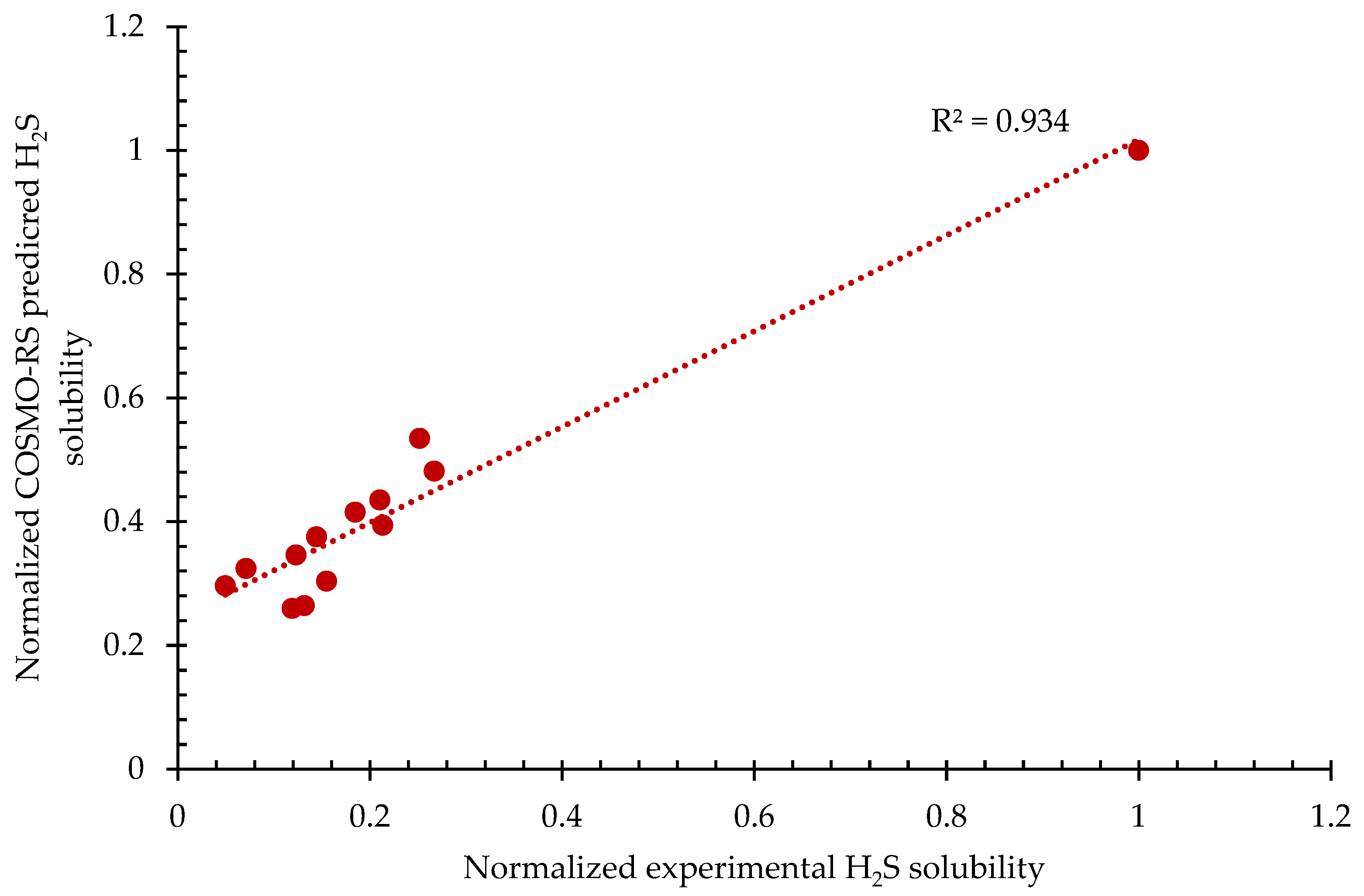
Table 1 portrays both the experimental solubility value and predicted solubility of H2S for 13 different ILs. From Table 1, it can be observed that [bmim][Cl] has the highest solubility value. The reason for this could be because of the Cl− anion. The Cl− anion is highly electronegative; hence, it increases the polarity of the whole IL system, thus making it a good hydrogen-bond acceptor. Therefore, it is favorable to form a strong hydrogen-bond interaction with the H2S since H2S is, by nature, a hydrogen-bond donor [50]. Additionally, from Table 1, it can be noticed that [emim][Tf2N] has the lowest predicted solubility, accounting for a solubility value of 0.9664 mol/kg. This could be due to the Tf2N anion, which is acidic in nature; therefore, less H2S interacts with the anion since H2S itself is acidic in nature. Furthermore, the trend of the predicted solubility generally follows: [bmim][Cl] > [emim][BF4] > [bmim][BF4] > [hmim][BF4] > [hmim][TfA] > [omim][BF4] > [bmim][TfO] > [bmim][Tf2N] > [hmim][Tf2N] > [HOemim][TfO] > [HOemim][PF6] > [emim][Tf2N]. It can also be observed that the distribution of solubility data from both experimental and predicted solubility values are different. Hence, both of these variables are normalized and plotted, as can be observed in Figure 1.
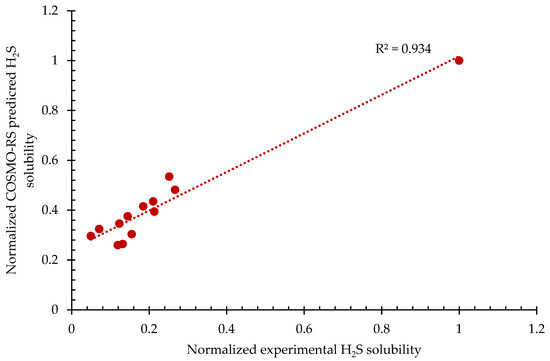
Figure 1.
Normalized COSMO-RS-predicted H2S solubility vs. normalized experimental H2S solubility.
The purpose of this subsection was only to provide validation between normalized COSMO-RS-predicted H2S solubility and normalized experimental H2S solubility, hence establishing accuracy. The establishment of accuracy allows for the utilization of COSMO-RS for this study. From Figure 1, it can be observed that the normalized COSMO-RS-predicted solubility value is in good agreement with the normalized experimental value with the coefficient of correlation of 93.4% accounting for 13 data points in total. By taking into consideration the factors that may lead to deviations, for example, the active nature of H2S, which may cause the formation of a strong hydrogen bond with the solvent during the absorption process, as well as human errors that are present during the handling of the experiment by researchers and COSMO-RS overprediction, the R2 value is still considered as remaining within an acceptable range, since it is lower than a 10% deviation. In addition, based on the statistical analysis of the data presented in Figure 1, it is also found that the p-value is 0.0000000777, which is lower than 0.05, thus implying that the 95% confidence level is statistically significance. Thus, it can be concluded that COSMO-RS-predicted results are reliable solubility data; therefore, it was appropriate to use COSMO-RS as a prescreening tool in this study.
3.2. The Establishment of the Correlation between Predicted H2S Solubility in ILs and Conversion Performance of H2S to Elemental Sulfur
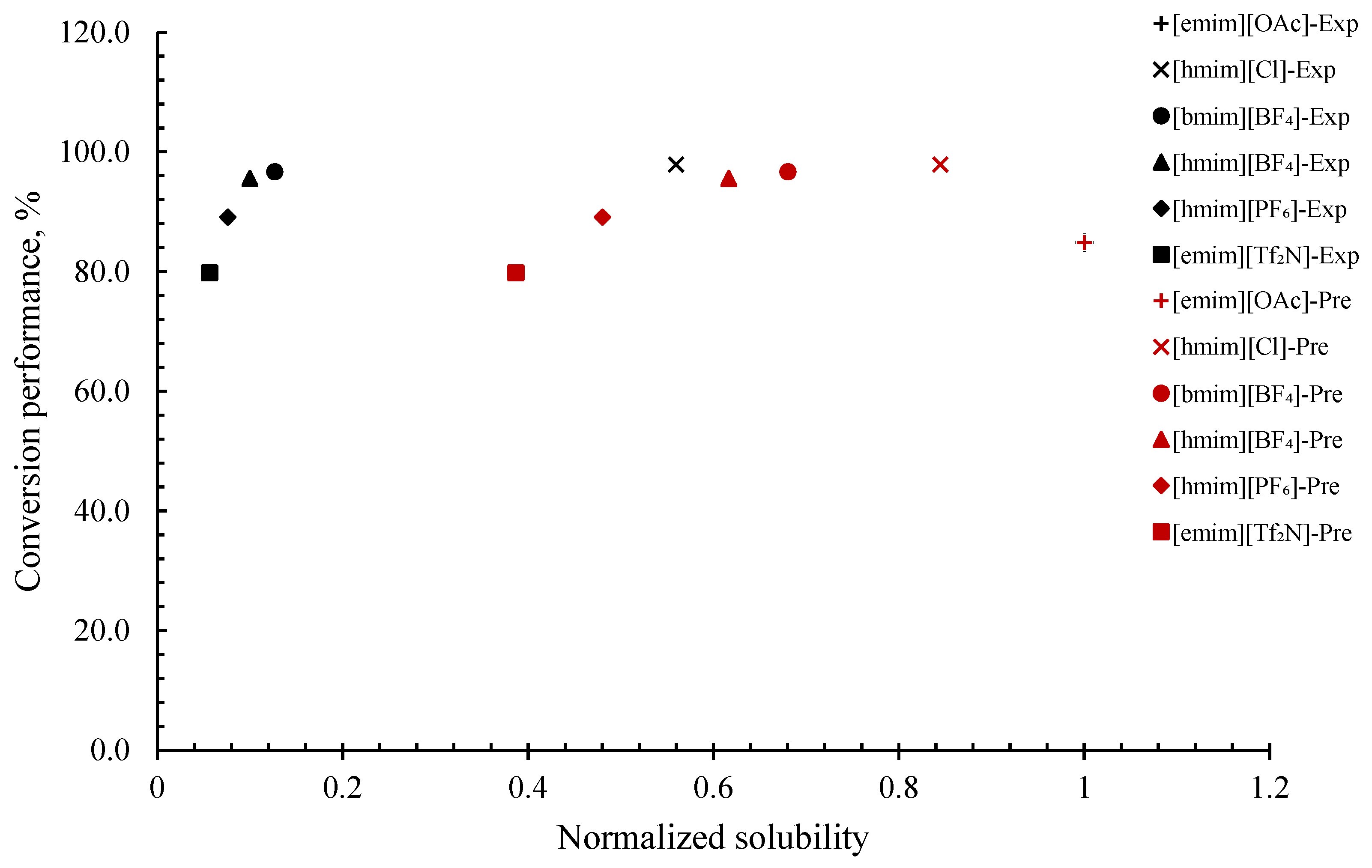
The correlation between H2S solubility and the conversion performance of H2S can be evaluated by interpreting the normalized COSMO-RS-predicted H2S solubility, normalized experimental H2S solubility, and the conversion ratio of H2S to elemental sulfur that was collected from the literature [35]. From Figure 2, it can be noticed that COSMO-RS tends to overpredict the H2S solubility value, which may be unwanted for researchers following accurate quantitative measurements. This can be observed from the COSMO-RS-predicted H2S solubility data of [hmim][Cl], [bmim][BF4], [hmim][BF4], [hmim][PF6], and [emim][Tf2N], which differ from the experimental data. Furthermore, it can also be noticed that, only for [emim][Cl], both the predicted and experimental H2S solubilities are strongly in agreement. Even though COSMO-RS tends to overpredict values, it does not neglect the fact that COSMO-RS is able to present similar normalized solubility trends for both predicted and experimental values, which is meaningful: [emim][OAc] > [hmim][Cl] > [bmim][BF4] > [hmim][BF4] > [hmim][PF6] > [emim][Tf2N]. Additionally, it can be noticed that the conversion ratio of H2S is generally dependent on the sequence of H2S solubility in ILs, indicating that the higher the solubility of H2S in ILs, the higher the conversion ratio of H2S to elemental sulfur. However, this only applies to all ILs studied, except [emim][OAc], which displayed the highest solubility value, but with a relatively lower conversion ratio of 84.9. This discrepancy may be due to either an experimental error or handling method error. The observed trend is in close agreement with the work reported by Huang et al. [35], which claimed that the ILs activity generally follows the sequence of gas solubility. Solubility is simply described as the ability of the solute to dissolve in the solvent to form a solution [59]. Solutions with sufficient dissolved solutes are essential in a chemical reaction that promotes the free movement of reactants throughout the liquid medium, resulting in an enhanced homogenous interaction of reactants. Thus, a good solubility provides a high concentration of H2S in the solvent, thus leading to a high collision of reactants in the liquid media, hence facilitating the reactions and forming the desired product [60], which in turn yields a high conversion value of H2S to elemental sulfur.
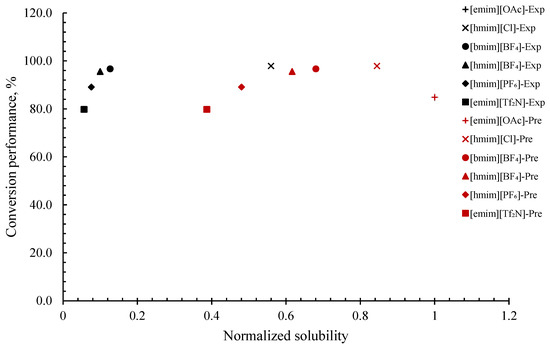
Figure 2.
Comparison of conversion performance of H2S to elemental sulfur vs. normalized solubility (experimental and COSMO-RS prediction) of H2S in various ILs.
Therefore, it can be concluded that H2S conversion performance is generally dependent on the sequence of H2S solubility in the ILs. COSMO-RS can be a valuable tool to estimate the conversion performance of H2S in ILs to elemental sulfur by predicting the solubility and investigating the resulting trends. However, due to the limitation of data in the literature, it can only be confirmed that this method can be utilized at low temperatures, 1 bar pressure, and is best for compounds taking physical absorption isotherms, for instance, ILs without carboxylate anions, non-phenolic ILs, and non-hydrophobic protic ILs [61,62,63].
3.3. Selection of the Best ILs to Be Paired with Metal
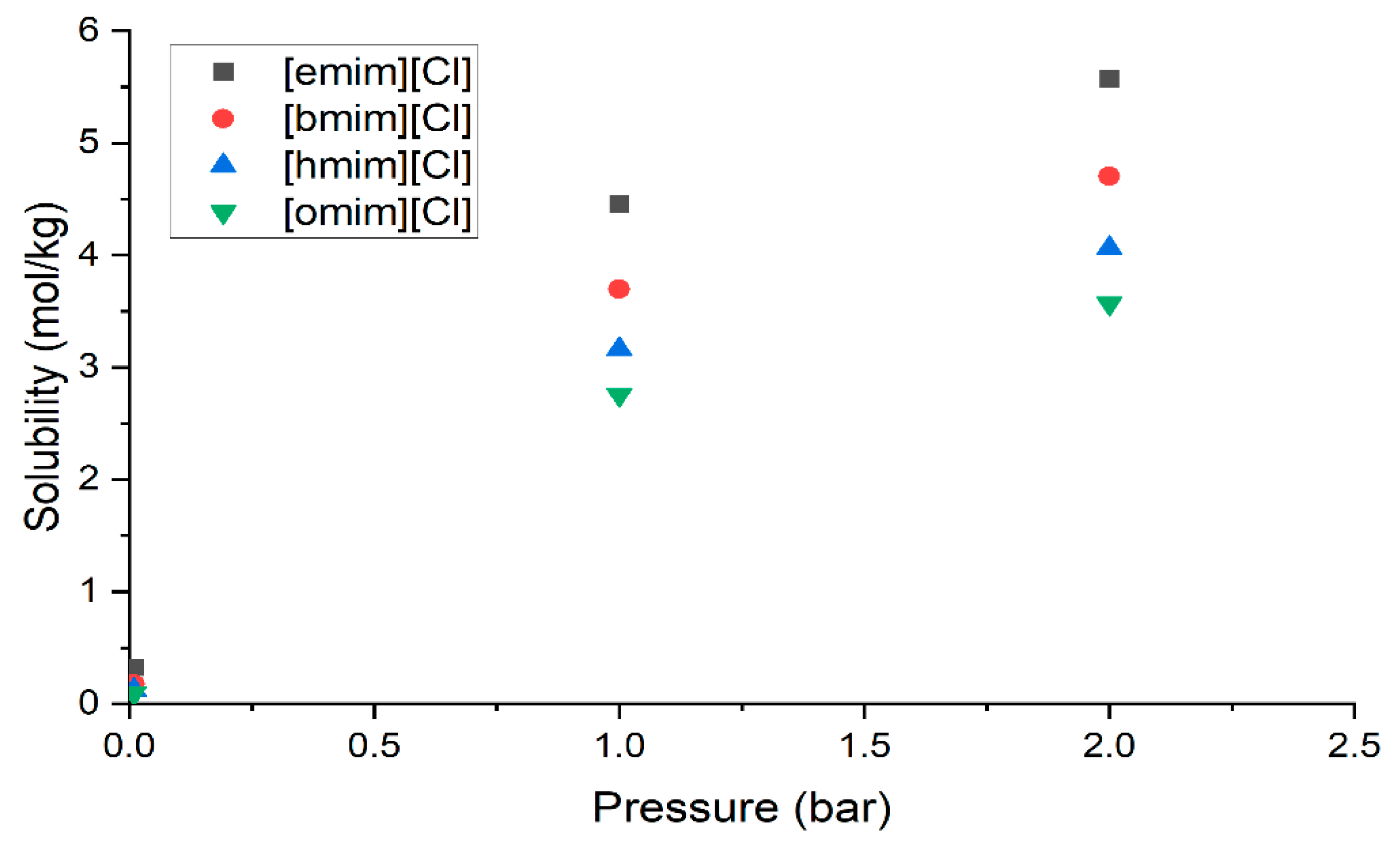
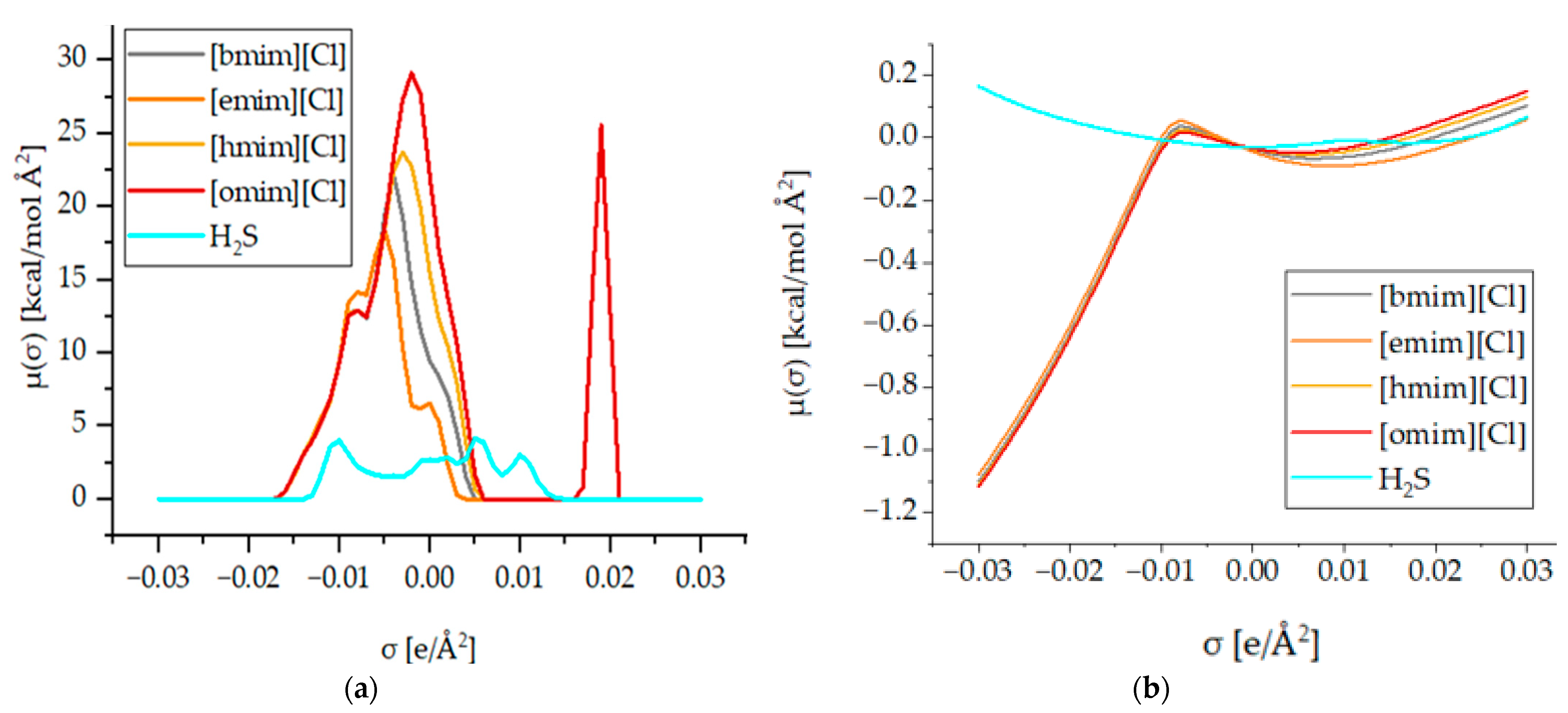
Although, [hmim][Cl] was observed to have the highest conversion performance, as shown in Figure 2, to further explore the best IL, the effect of alkyl chain length was evaluated, and the solubility data as functions of pressure are presented in Figure 3. According to the results, the solubility trend at any pressure follows the following decreasing order: [emim] > [bmim] > [hmim] > [omim]. These results can be further justified by analyzing the sigma profile and sigma potential of H2S and the ILs, which are shown in Figure 4a,b, respectively.
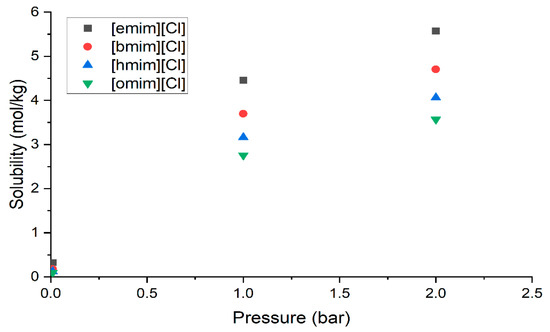
Figure 3.
Effect of alkyl chain length of cation on the solubility of ILs at various pressures and 25 °C.

Figure 4.
(a) Sigma profile of H2S and ILs, and (b) sigma potential of H2S and ILs.
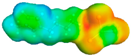
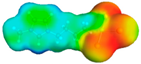
Figure 4a portrays the sigma profile of H2S and 1-alkyl-3-methylimidazolium chloride ILs. It can be observed that the H2S sigma profile falls in the range of −0.014 to +0.014 e/Å2 and there are two distinct peaks that appear at +0.005 and −0.01 e/Å2. Therefore, it can be said that H2S has dominant non-polar properties as well as hydrogen-bond donor properties. For the 1-alkyl-3-methylimidazolium chloride ILs, it can be observed that the sigma of the cations appears at a range of +0.006 to −0.017 e/Å2, which indicates its non-polar and hydrogen-bond donor properties. Moreover, the anion appears at the +0.016 to 0.021 + e/Å2 range, which indicates a strong hydrogen-bond acceptor. Additionally, it can be observed that [omim][Cl] has a more extended sigma profile, followed by [hmim][Cl], [bmim][Cl], and [emim][Cl], which means comparing the non-polar capacities of these ILs to each other: [omim][Cl] < [hmim][Cl] < [bmim][Cl] < [emim][Cl]. Figure 4b shows the sigma potential of H2S and the previously discussed ILs in the sigma profile. It can be observed that the sigma potential is in agreement with the sigma profile in which, at the non-polar region, H2S has the lowest negative µ(σ) value followed by the hydrogen-bond acceptor region, which means it has a high affinity to molecules with both properties. For the ILs, it can be noticed that the anion has an excellent affinity with the hydrogen-bond donor, while for the cation, they all have a high affinity towards non-polar and hydrogen-bond acceptor regions following the trend of [emim][Cl] > [bmim][Cl] > [hmim][Cl] > [omim][Cl].
Although the H2S solubility is an important parameter, in industrial practices, H2S gas mainly exists in a mixture with CO2. Thus, it is crucial to consider the selectivity of ILs towards H2S rather than CO2 for effective capture and conversion, since CO2 is the major constituent of the mixture, whereas H2S is a minor constituent of industrial gases. Therefore, it is important to ensure that the IL used is more selectively reactive to H2S and provides a better solubility performance. Table 2 shows the selectivity of the imidazolium-based ILs with varying alkyl chain-length cations. It can be observed that [bmim][Cl] has the highest selectivity towards H2S, followed by [emim][Cl]. Therefore, [bmim][Cl] is selected as the best IL for incorporating the transition metal.

Table 2.
Selectivity of imidazolium-based ILs with varying alkyl chainlength of the cation.
3.4. The Prediction of H2S Solubility and Viscosity for Direct H2S Conversion in IL-Incorporated Transition Metal, and the Selection of the Best IL-Incorporated Transition Metal
Table 3 shows the list of various ILTMs, their corresponding structures, sigma surfaces, and dynamic viscosities obtained from COSMO-RS. The sigma surface is represented in a few colors where red, blue, and green represent negative (−ve), positive (+ve), and neutral (0) charges on the molecular surface, respectively [64].

Table 3.
List of ILTMs structures, sigma surfaces, and predicted viscosity using COSMO-RS.
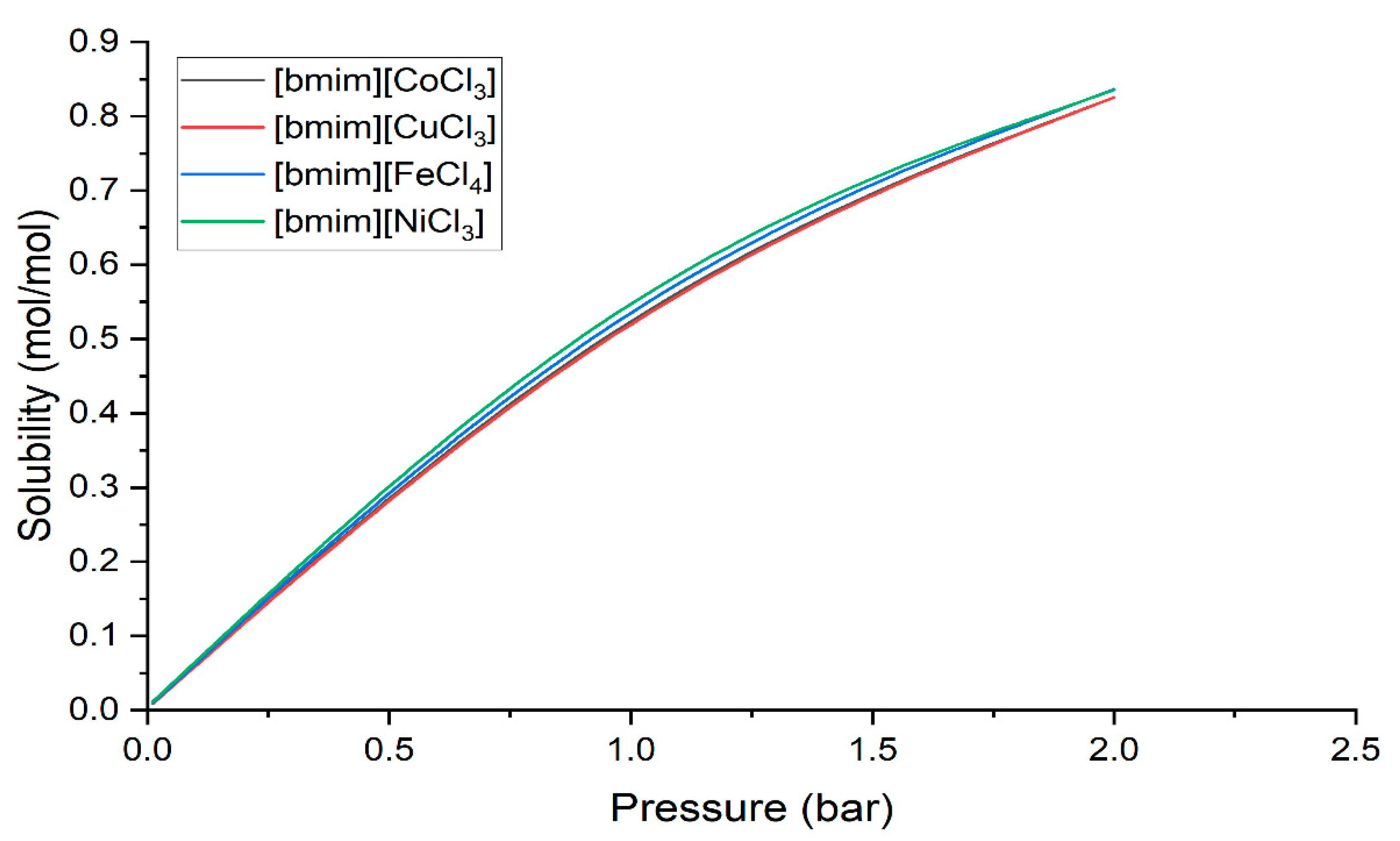
Table 4 portrays the solubility value of the H2S in five different ILTMs at various pressures, which is illustrated in Figure 5. It can be clearly observed that the H2S solubility trend follows a decreasing order: [bmim][NiCl3] > [bmim][FeCl4] > [bmim][CoCl3] > [bmim][CuCl3]. Therefore, it can be stated that the conversion performance also followed the same trend.

Table 4.
Predicted solubility value of H2S in ILTMs at different pressures using COSMO-RS.

Figure 5.
Predicted solubility trend of H2S in ILTMs as a function of pressure using COSMO-RS.
In addition to the solubility value, the viscosity of ILTMs is also another property that must be taken into consideration. Higher viscosities of the ILs may offer resistance to gas flow, increasing the gas–liquid, and hence deteriorating the conversion performance. Moreover, if the circulation of ILs is needed, they result in a higher transportation energy penalty. According to the predicted viscosity values presented in Table 3, it can be noticed that the order of viscosity is as follows: [bmim][NiCl3] > [bmim][CuCl3] > [bmim][CoCl3] > [bmim][FeCl4]. It can be concluded that ILs with higher viscosities are not suitable for the application mentioned in this work, which is at an ambient condition. Therefore, according to the results presented in this work, [bmim][FeCl4] is the best ILTM for the direct conversion of H2S to elemental sulfur, with a lower viscosity value of 21.81 mPa.s and relatively higher solubility performance. In addition, it is believed that the other formulation of 1-alkyl-3-imidazolium chloride and FeCl3 is also possible, provided that the viscosity value is considered as a limiting factor.
Figure 6 shows the four synthesized ILTMs with different transition metal chlorides. It can be observed that [bmim][NiCl3] is highly viscous, while [bmim][CoCl3] is less viscous when compared to [bmim][NiCl3]. [bmim][CuCl3] showed intermediate viscosity, whereas [bmim][FeCl4] displayed the least viscosity value among the rest of the ILTMs synthesized in this study. Hence, it appears that [bmim][FeCl4] is appropriate for the current application.

Figure 6.
Synthesized ILTMs: (a) [bmim][NiCl3], (b) [bmim][FeCl4], (c) [bmim][CoCl3], and (d) [bmim][CuCl3].
The experimental values for viscosity are presented in Table 5; however, the observation shows that the viscosity trend follows [bmim][NiCl3] > [bmim][CoCl3] > [bmim][CuCl3] > [bmim][FeCl4], which is dissimilar to the COSMO-Rs-predicted trend, as shown in Table 3. Moreover, the experimental values for viscosity are also not comparable to those of the predicted values. The possible reason behind this large deviation could be due to COSMO-RS’s limitation in predicting the physicochemical properties of the solvents containing metals. Another possible concern is that the COSMO-RS viscosity predictions require only defining one parameter, i.e., temperature, and computes at infinite dilutions. However, the experimental conditions, such as temperature, pressure, concentrations, and shear rate, have different contributions to the viscosity of the ILTMs. These factors could be the main reasons that led to the great deviations in the experimental and predicted viscosity values.

Table 5.
Experimental viscosity values for ILTMs measured at 298.15 K.
4. Conclusions
In this work, the experimental solubility data of 13 ILs were collected from the literature and used to validate the COSMO-RS-predicted values. The normalized predicted and experimental solubility values displayed a good correlation coefficient of 93.4%. This conclusion was determined taking consideration of the factors that may lead to deviations, such as COSMO-RS overprediction; hence, the R2 value was still considered as being within an acceptable range, since it was lower than a 10% deviation. Additionally, based on the statistical analysis, it was found that the p-value was 0.0000000777, which is less than 0.05, hence implying that the 95% confidence level was statistically significant. The COSMO-RS-predicted solubility values were furthered with the conversion ratio of H2S. The results reveal that the conversion of H2S to elemental sulfur is generally dependent on H2S solubility. Thus, COSMO-RS can be used as a pre-screening tool to estimate the conversion performance of H2S to elemental sulfur. Furthermore, ILs with chloride anions and varying alkyl chain lengths of cations, such as [emim][Cl], [bmim][Cl], [hmim][Cl], and [omim][Cl], were used for the prediction of solubility and selectivity using COSMO-RS. According to the results, [bmim][Cl] displayed better solubility and selectivity than H2S; therefore, it was used further as the base IL for the incorporation of transition metals to form ILTMs. The molecular structures of ILTMs were optimized in Turbomole and imported to the COSMOtherm program. The trend of the predicted H2S solubility of ILTMs at 1 bar pressure appear to follow the order: [bmim][NiCl3] > [bmim][FeCl4] > [bmim][CoCl3] > [bmim][CuCl3]. Furthermore, the ILTMs were synthesized and their viscosities were also predicted using COSMO-RS and measured experimentally at 298.15K. The predicted viscosity follows [bmim][NiCl3] > [bmim][CuCl3] > [bmim][CoCl3] > [bmim][FeCl4]. However, when compared to the experimental viscosity trend, the order of [bmim][CoCl3] interchanges with [bmim][CuCl3]. According to the overall findings in this study, it can be concluded that [bmim][FeCl4] appears to be an appropriate ILTM among the three other studied ILTMs, owing to its higher solubility, in addition to its lowest viscosity. Additionally, it is also believed that other formulations of 1-alkyl-3-methylimidazolium chloride and FeCl3 are also possible in capturing and converting H2S to elemental sulfur, providing that the viscosity is taken into consideration as a limiting factor.
5. Recommendation
Moreover, when the predicted and experimental viscosities were compared, large deviations were observed, which could be due to COSMO-RS’s limitation in predicting the physicochemical properties of the solvents containing metals. Another possible concern is that the COSMO-RS viscosity predictions require only defining one parameter, i.e., temperature, and computes at infinite dilutions. However, the experimental conditions, such as temperature, pressure, concentrations, and shear rate, have different contributions to the viscosity of ILTMs. As a recommendation, it is highly advised for researchers to synthesize their ILTMs and check their viscosity values via experimentation.
Author Contributions
Conceptualization, M.A.B., N.F.A.M., M.D.H.W. and A.I.; methodology, N.F.A.M.; software, N.F.A.M. and A.I.; validation, N.F.A.M.; formal analysis, N.F.A.M.; investigation, N.F.A.M.; resources, M.A.B.; data curation, N.F.A.M.; writing—original draft preparation, N.F.A.M.; writing—review and editing, M.A.B., M.D.H.W. and A.I.; visualization, N.F.A.M.; supervision, M.A.B.; project administration, N.F.A.M.; funding acquisition, M.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education (MOHE), grant number 015MA0-071.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the support received from the members of the Centre of Research in Ionic Liquids, Chemical Engineering Department, Universiti Teknologi PETRONAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bai, Y.; Bai, Q. Subsea Corrosion and Scale. In Subsea Engineering Handbook, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 455–487. [Google Scholar]
- Hantson, P.; Baud, F.J.; Garnie, R. Toxic Gases. In Human Toxicology; Elsevier: Amsterdam, The Netherlands, 1996; pp. 661–669. [Google Scholar]
- Rahnama-Moghadam, S.; Hillis, L.D.; Lange, R.A. Environmental Toxins and the Heart. In Heart and Toxins; Academic Press: Cambridge, MA, USA, 2015; pp. 75–132. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Chapter 8—Sulfur Recovery Processes. In Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 670–730. [Google Scholar]
- Stewart, M. Gas Sweetening. In Surface Production Operations, 3rd ed.; Gulf Professional Publishing: Houston, TX, USA, 2014; pp. 433–539. [Google Scholar]
- Khairulin, S.; Kerzhentsev, M.; Salnikov, A.; Ismagilov, Z.R. Direct Selective Oxidation of Hydrogen Sulfide: Laboratory, Pilot and Industrial Tests. Catalysts 2021, 11, 1109. [Google Scholar] [CrossRef]
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Natural Gas Treating. In Handbook of Natural Gas Transmission and Processing; Gulf Professional Publishing: Houston, TX, USA, 2019; pp. 231–269. [Google Scholar]
- Primavera, A.; Trovarelli, A.; Andreussi, P.; Dolcetti, G. The effect of water in the low-temperature catalytic oxidation of hydrogen sulfide to sulfur over activated carbon. Appl. Catal. A Gen. 1998, 173, 185–192. [Google Scholar] [CrossRef]
- Basu, R.; Clausen, E.C.; Gaddy, J.L. Biological conversion of hydrogen sulfide into elemental sulfur. Environ. Prog. 1996, 15, 234–238. [Google Scholar] [CrossRef]
- Sipma, J.; Janssen, A.J.H.; Pol, L.W.H.; Lettinga, G. Development of a novel process for the biological conversion of H2S and methanethiol to elemental sulfur. Biotechnol. Bioeng. 2003, 82, 1–11. [Google Scholar] [CrossRef]
- Khabazipour, M.; Anbia, M. Removal of Hydrogen Sulfide from Gas Streams Using Porous Materials: A Review. Ind. Eng. Chem. Res. 2019, 58, 22133–22164. [Google Scholar] [CrossRef]
- Hossain, M.; Park, H.; Choi, H. A Comprehensive Review on Catalytic Oxidative Desulfurization of Liquid Fuel Oil. Catalysts 2019, 9, 229. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Huang, Y.; Afzal, R.M.; Zhang, X.; Zhang, S. Predicting H2S solubility in ionic liquids by the quantitative structure–property relationship method using Sσ-profile molecular descriptors. RSC Adv. 2016, 6, 70405–70413. [Google Scholar] [CrossRef]
- Mokhatab, S.; Poe, W.A.; Mak, J.Y. Gas Processing Plant Automation. In Handbook of Natural Gas Transmission and Processing: Principles and Practices; Gulf Professional Publishing: Houston, TX, USA, 2018; pp. 615–642. [Google Scholar] [CrossRef]
- Mokhatab, S.; Mak, J.Y.; Valappil, J.V. (Eds.) LNG Plant and Regasification Terminal Operations. In Handbook of Liquefied Natural Gas; Gulf Professional Publishing: Houston, TX, USA, 2014. [Google Scholar]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Le Borgne, S.; Baquerizo, G. Microbial Ecology of Biofiltration Units Used for the Desulfurization of Biogas. ChemEngineering 2019, 3, 72. [Google Scholar] [CrossRef]
- Heim, T.; Mares, B.; Prosernat, V.S. Direct Oxidation Sulphur Recovery Process for Very Lean Acid Gas Applications. 2016. Available online: https://www.digitalrefining.com/article/1001263/direct-oxidation-sulphur-recovery-process-for-very-lean-acid-gas-applications#.YtUEcYRByMp (accessed on 30 May 2022).
- Sutradhar, M.; Pombeiro, A.J. Vanadium Complexes in Catalytic Oxidations; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Mai, N.L.; Ahn, K.; Koo, Y.-M. Methods for recovery of ionic liquids—A review. Process Biochem. 2014, 49, 872–881. [Google Scholar] [CrossRef]
- Lee, S.H.; Ha, S.H.; You, C.Y.; Koo, Y.M. Recovery of magnetic ionic liquid [bmim]FeCl4 using electromagnet. Korean J. Chem. Eng. 2007, 24, 436–437. [Google Scholar] [CrossRef]
- Chen, Y.; Woodley, J.; Kontogeorgis, G.; Gani, R. Integrated Ionic Liquid and Process Design involving Hybrid Separation Schemes. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; Volume 44. [Google Scholar]
- Rogers, R.D.; Seddon, K.R. Ionic Liquids--Solvents of the Future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef]
- Zhao, J.L.; Anderson, Q. 2.11—Ionic Liquids in Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 213–242. [Google Scholar]
- Dharaskar, S.A.; Wasewar, K.L.; Varma, M.N.; Shende, D.Z.; Yoo, C.K. Ionic Liquids:—The Novel Solvent for Removal of Dibenzothiophene from Liquid Fuel. Procedia Eng. 2013, 51, 314–317. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [Google Scholar] [CrossRef]
- Paulechka, Y.; Zaitsau, D.H.; Kabo, G.; Strechan, A. Vapor pressure and thermal stability of ionic liquid 1-butyl-3-methylimidazolium Bis (trifluoromethylsulfonyl) amide. Thermochim. Acta 2005, 439, 158–160. [Google Scholar] [CrossRef]
- Ratti, R. Ionic Liquids: Synthesis and Applications in Catalysis. Adv. Chem. 2014, 2014, 729842. [Google Scholar] [CrossRef]
- Sawant, A.; Raut, D.; Darvatkar, N.; Salunkhe, M. Recent developments of task-specific ionic liquids in organic synthesis. Green Chem. Lett. Rev. 2011, 4, 41–54. [Google Scholar] [CrossRef]
- Gu, Z.; Brennecke, J.F. Volume Expansivities and Isothermal Compressibilities of Imidazolium and Pyridinium-Based Ionic Liquids. J. Chem. Eng. Data 2002, 47, 339–345. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W. Oxidative Absorption of Hydrogen Sulfide by Iron-Containing Ionic Liquids. Energy Fuels 2014, 28, 5930–5935. [Google Scholar] [CrossRef]
- Cheng, H.; Li, N.; Zhang, R.; Wang, N.; Yang, Y.; Teng, Y.; Jia, W.; Zheng, S. Measuring and Modeling the Solubility of Hydrogen Sulfide in rFeCl3/[bmim]Cl. Processes 2021, 9, 652. [Google Scholar] [CrossRef]
- Wang, J.; Ding, R. Effect of Water Content on Properties of Homogeneous [bmim]Fe(III)Cl4–H2O Mixtures and Their Application in Oxidative Absorption of H2S. Inorganics 2018, 6, 11. [Google Scholar] [CrossRef]
- Huang, K.; Feng, X.; Zhang, X.-M.; Wu, Y.-T.; Hu, X.-B. The ionic liquid-mediated Claus reaction: A highly efficient capture and conversion of hydrogen sulfide. Green Chem. 2016, 18, 1859–1863. [Google Scholar] [CrossRef]
- Kamgar, A.; Esmaeilzadeh, F. Prediction of H2S solubility in [hmim][Pf6], [hmim][Bf4] and [hmim][Tf2N] using UNIQUAC, NRTL and COSMO-RS. J. Mol. Liq. 2016, 220, 631–634. [Google Scholar] [CrossRef]
- Klamt, A. The Beauty of Fluid Phase Thermodynamics from the Perspective of Quantum Chemistry; The Society of Chemical Engineers: Tokyo, Japan, 2004; pp. 1–10. [Google Scholar]
- Salleh, Z.; Wazeer, I.; Mulyono, S.; El-Blidi, L.; Hashim, M.A.; Hadj-Kali, M.K. Efficient removal of benzene from cyclohexane-benzene mixtures using deep eutectic solvents—COSMO-RS screening and experimental validation. J. Chem. Thermodyn. 2016, 104, 33–44. [Google Scholar] [CrossRef]
- Aminuddin, M.S.; Man, Z.; Khalil, M.A.B.; Abdullah, B. Screening of Metal Chloride Anion-based Ionic Liquids for Direct Conversion of Hydrogen Sulfide by COSMO-RS. E3S Web Conf. 2021, 287, 02003. [Google Scholar] [CrossRef]
- January, R. Thermal conductivity of graphene kirigami: Ultralow and strain robustness. Int. J. Pharma Bio Sci. 2013, 3, 1–3. [Google Scholar]
- Taheri, M.; Zhu, R.; Yu, G.; Lei, Z. Ionic liquid screening for CO2 capture and H2S removal from gases: The syngas purification case. Chem. Eng. Sci. 2020, 230, 116199. [Google Scholar] [CrossRef]
- Dan, M.; Yu, S.; Li, Y.; Wei, S.; Xiang, J.; Zhou, Y. Hydrogen sulfide conversion: How to capture hydrogen and sulfur by photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2019, 42, 100339. [Google Scholar] [CrossRef]
- Jalili, A.H.; Rahmati-Rostami, M.; Ghotbi, C.; Hosseini-Jenab, M.; Ahmadi, A.N. Solubility of H2S in Ionic Liquids [bmim][PF6], [bmim][BF4], and [bmim][Tf2N]. J. Chem. Eng. Data 2009, 54, 1844–1849. [Google Scholar] [CrossRef]
- Sakhaeinia, H.; Taghikhani, V.; Jalili, A.H.; Mehdizadeh, A.; Safekordi, A.A. Solubility of H2S in 1-(2-hydroxyethyl)-3-methylimidazolium ionic liquids with different anions. Fluid Phase Equilibria 2010, 298, 303–309. [Google Scholar] [CrossRef]
- Huang, K.; Wu, Y.-T.; Hu, X.-B. Effect of alkalinity on absorption capacity and selectivity of SO2 and H2S over CO2: Substituted benzoate-based ionic liquids as the study platform. Chem. Eng. J. 2016, 297, 265–276. [Google Scholar] [CrossRef]
- Nematpour, M.; Jalili, A.H.; Ghotbi, C.; Rashtchian, D. Solubility of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium trifluoromethanesulfonate. J. Nat. Gas Sci. Eng. 2016, 30, 583–591. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, B.; Liu, S.; Sun, X.; Zhu, X.; Fu, H. Theoretical and experimental investigation on the capture of H 2 S in a series of ionic liquids. J. Mol. Graph. Model. 2016, 68, 87–94. [Google Scholar] [CrossRef]
- Balchandani, S.; Singh, R. COSMO-RS Analysis of CO2 Solubility in N-Methyldiethanolamine, Sulfolane, and 1-Butyl-3-methyl-imidazolium Acetate Activated by 2-Methylpiperazine for Postcombustion Carbon Capture. ACS Omega 2020, 6, 747–761. [Google Scholar] [CrossRef]
- Salleh, M.Z.M.; Hadj-Kali, M.K.; Hashim, M.A.; Mulyono, S. Ionic liquids for the separation of benzene and cyclohexane—COSMO-RS screening and experimental validation. J. Mol. Liq. 2018, 266, 51–61. [Google Scholar] [CrossRef]
- Santiago, R.; Lemus, J.; Outomuro, A.X.; Bedia, J.; Palomar, J. Assessment of ionic liquids as H2S physical absorbents by thermodynamic and kinetic analysis based on process simulation. Sep. Purif. Technol. 2019, 233, 116050. [Google Scholar] [CrossRef]
- Bavoh, C.; Lal, B.; Nashed, O.; Khan, M.S.; Keong, L.K.; Bustam, M.A. COSMO-RS: An ionic liquid prescreening tool for gas hydrate mitigation. Chin. J. Chem. Eng. 2016, 24, 1619–1624. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Wei, F. Synthesis and Properties of Ultralong Carbon Nanotubes. In Nanotube Superfiber Materials; Elsevier: Amsterdam, The Netherlands, 2013; Chapter 4; pp. 87–136. [Google Scholar] [CrossRef]
- Rahmati-Rostami, M.; Ghotbi, C.; Hosseini-Jenab, M.; Ahmadi, A.N.; Jalili, A. Solubility of H2S in ionic liquids [hmim][PF6], [hmim][BF4], and [hmim][Tf2N]. J. Chem. Thermodyn. 2009, 41, 1052–1055. [Google Scholar] [CrossRef]
- Sakhaeinia, H.; Jalili, A.H.; Taghikhani, V.; Safekordi, A.A. Solubility of H2S in Ionic Liquids 1-Ethyl-3-methylimidazolium Hexafluorophosphate ([emim][PF6]) and 1-Ethyl-3-methylimidazolium Bis(trifluoromethyl)sulfonylimide ([emim][Tf2N]). J. Chem. Eng. Data 2010, 55, 5839–5845. [Google Scholar] [CrossRef]
- Safavi, M.; Ghotbi, C.; Taghikhani, V.; Jalili, A.H.; Mehdizadeh, A. Study of the solubility of CO2, H2S and their mixture in the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate: Experimental and modelling. J. Chem. Thermodyn. 2013, 65, 220–232. [Google Scholar] [CrossRef]
- Jalili, A.H.; Shokouhi, M.; Maurer, G.; Zoghi, A.T.; Ahari, J.S.; Forsat, K. Measuring and modelling the absorption and volumetric properties of CO2 and H2S in the ionic liquid 1-ethyl-3-methylimidazolium tetrafluoroborate. J. Chem. Thermodyn. 2018, 131, 544–556. [Google Scholar] [CrossRef]
- Zhao, Z.; Huang, Y.; Zhang, Z.; Fei, W.; Luo, M.; Zhao, Y. Experimental and simulation study of CO2 and H2S solubility in propylene carbonate, imidazolium-based ionic liquids and their mixtures. J. Chem. Thermodyn. 2019, 142, 106017. [Google Scholar] [CrossRef]
- Guo, S.; Song, B.; Ghalambor, A.; Lin, T.R. Chapter 15—Flow Assurance; Gulf Professional Publishing: Boston, MA, USA, 2014; pp. 179–231. [Google Scholar]
- Key, J.A.; Ball, D.W. Introductory Chemistry—1st Canadian Edition; BCcampus: Victoria, BC, Canada, 2014; pp. 195–200. Available online: http://uilis.unsyiah.ac.id/oer/files/original/dcded6f2f4b25f5b280bd6494723b8b3.pdf (accessed on 25 June 2021).
- Huang, K.; Zhang, X.-M.; Zhou, L.-S.; Tao, D.-J.; Fan, J.-P. Highly efficient and selective absorption of H2S in phenolic ionic liquids: A cooperative result of anionic strong basicity and cationic hydrogen-bond donation. Chem. Eng. Sci. 2017, 173, 253–263. [Google Scholar] [CrossRef]
- Huang, Y.T.; Zhang, K.; Hu, X.M.; Wu, X.B. Hydrophobic Protic Ionic Liquids Tethered with Tertiary Amine Group for Highly Efficient and Selective Absorption of H2S from CO2. AIChE J. 2016, 62, 4480–4490. [Google Scholar] [CrossRef]
- Huang, K.; Cai, D.N.; Chen, Y.L.; Wu, Y.T.; Hu, X.B.; Zhang, Z.B. Thermodynamic Validation of 1-Alkyl-3-methylimidazolium Carboxylates as Task-Specific Ionic Liquids for H2S Absorption. AIChE J. 2012, 59, 2227–2235. [Google Scholar] [CrossRef]
- Motlagh, S.R.; Harun, R.; Biak, D.R.A.; Hussain, S.A.; Ghani, W.A.W.A.K.; Khezri, R.; Wilfred, C.D.; Elgharbawy, A.A.M. Screening of Suitable Ionic Liquids as Green Solvents for Extraction of Eicosapentaenoic Acid (EPA) from Microalgae Biomass Using COSMO-RS Model. Molecules 2019, 24, 713. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).