Abstract
Mechanochemistry is a method that can cover the energy demand of reaction pathways between solid materials. This requires enough energy to maintain the reactions between the starting materials. This is called “high-energy milling”. In our case, a planetary ball mill provided the required energy. Using the Burgio-equation, the required energy is determinable; the energy released during a single impact of a milling ball (Eb), as well as during the whole milling process (Ecum). The aim of this work was the one-step production of BaTiO3 from BaO and TiO2 starting materials. Whereas during mechanochemical reactions it is possible to produce nanoparticles of up to 10 nm, the essence of this study is to develop the preparation of BaTiO3 with a perovskite structure even without subsequent heat treatment, since sintering at high temperatures is associated with a rapid increase in the size of the particles. By describing the synthesis parameters and their energy values (Eb and Ecum), it is possible to transpose experimental conditions, so that in the case of other types of planetary ball mills or grinding vessel made of other materials, the results can be used. In this study, the mechanical treatment was carried out with a Fritsch Pulverisette-6 planetary ball mill and the transformation of the starting materials was investigated by X-ray diffractometric, Raman and Energy-dispersive X-ray spectroscopic, and transmission electron microscopic measurements.
1. Introduction
Ceramics are produced and used in huge quantities all over the world due to the wide variety of probable applications. In total, there are only a few minerals with applications and technological uses that dominate the industrial application (e.g., quartz, calcium silicates, alumina, and titanium dioxide). These materials are characterized by both the crystal structure and the composition and are important due to their specific properties and applications. The question arose whether there is a structure that is multifunctional and crystallographically suitable for the development of useful properties. Considering the three-component crystal structures, there are only a dozen ceramics that are widely used. Among them, the A2BX4 spinel and ABX3 perovskite excel and perovskite is the only structure, the chemical modification of which results in an extremely wide range of phases with completely different properties [1]. Due to its unique electrical properties, the family of chemical compounds with perovskite-structure permits a wide range of electrotechnical applications: semiconductor dielectrics, superionic conductors, combined with ionic and electron conductivity for high-temperature superconductors [2].
The possibility of fine grinding, mechanical activations and chemical reactions that can be carried out in planetary ball mills has long been known. However, several factors affect the success of each milling, since the energy generated during milling must be in balance with the properties of the desired product, therefore predetermined, optimal parameters are very important. It is necessary to consider the material quality of the grinding vessel and balls; the rotational speed; the time of treatment; the applied atmosphere and temperature [3], the number of balls and the filling ratio of the balls and reactants; and the physical and chemical properties of the reactants [4]. These parameters are also very interdependent and play an important role in the development of optimal milling energy, thus achieving the best product yield in the shortest reaction time [5]. Obviously, higher milling energy can be achieved by increasing the rotational speed or using a grinding vessel of higher hardness. In our case, grinding vessels made of three different materials (silicon nitride, stainless steel, tungsten carbide) were used, although there are studies in which BaTiO3 was produced in zirconia grinding vessels [6,7]. The intensity of grinding increases the particle size of crystalline materials, or when powders are ground, compounds of different compositions can be formed with a temperature change. However, it should be noted that too much energy may cause the onset of secondary reactions, such as product degradation or transformation.
In the literature, many examples can be found in the production of barium titanate from barium-oxide [8] or barium-peroxide [9] and titanium-dioxide precursors. In many of these cases, mechanical activation is used [10]. It is important to note that this does not mean the transformation of the starting materials during grinding, but is only used for the thorough mixing of precursors, while the perovskite-structured barium titanate is formed during the subsequent heat treatment [8,9,10]. High-energy milling may be suitable for creating the conditions for the temperature required for subsequent calcination. Research of this kind began as early as the 1960s when Bowden and Yoffe introduced the “Hot-spot” theory [11]. Later, Weicher and Schiner experimentally demonstrated that the area carrying extra energy is about 1 mm2 with a temperature of 1000–1500 K, which keeps this state for about 10−4–10−3 s [12]. By providing this extra energy, it is also possible to develop BaTiO3 mechanochemically. It should be noted that, in addition to the mechanochemical process, BaTiO3 perovskite can also be produced in several other ways, including a wet-chemical [13], hydrothermal [14], or microwave-assisted hydrothermal reaction [15]. In contrast, the mechanochemical process has the advantage that it does not require an expensive solvent, it can be produced in one step, and, as we present later, there is no need for subsequent heat treatment [16]. The advantage of this is that, it avoids the increase in the size of the particles at high temperatures and avoids the undesirable transformation of the product, which in many cases can happen even during mechanical treatment [17].
In this study, we followed the formation of perovskite-structured barium titanate from BaO and TiO2 precursors with X-ray diffractometric (XRD) and Raman spectroscopic measurements. To do this, we used three grinding drums of different hardness and methodically changed the number of grinding balls, the rotational speed, and the time of grinding. Using the Energy-dispersive X-ray spectroscopic (EDS) technique, we measured the barium-titanium ratio of the powder mixture. Since BaO is water-soluble (~3.6 g/100 mL at 20 °C) as opposed to BaTiO3 and TiO2, which are practically insoluble in water, after washing with distilled water the ratio of the two metals must change.
2. Materials and Methods
2.1. The Ball-Milling Experiments
The grinding was carried out in a Fritsch Pulverisette-6 planetary ball mill. Such mills are suitable for high-energy milling, which allows them to provide the energy needed to form BaTiO3. In each case, grinding balls with a diameter of 10 mm were used in the three grinding vessels (80 mL) of the same material. To produce BaTiO3 2.00 g BaO (99.99%) and 1.04 g TiO2 (≥99%) were measured in all cases. The energy released during the millings has been predetermined. This was made possible by the energy model introduced by Burgio et al. [18]. The applicability of the model to our system has already been proven by our research team experimentally [19]. The Equation (1) can be used to determine two energy values: the Eb (1), which represents the total energy available during an impact event of a milling ball, and Ecum (2), which means the energy transferred to 1 g of the powder during the whole milling:
where K is the geometric constant of the mill, φb is the obstruction factor, is the density of the milling balls, db is the diameter of the balls, dv is the diameter of the grinding vessel, ωp and ωv is the rotational speed of the disc and the vessel and rp is the distance between the rotational axes of the disc and the vessel [19].
where f is the frequency of impacts, t is the milling time and mp is the mass of the measured sample. Thus, it is possible to produce our product in similar energy conditions using different parameters. This answers our question as to whether the perovskite structure can be formed in all three grinding vessels. In the experiments, we changed the rotational speed from 300 to 500 rpm, the number of grinding balls from 10 to 25. In all cases, the grinding took 3 h. For XRD measurements, samples were taken hourly, while for Raman and EDS measurements 3-h samples were used.
2.2. Characterization
The powder X-ray diffraction patterns were obtained using a Rigaku Miniflex II XRD (Rigaku Corporation, Tokyo, Japan) instrument operating with Cu Kα radiation (λ = 1.5406 Å). The 2Θ Bragg angles were scanned over a range of 5–90° at a rate of 1.0° min−1. Transmission Electron Microscope (TEM) analysis was performed with a FEI Tecnai G2 20 X-TWIN instrument with a point resolution of 0.26 nm. Samples were placed on holey carbon-coated copper grids of 300 mesh. Raman characterization was performed at the excitation wavelength of 532 nm, and a nominal laser power of 12.5 mW (Senterra Bruker Optik GmbH, Ettlingen, Germany). The spectral resolution was set to ca. 3–5 cm−1, and the interferometer resolution was 1.5 cm−1. The elemental composition of the prepared samples was characterized by energy-dispersive X-ray spectroscopy (Hitachi Co., Tokyo, Japan) operating at 20 kV, equipped with a Röntec energy dispersive spectrometer with a 12 mm working distance. Transmission Electron Microscope (TEM) analysis was performed by an FEI-Tecnai G2/20/X-TWIN (FEI Company, Hillsboro, OR, USA) instrument with a point resolution of 0.26 nm. Samples were placed on holey carbon-coated copper grids of 300 mesh.
3. Results
9 different settings were determined based on the energy model. These values are shown in the table below.
It can be seen that higher density grinding vessels and rotational speed increase the value of the Eb, although there are smaller overlaps, such as SiN500/25 and stainless steel SN300/25 settings.
In the case of Ecum values, this increase is not so gradual, since the number of grinding balls used increases the frequency of impacts. Therefore, a higher Ecum may be obtained for the lower density Si3N4 vessel, where 25 grinding balls have been inserted than for the FeNiCr vessel with a density of 2.3 times higher, where this value is lower due to slower rotation and fewer grinding balls.
Raman spectroscopic measurements were performed on all 9 samples at excitation wavelengths of 532 nm (Figure 1). On the spectra, the characteristic peaks of TiO2 and BaTiO3 are clearly observed. In the case of TiO2, characteristic peaks of the anatase structure were observed [20]. TiO2 Raman-active lattice vibrations assigned as follows: (A1) 516 cm−1 + (B1) 636 cm−1 + (B1) 395 cm−1 + (E) 635 cm−1 + (E) 144 cm−1 + (E) 198 cm−1 the weak band at 796 cm−1 was assigned as the first overtone of the B1 mode [21]. The Raman spectrum of BaTiO3 crystals assigned as follows: (A1) 186 cm−1 + (A1) 265 cm−1 + (B1) 303 cm−1 and an asymmetric broadband (A1/E) 520 cm−1 and a broad weak peek at (A1/E) 720 cm−1 [22]. Of the weak bands of BaO, only the most intense peak signal was detected at (A1) 193 cm−1 [23,24]. Due to the overlap between the starting materials and the vibrations of the product, the progress of conversion cannot be clearly stated only based on the most intense peak of BaTiO3 [25]. Therefore, it is possible to deduce from the decrease in the intensity of the individual vibrations of TiO2 the re-evaluation of the precursors [26].
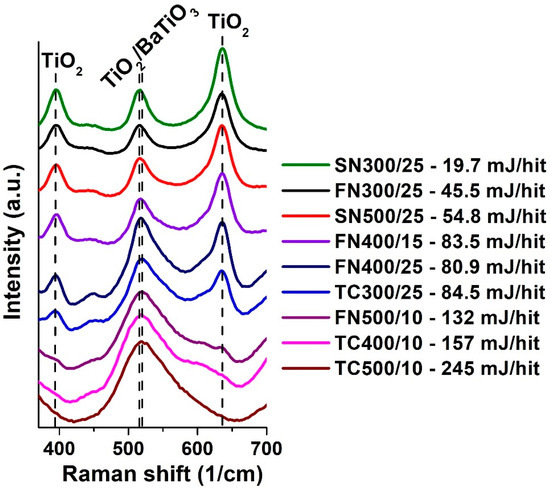
Figure 1.
Raman spectra of all samples treated at different ball-impact energy (Eb).
Figure 1 shows the Raman spectra of all samples between 370–700 1/cm. In this period there are three intensive peaks specific to TiO2 and one peak typical of BaTiO3. The peaks of 516 and 520 1/cm largely overlap with each other [26]. Nevertheless, by increasing the Eb, the change in intensity at the peaks of TiO2 395 and 636 1/cm can be traced, which gradually disappears towards with the increasing energies. In the case of samples treated at the highest Eb (FN500/10; TC400/10; TC500/10), these peaks are barely detectable, while the peak typical of BaTiO3 in this range dominates at 456 1/cm.
The rate of transformation of starting materials is well suited to the rate of the growing Eb. The only exceptions were the FN400/15 and FN400/25 samples, where even though in the latter case the Eb was 3.11% less, the rate of transformation was slightly higher. This phenomenon can be explained by an increase in the number of grinding balls and, with it, by a higher value of Ecum, which was 61.5% higher in case the of the sample FN400/25 (Table 1). This is supported by previous experience: the reaction rate is basically determined by the input energy, but the frequency of impacts also counts, especially if the energy transferred may be accumulating to some extent [19].

Table 1.
Values of Ecum and Eb for each grinding vessel. ωp: rotational speed of the grinding vessel. Nb: number of grinding balls.
In addition to Raman spectroscopic measurements, the formation of BaTiO3 has been measured by XRD in this case of different grinding vessels (Figure 2). The typical reflections of BaTiO3 between 2 theta 20–80° are listed below: 2 theta 22.04° (100); 31.44° (110); 38.76° (111); 45.08° (200); 50.72° (210); 56.02° (211); 65.68° (220); 70.20° (300); 74.66° (310); 78.98° (311) [27]. BaTiO3 produced at a sintering temperature above 1000 °C, is typically tetragonal, which is changed in hexagonal structure at 1400 °C [28]. The evolution of the starting materials and the reflections characteristic of BaTiO3 can be clearly tracked by increasing the milling energy [26]. With mechanochemical treatment, the energy required for the formation of the tetragonal structure was provided during the 3-h milling process, which was detected first in the SN500/25 sample. The Eb limit for the transformation of the precursors is therefore above 50 mJ/hit. Below this value, the perovskite structure does not form despite further 6 h of grinding. This is additional information compared to Raman results, as those measurements did not clearly show the beginning of the conversion due to the overlap of the peaks of the precursors and the product.
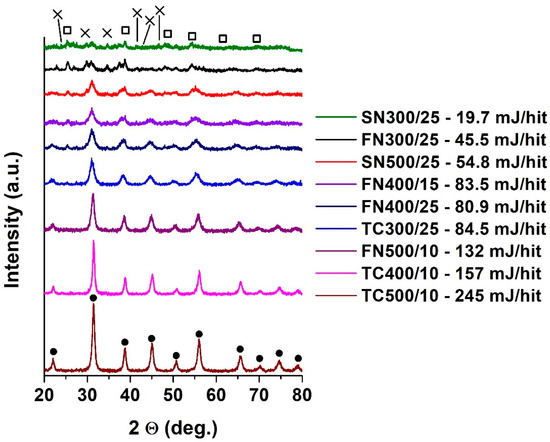
Figure 2.
XRD diffractograms of all samples treated at different ball-impact energy (Eb). ●: BaTiO3, □: TiO2, ×: BaO reflections.
As in the previous results, the exchange of FN400/25 and FN400/15 samples (by the value of Eb) based on intensities was observed in XRD measurements. This supports the hypothesis that, in addition to the appropriate Eb, sufficient treatment time (Ecum) should be ensured.
For better visualization of the XRD results, diffractograms were used to track the formation of BaTiO3 product at the intensity of most typical peaks at 2θ 31.4°, i.e., based on the fact that the intensity of this peak shows the increasing appearance of the product in the grinding vessel (Figure 3). As a result, a so-called “milling-map” was created [29]. A good correlation between the performed Eb and the reflection intensity is observed. The threshold for ball-impact energy required to produce the BaTiO3 is well-drawn, which is around 0.05 J/hit. In mechanochemistry, this is a typical limit, i.e., below Eb = 0.05 J/hit low-energy, while above it is high-energy milling [29,30]. The latter arises from the limiting factor of typical single-axis mills that, when using such a mill at too high a rotational speed, the balls already move together with the walls of the grinding vessel above a certain value. In a planetary ball mill, the complex rotation determined by the two axes, up to a very high rotational speed, does not prevent the grinding balls detach and impact into the walls of the grinding vessel [31]. Low-intensity reflections will only be replaced in samples ground with the highest impact energy with signals higher than 400 cps, which clearly show the presence of tetragonal BaTiO3.
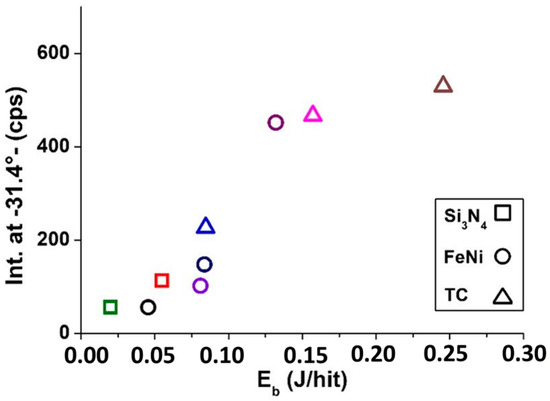
Figure 3.
XRD measurement-based (BaTiO3 peak intensity at 2θ 31.4°,) milling-map of BaTiO3 samples in different grinding vessels.
Based on the Raman and XRD results, the minimum Eb required for the formation of the perovskite structure can be determined (~0.05 J/hit). However, to be able to draw on the relationship between the Eb and Ecum and the amount of BaTiO3 converted, a quantification was also necessary, thus, the energy-dispersive X-ray spectrum of all 9 samples has been measured (Figure 4). Since the water solubility of BaO is ~36 g/L at 20 °C, assuming that the weighed 2.0 g is not converted to BaTiO3 at all, it can dissolve in a minimum of ~57 mL of water. In comparison, to prove that all Ba present in the form of BaO has been removed, all samples have been washed with 2 L of deionized water. High-intensity characteristic peaks corresponding to Ba (La—4.47; Bb1—4.83; Bb2—5.17 keV) and Ti (Ka—4.50; Kb—4,91 keV) elements were noticed in the EDS patterns of the nanoparticles [32]. The percentage of Ba and Ti relative to each other was plotted from the resulting values.
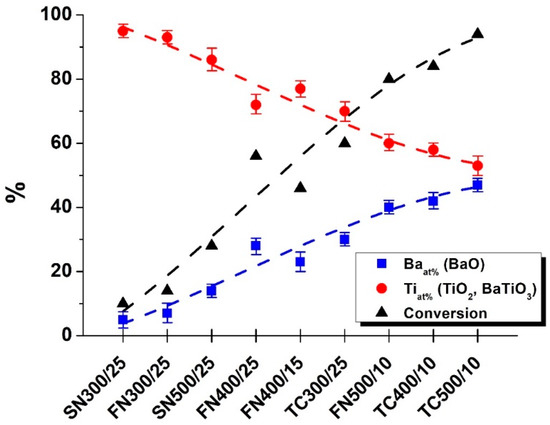
Figure 4.
The atomic percentage (at%) of Ba (■) and Ti (●) based on EDS measurements, and the calculated conversions (▲) of the precursors. Dashed lines in the figure are guides for the eye.
Figure 4 shows the changes in the percentage distribution of the two elements examined and the calculated conversions. As the reaction progresses, the barium content of the BaO precursor is gradually integrated into the water-insoluble BaTiO3, so that its amount will increase continuously in the sample that remains after distilled water wash. The absolute amount of titanium should not change, as it is still in water-insoluble form as a precursor and after being incorporated into BaTiO3, but its relative amount will decrease in relation to barium. If all starting materials were to be converted to BaTiO3, the Ba-Ti ratios would have to be 50–50%. In the sample milled with the highest Eb and Ecum (TC500/10), the conversion value was 94%. Further conversion trends can be seeded from the path of the pasted curve, as it gradually saturates. The 94% value could be increased by extending the milling time, but the 100% cannot be achieved due to the trapping of the precursors (on the wall of the grinding vessel, in particular at the junction of the lid and the vessel wall) [33].
Figure 5 summarizes conversion data calculated from EDS measurements, Eb and Ecum values as a function of each sample. The Eb values of the samples (FN400/25, FN400/15, TC300/25) within the area framed by the dotted line are almost the same, the differences are below 5%. In Ecum, however, there are significant differences due to the number of grinding balls and the rotational speed. This discrepancy can be tracked in the conversion of precursors, which follows the Ecum values. It can be concluded that the Eb necessary for the formation of BaTiO3 perovskite is available, but most of it is still lost during grinding (in the form of heat or friction). For this reason, the corrective effect of Ecum is necessary, which counteracts this relatively low Eb. For this reason, the conversion of starting materials follows the course of Ecum.
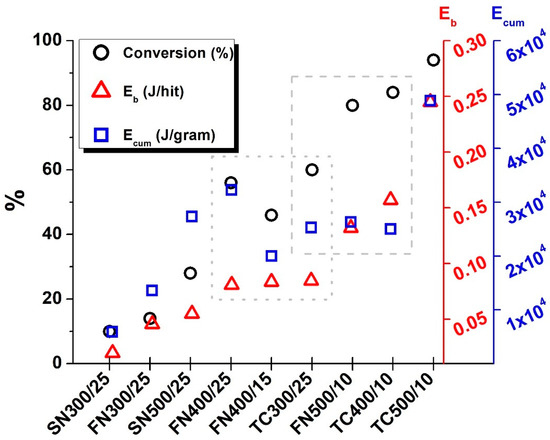
Figure 5.
The conversion of the precursors (○), the ball-impact (Eb, ∆) energy and the cumulative energy (Ecum, □) in case of all grinding sets. The areas with the dotted and dashed lines show the samples compared in the text.
In contrast, in the case of samples (TC300/25, FN500/10, TC400/10) framed by a dashed line (Figure 5), the values of Ecum are close to each other, with the largest difference being less than 5%. The Eb values are as follows: 0.0845; 0.132; 0.157 J/hit. The difference relative to the maximum value (TC400/10) is 46.2% for TC300/25 and 16.0% for FN500/10 samples. But the difference in the conversion of the starting materials is 28.6% for the TC300/25 and only 4.8% for the FN500/10 sample. From this, it can be concluded that, under the circumstances, further increases in the Eb will no longer bring such a significant increase in the transformation of the precursors. This is confirmed by the fact that, in the case of the TC500/10 compared to the TC400/10 sample, an increase in Eb by 56.1% and 95.3% in Ecum only causes an increase in conversion by 12%. Based on these results, the two values can be determined from the point of view of both Eb and the Ecum, between which the perovskite structured BaTiO3 is formed, and the speed of production can be influenced without compromising the quality of the product.
Figure 6 shows the TEM images of end-product in the case of SN300/25, FN300/25, and TC300/25 samples. By increasing the density of the grinding vessels, the morphology of the particles becomes sharper. While only a mixture of starting materials can be seen in the Si3N4 grinding vessel (SN300/25), as confirmed by the XRD results, individual particles can be distinguished in the samples made in the FeNi (FN300/25) and TC (TC300/25) grinding vessel. By the TEM images, size distribution histograms were made in the case of FN300/25 and TC300/25 samples. Due to the sufficient Eb, particle growth is inhibited during the formation of the BaTiO3 structure. It follows that, the size of the particles falls within the nano range. The conspicuous difference between the material produced in the two grinding vessels is that the increase in particle size was measurable in the TC vessel, which provides more impact energy (Eb). This phenomenon may have been caused by excessive Ecum, which thus led to the sintering of particles. This process is orders of magnitude greater in the case of subsequent heat treatment, while in this case, it allows the final size distribution of the product to be regulated. Overall, the average diameter of the BaTiO3 perovskite particles is 13.2 nm (FN300/25) and 19.2 nm (TC300/25).
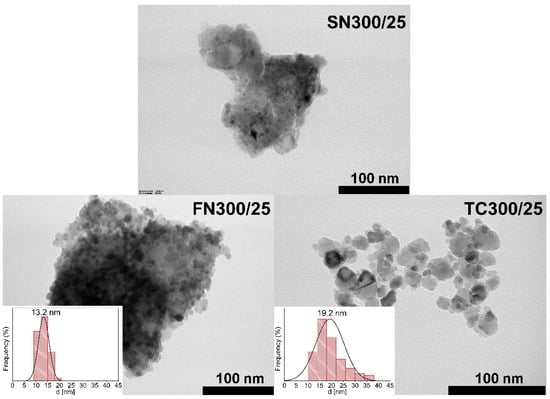
Figure 6.
TEM images of BaTiO3 perovskites synthesized in the Si3N4 (SN300/25), FeNi (FN300/25) and TC (TC300/25) grinding vessels.
4. Conclusions
The formation of BaTiO3 from BaO and TiO2 was studied in grinding vessels made of different materials, at different rotational speeds and a different number of grinding balls. Based on XRD and Raman measurements, the lowest ball-impact energy (Eb) with which perovskite-structured BaTiO3 can be formed under the studied experimental conditions has been determined (Eb = 50 mJ/hit). Above this value, within a wide range, the transformation of starting materials is almost continuously increasing. It has been shown that to produce the BaTiO3 perovskite in sufficient quantities, the synchronization of Eb and cumulative energy (Ecum) is essential. Based on the results obtained, the highest Eb value can be determined, which can be used to speed up the formation of the product (Eb = 160 mJ/hit). Above this value, the additional energy no longer contributes to increasing the rate of reaction. This excess energy often causes the product transformation, crystallization, or growth of the individual particles through the process of sintering. In this case, by accurately defining the grinding conditions, the parameters necessary for the transformation have been successfully determined without further transformation of the product. Choosing the necessary Eb and Ecum allows for the formation of the BaTiO3 structure, and in addition, increasing these energies, also allows to control of the final size. In addition to the controllability of mechanochemical perovskite-synthesis, the results support the goodness of the model for calculating Eb and Ecum. This allows the results of the experiments to be quantified, thereby converting the grinding parameters between grinding vessels of different materials or different types of planetary ball mills.
Author Contributions
G.K.: Conceptualization, Methodology, Formal analysis, Visualization, Validation, Writing—original draft. K.L.: measurements, laboratory work. C.D.: measurements, formal analysis. A.R.: Writing—review & editing. Á.K.: Supervision, Funding acquisition. Z.K.: Supervision, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the János Bolyai Research Fellowship of the Hungarian Academy of Sciences (BO/00835/19/7 for G.K. and BO/00384/21/7 for A.R.), and by the professional support of the New National Excellence Program of the Ministry of Innovation and Technology ÚNKP-21-5-SZTE-547 for G.K. and ÚNKP-21-5-SZTE-876 for A.R. Project No. TKP2021-NVA-19 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NVA funding scheme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Vijatovic, M.; Bobic, J.; Stojanovic, B. History and challenges of barium titanate: Part I. Sci. Sinter. 2008, 40, 155–165. [Google Scholar] [CrossRef]
- Jiang, B.; Iocozzia, J.; Zhao, L.; Zhang, H.; Harn, Y.-W.; Chen, Y.; Lin, Z. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties. Chem. Soc. Rev. 2019, 48, 1194–1228. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Brekalo, I.; Kaitner, B. Effect of atmosphere on solid-state amine–aldehyde condensations: Gas-phase catalysts for solid-state transformations. Chem. Commun. 2012, 48, 11683–11685. [Google Scholar] [CrossRef]
- Stolar, T.; Užarević, K. Mechanochemistry: An efficient and versatile toolbox for synthesis, transformation, and functionalization of porous metal–organic frameworks. CrystEngComm 2020, 22, 4511–4525. [Google Scholar] [CrossRef]
- Tan, D.; Loots, L.; Friščić, T. Towards medicinal mechanochemistry: Evolution of milling from pharmaceutical solid form screening to the synthesis of active pharmaceutical ingredients (APIs). Chem. Commun. 2016, 52, 7760–7781. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, B.D.; Jovalekić, C.; Vukotic, V.; Simoes, A.Z.; Varela, J.A. Ferroelectric Properties of Mechanically Synthesized Nanosized Barium Titanate. Ferroelectrics 2005, 319, 65–73. [Google Scholar] [CrossRef]
- Stojanovic, B.; Simoes, A.; Paiva-Santos, C.; Jovalekic, C.; Mitic, V.; Varela, J. Mechanochemical synthesis of barium titanate. J. Eur. Ceram. Soc. 2005, 25, 1985–1989. [Google Scholar] [CrossRef]
- Garbarz-Glos, B.; Bak, W.; Budziak, A.; Dulian, P.; Lisinka-Czekaj, A.; Czekaj, D. The application of the mechanochemical synthesis for the preparation of advanced ceramics based on barium titanate. Arch. Metall. Mater. 2020, 65, 1391–1396. [Google Scholar]
- van Hal, H.; Groen, W.; Maassen, S.; Keur, W. Mechanochemical synthesis of BaTiO3, Bi0.5Na0.5TiO3 and Ba2NaNb5O15 dielectric ceramics. J. Eur. Ceram. Soc. 2001, 21, 1689–1692. [Google Scholar] [CrossRef]
- Aydin, Z.; Turgut, S.; Akbas, H.Z. Structural Differences of BaTiO3 Ceramics Modified by Ultrasonic and Mechanochemical Methods. Sov. Powder Met. Met. Ceram. 2018, 57, 490–497. [Google Scholar] [CrossRef]
- Jost, W. Fast Reactions in Solids, von FP Bowden und AD Yoffe; Butterworths Scientific Publications: London, UK, 1958; Volume 71, p. 752. [Google Scholar]
- Weichert, R.; Schönert, K. On the temperature rise at the tip of a fast running crack. J. Mech. Phys. Solids 1974, 22, 127–133. [Google Scholar] [CrossRef]
- Gomes, M.A.; Lima, A.; Eguiluz, K.; Salazar-Banda, G.R. Wet chemical synthesis of rare earth-doped barium titanate nanoparticles. J. Mater. Sci. 2016, 51, 4709–4727. [Google Scholar] [CrossRef]
- Seo, K.W.; Kong, H.G. Hydrothermal preparation of BaTiO3 thin films. Korean J. Chem. Eng. 2000, 17, 428–432. [Google Scholar] [CrossRef]
- Sun, W.; Li, C.; Li, J.; Liu, W. Microwave-hydrothermal synthesis of tetragonal BaTiO3 under various conditions. Mater. Chem. Phys. 2006, 97, 481–487. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Naviglio, S.; Barrino, F. Antibacterial Properties of Sol–Gel Biomaterials with Different Percentages of PEG or PCL. Macromol. Symp. 2020, 389, 1900056. [Google Scholar] [CrossRef]
- Šagud, I.; Zanolla, D.; Perissutti, B.; Passerini, N.; Škorić, I. Identification of degradation products of praziquantel during the mechanochemical activation. J. Pharm. Biomed. Anal. 2018, 159, 291–295. [Google Scholar] [CrossRef]
- Burgio, N.; Iasonna, A.; Magini, M.; Martelli, S.; Padella, F. Mechanical Alloying of the Fe-Zr System—Correlation between Input Energy and End-Products. Il Nuovo Cimento D 1991, 13, 459–476. [Google Scholar] [CrossRef]
- Kozma, G.; Puskás, R.; Papp, I.; Bélteky, P.; Kónya, Z.; Kukovecz, Á. Experimental validation of the Burgio–Rojac model of planetary ball milling by the length control of multiwall carbon nanotubes. Carbon 2016, 105, 615–621. [Google Scholar] [CrossRef]
- Andonova, S.M.; Senturk, G.S.; Kayhan, E.; Ozensoy, E. Nature of the Ti-Ba Interactions on the BaO/TiO2/Al2O3 NOx Storage System. J. Phys. Chem. C 2009, 113, 11014–11026. [Google Scholar] [CrossRef]
- Zhang, W.F.; He, Y.L.; Zhang, M.S.; Yin, Z.; Chen, Q. Raman scattering study on anatase TiO2 nanocrystals. J. Phys. D-Appl. Phys. 2000, 33, 912–916. [Google Scholar] [CrossRef]
- Venkateswaran, U.D.; Naik, V.M.; Naik, R. High-pressure Raman studies of polycrystalline BaTiO3. Phys. Rev. B 1998, 58, 14256–14260. [Google Scholar] [CrossRef]
- Shiratori, Y.; Pithan, C.; Dornseiffer, J.; Waser, R. Raman scattering studies on nanocrystalline BaTiO3 Part I—Isolated particles and aggregates. J. Raman Spectrosc. 2007, 38, 1288–1299. [Google Scholar] [CrossRef]
- Hayashi, H.; Nakamura, T.; Ebina, T. In-situ Raman spectroscopy of BaTiO3 particles for tetragonal–cubic transformation. J. Phys. Chem. Solids 2013, 74, 957–962. [Google Scholar] [CrossRef]
- Lazarevic, Z.; Romčević, N.; Vijatović, M.; Paunović, N.; Romčević, M.; Stojanović, B.; Dohčević-Mitrović, Z. Characterization of Barium Titanate Ceramic Powders by Raman Spectroscopy. Acta Phys. Pol. A 2009, 115, 808–810. [Google Scholar] [CrossRef]
- Sydorchuk, V.; Zazhigalov, V.A.; Khalameida, S.; Wieczorek-Ciurowa, K. Mechanochemical synthesis of BaTiO3 using different forms of TiO2. Inorg. Mater. 2010, 46, 1126–1130. [Google Scholar] [CrossRef]
- Siddheswaran, R.; Šutta, P.; Novák, P.; Netrvalová, M.; Hendrych, A.; Životský, O. In-situ X-ray diffraction studies and magneto-optic Kerr effect on RF sputtered thin films of BaTiO3 and Co, Nb co-doped BaTiO3. Ceram. Int. 2016, 42, 3882–3887. [Google Scholar] [CrossRef]
- Sun, Q.; Gu, Q.; Zhu, K.; Jin, R.; Liu, J.; Wang, J.; Qiu, J. Crystalline Structure, Defect Chemistry and Room Temperature Colossal Permittivity of Nd-doped Barium Titanate. Sci. Rep. 2017, 7, 42274. [Google Scholar] [CrossRef] [Green Version]
- Rojac, T.; Kosec, M.; Malič, B.; Holc, J. The application of a milling map in the mechanochemical synthesis of ceramic oxides. J. Eur. Ceram. Soc. 2006, 26, 3711–3716. [Google Scholar] [CrossRef]
- Magini, M.; Iasonna, A.; Padella, F. Ball milling: An experimental support to the energy transfer evaluated by the collision model. Scr. Mater. 1996, 34, 13–19. [Google Scholar] [CrossRef]
- Abdellaoui, M.; Gaffet, E. The physics of mechanical alloying in a planetary ball mill: Mathematical treatment. Acta Met. Mater. 1995, 43, 1087–1098. [Google Scholar] [CrossRef]
- Raja, S.; Bheeman, D.; Rajamani, R.; Pattiyappan, S.; Sugamaran, S.; Bellan, C.S. Synthesis, Characterization and Remedial Aspect of BaTiO3 Nanoparticles Against Bacteria. Nanomed. Nanobiol. 2015, 2, 16–20. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Breitung-Faes, S.; Kwade, A. Experimental investigations and modelling of the ball motion in planetary ball mills. Powder Technol. 2011, 212, 224–230. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).