A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites
Abstract
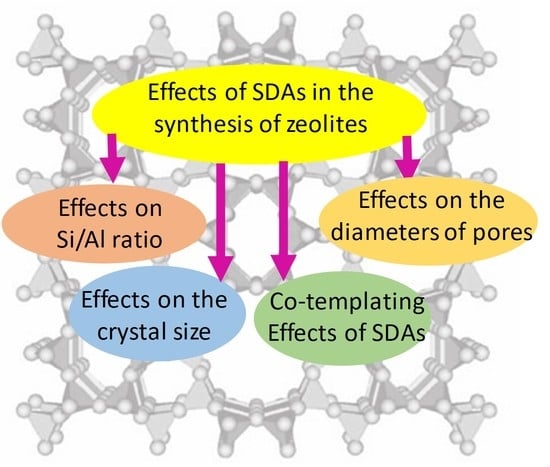
:1. Introduction
2. Effects of SDAs on the Chemical Composition of Zeolites (or Si/Al Ratio)
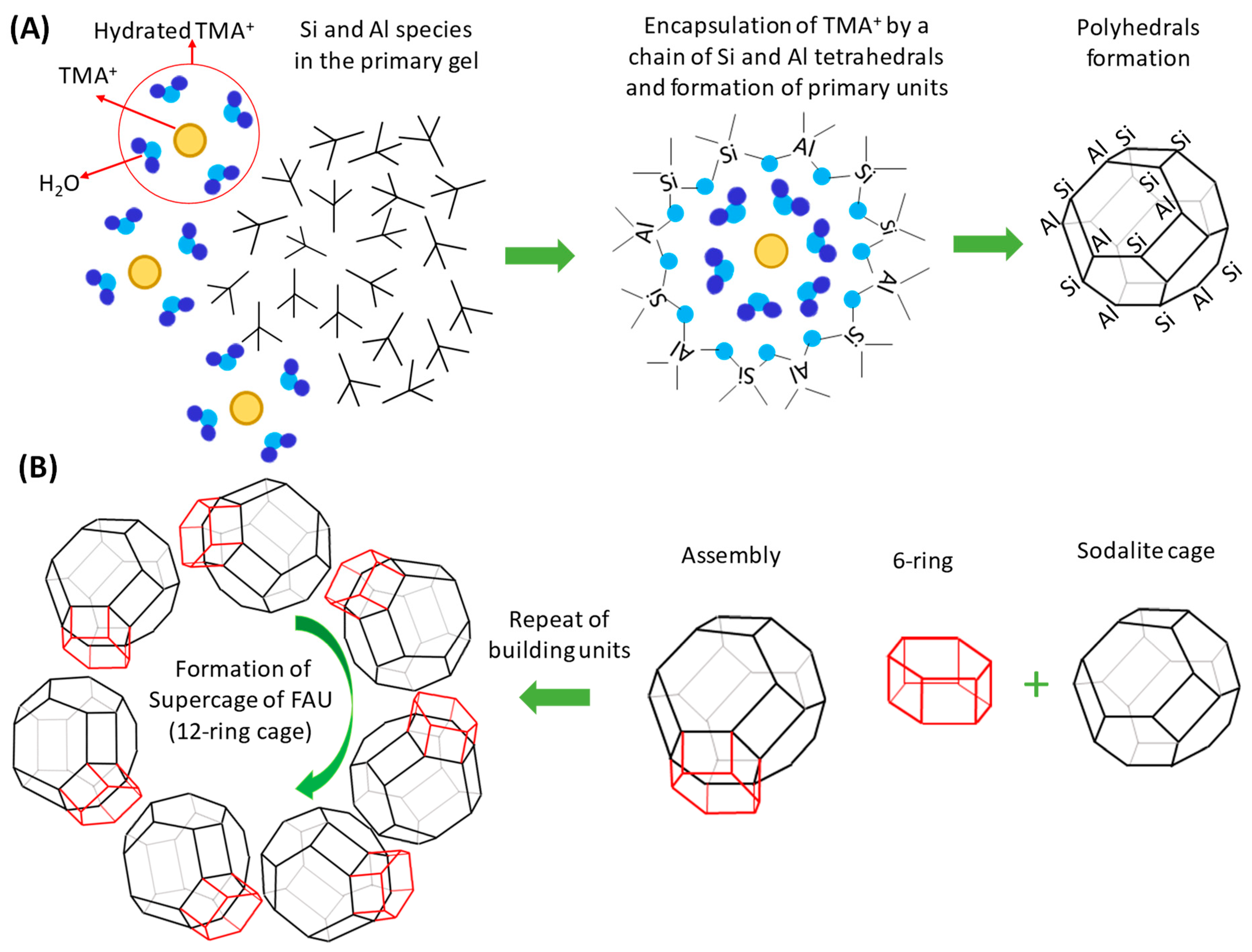
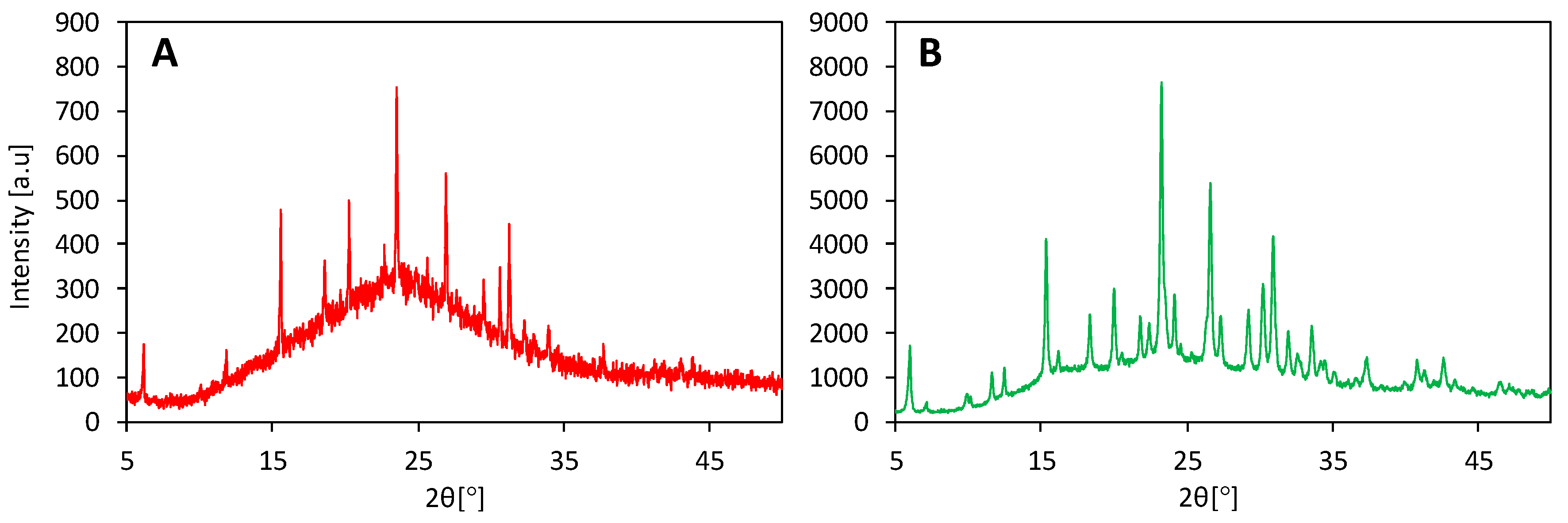
3. Effects of SDAs on the Zeolite Crystallization Process
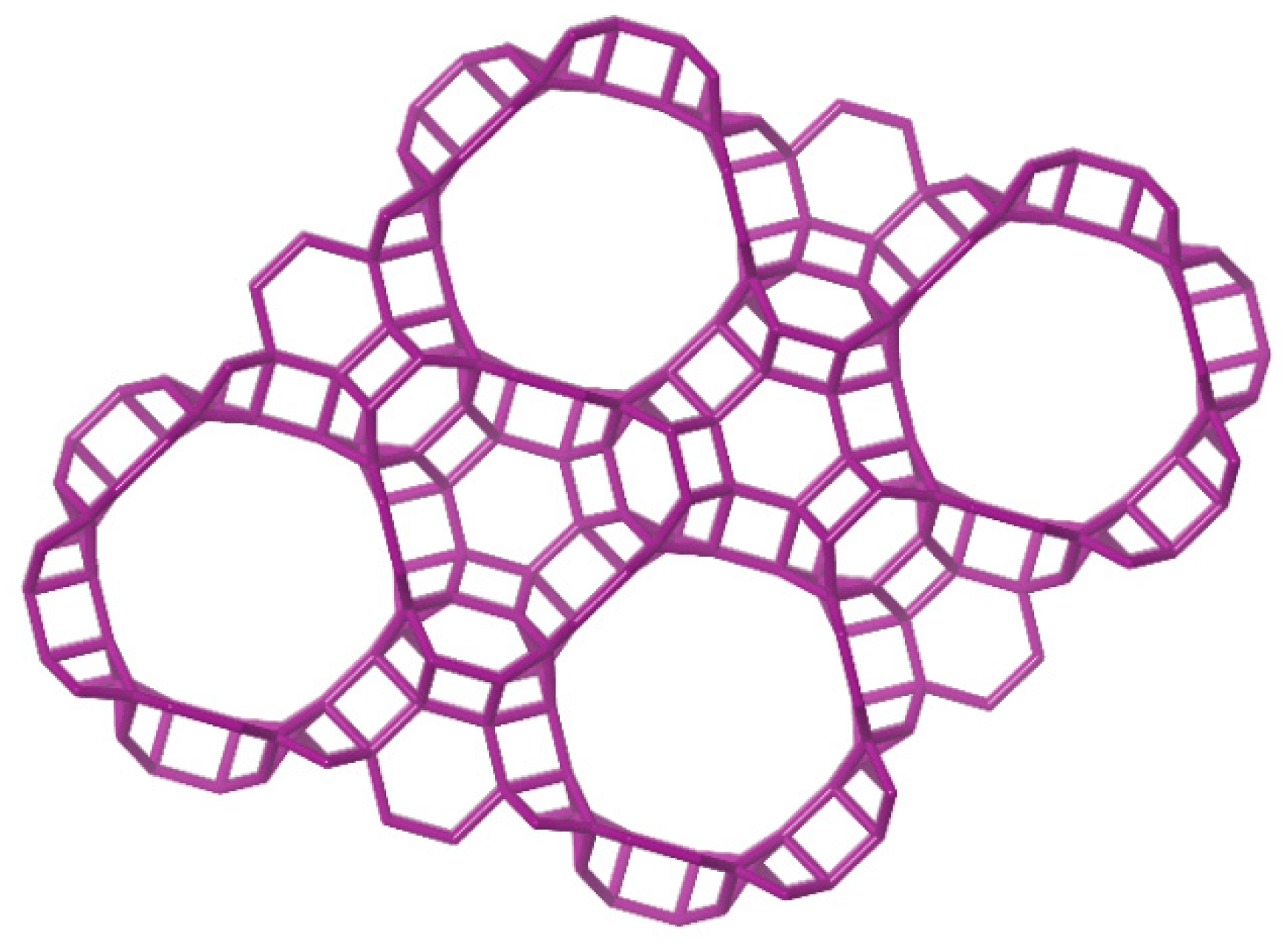
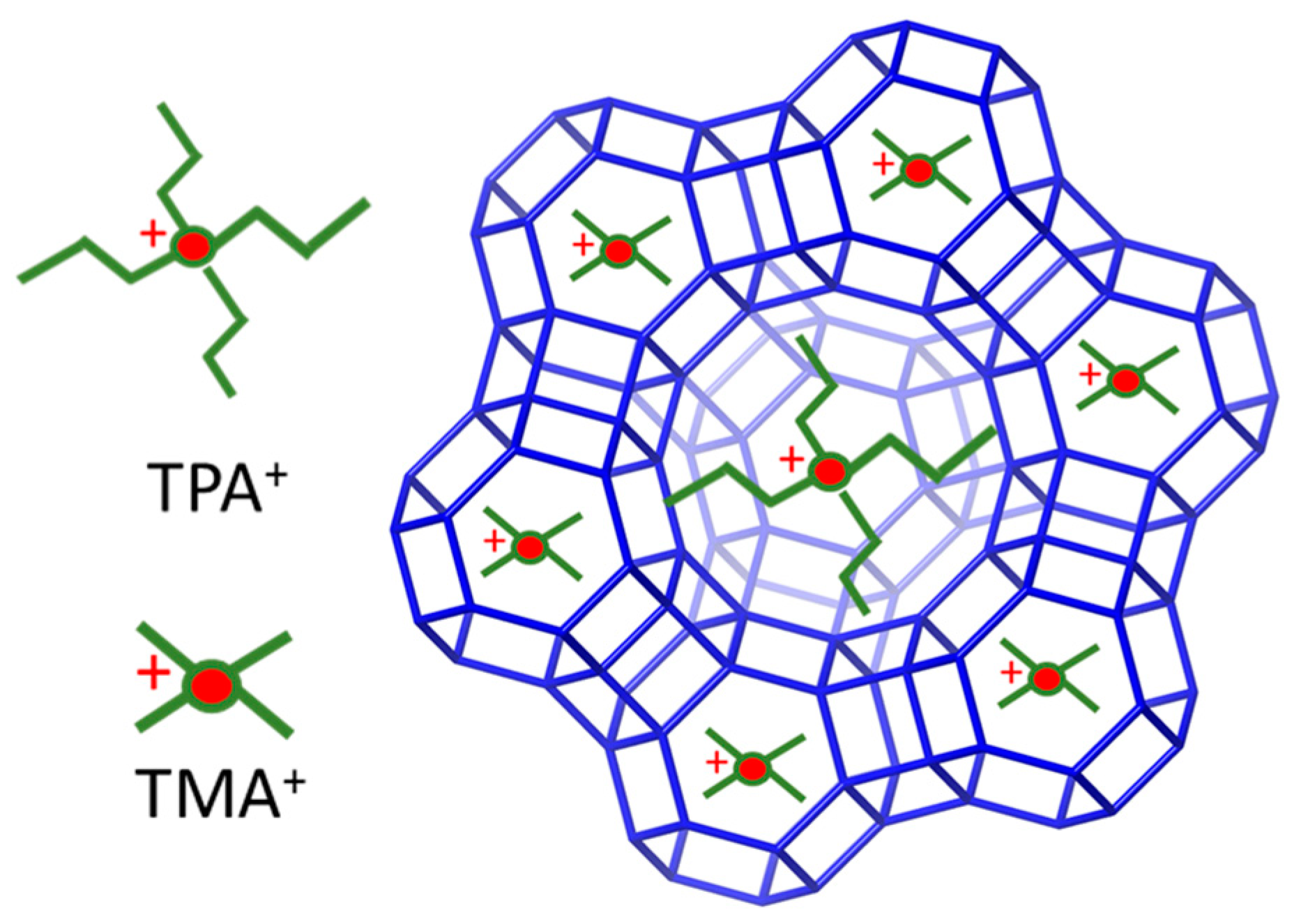
4. Application of the SDAs in the Formation of Microporous Zeolites
5. Co-Templating Technique Using Organic SDAs
6. Soft-Templating Technique for the Synthesis of Large Pore and Hierarchical Zeolites
7. Influence of SDAs on the Size of Zeolite Particles: Giant (Several Microns/Millimeter Scale) Crystals versus Miniaturized (Nanoscale) Zeolites Particles
8. Summary and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rhodes, C.J. Properties and applications of zeolites. Sci. Prog. 2010, 93, 223–284. [Google Scholar] [CrossRef] [PubMed]
- Rimer, J.D. Rational design of zeolite catalysts. Nat. Catal. 2018, 1, 488–489. [Google Scholar] [CrossRef]
- Weitkamp, J. Zeolites and catalysis. Solid State Ion. 2000, 131, 175–188. [Google Scholar] [CrossRef]
- Ackley, M.W.; Rege, S.U.; Saxena, H. Application of natural zeolites in the purification and separation of gases. Microporous Mesoporous Mater. 2003, 61, 25–42. [Google Scholar] [CrossRef]
- Feng, C.; Jiaqiang, E.; Han, W.; Deng, Y.; Zhang, B.; Zhao, X. Key technology and application analysis of zeolite adsorption for energy storage and heat-mass transfer process: A review. Renew. Sustain. Energy Rev. 2021, 144, 110954–110980. [Google Scholar] [CrossRef]
- Thompson, R.W. Recent Advances in the Understanding of Zeolite Synthesis. Mol. Sieves 1998, 1, 1–33. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and their synthesis. Zeolites 1981, 1, 130–140. [Google Scholar] [CrossRef]
- Barrer, R.M. Syntheses and reactions of mordenite. J. Chem. Soc. 1948, 435, 2158–2163. [Google Scholar] [CrossRef]
- Yu, J. Chapter 3 Synthesis of zeolites Introd. Zeolite Sci. Paractice 2007, 168, 39–103. [Google Scholar]
- Reed, T.B.; Breck, D.W. Crystalline Zeolites. II. Crystal Structure of Synthetic Zeolite, Type A. J. Am. Chem. Soc. 1956, 545, 5972–5977. [Google Scholar] [CrossRef]
- Breck, D.W. Crystalline Zeolite Y. U.S. Patent 3,130,007, 21 April 1964. [Google Scholar]
- Argauer, R.J.; Landolt, G.R. Crystalline Zeolite ZSM-5 and Method of Preparing the Same. U.S. Patent 3,702,886, 14 November 1972. [Google Scholar]
- Wadlinger, R.L.; Kerr, G.T.; Rosinski, E.J. Catalytic Composition of a Crystalline Zeolite. U.S. Patent 3,308,069, 7 March 1967. [Google Scholar]
- Karami, D.; Mahinpey, N. The synthesis of novel zeolite Y nanoparticles using mesoporous silica with a temperature controlling method. Can. J. Chem. Eng. 2014, 92, 671–675. [Google Scholar] [CrossRef]
- Rollmann, L.D.; Schlenker, J.L.; Lawton, S.L.; Kennedy, C.L.; Kennedy, G.J.; Doren, D.J. On the Role of Small Amines in Zeolite Synthesis. J. Phys. Chem. B 1999, 34, 7175–7183. [Google Scholar] [CrossRef]
- Burton, A. Recent trends in the synthesis of high-silica zeolites. Catal. Rev. 2018, 60, 132–175. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cao, H.; Yu, J. Toward a New Era of Designed Synthesis of Nanoporous Zeolitic Materials. ACS Nano 2018, 12, 4096–4104. [Google Scholar] [CrossRef]
- Davis, M.E.; Lobo, R.F. Zeolite and Molecular Sieve Synthesis. Chem. Mater. 1992, 4, 756–768. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef]
- Schüth, F. Engineering porous catalytic materials. Annu. Rev. Mater. Res. 2005, 35, 209–238. [Google Scholar] [CrossRef]
- Lee, H.; Zones, S.I.; Davis, M.E. Zeolite synthesis using degradable structure-directing agents and pore-filling agents. J. Phys. Chem. B 2005, 109, 2187–2191. [Google Scholar] [CrossRef]
- Sakai, M.; Hori, H.; Matsukata, M. Alkaline-treatment with pore-filling agent for defect-healing of zeolite membrane. Microporous Mesoporous Mater. 2022, 336, 111901–111906. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Díaz-Cabañas, M.J.; Baerlocher, C. Synthesis and structural characterization of MWW type zeolite ITQ-1, the pure silica analog of MCM-22 and SSZ-25. J. Phys. Chem. B 1998, 102, 44–511. [Google Scholar] [CrossRef]
- Ouyang, X.; Hwang, S.J.; Runnebaum, R.C.; Xie, D.; Wanglee, Y.J.; Rea, T.; Zones, S.I.; Katz, A. Single-step delamination of a MWW borosilicate layered zeolite precursor under mild conditions without surfactant and sonication. J. Am. Chem. Soc. 2014, 136, 1449–1461. [Google Scholar] [CrossRef] [Green Version]
- Pinar, A.B.; Wright, P.A.; Gómez-Hortigüela, L.; Pérez-Pariente, J. Synthesis of ferrierite zeolite with pyrrolidine as structure directing agent: A combined X-ray diffraction and computational study. Microporous Mesoporous Mater. 2010, 129, 164–172. [Google Scholar] [CrossRef]
- Zones, S.I.; Burton, A.W. Diquaternary structure-directing agents built upon charged imidazolium ring centers and their use in synthesis of one-dimensional pore zeolites. J. Mater. Chem. 2005, 15, 4215–4223. [Google Scholar] [CrossRef]
- Beck, L.W.; E Davis, M. Alkylammonium polycations as structure-directing agents in MFI zeolite synthesis. Microporous Mesoporous Mater. 1998, 22, 107–114. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Márquez-Álvarez, C.; Grande-Casas, M.; García, R.; Pérez-Pariente, J. Tailoring the acid strength of microporous silicoaluminophosphates through the use of mixtures of templates: Control of the silicon incorporation mechanism. Microporous Mesoporous Mater. 2009, 121, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Ren, J.; Sun, Y. Influence of template on Si distribution of SAPO-11 and their performance for n-paraffin isomerization. Microporous Mesoporous Mater. 2008, 114, 365–372. [Google Scholar] [CrossRef]
- Lewis, D.W.; Freeman, C.M.; Catlow, C.R.A. Predicting the templating ability of organic additives for the synthesis of microporous materials. J. Phys. Chem. 1995, 99, 11194–11202. [Google Scholar] [CrossRef]
- Yang, X.; Camblor, M.A.; Lee, Y.; Liu, H.; Olson, D.H. Synthesis and crystal structure of as-synthesized and calcined pure silica zeolite ITQ-12. J. Am. Chem. Soc. 2004, 126, 10403–10409. [Google Scholar] [CrossRef]
- Millini, R. Application of modeling in zeolite science. Catal. Today 1998, 41, 41–51. [Google Scholar] [CrossRef]
- Araya, A.; Lowe, B.M. Effect of organic species on the synthesis and properties of ZSM-5. Zeolites 1986, 6, 111–118. [Google Scholar] [CrossRef]
- Corma, A.; Navarro, M.T.; Rey, F.; Rius, J.; Valencia, S. Pure polymorph C of zeolite beta synthesized by using framework isomorphous substitution as a structure-directing mechanism. Angew. Chem. 2001, 113, 2337–2340. [Google Scholar] [CrossRef]
- Feast, S.; Rafiq, M.; Siddiqui, H.; Wells, R.P.; Willock, D.J.; King, F.; Rochester, C.H.; Bethell, D.; Bulman Page, f.c.; Hutchings, G.J. Enantioselective dehydration of butan-2-ol using zeolite Y modified with dithiane oxides. J. Catal. 1997, 167, 533–542. [Google Scholar] [CrossRef]
- Mizukami, F. Application of zeolite membranes, films and coatings. Stud. Surf. Sci. Catal. 1999, 125, 1–12. [Google Scholar] [CrossRef]
- Sastre, G.; Leiva, S.; Sabater, M.J.; Gimenez, I.; Rey, F.; Valencia, S.; Corma, A. Computational and Experimental Approach to the Role of Structure-Directing Agents in the Synthesis of Zeolites: The Case of Cyclohexyl Alkyl Pyrrolidinium Salts in the Synthesis of Zeolites. J. Phys. Chem. 2003, 107, 5432–5440. [Google Scholar] [CrossRef]
- Corma, A.; Díaz-Cabañas, M.J.; Rey, F.; Nicolopoulus, S.; Boulahya, K. ITQ-15: The first ultralarge pore zeolite with a bi-directional pore system formed by intersecting 14- and 12-ring channels, and its catalytic implications. Chem. Commun. 2004, 4, 1356–1357. [Google Scholar] [CrossRef]
- Corma, A.; Díaz-Cabañas, M.J.; Martı´nez-Triguero, J.; Rey, F.; Rius, J. A large-cavity zeolite with wide pore windows and potential as an oil refining catalyst. Lett. Nat. 2002, 418, 1277–1280. [Google Scholar] [CrossRef]
- Feliczak-guzik, A. Microporous and Mesoporous Materials, Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Turta, N.A.; De Luca, P.; Bilba, N.; Nagy, J.B.; Nastro, A. Synthesis of titanosilicate ETS-10 in presence of cetyltri-methylammonium bromide. Microporous Mesoporous Mater. 2008, 112, 425–431. [Google Scholar] [CrossRef]
- Pavel, C.C.; Nagy, J.B.; Bilba, N.; Nastro, A.; Perri, C.; Vuono, D.; De Luka, P.; Asaftei, I.V. Influence of the TAABr salts on the crystallization of ETS-10. Microporous Mesoporous Mater. 2004, 71, 77–85. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.G.; Garcia, J. Realizing the Commerncial Potential of Hierarchical Zeolites—Final Realizing the Commercial Potential of Hierarchical Zeolites: New Opportunities in Catalytic Cracking. ChemCatChem 2014, 6, 46–66. [Google Scholar] [CrossRef]
- Asgar Pour, Z.; Boer, D.G.; Fang, S.; Tang, Z.; Pescarmona, P.P. Bimetallic Zeolite Beta Beads with Hierarchical Porosity as Brønsted-Lewis Solid Acid Catalysts for the Synthesis of Methyl Lactate. Catalysts 2021, 11, 1346. [Google Scholar] [CrossRef]
- Garcia-Martinez, J.; Cazorla-Amorós, D.; Linares-Solano, A.; Lin, J. Synthesis and characterisation of MFI-type zeolites supported on carbon materials. Microporous Mesoporous Mater. 2001, 42, 255–268. [Google Scholar] [CrossRef]
- Tao, Y.; Kanoh, H.; Kaneko, K. ZSM-5 Monolith of Uniform Mesoporous Channels. J. Am. Chem. Soc. 2003, 125, 6044–6045. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, J.S.; Park, Y.S.; Yoon, K.B. Synthesis of Large Monolithic Zeolite Foams with Variable Macropore Architectures. Adv. Mater. 2001, 13, 1259–1263. [Google Scholar] [CrossRef]
- Wiśniewska, M.; Fijałkowska, G.; Nosal-Wiercińska, A.; Franus, M.; Panek, R. Adsorption mechanism of poly(vinyl alcohol) on the surfaces of synthetic zeolites: Sodalite, Na-P1 and Na-A. Adsorption 2019, 25, 567–574. [Google Scholar] [CrossRef] [Green Version]
- Panek, R.; Medykowska, M.; Szewczuk-Karpisz, K.; Wiśniewska, M. Comparison of Physicochemical Properties of Fly Ash Precursor, Na-P1(C) Zeolite–Carbon Composite and Na-P1 Zeolite—Adsorption Affinity to Divalent Pb and Zn Cations. Materials 2021, 14, 3018. [Google Scholar] [CrossRef]
- Panek, R.; Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Franus, M. Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite. Materials 2021, 14, 2832. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Pereira-Almao, P.R. Production of Highly Dispersed Ni within Nickel Silicate Materials with the MFI Structure for the Selective Hydrogenation of Olefins. Ind. Eng. Chem. Res. 2019, 58, 8597–8611. [Google Scholar] [CrossRef]
- Barrer, R.M.; Denny, P.J. Hydrothermal Chemistry of the silicates. Part IX. Nitrogenous Aluminosilicates. J. Chem. Soc. 1961, 971–982. [Google Scholar] [CrossRef]
- Kerr, G.T. Chemistry of Crystalline Aluminosilicates. II. The Synthesis and Properties of Zeolite ZK-4. Inorg. Chem. 1966, 5, 1537–1539. [Google Scholar] [CrossRef]
- Goepper, M.; Li, H.-X.; Davis, M.E. A possible role of alkali metal ions in the synthesis of pure-silica molecular sieves. J. Chem. Soc. Chem. Commun. 1992, 22, 1665–1666. [Google Scholar] [CrossRef] [Green Version]
- Lobo, R.F.; Zones, S.I.; Davis, M.E. Structure-direction in zeolite synthesis. J. Incl. Phenom. Mol. Recognit. Chem. 1995, 21, 47–78. [Google Scholar] [CrossRef]
- Martínez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Rey, F.; Rius, J.; Sabater, M.J.; Valencia, S. Supramolecular self-assembled molecules as organic directing agent for synthesis of zeolites. Nature 2004, 431, 287–290. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, H.; Yang, C.; Liu, S.; Ren, L.; Zhang, L.; Meng, X.; Yilmaz, B.; Müller, U.; Xiao, F.-S. Seed-directed synthesis of zeolites with enhanced performance in the absence of organic templates. Chem. Commun. 2011, 47, 3945–3947. [Google Scholar] [CrossRef]
- Moliner, M.; Rey, F.; Corma, A. Towards the Rational Design of Efficient Organic Structure-Directing Agents for Zeolite Synthesis. Angew. Chem. Int. Ed. 2013, 52, 13880–13889. [Google Scholar] [CrossRef]
- Yabushita, M.; Osuga, R.; Muramatsu, A. Control of location and distribution of heteroatoms substituted isomorphously in framework of zeolites and zeotype materials. CrystEngComm 2021, 23, 6226–6233. [Google Scholar] [CrossRef]
- Burton, A.W.; Zones, S.I.; Elomari, S. The chemistry of phase selectivity in the synthesis of high-silica zeolites. Curr. Opin. Colloid Interface Sci. 2005, 10, 211–219. [Google Scholar] [CrossRef]
- Corminboeuf, C.; Tran, F.; Weber, J. The role of density functional theory in chemistry: Some historical landmarks and applications to zeolites. J. Mol. Struct. Theochem 2006, 762, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Liu, Z.P. The Role of Zeolite Framework in Zeolite Stability and Catalysis from Recent Atomic Simulation. Top. Catal. 2022, 65, 59–68. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Li, S.; Jones, C.B.; Nimlos, C.T.; Wang, Y.; Kunkes, E.; Vattipalli, V.; Prasad, S.; Moini, A.; Schneider, W.F.; et al. Cooperative and Competitive Occlusion of Organic and Inorganic Structure-Directing Agents within Chabazite Zeolites Influences Their Aluminum Arrangement. J. Am. Chem. Soc. 2020, 142, 4807–4819. [Google Scholar] [CrossRef]
- Nimlos, C.T.; Hoffman, A.J.; Hur, Y.G.; Lee, B.J.; Di Iorio, J.R.; Hibbitts, D.D.; Gounder, R. Experimental and Theoretical Assessments of Aluminum Proximity in MFI Zeolites and Its Alteration by Organic and Inorganic Structure-Directing Agents. Chem. Mater. 2020, 32, 9277–9298. [Google Scholar] [CrossRef]
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Robson, H.; Lillerud, K.P. (Eds.) Verified Synthesis of Zeolitic Materials; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Goretsky, A.V.; Beck, L.W.; I Zones, S.; E Davis, M. Influence of the hydrophobic character of structure-directing agents for the synthesis of pure-silica zeolites. Microporous Mesoporous Mater. 1999, 28, 387–393. [Google Scholar] [CrossRef]
- Gies, H.; Marker, B. The structure-controlling role of organic templates for the synthesis of porosils in the systems SiO2/template/H2O. Zeolites 1992, 12, 42–49. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Corà, F.; Catlow, C.R.A.; Pérez-Pariente, J. Computational Study of the Structure-Directing Effect of Benzylpyrrolidine and Its Fluorinated Derivatives in the Synthesis of the Aluminophosphate AlPO-5. J. Am. Chem. Soc. 2004, 126, 12097–12102. [Google Scholar] [CrossRef]
- Davis, M.E. Zeolites from a Materials Chemistry Perspective. Chem. Mater. 2013, 26, 239–245. [Google Scholar] [CrossRef]
- Sastre, G.; Pulido, A.; Castañeda, R.; Corma, A. Effect of the Germanium Incorporation in the Synthesis of EU-1, ITQ-13, ITQ-22, and ITQ-24 Zeolites. J. Phys. Chem. B 2004, 108, 8830–8835. [Google Scholar] [CrossRef]
- Leon, S.; Sastre, G. Zeolite Phase Selectivity Using the Same Organic Structure-Directing Agent in Fluoride and Hydroxide Media. J. Phys. Chem. C 2022, 126, 2078–2087. [Google Scholar] [CrossRef]
- Strohmaier, K.G.; Vaughan, D.E.W. Structure of the First Silicate Molecular Sieve with 18-Ring Pore Openings, ECR-34. J. Am. Chem. Soc. 2003, 125, 16035–16039. [Google Scholar] [CrossRef] [PubMed]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 17 March 2022).
- Kubota, Y.; Helmkamp, M.M.; Zones, S.I.; Davis, M.E. Properties of organic cations that lead to the structure-direction of high-silica molecular sieves. Microporous Mater. 1996, 6, 213–229. [Google Scholar] [CrossRef] [Green Version]
- Liebau, F. Zeolites and clathrasils—Two distinct classes of framework silicates. Zeolites 1983, 3, 191–193. [Google Scholar] [CrossRef]
- Casci, J.L.; Barrie, M.; Whittam, T.V. Zeolite EU-1 and a Method of Making Zeolite EU-1. U.S. Patent 4,537,754, 11 June 1981. [Google Scholar]
- Schlenker, J.; Rohrbaugh, W.; Chu, P.; Valyocsik, E.; Kokotailo, G. The framework topology of ZSM-48: A high silica zeolite. Zeolites 1985, 5, 355–358. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Lee, G.; Harris, T.; Yuen, L.; Zones, S. Guest/host relationships in zeolite synthesis: Ring-substituted piperidines and the remarkable adamantane mimicry by 1-azonio spiro [5.5] undecanes. Microporous Mesoporous Mater. 1998, 22, 69–85. [Google Scholar] [CrossRef]
- Baerlocher, C.; Gramm, F.; Massüger, L.; McCusker, L.B.; He, Z.; Hovmöller, S.; Zou, X. Structure of the Polycrystalline Zeolite Catalyst IM-5 Solved by Enhanced Charge Flipping. Science 2007, 315, 1113–1116. [Google Scholar] [CrossRef]
- Jackowski, A.; Zones, S.; Burton, A. A study on zeolite synthesis from diquaternary ammonium compounds; the effect of changing end-group heterocycles in the HF/SiO2 synthesis of molecular sieves. Stud. Surf. Sci. Catal. 2008, 174, 111–116. [Google Scholar] [CrossRef]
- Gramm, F.; Baerlocher, C.; McCusker, L.B.; Warrender, S.J.; Wright, P.A.; Han, B.; Hong, S.B.; Liu, Z.; Ohsuna, T.; Terasaki, O. Complex zeolite structure solved by combining powder diffraction and electron microscopy. Nature 2006, 444, 79–81. [Google Scholar] [CrossRef]
- Jackowski, A.; Zones, S.I.; Hwang, S.-J.; Burton, A.W. Diquaternary Ammonium Compounds in Zeolite Synthesis: Cyclic and Polycyclic N-Heterocycles Connected by Methylene Chains. J. Am. Chem. Soc. 2009, 131, 1092–1100. [Google Scholar] [CrossRef]
- Zones, S. Synthesis of pentasil zeolites from sodium silicate solutions in the presence of quaternary imidazole compounds. Zeolites 1989, 9, 458–467. [Google Scholar] [CrossRef]
- Barrett, P.A.; Boix, T.; Puche, M.; Olson, D.H.; Jordan, E.; Koller, H.; Camblor, M.A. ITQ-12: A new microporous silica polymorph potentially useful for light hydrocarbon separations. Chem. Commun. 2003, 17, 2114–2115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zones, S.I.; Burton, A.W. Molecular Soeve SSZ-70 Composition of Matter and Synthesis Thereof. U.S. Patent 7,108,842 B2, 19 September 2006. [Google Scholar]
- Lorgouilloux, Y.; Dodin, M.; Paillaud, J.-L.; Caullet, P.; Michelin, L.; Josien, L.; Ersen, O.; Bats, N. IM-16: A new microporous germanosilicate with a novel framework topology containing d4r and mtw composite building units. J. Solid State Chem. 2009, 182, 622–629. [Google Scholar] [CrossRef]
- Parnham, E.R.; Morris, R.E. The Ionothermal Synthesis of Cobalt Aluminophosphate Zeolite Frameworks. J. Am. Chem. Soc. 2006, 128, 2204–2205. [Google Scholar] [CrossRef] [PubMed]
- Zones, S.I.; Nakagawa, Y.; Yuen, A.L.T.; Harris, T.V. Guest/Host Interactions in High Silica Zeolite Synthesis: [5.2.1.02.6]Tricyclodecanes as Template Molecule. J. Am. Chem. Soc. 1996, 118, 7558–7567. [Google Scholar] [CrossRef]
- Calabro, D.C.; Cheng, J.C.; Crane, R.A.; Kresge, C.T.; Dhingra, S.S.; Steckel, M.A.; Stern, D.L.; Weston, S.C. Synthetic Porous Crystalline MCM-68, Its Synthesis and Use. U.S. Patent 6,049,018, 11 April 2000. [Google Scholar]
- Dorset, D.L.; Weston, S.C.; Dhingra, S.S. Crystal Structure of Zeolite MCM-68: A New Three-Dimensional Framework with Large Pores. J. Phys. Chem. B 2006, 110, 2045–2050. [Google Scholar] [CrossRef]
- Lee, G.S.; Zones, S.I. Polymethylated [4.1.1] Octanes Leading to Zeolite SSZ-50. J. Solid State Chem. 2002, 167, 289–298. [Google Scholar] [CrossRef]
- Elomari, S.; Burton, A.; Medrud, R.C.; Grosse-Kunstleve, R. The synthesis, characterization, and structure solution of SSZ-56: An extreme example of isomer specificity in the structure direction of zeolites. Microporous Mesoporous Mater. 2009, 118, 325–333. [Google Scholar] [CrossRef]
- Elomari, S. Process for Preparing Zeolites Using Pyrrplidinium Cations. U.S. Patent 6,616,911 b2, 9 September 2003. [Google Scholar]
- Burton, A.; Elomari, S.; Medrud, R.C.; Chan, I.Y.; Chen, C.-Y.; Bull, L.M.; Vittoratos, E.S. The Synthesis, Characterization, and Structure Solution of SSZ-58: A Novel Two-Dimensional 10-Ring Pore Zeolite with Previously Unseen Double 5-Ring Subunits. J. Am. Chem. Soc. 2003, 125, 1633–1642. [Google Scholar] [CrossRef]
- Burton, A.; Elomari, S.; Chen, C.-Y.; Medrud, R.C.; Chan, I.Y.; Bull, L.M.; Kibby, C.; Harris, T.V.; Zones, S.I.; Vittoratos, E.S. SSZ-53 and SSZ-59: Two Novel Extra-Large Pore Zeolites. Chem. A Eur. J. 2003, 9, 5737–5748. [Google Scholar] [CrossRef]
- Elomari, S.; Burton, A.W.; Ong, K.; Pradhan, A.R.; Chan, I.Y. Synthesis and Structure Solution of Zeolite SSZ-65. Chem. Mater. 2007, 19, 5485–5492. [Google Scholar] [CrossRef]
- Jiang, J.; Yu, J.; Corma, A. Extra-Large-Pore Zeolites: Bridging the Gap between Micro and Mesoporous Structures. Angew. Chem. Int. Ed. 2010, 49, 3120–3145. [Google Scholar] [CrossRef] [PubMed]
- Delprato, F.; Delmotte, L.; Guth, J.; Huve, L. Synthesis of new silica-rich cubic and hexagonal faujasites using crown-etherbased supramolecules as templates. Zeolites 1990, 10, 546–552. [Google Scholar] [CrossRef]
- Pinar, A.B.; Marquez-Alvarez, C.; Grande-Casas, M.; Pérez-Pariente, J. Template-controlled acidity and catalytic activity of ferrierite crystals. J. Catal. 2009, 263, 258–265. [Google Scholar] [CrossRef] [Green Version]
- Marquez-Alvarez, C.; Pinar, A.B.; Garcia, R.; Grande-Casas, M.; Pérez-Pariente, J. Influence of Al Distribution and Defects Concentration of Ferrierite Catalysts Synthesized From Na-Free Gels in the Skeletal Isomerization of n-Butene. Top. Catal. 2009, 52, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.; Ribeiro, F.; Lourenço, J.; Gabelica, Z. An elegant way to increase acidity in SAPOs: Use of methylamine as co-template during synthesis. Stud. Surf. Sci. Catal. 2008, 174, 281–284. [Google Scholar] [CrossRef]
- Li, L.; Zhang, F. Effect of templates on synthesis of SAPO-41 and their catalytic performance in n-octane hydroisomerization. Stud. Surf. Sci. Catal. 2007, 170, 397–402. [Google Scholar]
- Chatelain, T.; Patarin, J.; Soulard, M.; Guth, J.; Schulz, P. Synthesis and characterization of high-silica EMT and FAU zeolites prepared in the presence of crown-ethers with either ethylene glycol or 1,3,5-trioxane. Zeolites 1995, 15, 90–96. [Google Scholar] [CrossRef]
- Pelrine, B.P. Synthesis of ZSM-39. U.S. Patent 4,259,306, 31 March 1981. [Google Scholar]
- Jacobs, P.A.; Martens, J.A. Synthesis of High-Silica Aluminosilicate Zeolites; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Schlenker, J.L.; Dwyer, F.G.; Jenkins, E.E.; Rohrbaugh, W.J.; Kokotailo, G.T.; Meier, W.M. Crystal structure of a synthetic high silica zeolite—ZSM-39. Nature 1981, 294, 340–342. [Google Scholar] [CrossRef]
- de Saldarriaga, L.S.; Saldarriaga, C.; Davis, M.E. Investigations into the nature of a silicoaluminophosphate with the faujasite structure. J. Am. Chem. Soc. 1987, 109, 2686–2691. [Google Scholar] [CrossRef]
- Franco, M.; Pérez-Pariente, J.; Misud, A.; Blasco, T.; Sanz, J. Crystallization kinetics of SAPO-37. Zeolites 1992, 12, 386–394. [Google Scholar] [CrossRef]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. SAPO-34 Seeds and Membranes Prepared Using Multiple Structure Directing Agents. Adv. Mater. 2008, 20, 729–732. [Google Scholar] [CrossRef]
- Xu, J.; Haw, K.-G.; Li, Z.; Pati, S.; Wang, Z.; Kawi, S. A mini-review on recent developments in SAPO-34 zeolite membranes and membrane reactors. React. Chem. Eng. 2020, 6, 52–66. [Google Scholar] [CrossRef]
- Masoumi, S.; Towfighi, J.; Mohamadalizadeh, A.; Kooshki, Z.; Rahimi, K. Tri-templates synthesis of SAPO-34 and its performance in MTO reaction by statistical design of experiments. Appl. Catal. A Gen. 2015, 493, 103–111. [Google Scholar] [CrossRef]
- Zones, S.I.; Hwang, S.-J.; Davis, M.E. Studies of the Synthesis of SSZ-25 Zeolite in a “Mixed-Template” System. Chem. Eur. J. 2001, 7, 1990–2001. [Google Scholar] [CrossRef]
- Zones, S.I.; Hwang, S.-J. Synthesis of High Silica Zeolites Using a Mixed Quaternary Ammonium Cation, Amine Approach: Discovery of Zeolite SSZ-47. Chem. Mater. 2001, 14, 313–320. [Google Scholar] [CrossRef]
- Blackwell, C.S.; Broach, R.W.; Gatter, M.G.; Holmgren, J.S.; Jan, D.-Y.; Lewis, G.J.; Mezza, B.J.; Mezza, T.M.; Miller, M.A.; Moscoso, J.G.; et al. Open-Framework Materials Synthesized in the TMA/TEA Mixed-Template System: The New Low Si/Al Ratio Zeolites UZM-4 and UZM-5. Angew. Chem. Int. Ed. 2003, 42, 1737–1740. [Google Scholar] [CrossRef]
- Zones, S.I.; Nakagawa, Y. Preparation of Zeolites Using Organic Template and Amine. U.S. Patent 5,785,947, 28 July 1998. [Google Scholar]
- Kalantari, N.; Farzi, A.; Delibaş, N.; Niaei, A.; Salari, D. Synthesis of multiple-template zeolites with various compositions and investigation of their catalytic properties. Res. Chem. Intermed. 2021, 47, 4957–4984. [Google Scholar] [CrossRef]
- Ma, H.; Lin, H.; Liu, X.; Lü, H.; Zhu, Z. In situ structural reconstruction triggers the hydrothermal synthesis of hierarchical Ti-Beta zeolites for oxidative desulfurization. Mater. Chem. Front. 2021, 5, 6101–6113. [Google Scholar] [CrossRef]
- Jia, X.; Khan, W.; Wu, Z.; Choi, J.; Yip, A.C. Modern synthesis strategies for hierarchical zeolites: Bottom-up versus top-down strategies. Adv. Powder Technol. 2018, 30, 467–484. [Google Scholar] [CrossRef]
- Wang, C.; Yang, M.; Tian, P.; Xu, S.; Yang, Y.; Wang, D.; Yuan, Y.; Liu, Z. Dual template-directed synthesis of SAPO-34 nanosheet assemblies with improved stability in the methanol to olefins reaction. J. Mater. Chem. A 2015, 3, 5608–5616. [Google Scholar] [CrossRef]
- Xu, H.; Wu, P. Two-dimensional zeolites in catalysis: Current state-of-the-art and perspectives. Catal. Rev. 2021, 63, 234–301. [Google Scholar] [CrossRef]
- Huang, L.; Guo, W.; Deng, P.; Xue, Z.; Li, Q. Investigation of Synthesizing MCM-41/ZSM-5 Composites. J. Phys. Chem. B 2000, 104, 2817–2823. [Google Scholar] [CrossRef]
- Na, K.; Choi, M.; Ryoo, R. Recent advances in the synthesis of hierarchically nanoporous zeolites. Microporous Mesoporous Mater. 2013, 166, 3–19. [Google Scholar] [CrossRef]
- Na, K.; Jo, C.; Kim, J.; Cho, K.; Jung, J.; Seo, Y.; Messinger, R.J.; Chmelka, B.F.; Ryoo, R. Directing Zeolite Structures into Hierarchically Nanoporous Architectures. Science 2011, 333, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, G.; Díaz, I.; Tsapatsis, M.; Jeong, H.-K.; Lee, A.Y.; Vlachos, D.G. Zeolite (MFI) Crystal Morphology Control Using Organic Structure-Directing Agents. Chem. Mater. 2004, 16, 5697–5705. [Google Scholar] [CrossRef]
- Patarin, J.; Soulard, M.; Kessler, H.; Guth, J.-L.; Baron, J. Characterization of siliceous MFI-type zeolites containing tetra-, tri-, and dipropylammonium fluoride species. Zeolites 1989, 9, 397–404. [Google Scholar] [CrossRef]
- Suzuki, K.; Kiyozumi, Y.; Shin, S.; Fujisawa, K.; Watanabe, H.; Saito, K.; Noguchi, K. Zeolite synthesis in the system pyrrolidine-Na2O-Al2O3-SiO2-H2O. Zeolites 1986, 6, 290–298. [Google Scholar] [CrossRef]
- Crea, F.; Nastro, A.; Nagy, J.; Aiello, R. Synthesis of silicalite 1 from systems with different TPABr/SiO2 ratios. Zeolites 1988, 8, 262–267. [Google Scholar] [CrossRef]
- Ahmed, S.; El-Faer, M.Z.; Abdillahi, M.M.; Siddiqui, M.A.; Barri, S.A. Investigation of the rapid crystallization method for the synthesis of MFI-type zeolites and study of the physicochemical properties of the products. Zeolites 1996, 17, 373–380. [Google Scholar] [CrossRef]
- Ferchiche, S.; Valcheva-Traykova, M.; Vaughan, D.E.; Warzywoda, J.; Sacco, A. Synthesis of large single crystals of templated Y faujasite. J. Cryst. Growth 2001, 222, 801–805. [Google Scholar] [CrossRef]
- Mintova, S.; Grand, J.; Valtchev, V. Nanosized zeolites: Quo Vadis? Comptes Rendus. Chim. 2016, 19, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Mintova, S.; Gilson, J.-P.; Valtchev, V. Advances in nanosized zeolites. Nanoscale 2013, 5, 6693–6703. [Google Scholar] [CrossRef] [PubMed]
- Alipour, S.M.; Halladj, R.; Askari, S. Effects of the different synthetic parameters on the crystallinity and crystal size of nanosized ZSM-5 zeolite. Rev. Chem. Eng. 2014, 30, 289–322. [Google Scholar] [CrossRef]



| Entry | Zeolite Topology | Zeolite Name | Obtained Si/Al | SDA |
|---|---|---|---|---|
| 1 | CHA | Chabazite | 2.1 | No SDA |
| SSZ-13 | 13.3 | N,N,N,trimethyl-1-adamantammonium | ||
| 2 | EDI | Linde Type F | 1.0 | No SDA |
| 1.5 | TMA+ | |||
| 3 | EMT | EMC-2 | 1.1 | No SDA |
| 3.8 | crown ether | |||
| 4 | FAU | Linde Type Y | 2.4 | No SDA |
| High Si EMC-1 | 3.8 | 15-crown-5 | ||
| 5 | KFI | ZK-5 | 3.4 | No SDA |
| High Silica KFI | 3.8 | 18-crown-6 | ||
| 6 | LTA | Linde Type A | 1.0 | No SDA |
| ZK-4 | 1.4 | TMA+ | ||
| Zeolite Alpha | 3.0 | TMA+ | ||
| 7 | OFF | Linde Type T | 3.5 | No SDA |
| Offretite | 3.8 | TMA+ |
| Entry | Zeolite Topology | Zeolite Name | SDA |
|---|---|---|---|
| 1 | EDI | Linde F | - |
| 2 | EDI | Nanosized Linde F | TEA+ |
| 3 | FAU | Linde Type Y | - |
| 4 | FAU | Nanosized Linde Type Y | TMA+ |
| 5 | LTA | Linde Type A | - |
| 6 | LTA | Nanosized Linde Type A | TMA+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asgar Pour, Z.; Sebakhy, K.O. A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites. Chemistry 2022, 4, 431-446. https://doi.org/10.3390/chemistry4020032
Asgar Pour Z, Sebakhy KO. A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites. Chemistry. 2022; 4(2):431-446. https://doi.org/10.3390/chemistry4020032
Chicago/Turabian StyleAsgar Pour, Zahra, and Khaled O. Sebakhy. 2022. "A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites" Chemistry 4, no. 2: 431-446. https://doi.org/10.3390/chemistry4020032
APA StyleAsgar Pour, Z., & Sebakhy, K. O. (2022). A Review on the Effects of Organic Structure-Directing Agents on the Hydrothermal Synthesis and Physicochemical Properties of Zeolites. Chemistry, 4(2), 431-446. https://doi.org/10.3390/chemistry4020032