Abstract
Different extraction pH values obtain polysaccharides with tailored structures and novel functionalities. This study investigated the influence of different extraction pH values (4.2, 6.8, and 9.2) on the physicochemical compositions and structural and functional properties of okra leaf polysaccharides (OLPs). The extraction yield (2.74–7.34%), molecular weights (68.5–85.4 kDa), total sugar contents (64.87–95.68%), degree of acetylation (18.28–22.88%), and methylation (8.97–15.20%) of OLPs varied significantly (p < 0.05). The monosaccharide composition reflected OLPs as pectic polysaccharides, with varied compositions of galacturonic acid, galactose, rhamnose, and arabinose. However, the differences in their sugar molar ratios, such as their side-chain and backbone chain compositions, greatly affected their functional properties. Additionally, notable differences due to extraction pH were observed in physical properties, thermal stability, and crystallinity. However, FTIR and NMR spectra revealed that extraction pH had negligible effects on the primary structure of OLPs. All OLPs showed non-Newtonian fluid behavior in the aqueous system with different apparent viscosities correlating with their molecular weights. Furthermore, the OLPs fractions stabilized oil-in-water emulsions differently and had distinct radical scavenging activities related to their compositions. This study provides a basis for selecting appropriate extraction pH to prepare OLPs with specific characteristics and applications in food-related disciplines.
1. Introduction
Polysaccharides are essential macromolecules existing as cell wall materials, exudates, and extracellular substances in plants and microorganisms. In recent years, there has been increasing research on extracting plant-based polysaccharides [1]. Most studies have demonstrated their unique functionalities and multiple biological activities, such as antioxidant and diverse pharmacological potentials [2,3,4]. Plant polysaccharides are used as texture modifiers, gelling agents, thickeners, emulsifiers, stabilizers, encapsulants, syneresis inhibitors, and film/coating agents [5,6,7]. Compared with synthetic polymers, their acceptability as a consumer-friendly material/ingredient benefits from their natural sources and exceptional functionalities, leading to their exponential demand in food-related fields, including medical, biomedical, pharmaceutical, and cosmetic industries [8,9].
The okra (Abelmoschus esculentus) plant is an annual crop of African origin and is grown in other tropical, subtropical, and temperate areas of the world [10]. The fruit/pod of okra is the most popular plant part, widely consumed as a vegetable and used in traditional folk medicine for treating diseases [2]; it has long been identified as a rich source of natural polysaccharides [6,11,12,13]. Moreover, functional polysaccharides with potent antioxidant and immunomodulating activities have been equally obtained from okra flowers [4,14]. In our previous study [15], we achieved the extraction of a water-soluble pectic polysaccharide from okra leaves (OLPs), with a similar structural composition to polysaccharides previously obtained from the pods and flowers of okra [2,14]. Typically, okra polysaccharides are pectic polysaccharides containing covalently linked homogalacturonan (HG) and rhamnogalacturonan I (RG-I) as main structural units, with side-chain substitution of galactans and arabinogalactans at the O-4 position of l-rhamnose residues [16]. HG is a linear chain of repeating units of 1,4-linked α-d-galacturonic acid residues, partially esterified or unesterified at the C-6 carboxyl group [17]. Moreover, the RG-I region is a branched-chain structure with a backbone of alternating units of α-(1,2)-linked rhamnosyl and α-(1,4)-linked galacturonic acid residues [18]. However, structural variations may exist depending on the extraction method [6,19,20].
Polysaccharides prepared by different extraction techniques (based on the technology or solvent used) exhibit notable differences in physicochemical compositions and macromolecular and structural characteristics, affecting their functional and biological properties [7,20,21,22]. In several studies, researchers have investigated the different effects of extraction technologies, such as conventional heating, ultrasound, microwave, enzyme, and high-pressure extraction, on the characteristics of polysaccharides [19,20,23,24]. Additionally, various solvent-based extraction methods, including those using deionized water, buffers, acidic and alkaline solutions, chelating agents, and deep eutectic solvents, extracted polysaccharides with unique properties [6,11,18,25,26]. In these studies, the extraction pH is often specified in detail, indicating its vital role in influencing the primary properties of polysaccharides, such as yield, molecular weight, and monosaccharide compositions [6,27]. Thus, selecting a suitable pH is essential for preparing polysaccharides with tailored functionalities.
The available studies have extensively investigated the impact of a range of solvent pH values (2–12) on the characteristics of polysaccharides from okra pods and indicated that tailored structural characteristics and novel functionalities could be achieved by adjusting the extraction pH [6,11]. However, there is no information on the characteristics of OLPs extracted at different extraction pH values. Therefore, in this present study, we extracted three polysaccharides from okra leaf using mildly acidic, neutral, and mildly alkaline extraction solutions at pH values of 4.2, 6.8, and 9.2, respectively, and examined their physicochemical and functional characteristics for their possible food-related applications.
2. Materials and Methods
2.1. Material and Chemical Reagents
Fresh okra leaves were collected (Danggin, Chungcheongnam-do Korea) and dried at 40 °C for 24 h. Dried leaves were crushed and sieved into a fine powder by passing through a 425μm sieve and kept at −20 °C until use. Sodium dodecyl sulfate (SDS), 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), diammonium salt (ABTS), and all monosaccharide standards were purchased from Sigma-Aldrich (St Louis, MO, USA). Other chemical reagents were of analytical grade (Duksan chemicals, Ansan, Korea).
2.2. Polysaccharides Extraction by pH
Polysaccharides were extracted from okra leaf powder using the ultrasonic-assisted extraction method described in our previous study [15]. The pH buffer solutions used in this study were prepared using 5 mm solutions of monobasic and dibasic sodium phosphate buffer salts to obtain final pH values of 4.2, 6.8, and 9.2. Before extraction, okra leaf powder was washed thrice in 85% ethanol (1:10 w/v) at 70 °C for 1 h to remove pigments and other impurities and obtain alcohol-insoluble residues. The dried powder was mixed with the prepared buffer solutions (1:20 g/mL) in a 2 L flask and extracted at 40 °C for 30 min. A bath-type ultrasonic machine (KHC-1SUMP, Kyung il Ultrasonic, Ansan, Korea) with an internal dimension of 45 × 45 × 49 was used, flasks containing mixtures were immersed into the ultrasonic bath, and the ultrasonic conditions were fixed at 100 kHz, 100% amplitude, and 600 W. After the extraction process, the mucilage supernatant was recovered by centrifugation, concentrated, and precipitated using three volumes of 95% ethanol (4 °C, overnight). The precipitated mass was washed exhaustively in ethanol, reconstituted in distilled water for dialysis (13 kDa, 48 h), and lyophilized. Polysaccharide powders were obtained after crushing, such that powders obtained from extraction pH values of 4.2, 6.8, and 9.2 were denoted as OLP4, OLP7, and OLP9, respectively. The extraction yield was expressed as the % ratio of yield in grams of dried polysaccharide to okra leaf powder.
2.3. Physicochemical Compositions
The physical parameters of powder bulk density, color values (L*, a*, and b*), water solubility, and water holding capacity (WHC) at ambient temperature were measured using a Chroma Meter (CR-300, Minolta Co., Osaka, Japan) and standard methods as described in a previous study [5]. Chemical properties, such as total protein content [28], total sugar [29], and uronic acid [30] compositions, were determined using colorimetric methods. The degree of acetylation (DA) and methylation (DM) was determined using the HPLC method of Voragen, Schols, and Pilnik [31] and expressed as moles of acetic acids and methanol per 100 moles of uronic acid, respectively [19].
2.4. Molecular Weight Analysis
OLPs (10 mg/mL) were dissolved in 20 mm NaNO3 containing 0.02% sodium azide and filtered with a 0.45 μm membrane for molecular weight determination. The filtrate was injected into the gel permeation chromatography (GPC) system (Breeze Systems, Waters, Milford, MA, USA), coupled with a refractive detector index (Waters 2414, Milford, MA, USA) and four Waters Ultrahydrogel Columns (linear, 120, 250, 500) arranged serially. Elution was performed at 30 °C and a 0.8 mL/min flow rate with 20 mm NaNO3. Pullulan standards (642–6.1 kDa) were used as a calibration curve for molecular weight determination, and data acquisition was performed with Empower® 2 Chromatography Data Software (Waters, Milford, MA, USA).
2.5. Monosaccharide Analysis
For monosaccharide analysis, 10 mg of polysaccharide powder was hydrolyzed in 2 mL of 2M TFA at 121 °C for 2 h. TFA was removed from the hydrolysate via vacuum evaporation with methanol thrice, redissolved in HPLC-grade water, and filtered through a 0.45 μm membrane. High-performance liquid chromatography (HPLC 1260 series, Agilent Technologies, Santa Clara, CA, USA) equipped with a refractive index (RI) detector and a Rezex ROA–organic acid H+ (8%) (150 mm × 4.6 mm) column (Phenomenex Inc., Torrance, CA, USA) was used for this analysis. Column elution was performed at 50 °C and a flow rate of 0.6 mL/min using 5 mM H2SO4. Constituent sugars were quantified using their respective monosaccharide standards (Sigma Aldrich, Burlington, MA, USA), including glucose (Glc), galactose (Gal), arabinose (Ara), rhamnose (Rha), and Galacturonic acid (GalA).
2.6. Structural Characteristics of OLPs
The structural features of OLPs were observed using FTIR and NMR spectroscopy and X-ray diffractogram. OLP powder (2 mg) was mixed with KBr (100 mg), and spectra reading was recorded (4000 cm−1 to 400 cm−1) at 4 cm−1 resolution by an FTIR spectrophotometer (Frontier, PerkinElmer, Waltham, MA, USA). The degree of esterification (FTIR-DE) was estimated based on the band area of esterified uronic acids (1726 cm−1) and unesterified uronic acid band areas (1626 cm−1) as described previously [20,32]. The spectra were analyzed using Origin v. 2019b software (OriginLab Corporation, Northampton, MA, USA).
For the NMR experiment, OLPs were dissolved in D2O and freeze-dried; this process was repeated thrice and then redissolved in 0.5 mL of D2O. Clear OLP solutions were used for proton NMR spectra acquisition at 25 °C using a Bruker AVANCE III-500 MHz (Billerica, MA, USA) spectrometer. Mnova (v.14.1.2, Mestrelab Research, Santiago de Compostela, Spain) software was used to process data. The X-ray diffraction patterns of OLPs were acquired using an XRD diffractometer (Malvern, Panalytical Empyrean) with the following working conditions: Cu 1.8KW (Max. 60kV 60 mA), an angular range of 5–90° (2θ) at room temperature.
2.7. Functional Properties of OLPs
2.7.1. Viscosity and Flow Properties
The apparent viscosity and flow curves of OLP solutions (1% w/v) at different shear rates (γ) were measured using a viscometer (DV-II + PRO, Brookfield, MA, USA), coupled with a spindle (# 42). The temperature was maintained at 25 °C throughout the experiment using a heating–cooling bath circulator (RW-0525G, Jeiotech, Korea). The obtained data were then fitted into the power-law model equation to describe the flow characteristics of OLPs (Equation (1)).
In Equation (1), τ and γ denote shear stress (mPa) and shear rate (s−1), respectively, and the model parameters K (flow consistency index, mPa·s) and n (flow behavior index) values were computed using the Microsoft Excel solver (Microsoft Corporation, Redmond, WA, USA).
2.7.2. Emulsifying Properties
The emulsifying properties of OLPs were investigated using the centrifugal [33] and turbidimetric methods developed by Pearce and Kinsella [34]. Briefly, 10 mL solutions of OLPs (1% w/v) were homogenized (PT-1200C, KINEMATICA AG, Malters, Switzerland) with an equal volume of sunflower oil (φ = 0.5) under a high-shearing rate of 20,000 rpm for 1 min and centrifuged at 4000 g for 10 min. Emulsion capacity (EC) was calculated using Equation (2). The emulsion stability (ES) was determined by heating the emulsion at 80 °C for 30 min, and tubes containing emulsions were centrifuged after cooling to room temperature. ES was calculated using Equation (3).
For turbidimetric determination, well-diluted emulsions in 0.1% SDS solution were measured using a UV spectrophotometer (Shimadzu Co. UV-2550, Tokyo, Japan) at 500 nm (A500), after producing the emulsion and after 1 h at room temperature. The spectrophotometric readings were used to calculate (Equations (4) and (5)) the emulsion activity index (EAI), defined as the ability of OLPs to form an oil-in-water dispersion, and the ES index (ESI) of the emulsion after 1 h.
In the above equations, Ev is the emulsion volume. Tv is the total volume, and Fev and Iev are the final emulsions and initial emulsion volumes after heat treatment.
where DF, C, and φ represent dilution factor, OLP concentration (g/mL), and volumetric oil fraction. A0 and A1 are absorbances at time 0 and after 1 h, respectively.
2.8. Thermal Properties of OLPs
The thermal properties of OLPs were evaluated using a differential scanning calorimeter (DSC, TA Instruments, New Castle, DE, USA) and a thermogravimetric analyzer (TGA SDT 650, TA Instruments, New Castle, DE, USA) to monitor the thermal stability and mass loss of OLPs during thermal treatment. For DSC analysis, about 10 mg of OLP powder was weighed and hermetically sealed in a DSC stainless steel pan, while an empty pan was used as a reference. Then, the pans were heated from 20 °C to 400 °C at 10 °C/min [35]. The enthalpy was calculated by integrating the area under the curve between onset and end-set temperatures using software associated with the hardware (Trios software v5.0.0 TA instruments, New Castle, DE, USA). For TGA analysis, about 10 mg of OLP powder was weighed into a 40 μL platinum pan, and the mass loss was recorded at temperatures between 30 °C and 400 °C with an increment at a rate of 10 °C/min. These tests were conducted under nitrogen flushing at 50 mL/min.
2.9. Antioxidant Activity
2.9.1. ABTS Radical Scavenging Activity
The ABTS radical scavenging assays (ABTS-RSA) were determined [36] and applied to the antioxidant activity of OLPs. Briefly, 50 μL of OLP solution dissolved in distilled water at final concentrations of 1–10 mg/mL or distilled water as control was mixed with 950 μL of ABTS radical cation solution and kept in the dark at 30 °C for 30 min. The absorbance readings were taken at 734 nm using a UV spectrophotometer (UV-2550, Shimazu Co., Tokyo, Japan), and ABTS-RSA was calculated using Equation (6) [37].
A0 and A1 represent the absorbances of the control and sample, respectively.
2.9.2. Ferric Reducing Antioxidant Power
The ferric reduction power (FRAP) was used to assess the ability of OLPs to reduce iron (III). Briefly, 100 µL of various concentrations (1–10 mg/mL) of OLP solution with 900 µL of FRAP reagent in the dark at room temperature for 30 min [7]. FRAP reagent consisted of 300 mM acetate buffer (pH 3.5), 10 mM TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine) solution in 40 mM HCl, and 20 mM ferric chloride solution in 40 mM HCl at a ratio of 10:1:1, respectively. The absorbance was measured using a UV spectrophotometer at 593 nm, and the results were expressed as the absorbance (abs) at 593 nm.
2.10. Statistical Analysis
All the experiments were performed in triplicate, and the results are presented as mean ± standard deviation (SD). Means were separated by Tukey’s test using SPSS v.22 statistical software (SPSS Inc., Chicago, IL, USA) at 5% significance (p < 0.05).
3. Results and Discussion
3.1. Physicochemical and Molecular Characteristics of OLPs
The extraction yields, physicochemical characteristics, and molecular weights of OLPs varied significantly (p < 0.05) in terms of extraction pH (Table 1). Extraction yield (2.74–7.34%), total sugars (64.87–95.68%), and total protein (0.18–0.45%) contents increased toward an alkaline condition as pH increased (OLP4 < OLP7 < OLP9). This result agrees with previous studies [6,38], which reported similar higher yields, sugar, and protein contents under alkaline extraction conditions (< pH 12). A higher yield in OLP9 may be due to the better solubility of OLPs under alkaline conditions [6] and the additional recovery of insoluble cell wall polysaccharides and their conversion to soluble polysaccharides by alkaline hydrolysis of hydrogen bonds between cellulose and hemicellulose [25]. Additionally, the decreased total sugar content in OLP4 may be due to the partial hydrolysis of some sugars at acidic pH accompanied by ultrasonic cavitation [24,25]. Moreover, higher total sugar content in OLP9 indicated alkaline extraction is well applicable to obtain OLPs with increased polysaccharide content. Similar to this present study, low protein content has been reported for OLPs [15], suggesting little or no protein contribution to the structure of OLPs. Higher protein content in OLP9 is due to the ease in the hydrolysis of amide bonds in protein by alkaline solution, resulting in a higher amount of soluble protein in the extract, available for co-precipitation with the polysaccharide [21].

Table 1.
Physicochemical properties and molecular weights of polysaccharides from okra leaves (OLPs).
All samples showed a high DA (18.28–22.88%) and low DM (8.97–11.38%), with OLP7 having higher values for both parameters. This variation could be due to the extraction of unesterified homogalacturonan by acid- and base-catalyzed hydrolysis of esters [11]. High DA and low DM are typical of okra polysaccharides [12]. Furthermore, the result of DM estimated by FTIR peak areas [20,32] showed lower values for DM, compared with the HPLC method; however, similar trends were observed (OLP7 > OLP4 > OLP9).
The total polyphenol content of OLPs was in the range of 1.37–2.90 mg GAE/g, indicating a low coextraction of polyphenols that may exist as impurities [15]. Moreover, it further suggests that these polyphenols were more soluble at neutral pH than acidic and alkaline solutions. According to extraction solution pH, significant differences (p < 0.05) in powder color values (L*-lightness, a*-redness, b*-yellowness), bulk densities (277.07–348.05 kg/m3), solubility (64.92–95.72%), and water-holding capacity (4.48–9.11 g/g) were observed in OLPs. OLP4 had a light and creamy-powder color and the highest bulk density value of 348.05 kg/m3; however, it was less soluble in water (64.92%) than other samples at room temperature. Conversely, OLP9 had the highest water solubility and similar bulk densities to OLP7. This result indicated an inverse relationship between bulk densities and water solubility of OLPs at room temperature. Moreover, a high WHC value of OLP7 at 9.11 g/g indicated that 1 g of OLP7 could absorb a 9.11 g mass of water. Overall, based on the results, differences in extraction pH affected the physical properties of OLPs.
The molecular weight result reflected differences in the average molecular weights (Mw) of OLPs with values of 68.5, 72.1, and 85.4 kDa for OLP4, OLP7, and OLP9, respectively (Table 1). The polydispersity index values of OLPs were similar (1.08–1.09), indicating their narrow molecular distribution and uniform dispersity in an aqueous solution [3]. This result suggested that OLPs with a high molar mass can be obtained by alkaline extraction. Moreover, a lower Mw value observed in OLP4 could be attributed to the partial hydrolysis of OLPs under acidic pH extraction [11]. The molecular parameters of polysaccharides are related to the corresponding functional properties of OLPs [15].
3.2. Monosaccharide Composition and Molar Ratios of OLPs
Monosaccharide analysis provided insights into the constituent sugars of the OLP structure (Table 2). OLPs comprised varying amounts of galactose (25.03–39.58 mol%), rhamnose (4.86–12.85 mol%), and arabinose (14.51–18.73 mol%) as the main neutral sugars and galacturonic acid (36.83–47.61 mol%), indicating that all OLPs are acidic polysaccharides [39]. Similar to this study, polysaccharides obtained from the pods [12,18], leaves [15], and flowers [4,14] of okra plants have been reported to contain pectic polysaccharides. Notable variations in molar percentages were observed in OLPs due to extraction pH. Such differences are associated with different extraction protocols, including extraction pH [6,11,40], with possible effects on their functional properties and biological activities [25]. For instance, the higher molar content of galactose and arabinose in OLP4 than those in other samples suggests a higher contribution of neutral sugars to their structure. Moreover, the high molar composition of GalA in OLP9 correlates with the low content of Gal and Ara sugars, indicating that alkaline pH resulted in the extraction of OLPs with low, neutral sugar composition [6,38].

Table 2.
Monosaccharide composition and molar ratios of polysaccharides from okra leaves (OLPs).
The molar ratios of monosaccharides composed in OLPs were further used to describe the structural conformation of OLPs (Table 2). The different molar ratio (R1–R3) values informed changes in side-chain and backbone structures of OLPs according to extraction pH. The R1, R2, and R3 illustrated the contribution of rhamnogalacturonan I (RG-I) to backbone structure, side-chain length of RG-I, and linearity of pectic polysaccharides, respectively [41,42]. The backbone structure of pectic polysaccharides may contain homogalacturonan (HG) and rhamnogalacturonan I (RG-I) covalently linked together. HG consists of repeating units of esterified and unesterified GalA residues, whereas RG-I consists of alternating units of Rha and GalA [18]. The R1 value of 0.13–0.29 confirms the presence of RG-I in the backbone structure of all OLPs (0.05 < R1 <1.00) in varied proportions depending on extraction pH [43]. OLP4 had the highest R2 value of 12.00 and the lowest R3 value of 0.58, indicating a long side-chain branching, i.e., arabinan, galactan, and arabinogalactan [38], and less backbone linearity, compared with other samples. Conversely, OLP9 exhibited fewer side-chain lengths (R2, 3.08) and a more linear backbone structure (R3, 0.91) than other samples. Therefore, it could be assumed that the neutral side-chain sugars along RG-I regions of OLPs were more easily degraded by sonication at alkaline pH. Similar observations have been reported in previous studies [40].
3.3. Structural Characteristics of OLP
3.3.1. FTIR Characteristics
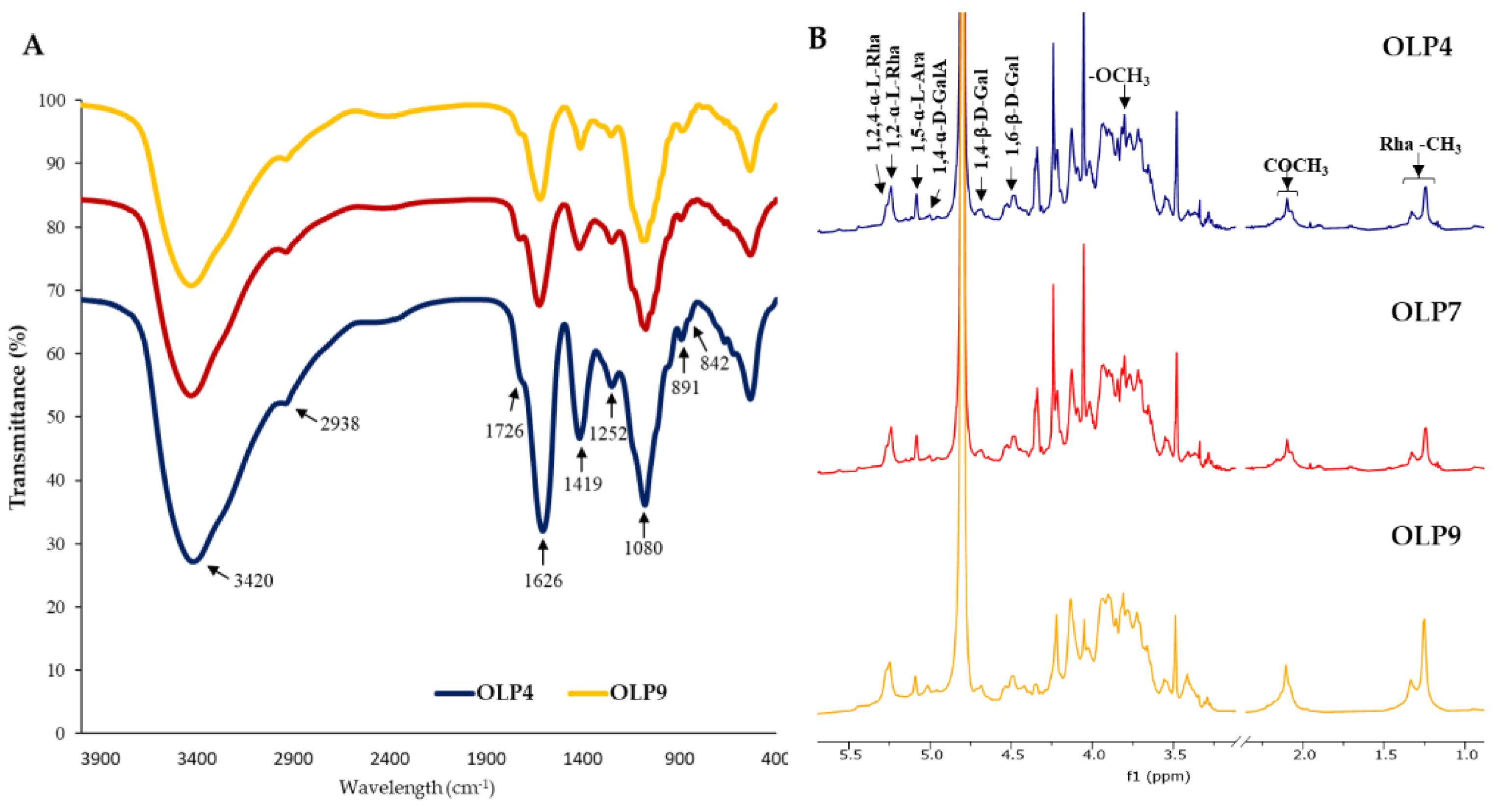
Figure 1A shows that all OLPs had identical FTIR absorption bands, indicating to some extent similarities in their structural features. The broad and intense peak of the hydroxyl group (−OH) at 3420 cm−1, the weak and small C–H group peak at 2938 cm−1, and the α- and β-glycosidic linkage peaks at 842 and 891 cm−1 are typical polysaccharide peaks [16,21,44]. The small peaks at 1726 and the intense and sharp peaks at 1626 correspond to C=O stretching of methyl-esters of carbonyl groups (CH3COO−) and stretching of carboxylic acid (−COOH), respectively [21]. These peak areas estimate the degree of esterification of polysaccharides [20,32]. Relative to the sharp peaks at 1626, the smaller peaks at 1726 confirmed low methylation in all OLPs (Table 1). However, compared with the HPLC determination, this method may not give a reliable estimation of methoxylation due to the broad stretching response of O–H at 3600–2500 cm−1, masking the activity of O–CH3 [45]. Additionally, the absorption peaks at 1419 cm−1 resulting from the C=O stretching vibration confirm the presence of uronic acid [46]. The absorption peaks at 1252 cm−1 correspond to the C–O stretching of the acetyl group [46,47]. The FTIR peaks conform well with the characteristic peaks of pectic polysaccharides in previous studies [12,48] and reflect no noticeable structural changes in OLPs.
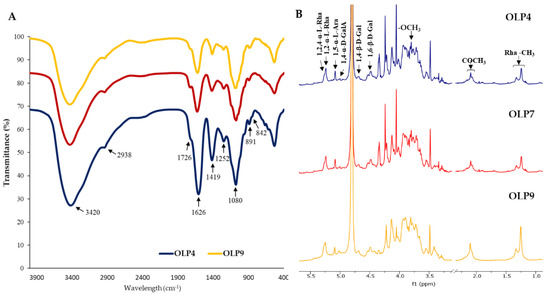
Figure 1.
FTIR (A) and 1H NMR (B) spectra of polysaccharides from okra leaves (OLPs).
3.3.2. NMR Characteristics
The 1H-NMR spectra of all OLPs exhibited similar characteristic peaks, consistent with the FTIR spectra, indicating no notable alteration in the primary structure of OLPs with the different extraction pH (Figure 1B). The main glycosidic linkages could be retained in polysaccharides extracted under various conditions [49,50]. The well-resolved α-anomeric protons (5.5–4.75 ppm) and β-anomeric protons (4.75–4.25 ppm) peaks observed in the spectra of leaf powder (Figure S1) and OLPs (Figure 1B) are attributed to the glycosidic regions of polysaccharides [51]. Other clustered proton peaks around 3.25–4.45 ppm were attributed to H-2–H-6 of constituent sugar residues [14,52].
The peaks at 5.27 and 5.25 and 1.27 and 1.25 ppm (Rha-CH3) were assigned to H-1 and H-6 of 1,2,4-ɑ-L-Rha and 1,2-ɑ-L-Rha, respectively, confirming the presence of branched and unbranched rhamnose residues in OLPs [15,18]. The H-1 peak of 1,5-ɑ-L-Ara was observed at 5.11 ppm. The H-1 peak of 1,4-ɑ-D-GalA and the methyl (−CH3) peak of esterified H-6 were observed around 5.02 and 3.80 ppm [19,44]. Additionally, two proton peaks of O-2 and O-3 acetyl groups attached to GalA residues were observed around 2.10–2.12 ppm [12]. The H-1 of 1,4-β-D-Gal and 1,6-β-D-Gal linkages were assigned to peaks at 4.69 and 4.48 ppm, respectively [2,50]. The NMR result was generally applicable to confirm the main sugar residues in OLPs, as seen in Table 2, which agrees with previous studies on okra plant polysaccharides [11,14,15].
3.3.3. X-ray Diffraction
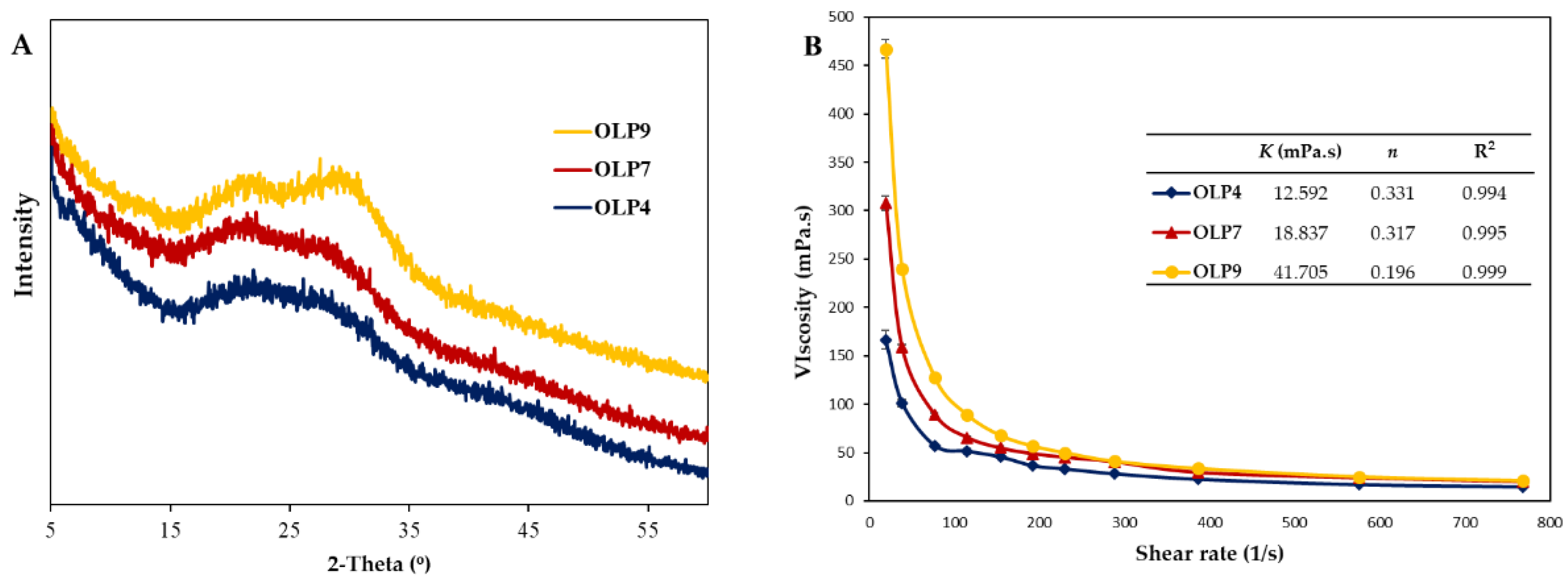
X-ray, an essential non-destructive tool, provided information on the amorphous or crystalline structure of OLPs (Figure 2A). According to the pattern, OLPs mainly consisted of amorphous nature, with wide and narrow peaks indicating amorphous and crystalline regions [53]. All OLPs showed similar XRD patterns and characteristic peaks around 21.5°, 27.5°, and 29.8° at 2θ, indicating the semicrystalline nature of the polysaccharides [54]. However, slight changes in their peak intensities were observed according to extraction pH, indicating variations in crystalline nature. The broad diffraction peaks pronounced in OLP4 showed the destructive effects of acidic extraction pH on crystalline regions [25]. In agreement with this study, alkaline-extracted pectin from citrus peels was reported to contain more crystalline structures than pectins obtained by other methods [26]. The presence of crystalline structures in pectin is due to their highly complex structures containing heterogeneous and alternating sugar units that differ in their molecular organization [55].
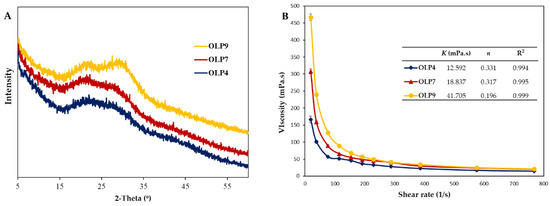
Figure 2.
X-ray diffractogram (A) and apparent viscosity at different shear rates (B) of polysaccharides from okra leaves (OLPs). In Figure 2B (inset), K and n denote the power-law model’s flow consistency and flow behavior index (Equation (1)).
3.4. Functional Properties
3.4.1. Viscosity and Flow Characteristics
Polysaccharides’ viscosity and flow behavior in an aqueous system is an essential functional property critically considered before its application in food-related systems [1]. The apparent viscosity of OLPs at different shear rates (0–800 s−1) showed distinct differences, especially at lower shear rates (below 100 s−1, Figure 2B). The apparent viscosities at 76.8 s−1 were 57.27, 89.22, and 127.68 mPa/s, whereas, at a higher shearing rate of 768 s−1, apparent viscosities were 14.87, 20.13, and 20.98 mPa/s for OLP4, OLP7, and OLP9, respectively.
Furthermore, the power-law model equation describes the flow characteristics, and the parameters are summarized in Figure 2B (inset). The coefficient value R2 at 0.994–0.999 indicates an excellent fitting of experimental data to the model. The flow consistency index value (K) was in the range of 12.592–41.705 mPa/s, which correlated with their apparent molecular weights. Moreover, all OLPs exhibited non-Newtonian fluid properties (n ≤1), as indicated by their dimensionless flow behavior index (n = 0.196–0.331). A high value for n is related to a low level of pseudo-plasticity and vice versa. These results reflect apparent changes in the flow behavior of OLPs according to extraction pH. Previous studies have also reported differences in viscometrical characteristics of polysaccharides influenced by the extraction methods [11,19,56]. The observed differences in OLPs may well be attributed to their corresponding molecular weights, uronic acid, and protein contents, among other structural factors [1,57,58]. Based on these parameters, OLP9 could be used as a natural thickener, whereas OLP4 may be applicable for processes requiring low-viscosity polymers, such as spray coating.
3.4.2. Emulsifying Ability and Stability
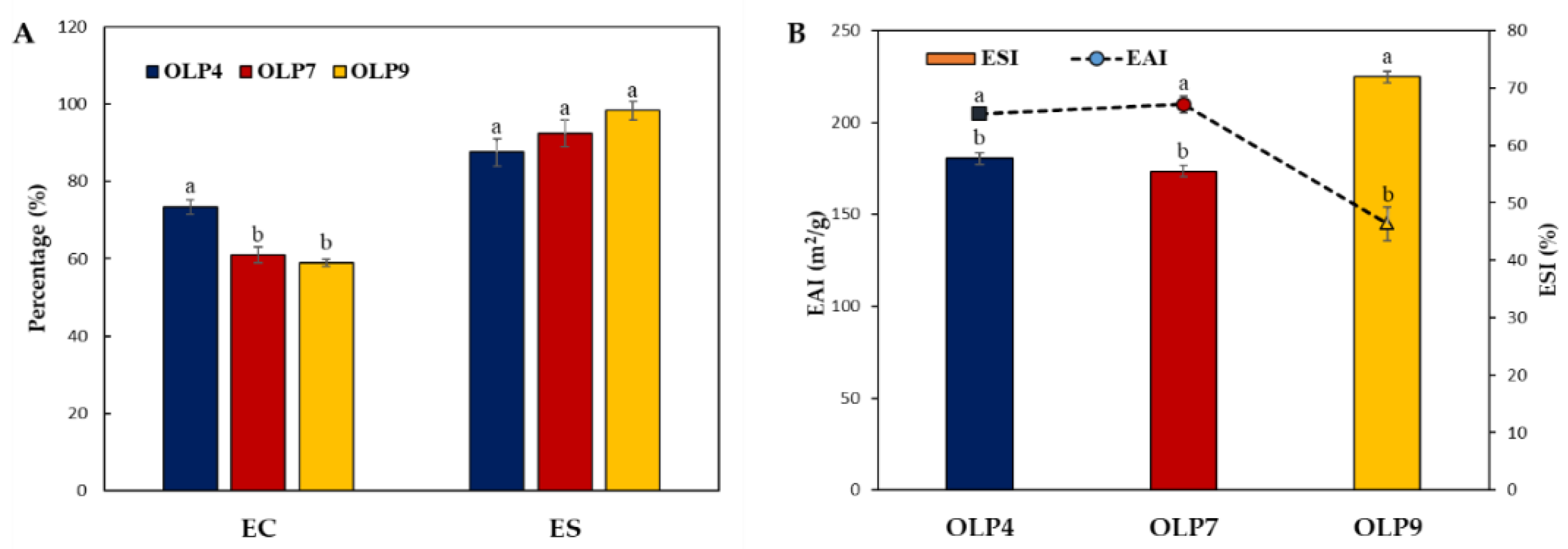
The emulsifying properties of OLPs were examined using centrifugal and turbidimetric methods (Figure 3). The former estimates the % ratio of the emulsified layer to the total volume, showing significant differences (p < 0.05) in the EC, 58.94–73.48%, and stability ES, 87.50–98.28% of OLPs (Figure 3A). Additionally, the values for EAI and ESI of OLPs were in the range of 144.86–209.75 m2/g and 57.70–71.95% (Figure 3B), respectively. EAI measures the fraction of oil enveloped by the continuous phase, and the ESI measures the ability of the emulsions to resist instability due to creaming, flocculation, or coalescence over a specific period [34].
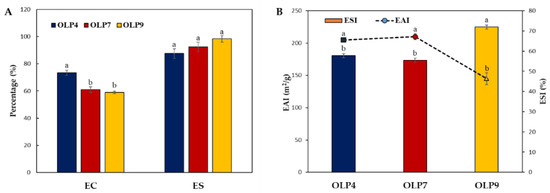
Figure 3.
Emulsifying properties of polysaccharides from okra leaves (OLPs). Centrifugal (A) and turbidimetric (B) estimation of emulsifying activity (EC and EAI) and stability (ES and ESI). a,b Superscripts represent significant differences (Tukey’s test, p < 0.05).
Notably, rapid emulsion formation was observed in OLP4 emulsion. However, a more stable emulsion was formed with OLP9, as observed in both emulsion assays (ES and ESI). Generally, all OLPs showed comparably good emulsifying properties, similar to previous studies [5,15,59]. The molecular weight, neutral sugar and side-chain composition, degree of esterification, and protein and acetyl group contents have been shown to influence the emulsifying properties of polysaccharides [57]. The rapid emulsion formation in OLP4 could be attributed to its extensively branched structure (Table 2), which positively influences emulsion formation by polysaccharides. Moreover, increased ES via the inhibition of creaming, droplet aggregation, and coalescence can be achieved in a continuous phase with higher viscosity by reducing the kinetic mobility of oil droplets [58]. This effect is more evident for polysaccharides with high molecular mass than those with low molecular mass, which explains the result observed for OLP9 in this study. Additionally, stabilization by steric and electrostatic repulsive force can be achieved by the higher amount of protein absorbed on the surface of oil droplets and increased charged groups along the polysaccharide chain [58,60].
3.4.3. Thermal Stability Properties
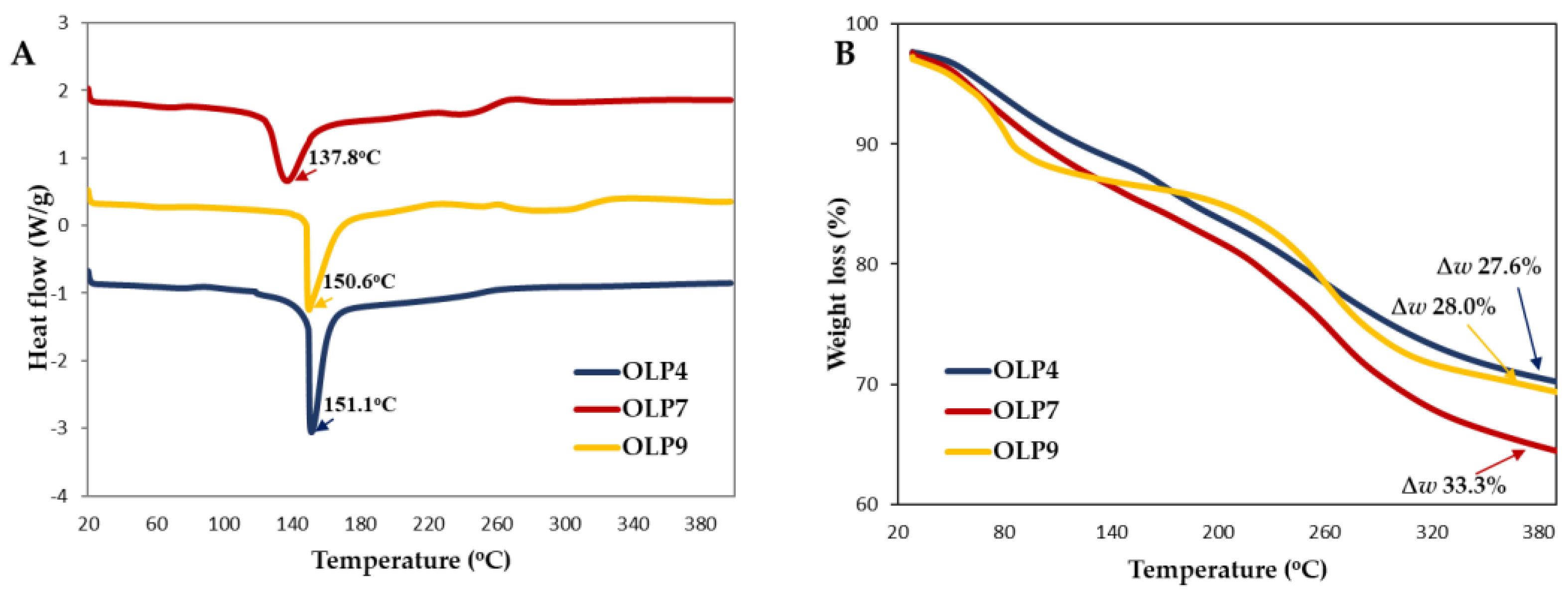
The differential effects of extraction pH on the thermal stability of OLPs were expressed using DSC and TGA curves (Figure 4). The thermal stability of polysaccharides helps predict their industrial applications [8]. In the DSC curve (Figure 4A), prominent endothermic peaks were observed at 137.8 °C in OLP7 and around 150–151°C in OLP4 and OLP9, corresponding to dehydration or degradation of polysaccharides [5,61]. The enthalpy changes during the reaction were 606.46, 581.76, and 459.76 J/g for OLP4, OLP7, and OLP9, respectively.
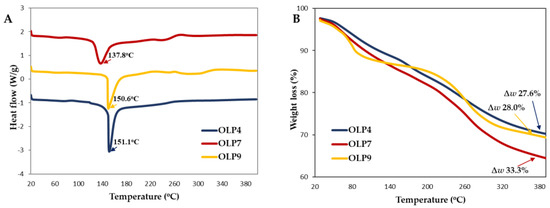
Figure 4.
Thermal stability properties of polysaccharides from okra leaves (OLPs). DSC (A) and TGA (B) curves. ∆w represents % weight loss.
Furthermore, as shown in Figure 4B, OLPs underwent stages of weight loss between 20 and 400 °C, corresponding to mass loss due to initial dehydration of free and bound water (50–155 °C), and the decomposition and chemical changes in the functional group of polysaccharides occurred in the temperature range of 211–330 °C [14]. At the end of the reaction, different total weight losses (∆w) were recorded at 27.6%, 33.3%, and 28.0% for OLP4, OLP7, and OLP9, respectively, lower than the reported values in the literature [14,15]. Overall, the results suggested that the thermal stability of OLPs was influenced by extraction pH. However, all OLPs are remarkably thermostable [8]. Differences in thermal properties of pectic polysaccharides are attributed to their physicochemical and structural characteristics, including constituent sugars, linearity and side-chain branching, and crystallinity [47].
3.5. Antioxidant Activity
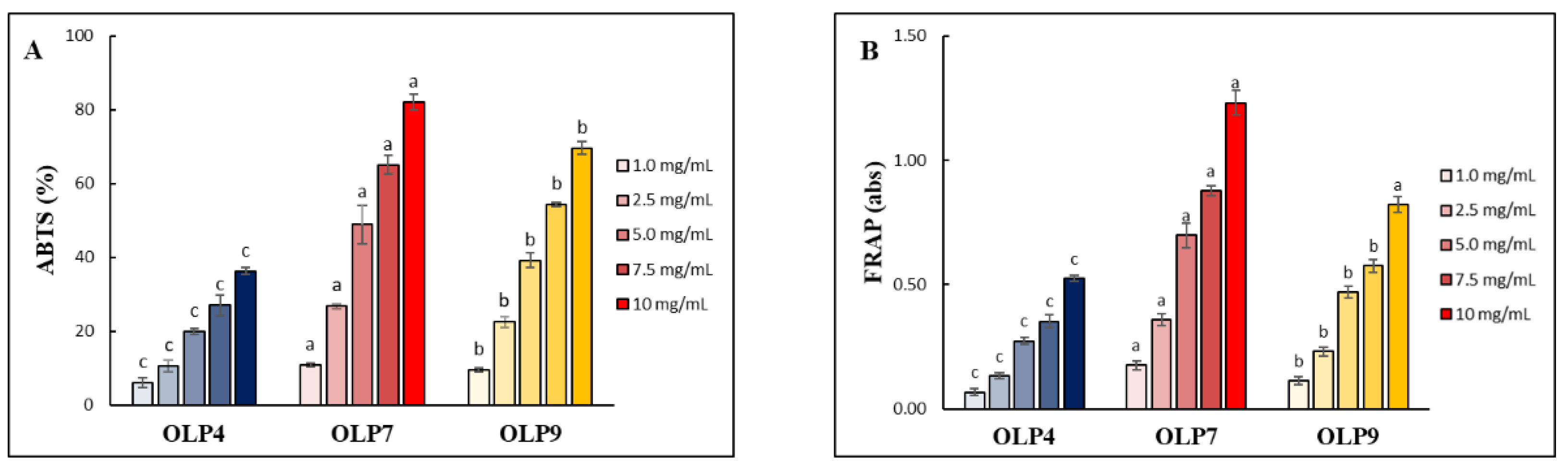
The ABTS radical scavenging assay (ABTS-RSA), widely employed to access the antioxidant activity of water-soluble polysaccharides, was used to evaluate the antioxidant activities of OLPs at various concentrations (1–10 mg/mL). All OLPs showed concentration-dependent antioxidant activities (Figure 5A), with half-maximal inhibitory (IC50) values of 14.17, 5.62, and 6.87 mg/mL for OLP4, OLP7, and OLP9, respectively, indicating notable differences in antioxidative properties of OLPs under different extraction pH. Furthermore, OLPs displayed good reducing powers (Figure 5B). At the concentration of 2.5 mg/mL, the absorbance of OLPs ranged from 0.13 to 0.36, in which a higher absorbance value indicates higher reducing power [22]. Due to compositional variations, differences in antioxidant activities of polysaccharides extracted from the same material under different extraction conditions have been well documented [20,21]. This study’s ABTS-RSA values reported for OLPs are close to the reported values for polysaccharides obtained from okra plants and other plant leaves [3,14,19,22]. Moreover, the value reported for FRAP in this study was comparably higher than the values (0.10–0.15) reported for okra-pod polysaccharides obtained by different extraction methods [20].
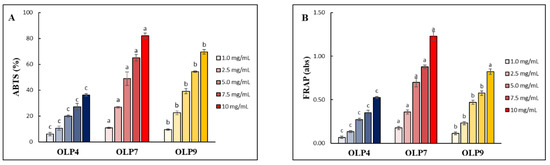
Figure 5.
(A) ABTS radical scavenging activity (ABTS-RSA) and (B) ferric reducing antioxidant power (FRAP) of polysaccharides from okra leaves (OLPs) at various concentrations. The color intensity in the graph denotes increasing concentration from 1.0 to 10 mg/mL. a–c Superscripts represent significant differences (Tukey’s test, p < 0.05).
In both antioxidant assays, more potent antioxidant activity in OLP7, compared with other samples, is likely due to their compositional variations, including their higher polyphenol content (Table 1). The underlying mechanisms of polysaccharide antioxidant activities are attributed to their electron- or hydrogen-ion- (H+) donating capacity influenced by their neutral sugars, total uronic acid, and unesterified carboxylic acids [9,20,33]. Additionally, the presence of coextracted phenolic compounds has equally been reported to enhance the antioxidant activities of polysaccharides [15,33].
4. Conclusions
This study characterized okra leaf polysaccharides (OLPs) obtained under mildly acidic to mildly alkaline pH (4.2, 6.8, and 9.2) extraction and examined their functional properties for their intended uses. According to extraction pH, significant variations in physicochemical properties, monosaccharide compositions, functional properties, thermal stability, and antioxidant activities of OLPs were observed. However, FTIR and NMR data revealed similarities in structural characteristics of all OLPs. Thus, it appeared that the effects of extraction pH on primary structures of OLPs were negligible. The observed variations in functional properties of OLPs were systematically related to their physicochemical differentiation, variation in the molar ratio of the monosaccharides, and molecular weights. Based on the functional properties, OLP9 exhibited superior rheological properties, produced more stable emulsions, and showed good thermal properties and antioxidant activity. The results of this study are applicable in preparing OLPs with specific characteristics and functions via tailored pH extraction.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry4020030/s1, Figure S1: 1H NMR Spectra of okra leave powder before extraction.
Author Contributions
Conceptualization, I.F.O. and W.Y.L.; methodology, I.F.O.; validation, I.F.O., J.J.P., and D.H.; formal analysis, I.F.O., J.J.P., and D.H.; investigation, I.F.O.; data curation, I.F.O.; writing—original draft preparation, I.F.O.; writing—review and editing, I.F.O., J.J.P., D.H., and W.Y.L.; visualization, I.F.O.; resources and supervision, W.Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The first author I.F. Olawuyi, wishes to acknowledge the scholarship funds received from Samsung Dream Scholarship Foundation, Korea.
Conflicts of Interest
There are no conflict of interest to declare.
References
- Olawuyi, I.F.; Kim, S.R.; Lee, W.Y. Application of plant mucilage polysaccharides and their techno-functional properties’ modification for fresh produce preservation. Carbohydr. Polym. 2021, 272, 118371. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhang, W.; Liu, L.; Cheng, Y.; Guo, Y.; Yao, W.; Qian, H. Fractionation, characterization and anti-fatigue activity of polysaccharides from Brassica rapa L. Process Biochem. 2021, 106, 163. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, T.; Feng, W.; Wang, W.; Zou, Y.; Zheng, D.; Takase, M.; Li, Q.; Wu, H.; Yang, L. Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus. Carbohydr. Polym. 2014, 106, 335. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Adhikari, R.; Kasapis, S.; Adhikari, B. Molecular and functional characteristics of purified gum from Australian chia seeds. Carbohydr. Polym. 2016, 136, 128. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, P.; Wang, W.; Wang, M. The influence of extraction pH on the chemical compositions, macromolecular characteristics, and rheological properties of polysaccharide: The case of okra polysaccharide. Food Hydrocoll. 2020, 102, 105586. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70. [Google Scholar] [CrossRef] [Green Version]
- Archana, G.; Sabina, K.; Babuskin, S.; Radhakrishnan, K.; Fayidh, M.A.; Babu, P.A.S.; Sivarajan, M.; Sukumar, M. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr. Polym. 2013, 98, 89. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxidative Med. Cell. Longev. 2016, 2016, 5692852. [Google Scholar] [CrossRef] [Green Version]
- Gemede, H.F.; Ratta, N.; Haki, G.D.; Woldegiorgis, A.Z.; Beyene, F. Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. J. Food Process Technol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Alba, K.; Laws, A.P.; Kontogiorgos, V. Isolation and characterization of acetylated LM-pectins extracted from okra pods. Food Hydrocoll. 2015, 43, 726. [Google Scholar] [CrossRef] [Green Version]
- Kpodo, F.; Agbenorhevi, J.K.; Alba, K.; Bingham, R.J.; Oduro, I.; Morris, G.; Kontogiorgos, V. Pectin isolation and characterization from six okra genotypes. Food Hydrocoll. 2017, 72, 323. [Google Scholar] [CrossRef] [Green Version]
- Raj, V.; Shim, J.-J.; Lee, J. Grafting modification of okra mucilage: Recent findings, applications, and future directions. Carbohydr. Polym. 2020, 246, 116653. [Google Scholar] [CrossRef]
- Zhang, W.; Xiang, Q.; Zhao, J.; Mao, G.; Feng, W.; Chen, Y.; Li, Q.; Wu, X.; Yang, L.; Zhao, T. Purification, structural elucidation and physicochemical property of a polysaccharide from Abelmoschus esculentus L. (okra) flowers. Int. J. Biol. Macromol. 2020, 155, 740–750. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Lee, W.Y. Structural characterization, functional properties and antioxidant activities of polysaccharide extract obtained from okra leaves (Abelmoschus esculentus). Food Chem. 2021, 354, 129437. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, Y.-B.; Chen, T.-T.; Wang, Z.-W.; Yan, J.-K. Innovative preparation, physicochemical characteristics and functional properties of bioactive polysaccharides from fresh okra (Abelmoschus esculentus (L.) Moench). Food Chem. 2020, 320, 126647. [Google Scholar] [CrossRef]
- Gutierrez-Alvarado, K.; Chacón-Cerdas, R.; Starbird-Perez, R. Pectin Microspheres: Synthesis Methods, Properties, and Their Multidisciplinary Applications. Chemistry 2022, 4, 121–136. [Google Scholar] [CrossRef]
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Res. 2009, 344, 1824. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Kim, S.R.; Hahn, D.; Lee, W.Y. Influences of combined enzyme-ultrasonic extraction on the physicochemical characteristics and properties of okra polysaccharides. Food Hydrocoll. 2020, 100, 105396. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, S.; Fu, Y.; Nie, X.-R.; Liu, W.; Su, Y.; Han, Q.-H.; Zhao, L.; Zhang, Q.; Lin, D.-R. Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 2019, 127, 178. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydr. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, F.; Ding, Y.; Li, H.-Y.; Xiang, X.-R.; Ye, Q.; Zhang, J.; Zhao, L.; Qin, W.; Gan, R.-Y. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int. J. Biol. Macromol. 2020, 146, 508. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.; Yadav, S.K. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar]
- Yuliarti, O.; Matia-Merino, L.; Goh, K.K.; Mawson, J.; Williams, M.A.; Brennan, C. Characterization of gold kiwifruit pectin from fruit of different maturities and extraction methods. Food Chem. 2015, 166, 479. [Google Scholar] [CrossRef]
- Dou, Z.-M.; Chen, C.; Huang, Q.; Fu, X. Comparative study on the effect of extraction solvent on the physicochemical properties and bioactivity of blackberry fruit polysaccharides. Int. J. Biol. Macromol. 2021, 183, 1548. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Liao, J.-S.; Qi, J.-R.; Jiang, W.-X.; Yang, X.-Q. Structural and physicochemical properties of pectin-rich dietary fiber prepared from citrus peel. Food Hydrocoll. 2021, 110, 106140. [Google Scholar] [CrossRef]
- Methacanon, P.; Krongsin, J.; Gamonpilas, C. Pomelo (Citrus maxima) pectin: Effects of extraction parameters and its properties. Food Hydrocoll. 2014, 35, 383. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157. [Google Scholar] [CrossRef]
- Voragen, A.; Schols, H.; Pilnik, W. Determination of the degree of methylation and acetylation of pectins by HPLC. Food Hydrocoll. 1986, 1, 65. [Google Scholar] [CrossRef]
- Kyomugasho, C.; Christiaens, S.; Shpigelman, A.; Van Loey, A.M.; Hendrickx, M.E. FT-IR spectroscopy, a reliable method for routine analysis of the degree of methylesterification of pectin in different fruit-and vegetable-based matrices. Food Chem. 2015, 176, 82. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Martinez, M.M.; Yang, B.; Guo, M. Fine structure, physicochemical and antioxidant properties of LM-pectins from okra pods dried under different techniques. Carbohydr. Polym. 2020, 241, 116272. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716. [Google Scholar] [CrossRef]
- Khemakhem, I.; Abdelhedi, O.; Trigui, I.; Ayadi, M.A.; Bouaziz, M. Structural, antioxidant and antibacterial activities of polysaccharides extracted from olive leaves. Int. J. Biol. Macromol. 2018, 106, 425. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239. [Google Scholar] [CrossRef]
- Olawuyi, I.F.; Park, J.; Lee, W.Y. Effect of extraction conditions on ultrasonic-assisted extraction of polyphenolic compounds from okra (Abelmoschus esculentus L.) leaves. Korean J. Food Preserv. 2020, 27, 476. [Google Scholar] [CrossRef]
- Wandee, Y.; Uttapap, D.; Mischnick, P. Yield and structural composition of pomelo peel pectins extracted under acidic and alkaline conditions. Food Hydrocoll. 2019, 87, 237. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Zhu, R.; Yu, J.; Lu, W.; Pan, C.; Yao, W.; Gao, X. Structure characterization, chemical and enzymatic degradation, and chain conformation of an acidic polysaccharide from Lycium barbarum L. Carbohydr. Polym. 2016, 147, 114. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wang, C.; Qiu, W.-Y.; Chen, T.-T.; Yang, Y.; Wang, W.-H.; Zhang, H.-N. Ultrasonic treatment at different pH values affects the macromolecular, structural, and rheological characteristics of citrus pectin. Food Chem. 2021, 341, 128216. [Google Scholar] [CrossRef]
- Alba, K.; Offiah, V.; Laws, A.P.; Falade, K.O.; Kontogiorgos, V. Baobab polysaccharides from fruits and leaves. Food Hydrocoll. 2020, 106, 105874. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Lv, X.; Wang, X.; Wang, X.; Cui, J.; Yan, M. Extractions and rheological properties of polysaccharide from okra pulp under mild conditions. Int. J. Biol. Macromol. 2020, 148, 510. [Google Scholar] [CrossRef] [PubMed]
- Schols, H.; Voragen, A. Complex pectins: Structure elucidation using enzymes. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 14, pp. 3–19. [Google Scholar]
- Wu, D.; Zheng, J.; Hu, W.; Zheng, X.; He, Q.; Linhardt, R.J.; Ye, X.; Chen, S. Structure-activity Relationship of Citrus Segment Membrane RG-I Pectin against Galectin-3: The Galactan is Not the Only Important Factor. Carbohydr. Polym. 2020, 245, 116526. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Goff, H.D. Structural characterization, degree of esterification and some gelling properties of Krueo Ma Noy (Cissampelos pareira) pectin. Carbohydr. Polym. 2004, 58, 391. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97. [Google Scholar] [CrossRef]
- Peng, P.; Peng, F.; Bian, J.; Xu, F.; Sun, R.-C.; Kennedy, J.F. Isolation and structural characterization of hemicelluloses from the bamboo species Phyllostachys incarnata Wen. Carbohydr. Polym. 2011, 86, 883. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195. [Google Scholar] [CrossRef]
- Wu, D.-T.; He, Y.; Fu, M.-X.; Gan, R.-Y.; Hu, Y.-C.; Peng, L.-X.; Zhao, G.; Zou, L. Structural characteristics and biological activities of a pectic-polysaccharide from okra affected by ultrasound assisted metal-free Fenton reaction. Food Hydrocoll. 2022, 122, 107085. [Google Scholar] [CrossRef]
- Li, W.; Wu, D.-T.; Li, F.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Structural and biological properties of water soluble polysaccharides from lotus leaves: Effects of drying techniques. Molecules 2021, 26, 4395. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, G.; Yu, Z.; Song, X.; Li, X.; Yang, Y.; Wang, L.; Liu, L.; Dai, J. Purification, characterization and antiglycation activity of a novel polysaccharide from black currant. Food Chem. 2016, 199, 694. [Google Scholar] [CrossRef]
- Alba, K.; Kontogiorgos, V. Techniques for the chemical and physicochemical characterization of polysaccharides. In Handbook of Hydrocolloids, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–74. [Google Scholar]
- Xiong, F.; Li, X.; Zheng, L.; Hu, N.; Cui, M.; Li, H. Characterization and antioxidant activities of polysaccharides from Passiflora edulis Sims peel under different degradation methods. Carbohydr. Polym. 2019, 218, 46. [Google Scholar] [CrossRef]
- Song, T.; Cai, W.; Wang, F.; Lv, G. Effects of different depolymerisation methods on the physicochemical and antioxidant properties of polysaccharides derived from Sparassis latifolia. Process Biochem. 2021, 110, 110. [Google Scholar]
- Wang, R.-s.; He, X.-h.; Lin, H.; Liang, R.-h.; Liang, L.; Chen, J.; Liu, C.-m. Solubility difference between pectic fractions from creeping fig seeds. Polymers 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontogiorgos, V.; Margelou, I.; Georgiadis, N.; Ritzoulis, C. Rheological characterization of okra pectins. Food Hydrocoll. 2012, 29, 356. [Google Scholar] [CrossRef]
- Kontogiorgos, V. Polysaccharides at fluid interfaces of food systems. Adv. Colloid Interface Sci. 2019, 270, 28. [Google Scholar] [CrossRef]
- Williams, P.A.; Phillips, G.O. Introduction to food hydrocolloids. In Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–26. [Google Scholar]
- Gemede, H.F.; Haki, G.D.; Beyene, F.; Rakshit, S.K.; Woldegiorgis, A.Z. Indigenous Ethiopian okra (Abelmoschus esculentus) mucilage: A novel ingredient with functional and antioxidant properties. Food Sci. Nutr. 2018, 6, 563. [Google Scholar] [CrossRef] [Green Version]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ahmed, M.I.; Ma, M.; Wang, H. Preparation, deproteinization, characterization, and antioxidant activity of polysaccharide from Medemia argun fruit. Int. J. Biol. Macromol. 2020, 155, 919. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).