Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6
Abstract
:1. Introduction
2. Experimental
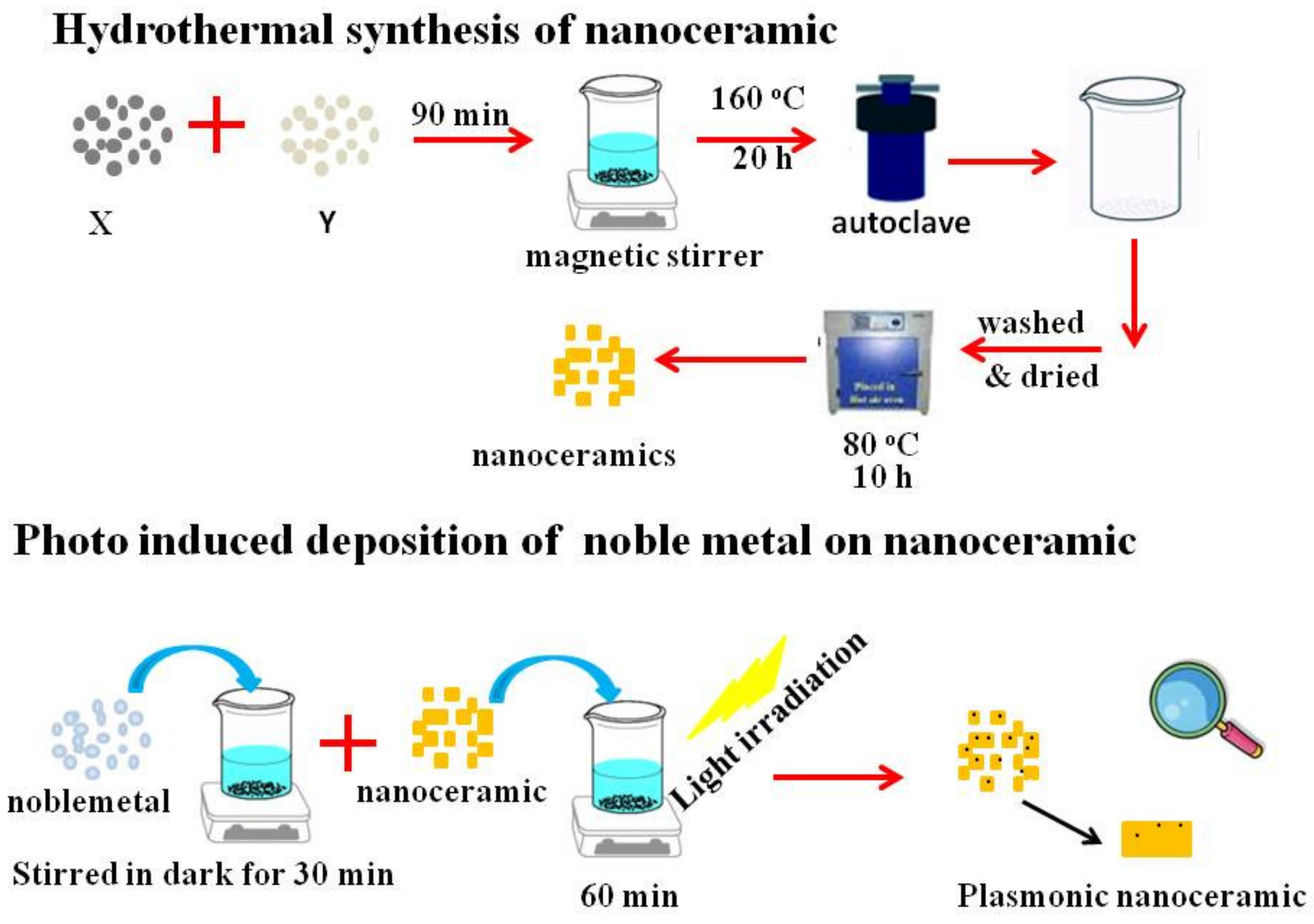
2.1. Synthesis of Bi2WO6
2.2. Synthesis of Plasmonic Ag/Bi2WO6
2.3. Characterization
2.4. Photocatalytic Experiment
Degradation of RhB and MB
2.5. Electrochemical Analysis
3. Results and Discussions
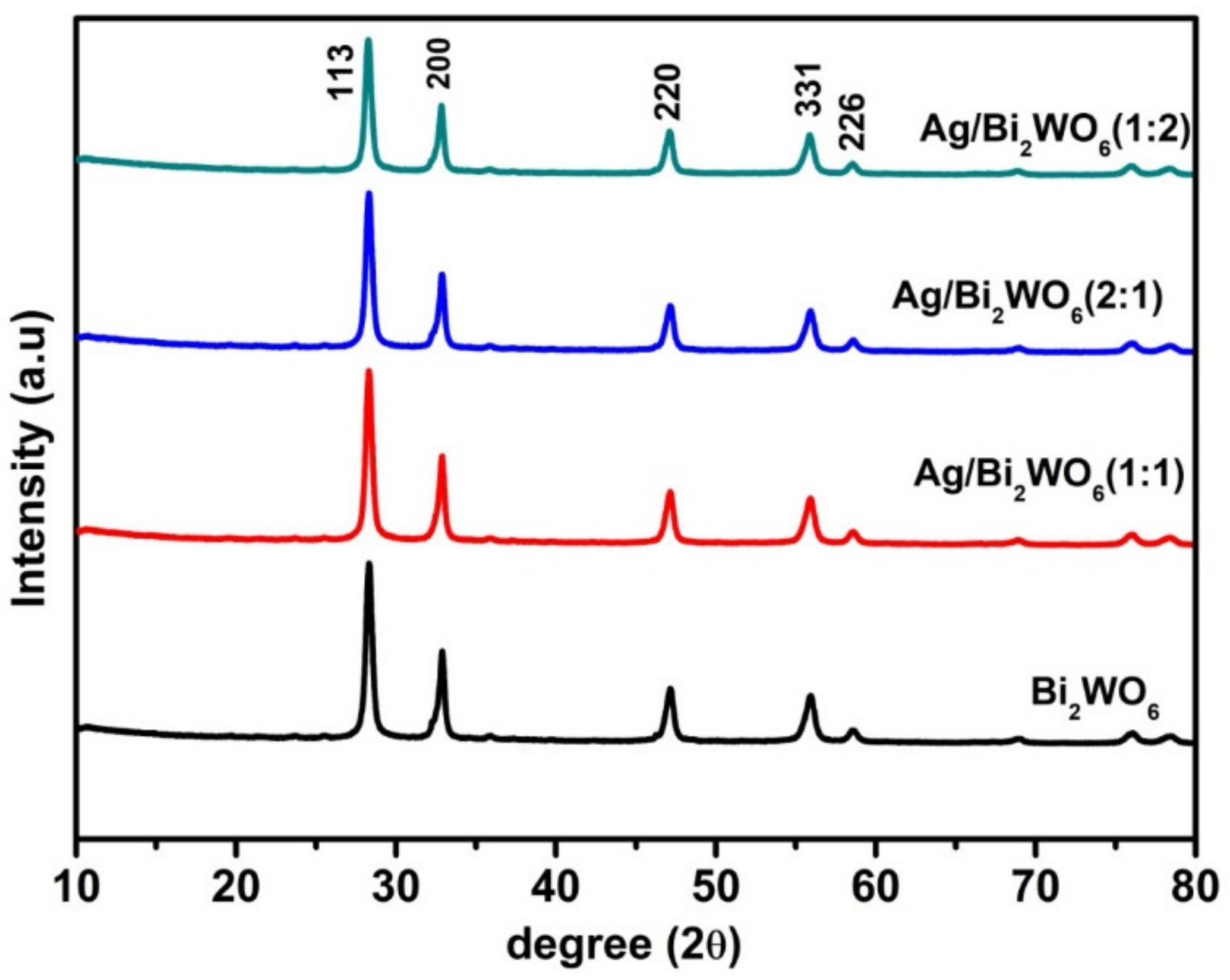
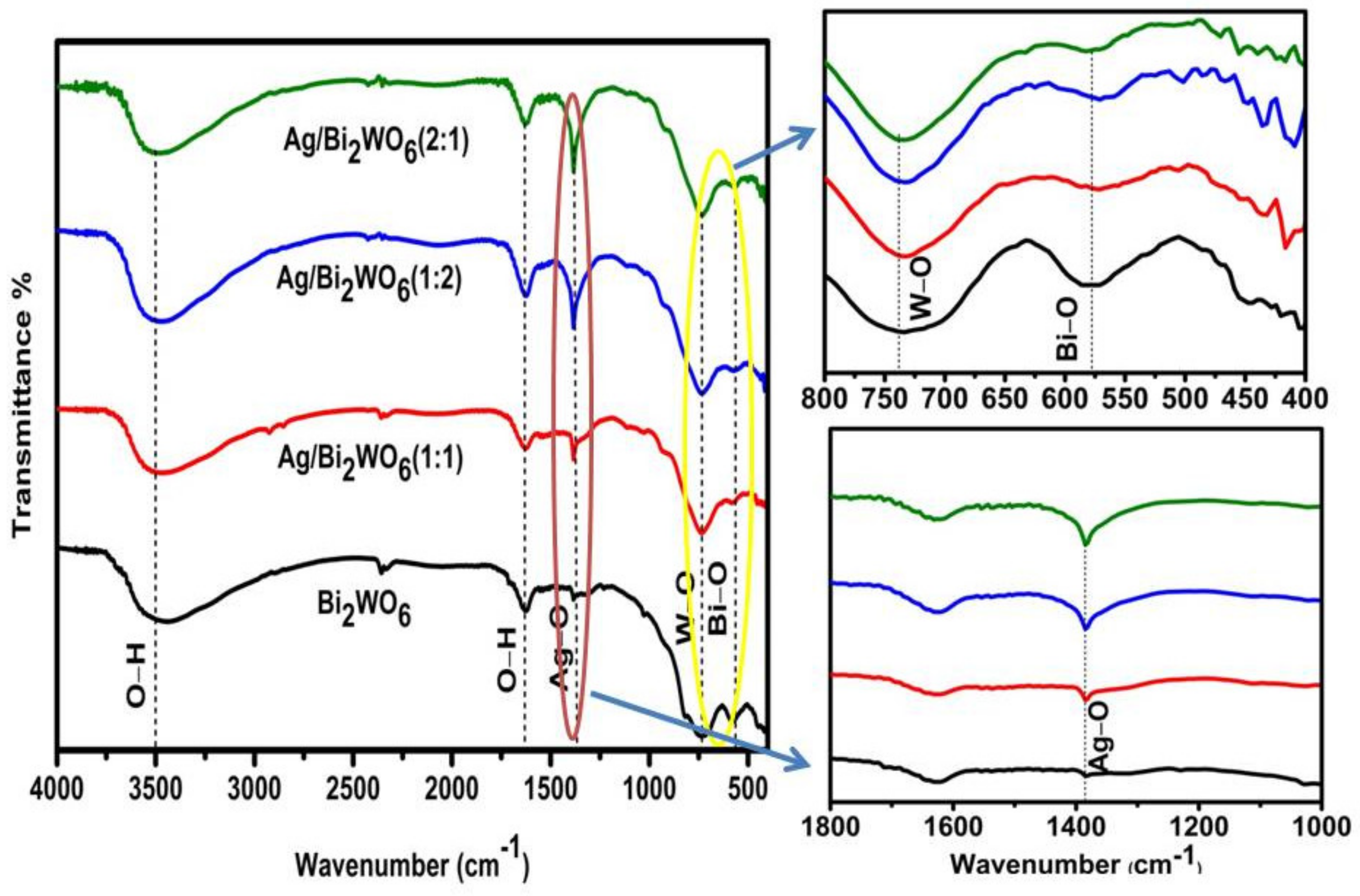
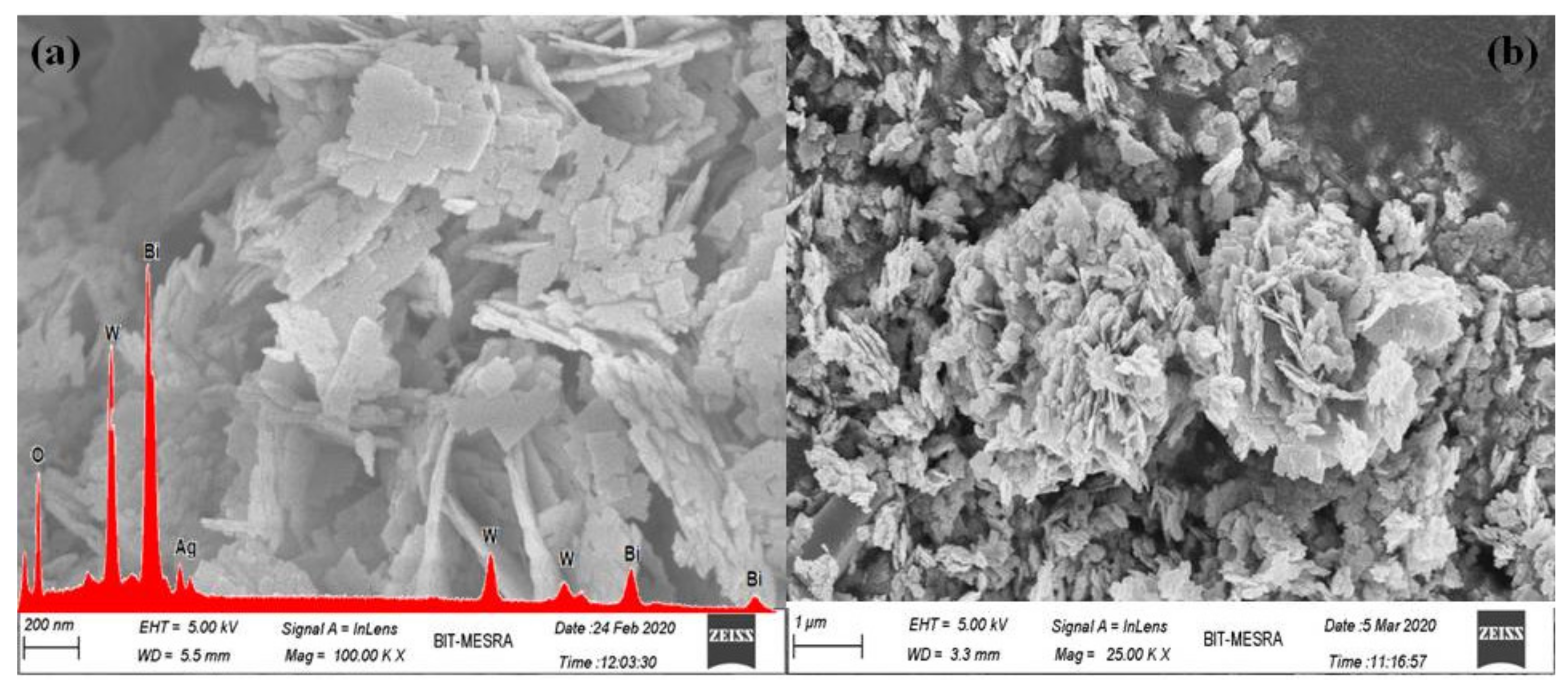
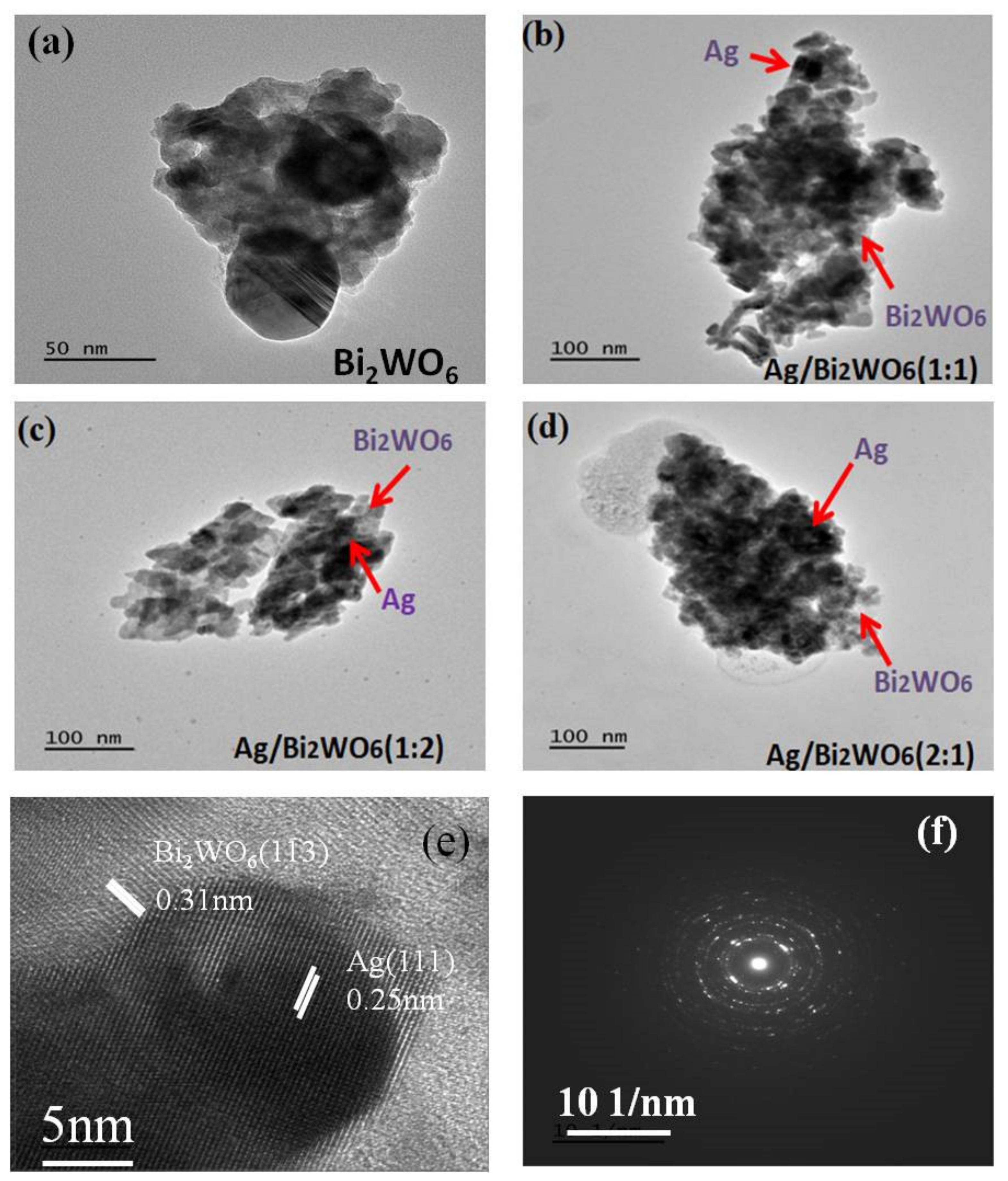
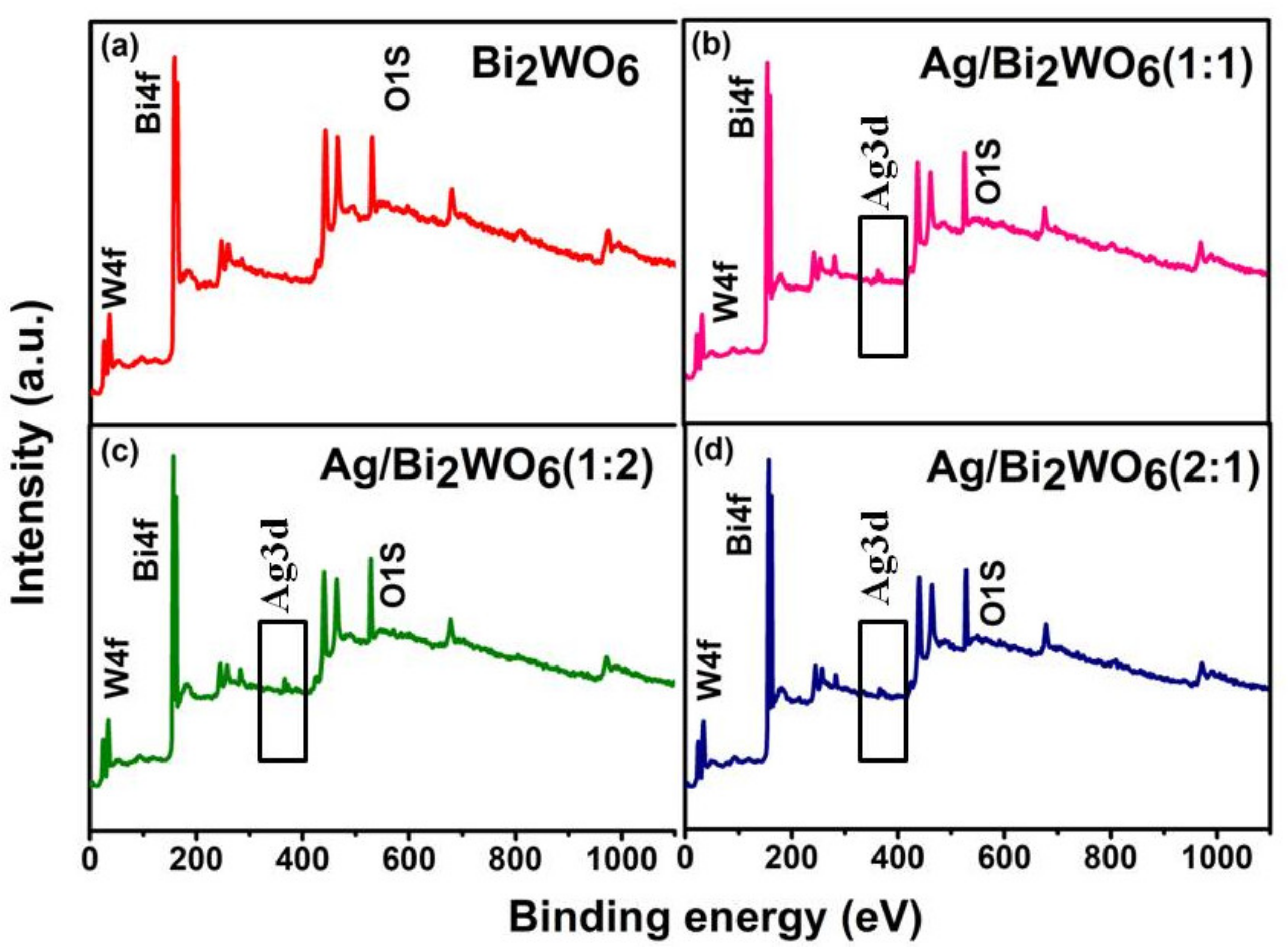
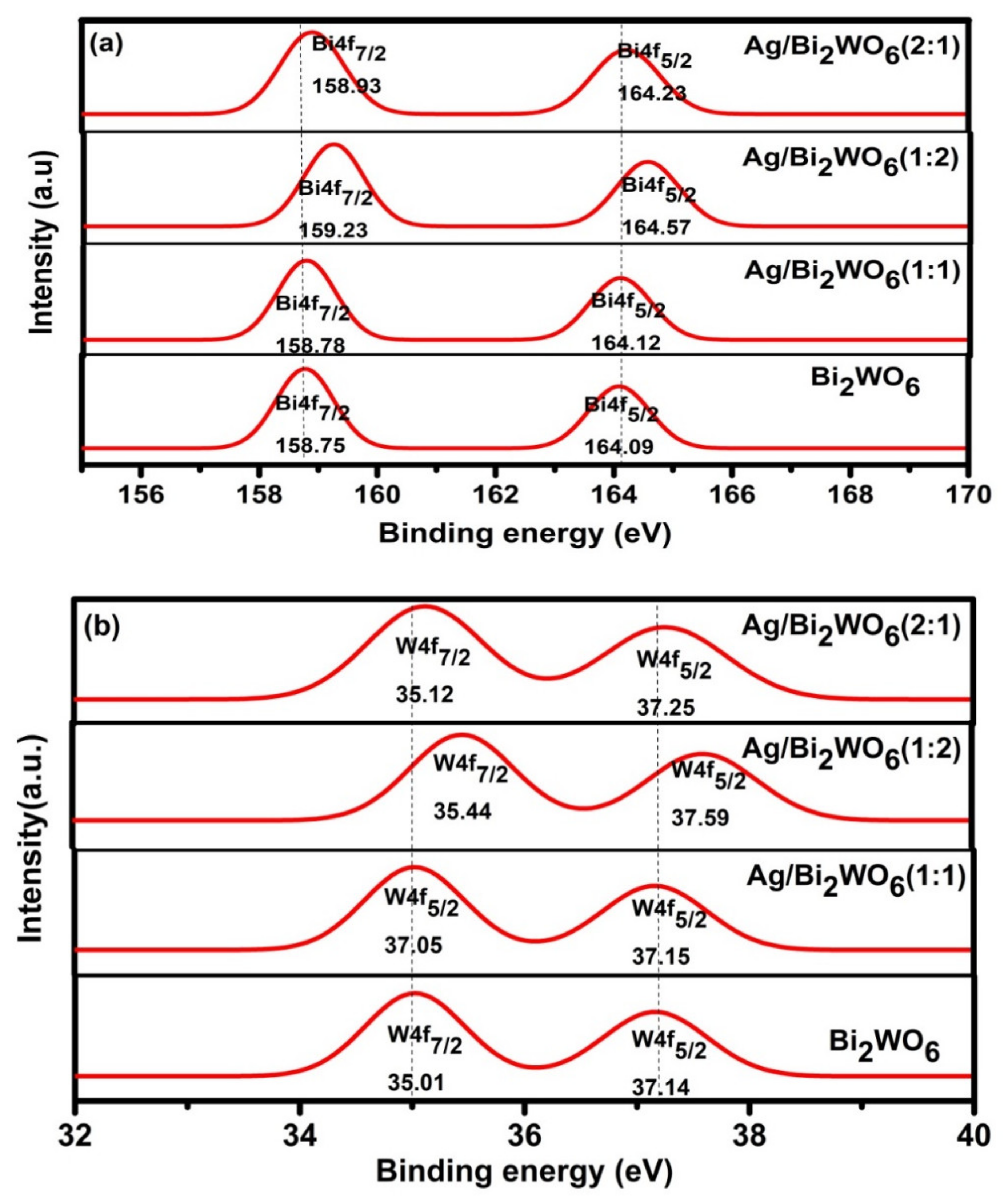
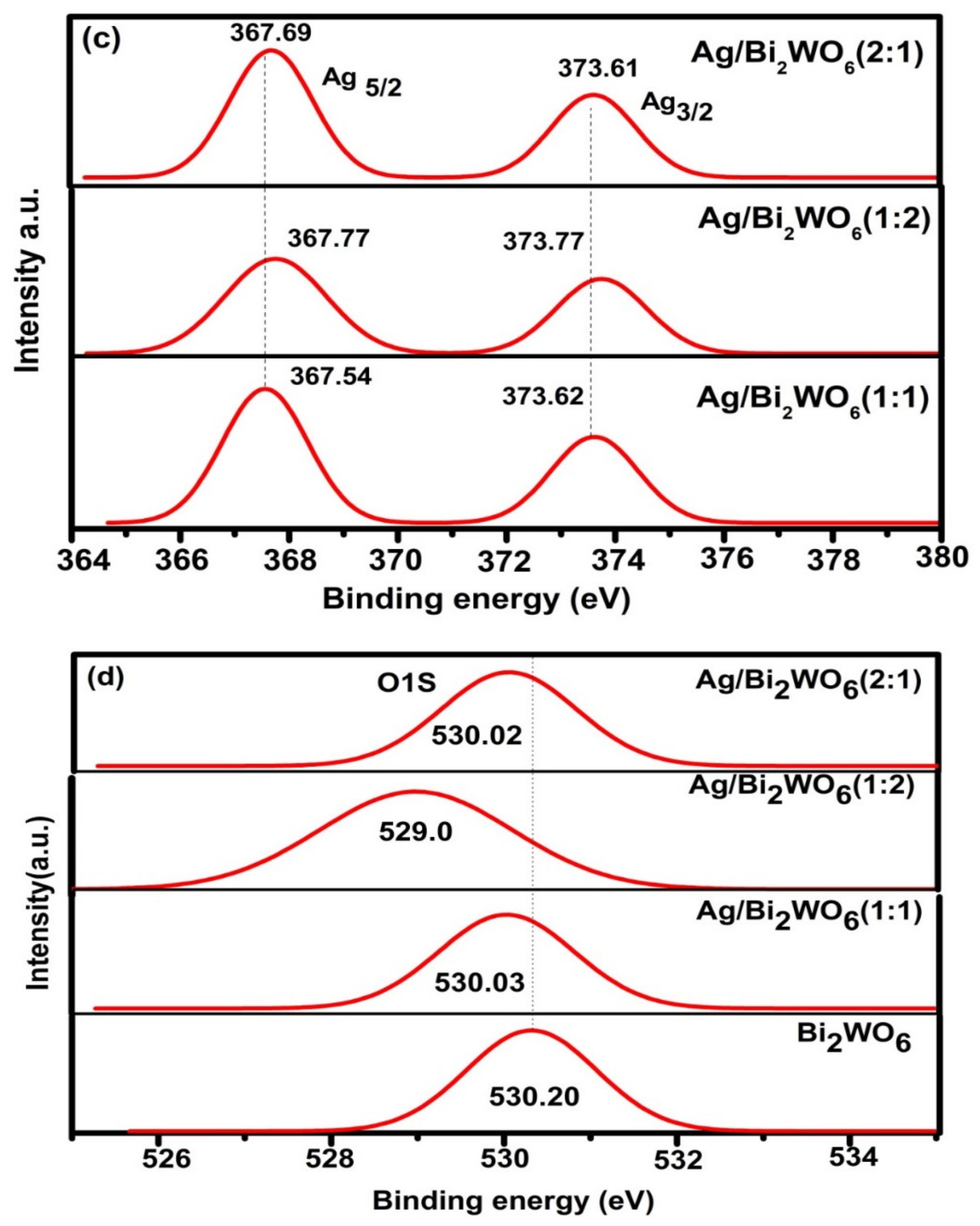
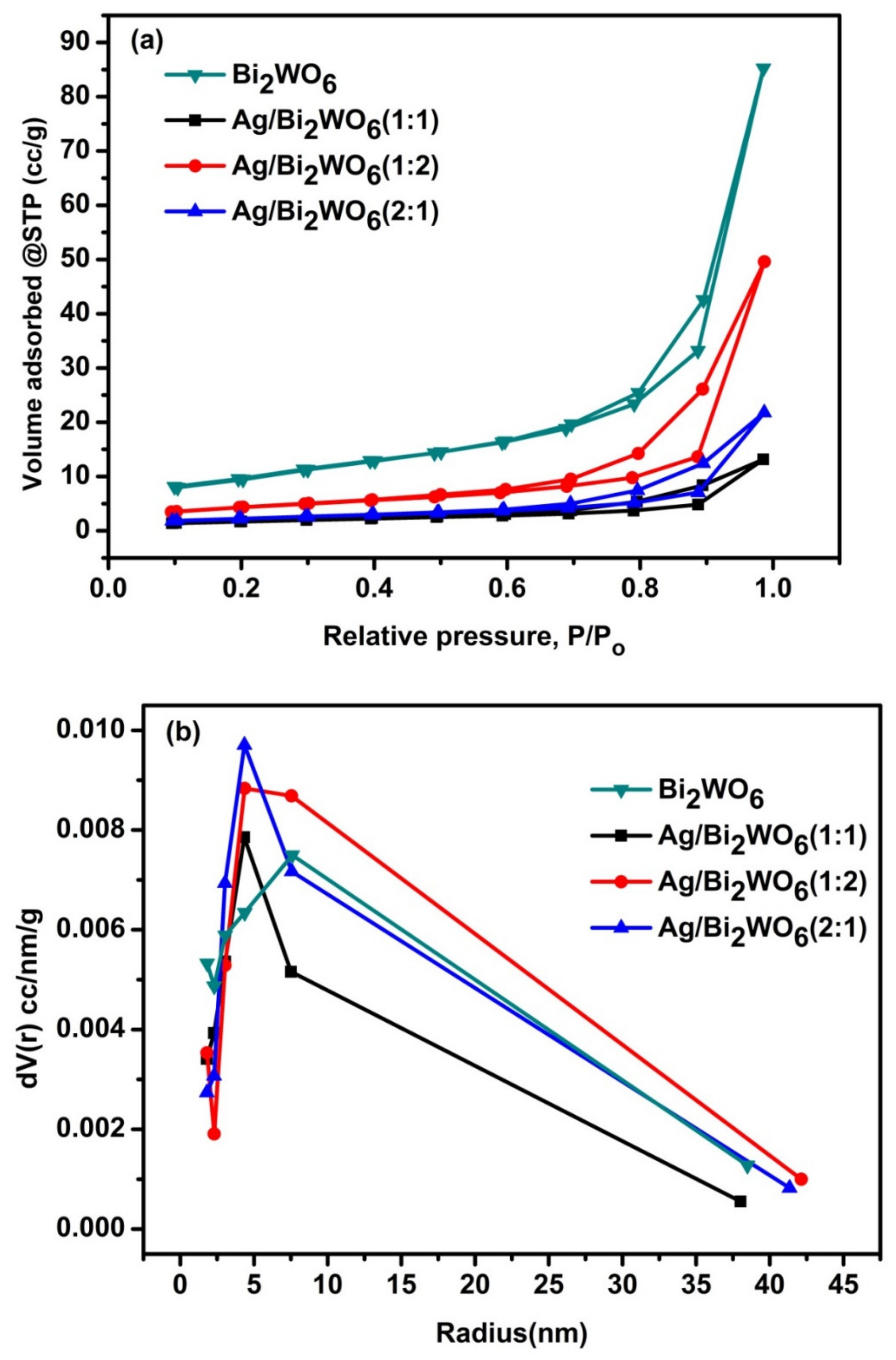
3.1. Structural Study
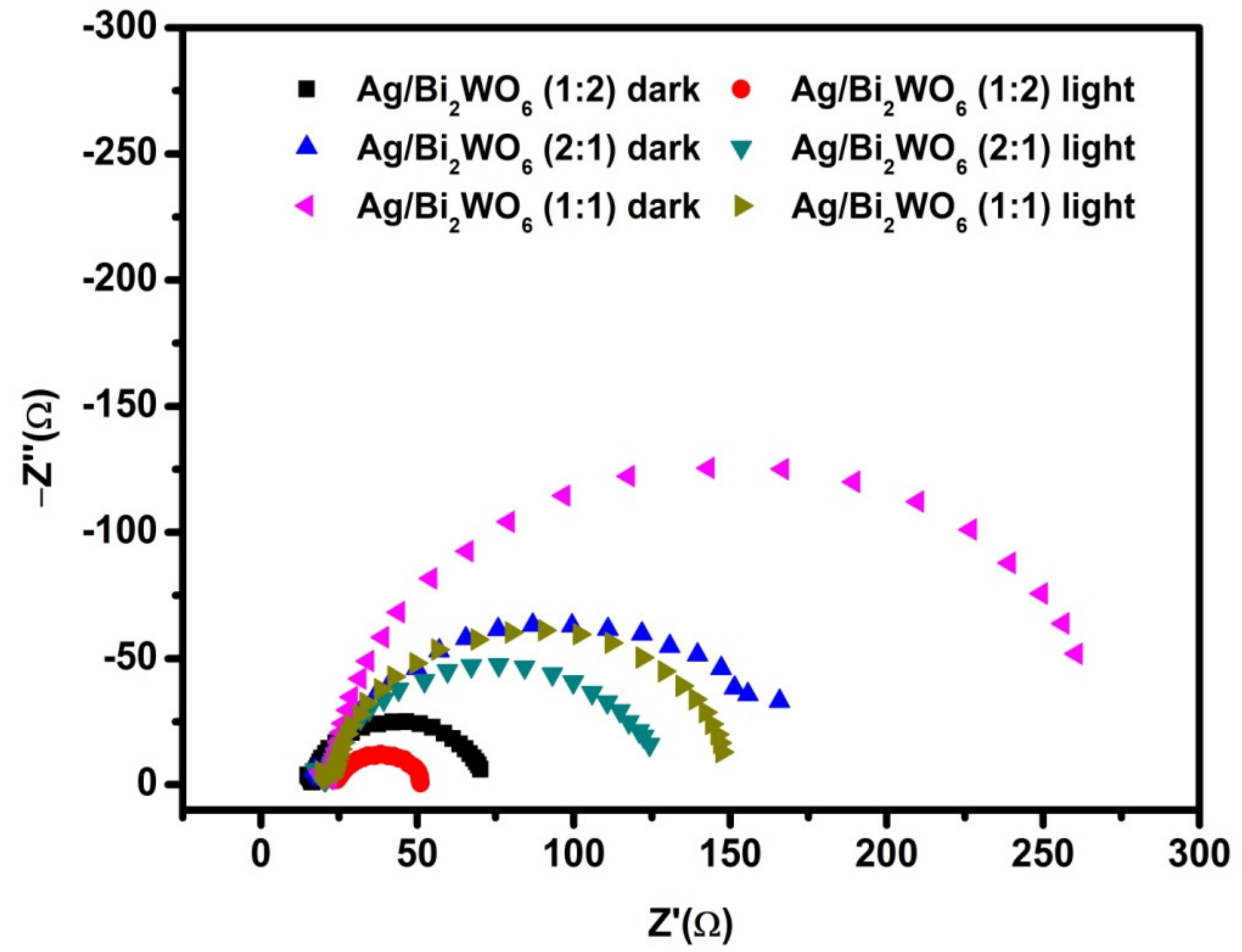
3.2. Electrochemical Properties
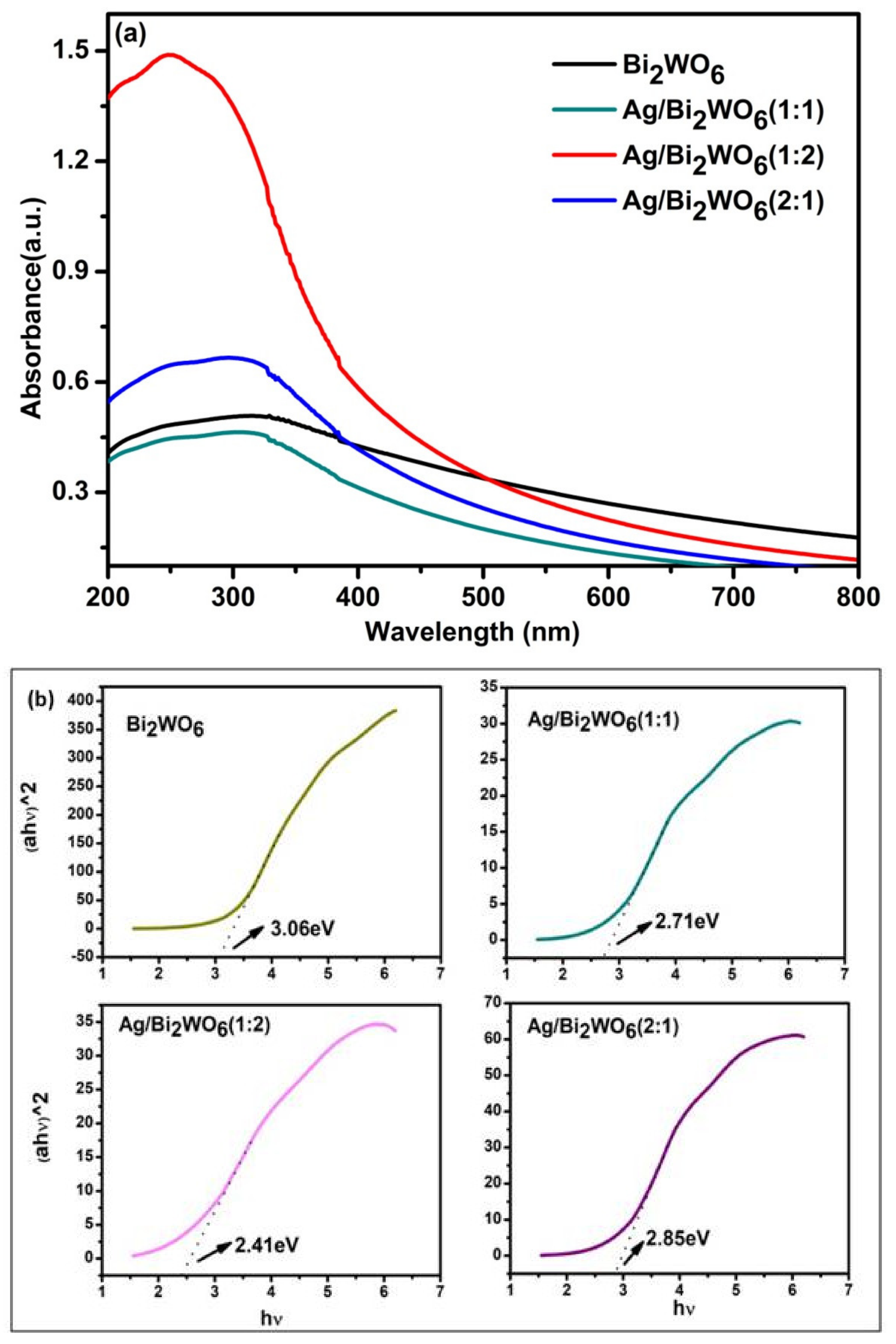
3.3. Optical Properties
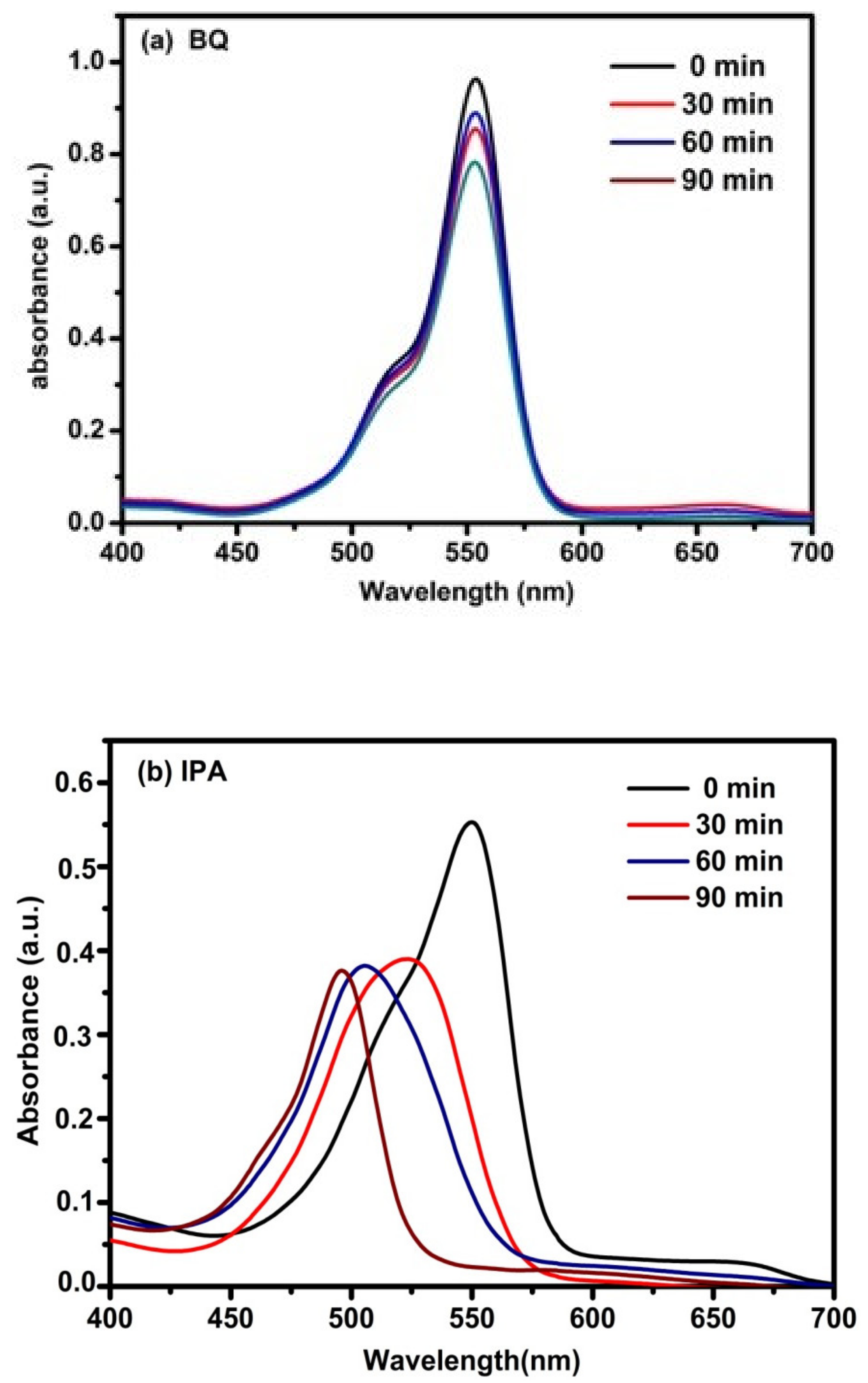
3.4. Photocatalytic Activity of RhB and MB
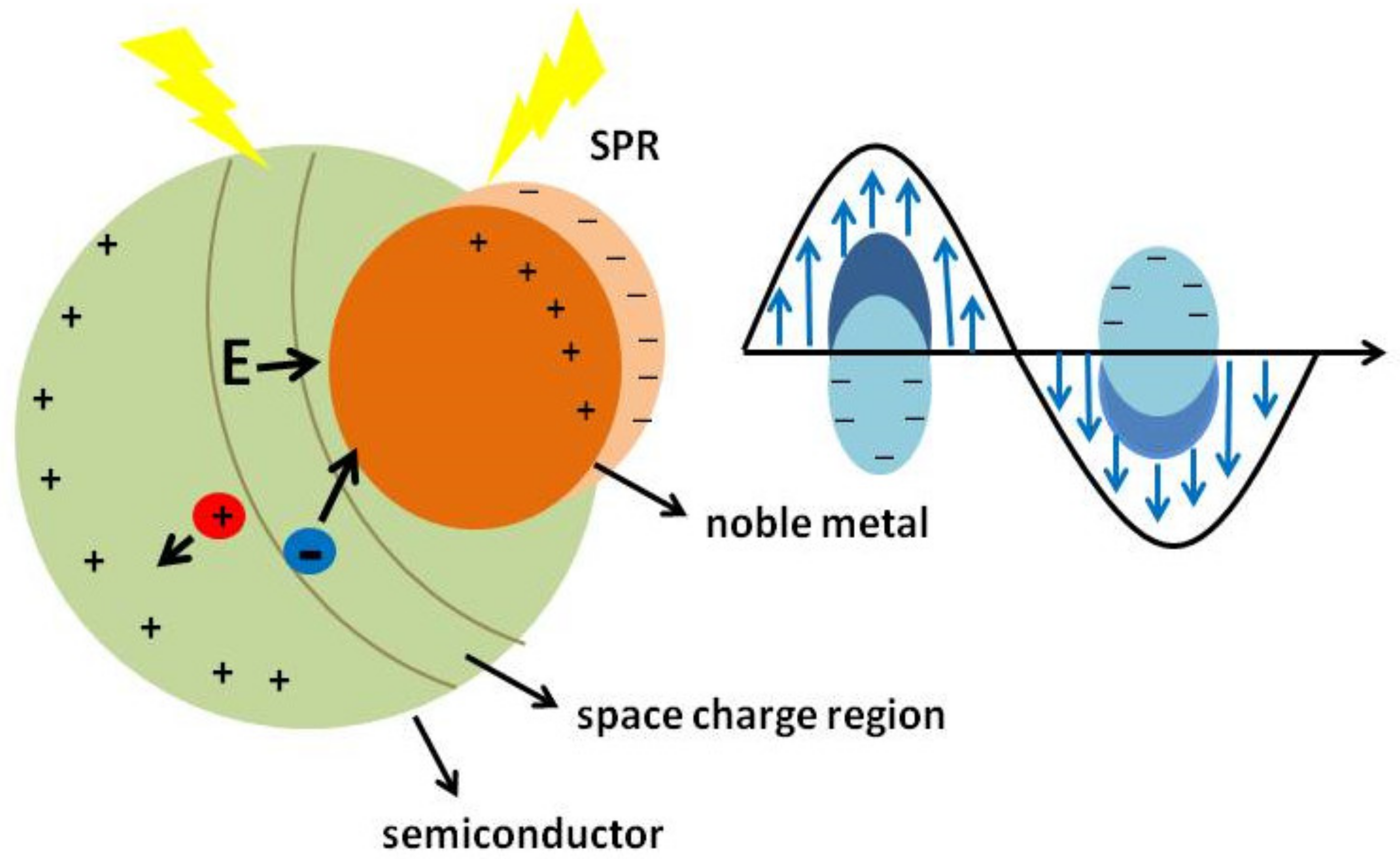
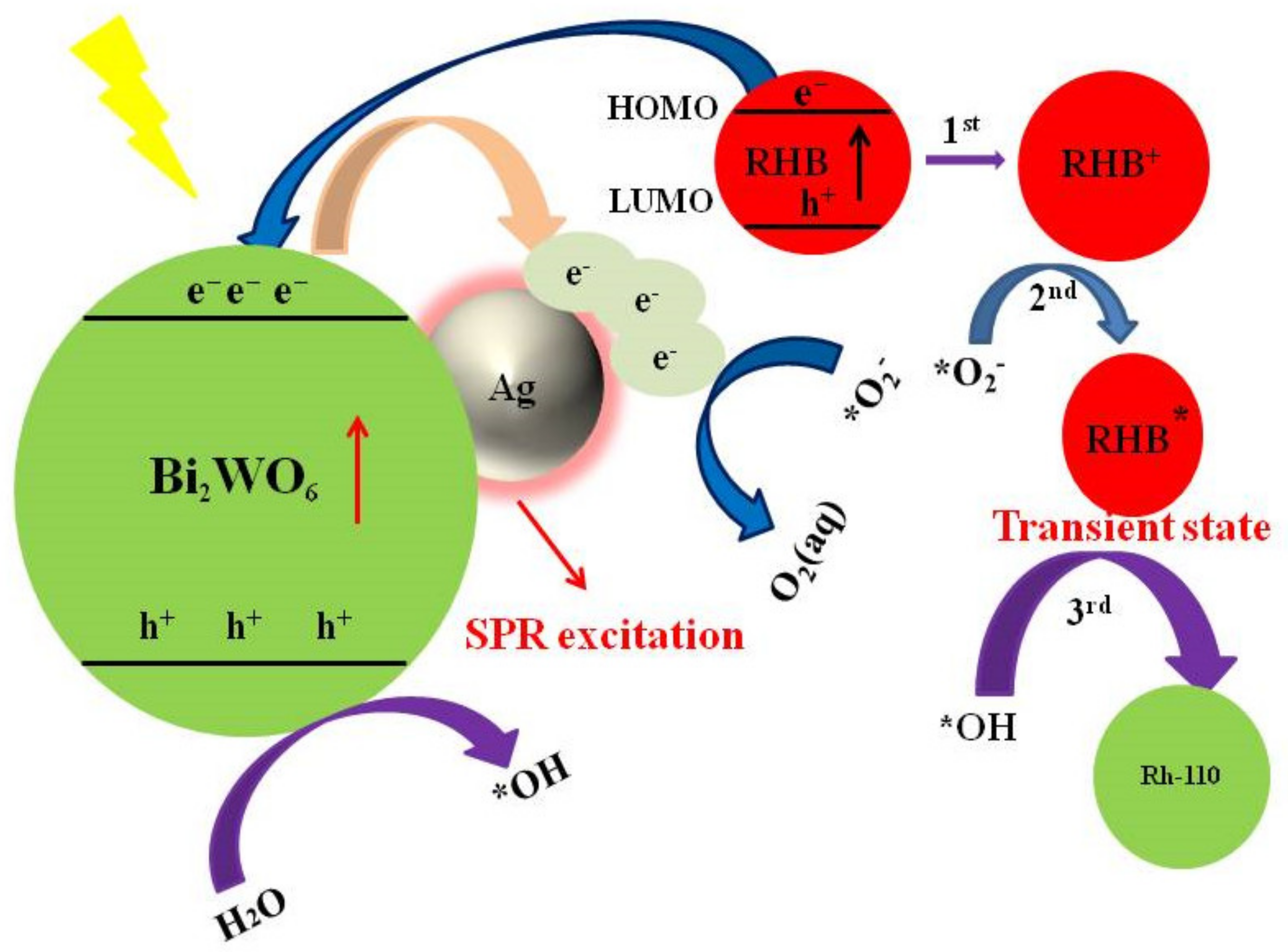
3.5. Proposed Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- He, C.; Shu, D.; Su, M.; Xia, D.; Asi, M.A.; Lin, L.; Xiong, Y. Photocatalytic activity of metal (Pt, Ag, and Cu)-deposited TiO2 photoelectrodes for degradation of organic pollutants in aqueous solution. Desalination 2010, 253, 88–93. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Cheng, B.; Ong, H.C. Microwave-Hydrothermal Preparation and Visible-Light Photoactivity of Plasmonic Photocatalyst Ag-TiO2 Nanocomposite Hollow Spheres. Chem. Asian J. 2010, 5, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhan, X.; Wang, F.; Wang, Q.; Xu, K.; Liu, Q.; Jiang, C.; Wang, Z.; He, J. Construction of CuInS2/Ag sensitized ZnO nanowire arrays for efficient hydrogen generation. RSC Adv. 2015, 5, 81723–81727. [Google Scholar] [CrossRef]
- Bajaj, G.; Soni, R. Nanocomposite ZnO/Au formation by pulsed laser irradiation. Appl. Surf. Sci. 2010, 256, 6399–6402. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.; Zheng, P.; Meng, F.; Chu, D.; Wu, N. Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat. Commun. 2013, 4, 2651. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, T.; Gong, J. Mechanistic Understanding of the Plasmonic Enhancement for Solar Water Splitting. Adv. Mater. 2015, 27, 5328–5342. [Google Scholar] [CrossRef]
- Xu, J.; Yang, W.-M.; Huang, S.-J.; Yin, H.; Zhang, H.; Radjenovic, P.; Yang, Z.-L.; Tian, Z.-Q.; Li, J.-F. CdS core-Au plasmonic satellites nanostructure enhanced photocatalytic hydrogen evolution reaction. Nano Energy 2018, 49, 363–371. [Google Scholar] [CrossRef]
- Boerigter, C.; Campana, R.; Morabito, M.; Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 2016, 7, 10545. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ye, M.; Iocozzia, J.; Lin, C.; Lin, Z. Plasmon-Mediated Solar Energy Conversion via Photocatalysis in Noble Metal/Semiconductor Composites. Adv. Sci. 2016, 3, 1600024. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.B.; Choi, S.; Kim, J.; Nam, Y.S. Plasmonically-assisted nanoarchitectures for solar water splitting: Obstacles and breakthroughs. Nano Today 2017, 16, 61–81. [Google Scholar] [CrossRef]
- Li, J.; Cushing, S.; Meng, F.; Senty, T.R.; Bristow, A.D.; Wu, N. Plasmon-induced resonance energy transfer for solar energy conversion. Nat. Photon. 2015, 9, 601–607. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Song, L.; Xiong, Y. Coupling Solar Energy into Reactions: Materials Design for Surface Plasmon-Mediated Catalysis. Small 2015, 11, 3873–3889. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Meng, G.; Zheng, P.; Huang, Q.; Li, Z.; Hu, X.; Wang, X.; Huang, Z.; Li, F.; Wu, N. A Hierarchically Ordered Array of Silver-Nanorod Bundles for Surface-Enhanced Raman Scattering Detection of Phenolic Pollutants. Adv. Mater. 2016, 28, 4871–4876. [Google Scholar] [CrossRef] [PubMed]
- Lange, H.; Juárez, B.H.; Carl, A.; Richter, M.; Bastús, N.G.; Weller, H.; Thomsen, C.; von Klitzing, R.; Knorr, A. Tunable Plasmon Coupling in Distance-Controlled Gold Nanoparticles. Langmuir 2012, 28, 8862–8866. [Google Scholar] [CrossRef]
- Ingram, D.B.; Christopher, P.; Bauer, J.L.; Linic, S. Predictive Model for the Design of Plasmonic Metal/Semiconductor Composite Photocatalysts; American Chemical Society: Washington, DC, USA, 2011; pp. 1441–1447. [Google Scholar]
- Zhang, W.; Ma, Y.; Zhu, X.; Liu, S.; An, T.; Bao, J.; Hu, X.; Tian, H. Fabrication of Ag decorated g-C3N4/LaFeO3 Z-scheme heterojunction as highly efficient visible-light photocatalyst for degradation of methylene blue and tetracycline hydrochloride. J. Alloys Compd. 2021, 864, 158914. [Google Scholar] [CrossRef]
- Liu, M.; Xue, X.; Yu, S.; Wang, X.; Hu, X.; Tian, H.; Chen, H.; Zheng, W. Improving Photocatalytic Performance from Bi2WO6@MoS2/graphene Hybrids via Gradual Charge Transferred Pathway. Sci. Rep. 2017, 7, 3637. [Google Scholar] [CrossRef]
- Tian, H.; Wan, C.; Xue, X.; Hu, X.; Wang, X. Effective Electron Transfer Pathway of the Ternary TiO2/RGO/Ag Nanocomposite with Enhanced Photocatalytic Activity under Visible Light. Catalysts 2017, 7, 156. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Z.; Zhang, M.; Guo, L. Piezotronics enhanced photocatalytic activities of Ag-BaTiO3 plasmonic photocatalysts. J. Alloys Compd. 2019, 801, 483–488. [Google Scholar] [CrossRef]
- Kisielewska, A.; Batory, D.; Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Piwoński, I. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials 2020, 13, 4653. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Kusior, A.; Michalec, K.; Jeleń, P.; Radecka, M. Shaped Fe2O3 nanoparticles—Synthesis and enhanced photocatalytic degradation towards RhB. Appl. Surf. Sci. 2018, 476, 342–352. [Google Scholar] [CrossRef]
- Liang, C.; Guo, H.; Zhang, L.; Ruan, M.; Niu, C.-G.; Feng, H.-P.; Wen, X.-J.; Tang, N.; Liu, H.-Y.; Zeng, G.-M. Boosting molecular oxygen activation ability in self-assembled plasmonic p-n semiconductor photocatalytic heterojunction of WO3/Ag@Ag2O. Chem. Eng. J. 2019, 372, 12–25. [Google Scholar] [CrossRef]
- Seddigi, Z.; Gondal, M.A.; Rashid, S.; Abdulaziz, M.; Ahmed, S. Facile synthesis and catalytic performance of nanosheet—nanorods g-C3N4-Bi2WO6 heterojunction catalyst and effect of silver nanoparticles loading on bare Bi2WO6 and g-C3N4-Bi2WO6 for N -deethylation process. J. Mol. Catal. A Chem. 2016, 420, 167–177. [Google Scholar] [CrossRef]
- Tang, J.; Zou, Z.; Katagiri, M.; Kako, T.; Ye, J. Photocatalytic degradation of MB on MIn2O4 (M=alkali earth metal) under visible light: Effects of crystal and electronic structure on the photocatalytic activity. Catal. Today 2004, 93–95, 885–889. [Google Scholar] [CrossRef]
- Singh, J.; Sahu, K.; Satpati, B.; Shah, J.; Kotnala, R.; Mohapatra, S. Facile synthesis, structural and optical properties of Au-TiO2 plasmonic nanohybrids for photocatalytic applications. J. Phys. Chem. Solids 2019, 135, 109100. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.-H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B Environ. 2018, 244, 11–24. [Google Scholar] [CrossRef]
- Xu, F.; Xu, C.; Chen, H.; Wu, D.; Gao, Z.; Ma, X.; Zhang, Q.; Jiang, K. The synthesis of Bi2S3/2D-Bi2WO6 composite materials with enhanced photocatalytic activities. J. Alloys Compd. 2018, 780, 634–642. [Google Scholar] [CrossRef]
- Lee, G.-J.; Zheng, Y.-C.; Wu, J.J. Fabrication of hierarchical bismuth oxyhalides (BiOX, X = Cl, Br, I) materials and application of photocatalytic hydrogen production from water splitting. Catal. Today 2018, 307, 197–204. [Google Scholar] [CrossRef]
- Xu, Y.-C.; Song, C.; Ding, X.-Y.; Zhao, Y.; Xu, D.-G.; Zhang, Q.-P.; Zhou, Y.-L. Tailoring lattices of Bi2WO6 crystals via Ce doping to improve the shielding properties against low-energy gamma rays. J. Phys. Chem. Solids 2019, 127, 76–80. [Google Scholar] [CrossRef]
- Wong-Ng, W.; McMurdie, H.; Hubbard, C.; Mighell, A. JCPDS-ICDD Research Associateship (cooperative program with NBS/NIST). J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Xue, P.; Wang, R.; Liu, L.; Liu, E.; Bai, X.; Fan, J.; Hu, X. Synergistic effects in simultaneous photocatalytic removal of Cr(VI) and tetracycline hydrochloride by Z-scheme Co3O4/Ag/Bi2WO6 heterojunction. Appl. Surf. Sci. 2019, 483, 677–687. [Google Scholar] [CrossRef]
- McDowell, N.A.; Knight, K.S.; Lightfoot, P. Unusual High-Temperature Structural Behaviour in Ferroelectric Bi2WO6. Chem.—Eur. J. 2006, 12, 1493–1499. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, G.; Zhen, Y.; Fu, F.; Li, D. Monodispersed Ag nanoparticles loaded on the surface of spherical Bi2WO6 nanoarchitectures with enhanced photocatalytic activities. J. Mater. Chem. 2012, 22, 4751–4758. [Google Scholar] [CrossRef]
- Sayadi, M.; Ahmadpour, N.; Homaeigohar, S. Photocatalytic and Antibacterial Properties of Ag-CuFe2O4@WO3 Magnetic Nanocomposite. Nanomaterials 2021, 11, 298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, W.; Chen, Z.; Zhou, L.; Xu, H.; Zhu, W. Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 2007, 17, 2526–2532. [Google Scholar] [CrossRef]
- Wu, Q.-S.; Cui, Y.; Yang, L.-M.; Zhang, G.-Y.; Gao, D.-Z. Facile in-situ photocatalysis of Ag/Bi2WO6 heterostructure with obviously enhanced performance. Sep. Purif. Technol. 2015, 142, 168–175. [Google Scholar] [CrossRef]
- Shen, J.; Xue, J.; Chen, Z.; Ni, J.; Tang, B.; He, G.; Chen, H. One-step hydrothermal synthesis of peony-like Ag/Bi2WO6 as efficient visible light-driven photocatalyst toward organic pollutants degradation. J. Mater. Sci. 2018, 53, 4848–4860. [Google Scholar] [CrossRef]
- Shen, J.; Xue, J.; He, G.; Ni, J.; Chen, Z.; Tang, B.; Zhou, Z.; Chen, H. Construction of 3D marigold-like Bi2WO6/Ag2O/CQDs heterostructure with superior visible-light active photocatalytic activity toward tetracycline degradation and selective oxidation. J. Mater. Sci. 2018, 53, 12040–12055. [Google Scholar] [CrossRef]
- Adebayo-Tayo, B.; Salaam, A.; Ajibade, A. Green synthesis of silver nanoparticle using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon 2019, 5, e02502. [Google Scholar] [CrossRef] [Green Version]
- Di, J.; Xia, J.; Ge, Y.; Li, H.; Ji, H.; Xu, H.; Zhang, Q.; Li, H.; Li, M. Novel visible-light-driven CQDs/Bi2WO6 hybrid materials with enhanced photocatalytic activity toward organic pollutants degradation and mechanism insight. Appl. Catal. B Environ. 2015, 168–169, 51–61. [Google Scholar] [CrossRef]
- Tian, J.; Sang, Y.; Yu, G.; Jiang, H.; Mu, X.; Liu, H. A Bi2WO6-Based Hybrid Photocatalyst with Broad Spectrum Photocatalytic Properties under UV, Visible, and Near-Infrared Irradiation. Adv. Mater. 2013, 25, 5075–5080. [Google Scholar] [CrossRef]
- Jonjana, S.; Phuruangrat, A.; Thongtem, S.; Thongtem, T. Preparation and enhanced photocatalytic performance of AgCl/Bi2MoO6 heterojunction. Mater. Lett. 2016, 179, 162–165. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Dumrongrojthanath, P.; Thongtem, S.; Thongtem, T. Hydrothermal synthesis of I-doped Bi2WO6 for using as a visible-light-driven photocatalyst. Mater. Lett. 2018, 224, 67–70. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Siri, S.; Wadbua, P.; Thongtem, S.; Thongtem, T. Microwave-assisted synthesis, photocatalysis and antibacterial activity of Ag nanoparticles supported on ZnO flowers. J. Phys. Chem. Solids 2018, 126, 170–177. [Google Scholar] [CrossRef]
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Lv, Y.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater. 2010, 182, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, D.; Wang, P.; Ao, Y.; Hou, J.; Qian, J. Effect of oxygen vacancy on enhanced photocatalytic activity of reduced ZnO nanorod arrays. Appl. Surf. Sci. 2015, 325, 112–116. [Google Scholar] [CrossRef]
- Lia, B.; Tana, L.; Liua, X.; Lia, Z.; Cuib, Z.; Lianga, Y.; Zhub, S.; Yangb, X.; Yeung, K.; Wuab, S. Superimposed surface plasma resonance effect enhanced the near-infrared photocatalytic activity of Au@Bi2WO6 coating for rapid bacterial killing. J. Hazard. Mater. 2019, 380, 120818. [Google Scholar] [CrossRef]
- Lu, C.; Li, X.; Wu, Q.; Li, J.; Wen, L.; Dai, Y.; Huang, B.; Li, B.; Lou, Z. Constructing Surface Plasmon Resonance on Bi2WO6 to Boost High-Selective CO2 Reduction for Methane. ACS Nano 2021, 15, 3529–3539. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Liu, E.; Wan, J.; Pan, C.; Ma, Y.; Hu, X.; Fan, J. Highly Efficient and Stable Au/Bi2MoO6/Bi2WO6 Heter-ostructure with Enhanced Photocatalytic Activity for NO Gas Removal under Visible Light Irradiation. J. Phys. D Appl. Phys. 2017, 50, 145103. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Guo, P.; Wang, M.; Su, J.; Zhao, D.; Guo, L. Plasmonic Ag@SiO2 core/shell structure modified g-C3N4 with enhanced visible light photocatalytic activity. J. Mater. Res. 2013, 29, 64–70. [Google Scholar] [CrossRef]
- Yu, L.; Ye, Z.; Li, J.; Ma, C.; Ma, C.; Liu, X.; Wang, H.; Tang, L.; Huo, P.; Yan, Y. Photocatalytic Degradation Mechanism of Tetracycline by Ag@ZnO/C Core–Shell Plasmonic Photocatalyst Under Visible Light. Nano 2018, 13, 1850065. [Google Scholar] [CrossRef]
- Liang, H.; Liu, S.; Zhang, H.; Wang, X.; Wang, J. New insight into the selective photocatalytic oxidation of RhB through a strategy of modulating radical generation. RSC Adv. 2018, 8, 13625–13634. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Jia, Z.; Zhang, H.; Wang, X.; Wang, J. Photocatalysis oxidation activity regulation of Ag/TiO2 composites evaluated by the selective oxidation of Rhodamine B. Appl. Surf. Sci. 2017, 422, 1–10. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Zhang, L.; Zhu, Y. Synthesis, characterization and photocatalytic properties of nanosized Bi2WO6, PbWO4 and ZnWO4 catalysts. Mater. Res. Bull. 2007, 42, 696–706. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Serpone, N. Photoassisted Degradation of Dye Pollutants. V. Self-Photosensitized Oxidative Trans-formation of Rhodamine B under Visible Light Irradiation in Aqueous TiO2 Dispersions. J. Phys. Chem. B 1998, 5647, 5845–5851. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, C.; Gu, F.L.; Wu, H.; Guo, Q. Significant visible-light photocatalytic enhancement in Rhodamine B degradation of silver orthophosphate via the hybridization of N-doped graphene and poly(3-hexylthiophene). J. Hazard. Mater. 2016, 315, 23–34. [Google Scholar] [CrossRef] [PubMed]


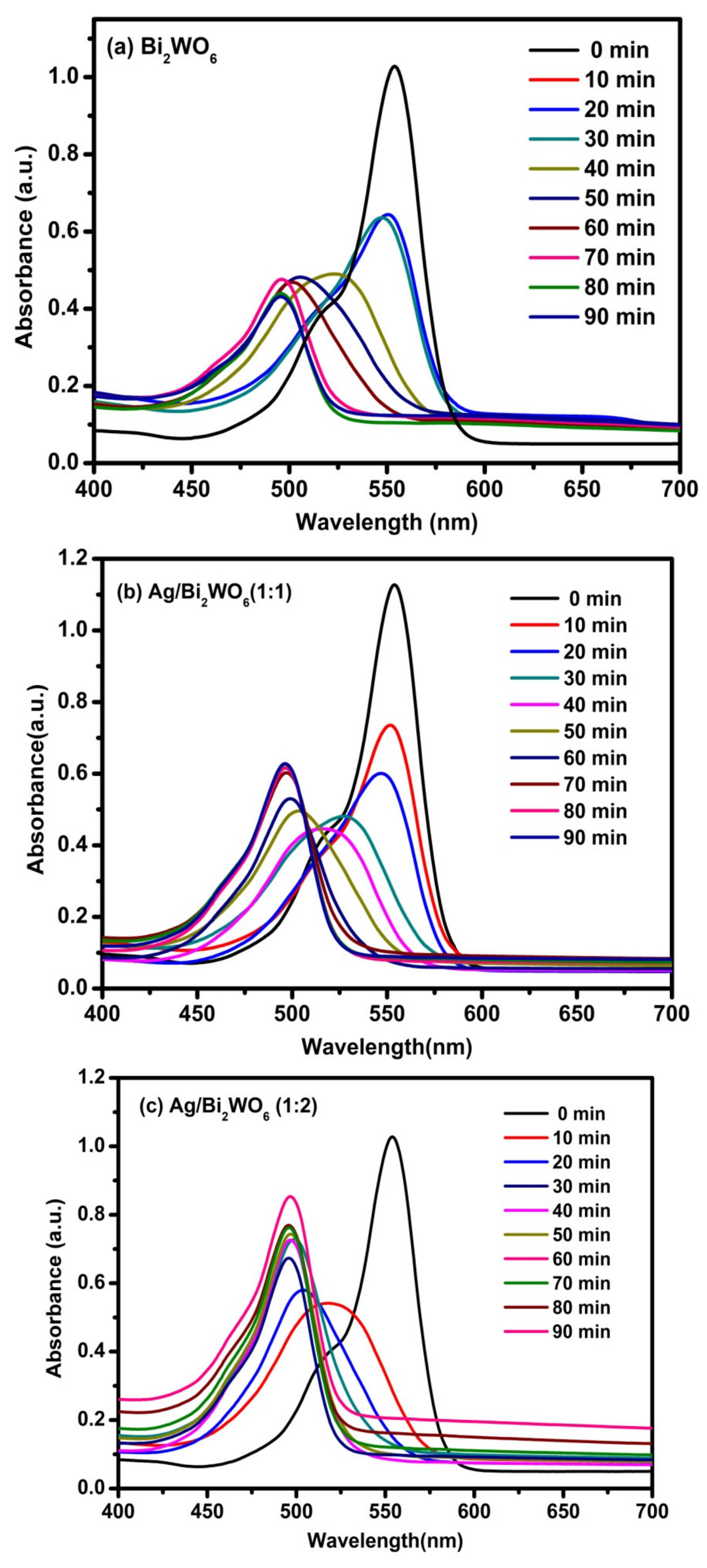
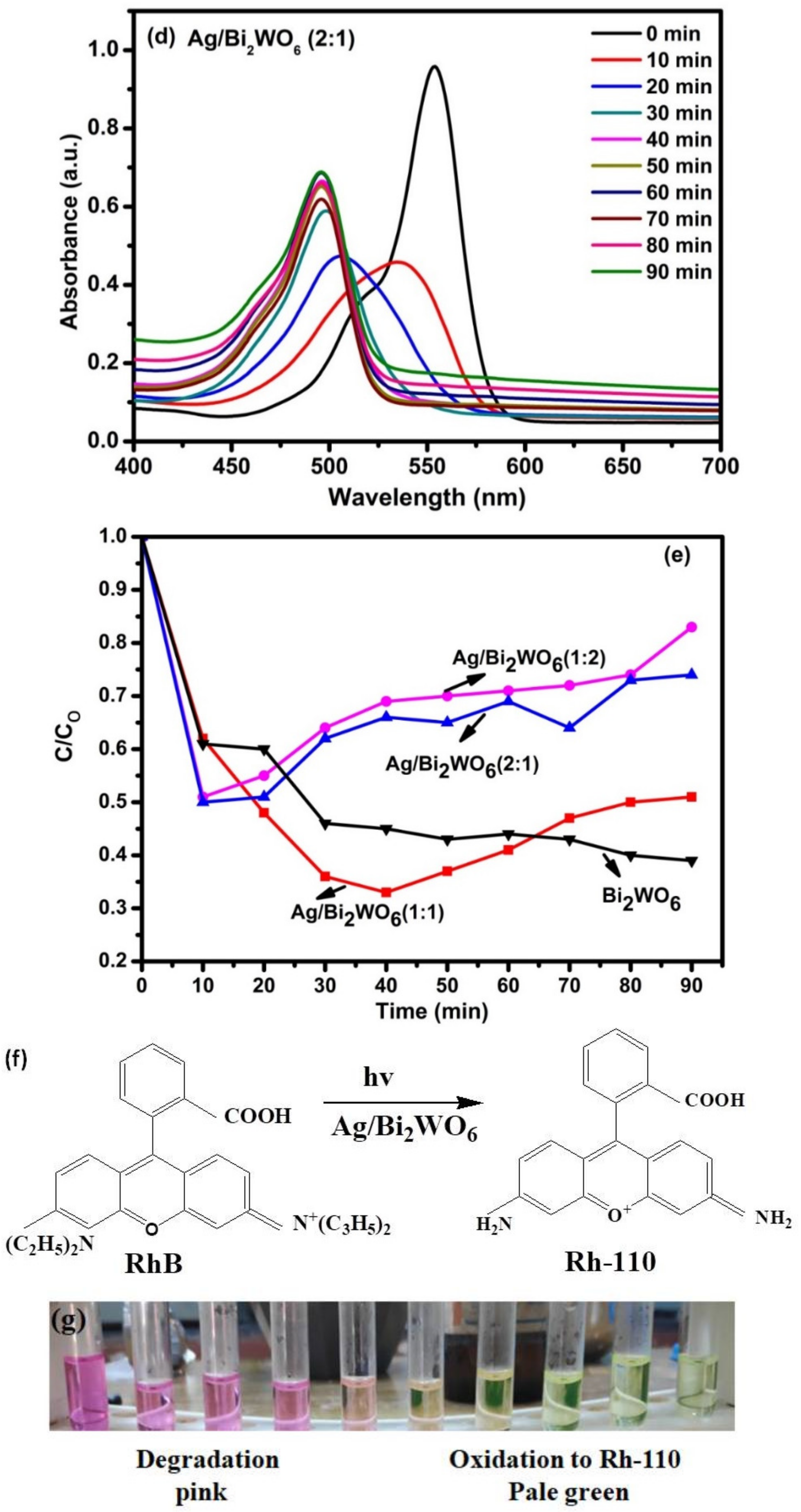

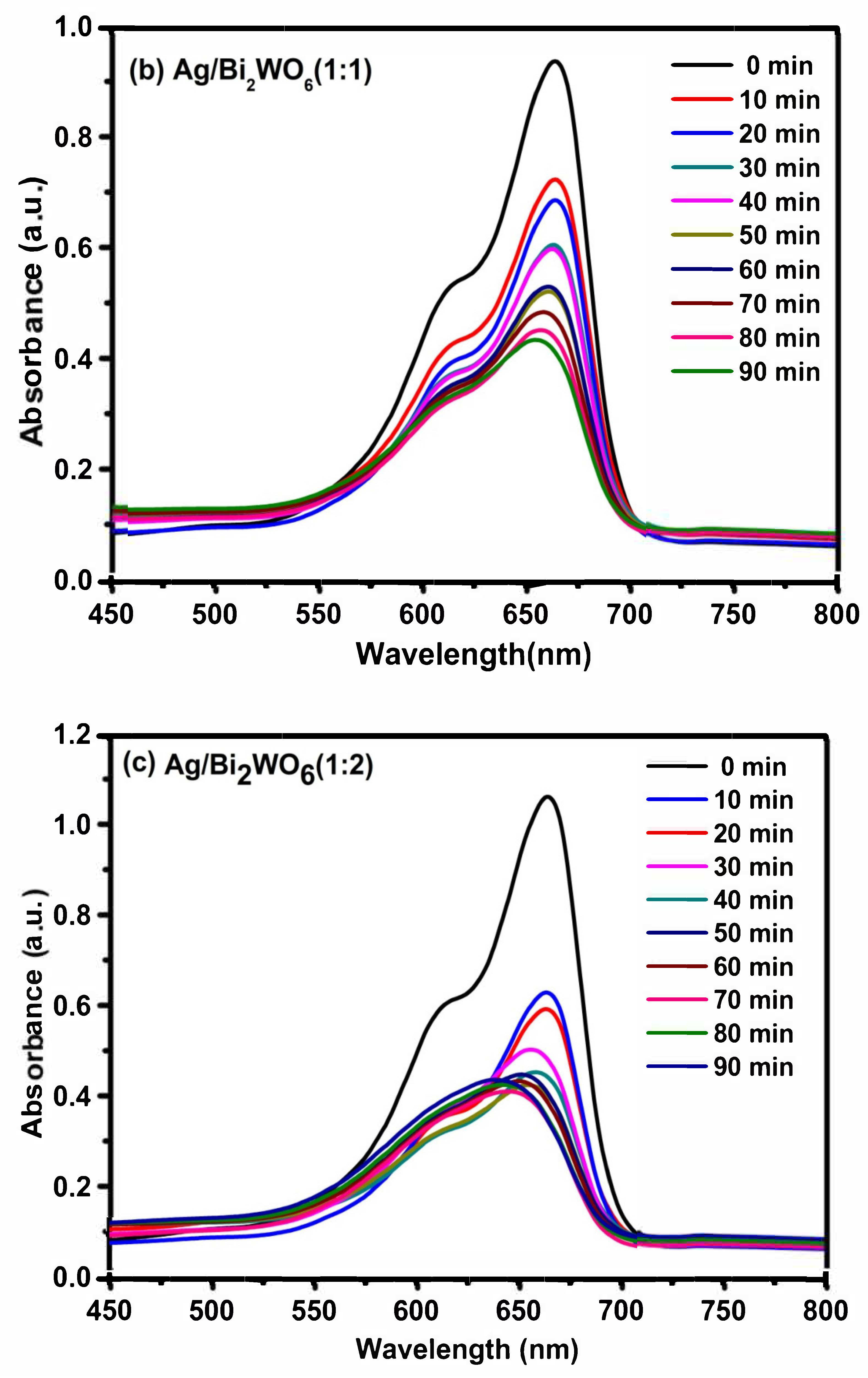

| Catalyst | Organic Pollutant | Light Source | Time (Min) | Degradation % | Reference |
|---|---|---|---|---|---|
| Ag-BaTiO3 | RhB | visible | 75 | 83 | [20] |
| TiO3 | RhB | visible | 75 | 63 | [20] |
| Nano-disc Fe2O3 | RhB | visible | 45 | 13 | [23] |
| TiO2-GO-AgNs | RhB | visible | 300 | 93 | [21] |
| Ag2CO3-WO3 | RhB | visible | 120 | 64.6 | [24] |
| g-C3N4 | RhB | visible | 90 | 24 | [25] |
| WO3-g-C3N4 | RhB | visible | 90 | 62 | [25] |
| Bi2WO6 | RhB | visible | 10 | 40 | Present work |
| Ag/Bi2WO6 (1:1) | RhB | visible | 10 | 40 | Present work |
| Ag/Bi2WO6 (1:2) | RhB | visible | 10 | 50 | Present work |
| Ag/Bi2WO6 (2:1) | RhB | visible | 10 | 50 | Present work |
| TiO2 | MB | visible | 120 | 40 | [26] |
| BaIn2O4 | MB | visible | 120 | 53 | [26] |
| SrIn2O4 | MB | visible | 120 | 67 | [26] |
| Au/TiO2 | MB | visible | 20 | 94 | [27] |
| Ag-ZnO | MB | visible | 40 | 100 | [22] |
| Bi2WO6 | MB | visible | 90 | 38 | Present work |
| Ag/Bi2WO6 (1:1) | MB | visible | 90 | 53 | Present work |
| Ag/Bi2WO6 (1:2) | MB | visible | 90 | 68 | Present work |
| Ag/Bi2WO6 (2:1) | MB | visible | 90 | 43 | Present work |
| Catalyst | Specific Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cc/g) |
|---|---|---|---|
| Bi2WO6 | 26.40 | 15.18 | 0.12 |
| Ag/Bi2WO6 (1:1) | 26.02 | 8.70 | 0.11 |
| Ag/Bi2WO6 (1:2) | 20.84 | 8.69 | 0.07 |
| Ag/Bi2WO6 (1:1) | 20.43 | 8.73 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, S.; Rout, S.K. Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6. Chemistry 2022, 4, 272-296. https://doi.org/10.3390/chemistry4020022
Khanam S, Rout SK. Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6. Chemistry. 2022; 4(2):272-296. https://doi.org/10.3390/chemistry4020022
Chicago/Turabian StyleKhanam, Shomaila, and Sanjeeb Kumar Rout. 2022. "Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6" Chemistry 4, no. 2: 272-296. https://doi.org/10.3390/chemistry4020022
APA StyleKhanam, S., & Rout, S. K. (2022). Enhanced Photocatalytic Oxidation of RhB and MB Using Plasmonic Performance of Ag Deposited on Bi2WO6. Chemistry, 4(2), 272-296. https://doi.org/10.3390/chemistry4020022







