(18-Crown-6)-bis(tetrahydrofuran)-potassium Anthracenide: The Salt of a Free Radical Anion Crystallizing as a Kryptoracemate
Abstract
:1. Introduction
2. Results and Discussion
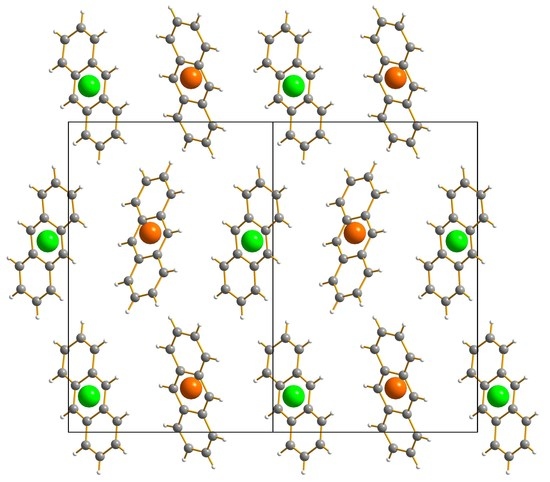
2.1. Overview
2.2. Kryptoracemic Assignment of the Structure: CheckCIF and the Flack x Parameter
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- BUFSIE
- DIHFIJ
- MURLIX
- QIBKIY
- WIPXEY
References
- Groom, C.R.; Bruno, J.; Lightfoot, M.P.; Ward, S.C. CCDC = The Cambridge Structural Database, 2016. Acta Cryst. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Rosokha, S.V.; Kochi, J.K. The question of aromaticity in open-shell cations and anions as ion-radical offsprings of polycyclic aromatic and antiaromatic hydrocarbons. J. Org. Chem. 2006, 71, 9357–9365. [Google Scholar] [CrossRef] [PubMed]
- Brennessel, W.W.; Jilek, R.E.; Ellis, J.E. Bis(1,2,3,4-n4-anthracene)ferrate(1-): A Paramagnetic Homoleptic Polyarene Transition-Metal Anion. Angew. Chem. Int. Ed. 2007, 46, 6132–6136. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.; Metta-Magana, A.J.; Fortier, S. Isolation of gravimetrically quantifiable alkali metal arenides using 18-crown-6. New J. Chem. 2016, 40, 1923–1926. [Google Scholar] [CrossRef]
- MERCURY: A graphics routine of CSD generated the overlay cifs subsequently used in DIAMOND.
- Putz, H.; Brandenburg, K. DIAMOND—A Graphics Program; v8.5.10.; GbR, Kreuzherrenstr: Bonn, Germany, 2019; Volume 102, p. 5322. [Google Scholar]
- Bernal, I. ACA Annual Meeting. Montreal, QC, Canada, 23–28 July 1995, Abstract 4a.1.e.
- Bernal, I.; Cai, J.; Myrczek, J. The phenomenon of kryptoracemic crystallization. 2. counterion control of crystallization pathway selection. 5. the crystallization of (+/−)[Co(en)(3)][oxalato]Cl·3H(2)O(I), (+/−)-[Co(en)(3)][oxalato]Br·3H(2)O(II) and (+/−)-([Co(en)(3)](oxalato)I)(2)·3H(2)O(III). Acta Chem. Hung. 1995, 132, 451–474. [Google Scholar]
- Bernal, I.; Somoza, F.; Banh, V. Kryptoracemic Crystallization. Part 4. Synthesis and X-Ray Structure of the Conglomerate [Co(en)2Ox]F.11.5H2O(I). J. Coord. Chem. 1997, 42, 1–10. [Google Scholar] [CrossRef]
- Bernal, I.; Watkins, S.F. A list of organometallic kryptoracemates. Acta Cryst. 2015, 71, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Fabian, L.; Brock, C.P. A list of organic kryptoracemates. Acta Cryst. 2010, 66, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Clevers, S.; Coquerel, G. Kryptoracemic Compound Hunting and Frequency in the Cambridge Structural Database. Cryst. Eng. Comm. 2020, 22, 7407–7419. [Google Scholar] [CrossRef]
- Parsons, S. Methods for determination of absolute structure using X-ray crystallography are described, with an emphasis on applications for absolute configuration assignment of enantiopure light-atom organic compounds. Tetrahedon Asymmetry 2017, 28, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Flack, H.D.; Bernardinelli, G. Reporting and evaluating absolute-structure and absolute-configuration determinations. J. Appl. Cryst. 2000, 33, 1143–1148. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, I.; Lalancette, R.A. (18-Crown-6)-bis(tetrahydrofuran)-potassium Anthracenide: The Salt of a Free Radical Anion Crystallizing as a Kryptoracemate. Chemistry 2022, 4, 137-145. https://doi.org/10.3390/chemistry4010012
Bernal I, Lalancette RA. (18-Crown-6)-bis(tetrahydrofuran)-potassium Anthracenide: The Salt of a Free Radical Anion Crystallizing as a Kryptoracemate. Chemistry. 2022; 4(1):137-145. https://doi.org/10.3390/chemistry4010012
Chicago/Turabian StyleBernal, Ivan, and Roger A. Lalancette. 2022. "(18-Crown-6)-bis(tetrahydrofuran)-potassium Anthracenide: The Salt of a Free Radical Anion Crystallizing as a Kryptoracemate" Chemistry 4, no. 1: 137-145. https://doi.org/10.3390/chemistry4010012
APA StyleBernal, I., & Lalancette, R. A. (2022). (18-Crown-6)-bis(tetrahydrofuran)-potassium Anthracenide: The Salt of a Free Radical Anion Crystallizing as a Kryptoracemate. Chemistry, 4(1), 137-145. https://doi.org/10.3390/chemistry4010012