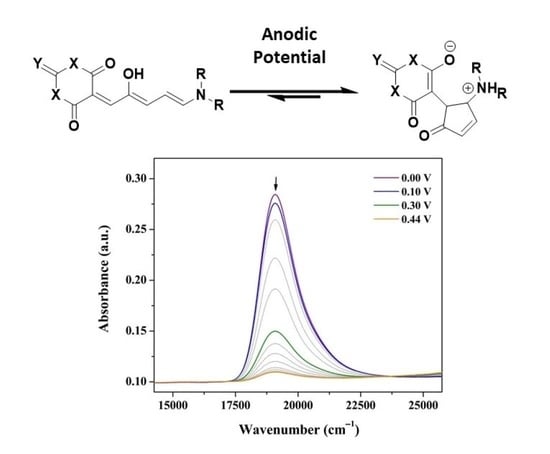
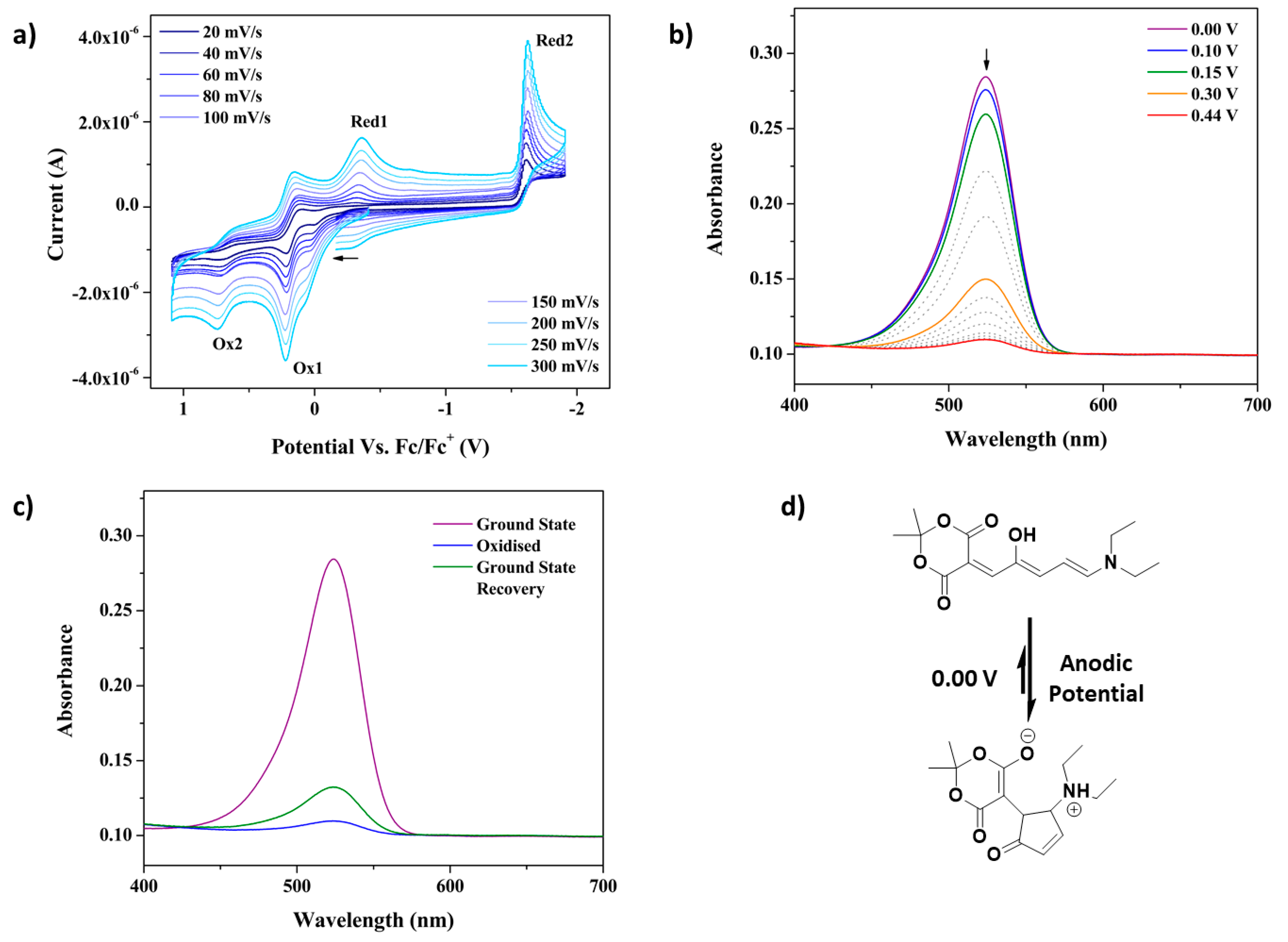
Electrochemical Switching of First-Generation Donor-Acceptor Stenhouse Adducts (DASAs): An Alternative Stimulus for Triene Cyclisation
Abstract
:Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natali, M.; Giordani, S. Molecular Switches as Photocontrollable “Smart” Receptors. Chem. Soc. Rev. 2012, 41, 4010–4029. [Google Scholar] [CrossRef] [Green Version]
- Tylkowski, B.; Trojanowska, A.; Marturano, V.; Nowak, M.; Marciniak, L.; Giamberini, M.; Ambrogi, V.; Cerruti, P. Power of Light—Functional Complexes Based on Azobenzene Molecules. Coord. Chem. Rev. 2017, 351, 205–217. [Google Scholar] [CrossRef]
- Avella-Oliver, M.; Morais, S.; Puchades, R.; Maquieira, Á. Towards Photochromic and Thermochromic Biosensing. TrAC-Trends Anal. Chem. 2016, 79, 37–45. [Google Scholar] [CrossRef]
- Gong, L.L.; Yao, W.T.; Liu, Z.Q.; Zheng, A.M.; Li, J.Q.; Feng, X.F.; Ma, L.F.; Yan, C.S.; Luo, M.B.; Luo, F. Photoswitching Storage of Guest Molecules in Metal-Organic Framework for Photoswitchable Catalysis: Exceptional Product, Ultrahigh Photocontrol, and Photomodulated Size Selectivity. J. Mater. Chem. A 2017, 5, 7961–7967. [Google Scholar] [CrossRef]
- Terao, F.; Morimoto, M.; Irie, M. Light-Driven Molecular-Crystal Actuators: Rapid and Reversible Bending of Rodlike Mixed Crystals of Diarylethene Derivatives. Angew. Chemie-Int. Ed. 2012, 51, 901–904. [Google Scholar] [CrossRef]
- Feringa, B.L. In Control of Motion: From Molecular Switches to Molecular Motors. Acc. Chem. Res. 2001, 34, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Andréasson, J.; Pischel, U. Storage and Processing of Information Using Molecules: The All-Photonic Approach with Simple and Multi-Photochromic Switches. Isr. J. Chem. 2013, 53, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Helmy, S.; Oh, S.; Leibfarth, F.A.; Hawker, C.J.; Read De Alaniz, J. Design and Synthesis of Donor-Acceptor Stenhouse Adducts: A Visible Light Photoswitch Derived from Furfural. J. Org. Chem. 2014, 79, 11316–11329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helmy, S.; Leibfarth, F.; Oh, S.; Poelma, J.; Hawker, C.J.; Read De Alaniz, J. Photoswitching Using Visible Light: A New Class of Organic Photochromic Molecules. J. Am. Chem. Soc. 2014, 136, 8169–8172. [Google Scholar] [CrossRef] [Green Version]
- Lerch, M.M.; Szymański, W.; Feringa, B.L. The (Photo)Chemistry of Stenhouse Photoswitches: Guiding Principles and System Design. Chem. Soc. Rev. 2018, 47, 1910–1937. [Google Scholar] [CrossRef] [PubMed]
- Mallo, N.; Foley, E.D.; Iranmanesh, H.; Kennedy, A.D.W.; Luis, E.T.; Ho, J.; Harper, J.B.; Beves, J.E. Structure-Function Relationships of Donor-Acceptor Stenhouse Adduct Photochromic Switches. Chem. Sci. 2018, 9, 8242–8252. [Google Scholar] [CrossRef] [Green Version]
- Mallo, N.; Tron, A.; Andréasson, J.; Harper, J.B.; Jacob, L.S.D.; McClenaghan, N.D.; Jonusauskas, G.; Beves, J.E. Hydrogen-Bonding Donor-Acceptor Stenhouse Adducts. ChemPhotoChem 2020, 4, 407–412. [Google Scholar] [CrossRef]
- Bléger, D.; Hecht, S. Visible-Light-Activated Molecular Switches. Angew. Chemie-Int. Ed. 2015, 54, 11338–11349. [Google Scholar] [CrossRef]
- Poelma, S.O.; Oh, S.S.; Helmy, S.; Knight, A.S.; Burnett, G.L.; Soh, H.T.; Hawker, C.J.; Read De Alaniz, J. Controlled Drug Release to Cancer Cells from Modular One-Photon Visible Light-Responsive Micellar System. Chem. Commun. 2016, 52, 10525–10528. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Cao, Z.; Wu, B.; Zhang, Q.; Wang, G. Polymer Dots of DASA-Functionalized Polyethyleneimine: Synthesis, Visible Light/PH Responsiveness, and Their Applications as Chemosensors. Sensors Actuators, B Chem. 2018, 254, 385–392. [Google Scholar] [CrossRef]
- Balamurugan, A.; Lee, H. A Visible Light Responsive On–Off Polymeric Photoswitch for the Colorimetric Detection of Nerve Agent Mimics in Solution and in the Vapor Phase. Macromolecules 2016, 49, 2568–2574. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; Velema, W.A.; Szymanski, W.; Feringa, B.L. Orthogonal Photoswitching in a Multifunctional Molecular System. Nat. Commun. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinawang, G.; Wu, B.; Wang, J.; Li, S.; He, Y. Polystyrene Based Visible Light Responsive Polymer with Donor–Acceptor Stenhouse Adduct Pendants. Macromol. Chem. Phys. 2016, 217, 2409–2414. [Google Scholar] [CrossRef]
- Singh, S.; Friedel, K.; Himmerlich, M.; Lei, Y.; Schlingloff, G.; Schober, A. Spatiotemporal Photopatterning on Polycarbonate Surface through Visible Light Responsive Polymer Bound DASA Compounds. ACS Macro Lett. 2015, 4, 1273–1277. [Google Scholar] [CrossRef]
- Safar, P.; Povazanec, F.; Pronayova, N.; Baran, P.; Kickelbick, G.; Kosizek, J.; Breza, M. No Title. Collect. Czech. Chem. Commun. 2000, 65, 1911–1938. [Google Scholar]
- Klajn, R. Spiropyran-Based Dynamic Materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukyanov, B.S.; Lukyanova, M.B. Spiropyrans: Synthesis, Properties, and Application. (Review)*. Chem. Heterocycl. Compd. 2005, 41, 281–311. [Google Scholar] [CrossRef]
- Saad, A.; Oms, O.; Marrot, J.; Dolbecq, A.; Hakouk, K.; El Bekkachi, H.; Jobic, S.; Deniard, P.; Dessapt, R.; Garrot, D.; et al. Design and Optical Investigations of a Spironaphthoxazine/Polyoxometalate/ Spiropyran Triad. J. Mater. Chem. C 2014, 2, 4748–4758. [Google Scholar] [CrossRef]
- Wagner, K.; Robert, B.; Zanoni, M.; Gambhir, S.; Dennany, L.; Breukers, R.; Higgins, M.; Wagner, P.; Diamond, D.; Wallace, G.G.; et al. A Multiswitchable Poly(Terthiophene) Bearing a Spiropyran Functionality: Understanding Photo- and Electrochemical Control. J. Am. Chem. Soc. 2011, 133, 5453–5462. [Google Scholar] [CrossRef] [Green Version]
- Goulet-Hanssens, A.; Utecht, M.; Mutruc, D.; Titov, E.; Schwarz, J.; Grubert, L.; Bléger, D.; Saalfrank, P.; Hecht, S. Electrocatalytic Z → E Isomerization of Azobenzenes. J. Am. Chem. Soc. 2017, 139, 335–341. [Google Scholar] [CrossRef]
- Goulet-Hanssens, A.; Rietze, C.; Titov, E.; Abdullahu, L.; Grubert, L.; Saalfrank, P.; Hecht, S. Hole Catalysis as a General Mechanism for Efficient and Wavelength-Independent Z → E Azobenzene Isomerization. Chem 2018, 4, 1740–1755. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, Y.; Peng, L.; Yan, D.; Wei, M. A Switchable Electrochromism and Electrochemiluminescence Bifunctional Sensor Based on the Electro-Triggered Isomerization of Spiropyran/Layered Double Hydroxides. Chem. Commun. 2017, 53, 8862–8865. [Google Scholar] [CrossRef]
- Garling, T.; Tong, Y.; Darwish, T.A.; Wolf, M.; Kramer Campen, R. The Influence of Surface Potential on the Optical Switching of Spiropyran Self Assembled Monolayers. J. Phys. Condens. Matter 2017, 29, 414002–414010. [Google Scholar] [CrossRef] [Green Version]
- Areephong, J.; Logtenberg, H.; Browne, W.R.; Feringa, B.L. Symmetric Six-Fold Arrays of Photo- and Electrochromic Dithienylethene Switches. Org. Lett. 2010, 12, 2132–2135. [Google Scholar] [CrossRef] [Green Version]
- Peters, A.; Branda, N.R. Electrochromism in Photochromic Dithienylcyclopentenes. J. Am. Chem. Soc. 2003, 125, 3404–3405. [Google Scholar] [CrossRef]
- Lerch, M.M.; Wezenberg, S.J.; Szymanski, W.; Feringa, B.L. Unraveling the Photoswitching Mechanism in Donor-Acceptor Stenhouse Adducts. J. Am. Chem. Soc. 2016, 138, 6344–6347. [Google Scholar] [CrossRef]
- Hemmer, J.R.; Poelma, S.O.; Treat, N.; Page, Z.A.; Dolinski, N.D.; Diaz, Y.J.; Tomlinson, W.; Clark, K.D.; Hooper, J.P.; Hawker, C.; et al. Tunable Visible and Near Infrared Photoswitches. J. Am. Chem. Soc. 2016, 138, 13960–13966. [Google Scholar] [CrossRef] [Green Version]
- Lui, B.F.; Tierce, N.T.; Tong, F.; Sroda, M.M.; Lu, H.; Read de Alaniz, J.; Bardeen, C.J. Unusual Concentration Dependence of the Photoisomerization Reaction in Donor–Acceptor Stenhouse Adducts. Photochem. Photobiol. Sci. 2019, 18, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Pu, S.; Liu, G.; Chen, B. Photochromism of Asymmetrical Diarylethenes with a Pyrimidine Unit: Synthesis and Substituent Effects. Dye. Pigment. 2014, 102, 159–168. [Google Scholar] [CrossRef]
- Bull, J.N.; Carrascosa, E.; Mallo, N.; Scholz, M.S.; Da Silva, G.; Beves, J.E.; Bieske, E.J. Photoswitching an Isolated Donor-Acceptor Stenhouse Adduct. J. Phys. Chem. Lett. 2018, 9, 665–671. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepherd, N.D.; Moore, H.S.; Beves, J.E.; D’Alessandro, D.M. Electrochemical Switching of First-Generation Donor-Acceptor Stenhouse Adducts (DASAs): An Alternative Stimulus for Triene Cyclisation. Chemistry 2021, 3, 728-733. https://doi.org/10.3390/chemistry3030051
Shepherd ND, Moore HS, Beves JE, D’Alessandro DM. Electrochemical Switching of First-Generation Donor-Acceptor Stenhouse Adducts (DASAs): An Alternative Stimulus for Triene Cyclisation. Chemistry. 2021; 3(3):728-733. https://doi.org/10.3390/chemistry3030051
Chicago/Turabian StyleShepherd, Nicholas D., Harrison S. Moore, Jonathon E. Beves, and Deanna M. D’Alessandro. 2021. "Electrochemical Switching of First-Generation Donor-Acceptor Stenhouse Adducts (DASAs): An Alternative Stimulus for Triene Cyclisation" Chemistry 3, no. 3: 728-733. https://doi.org/10.3390/chemistry3030051
APA StyleShepherd, N. D., Moore, H. S., Beves, J. E., & D’Alessandro, D. M. (2021). Electrochemical Switching of First-Generation Donor-Acceptor Stenhouse Adducts (DASAs): An Alternative Stimulus for Triene Cyclisation. Chemistry, 3(3), 728-733. https://doi.org/10.3390/chemistry3030051