Conjugate Nanoparticles in Cancer Theranostics
Abstract
1. Introduction
2. Core Nanomaterial Composition and Functionalization
2.1. Magnetic Nanoparticles
2.2. Gold-Based Nanostructures
2.3. Silica-Based Platforms
2.4. Carbon-Based Nanomaterials
2.5. Organic π-Conjugated and Polymer-Based Nanoparticles
3. Surface Functionalization Strategies
3.1. PEGylation and Polymer Coatings
3.2. Targeting Ligands
3.3. Stimuli-Responsive Moieties
3.4. Fluorescent and Imaging Enhancers
3.5. Biomimetic and Bioinspired Interfaces
4. Physicochemical Profiling of Nanoparticles
4.1. Particle Size and Morphology: Defining Biological Fate
4.2. Surface Charge (Zeta Potential): Indicator of Stability and Cellular Interaction
4.3. Drug Loading and Controlled Release: Efficacy with Precision
4.4. Magnetic and Relaxometric Properties: MRI and Hyperthermia Readiness
4.5. Surface Functionalization and Ligand Conjugation: Enhancing Target Specificity
4.6. Optical and Photothermal Properties: Imaging and Therapy Integration
4.7. Structural and Elemental Validation: Confirming Material Integrity
4.8. Stability and Biocompatibility: Ensuring Functional Performance
5. Mechanisms of Drug Loading and Release
5.1. pH-Responsive Drug Release
5.2. Redox-Responsive and GSH-Triggered Release
5.3. Prodrug Strategies: Covalent Conjugation and Stimuli-Cleavable Linkers
5.4. Physical Encapsulation and Sustained Release
5.5. Light-, Heat-, and Ultrasound-Triggered Release
5.6. Biochemical Triggering: Enzyme- and miRNA-Responsive Systems
5.7. Radiolabeled Drug Delivery and Internal Radiation Triggers
6. Targeting Strategies in Nanomedicine
6.1. Active Targeting via Ligand–Receptor Interactions
6.2. Passive Targeting via the EPR Effect
6.3. Stimuli-Responsive Targeting
6.4. Magnetic Targeting
6.5. Organelle-Targeted Delivery
6.6. Biomimetic and Homologous Targeting
6.7. Dual and Multimodal Targeting Strategies
7. Therapeutic Modalities Enabled by Nanocarriers
7.1. Chemotherapy: Targeted and Controlled Cytotoxicity
7.2. PTT: Heat-Induced Tumor Ablation
7.3. PDT: Light-Triggered Oxidative Stress
7.4. Radiotherapy and Radiosensitization: Precision Radiation Enhancement
7.5. Magnetic Hyperthermia: Magnetically Controlled Heat Therapy
7.6. Gene Therapy and Gene Silencing: Molecular-Level Precision
7.7. Sonodynamic and Catalytic Therapies: Deep-Tissue and Oxidative Strategies
7.8. Multimodal and Theranostic Platforms: Integrated Therapy and Imaging
8. Imaging and Diagnostic Functions of Nanoplatforms
8.1. FI: High Sensitivity and Molecular Resolution
8.2. MRI: Deep Tissue Contrast and Functional Guidance
8.3. PAI: Optical Contrast with Ultrasound Precision
8.4. Nuclear Imaging (PET/SPECT) and CT: Whole-Body Quantification and Clinical Translation
8.5. Multimodal Imaging: Combining Modalities for Diagnostic Synergy
8.6. Stimuli-Responsive Imaging: Smart Activation in Tumor Microenvironments
9. In Vitro Evaluation and Biocompatibility
9.1. Assessing Biocompatibility: Standard Assays and Normative Models
9.2. Selective Cytotoxicity and Tumor-Specific Action
9.3. Confirming Safety in Normal Cells and In-Vivo Models
9.4. Visualization-Integrated Viability Monitoring
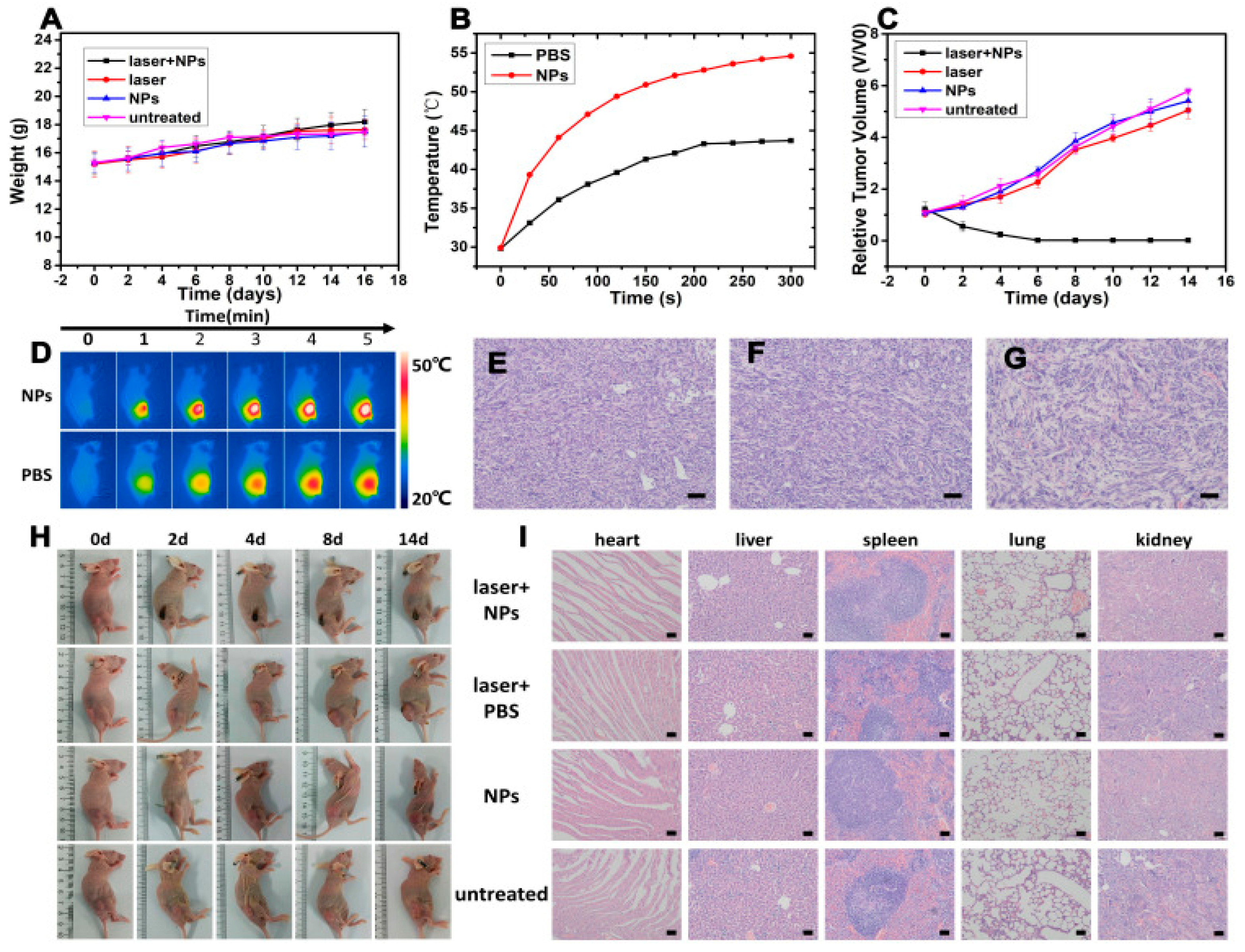
10. In-Vivo Performance and Biodistribution
10.1. Tumor-Selective Accumulation and Retention
10.2. Multimodal Imaging for Real-Time Biodistribution and Therapy Guidance
10.3. In-Vivo Therapeutic Efficacy: Tumor Suppression and Ablation
10.4. Biosafety and Systemic Clearance
10.5. Advanced Targeting Strategies and Personalized Delivery
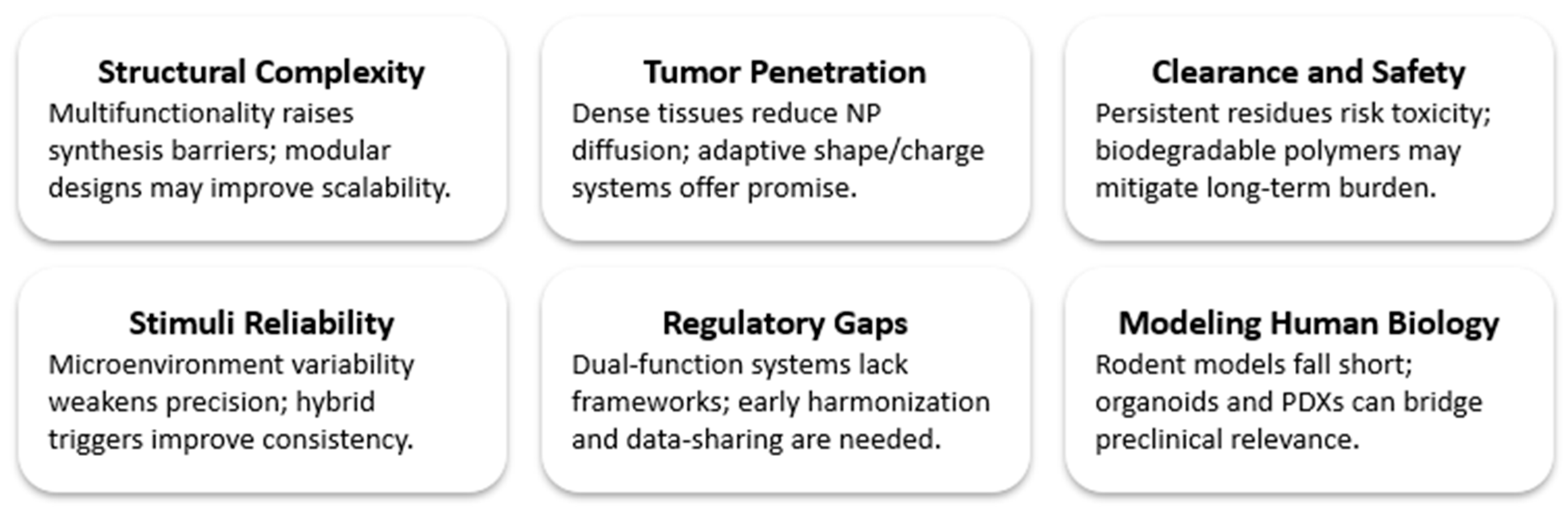
11. Challenges and Translational Strategies in Nanotheranostics
11.1. Current Limitations
11.2. Strategic Pathways Forward
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Abrishami, A.; Bahrami, A.R.; Nekooei, S.; Saljooghi, A.S.; Matin, M.M. Hybridized quantum dot, silica, and gold nanoparticles for targeted chemo-radiotherapy in colorectal cancer theranostics. Commun. Biol. 2024, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Arkaban, H.; Ebrahimi, A.K.; Yarahmadi, A.; Zarrintaj, P.; Barani, M. Development of a multifunctional system based on CoFe2O4@polyacrylic acid NPs conjugated to folic acid and loaded with doxorubicin for cancer theranostics. Nanotechnology 2021, 32, 305101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, X.; Nie, W.; Feng, W.; Zhang, Q.; Wang, W.; Zhang, Y.; Chen, Z.; Huang, P.; He, C. Marriage of Albumin–Gadolinium Complexes and MoS2 Nanoflakes as Cancer Theranostics for Dual-Modality Magnetic Resonance/Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 17786–17798. [Google Scholar] [CrossRef]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.-A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef]
- Gao, L.; Yu, J.; Liu, Y.; Zhou, J.; Sun, L.; Wang, J.; Zhu, J.; Peng, H.; Lu, W.; Yu, L.; et al. Tumor-penetrating Peptide Conjugated and Doxorubicin Loaded T1-T2 Dual Mode MRI Contrast Agents Nanoparticles for Tumor Theranostics. Theranostics 2018, 8, 92–108. [Google Scholar] [CrossRef]
- Muthuraj, B.; Mukherjee, S.; Patra, C.R.; Iyer, P.K. Amplified Fluorescence from Polyfluorene Nanoparticles with Dual State Emission and Aggregation Caused Red Shifted Emission for Live Cell Imaging and Cancer Theranostics. ACS Appl. Mater. Interfaces 2016, 8, 32220–32229. [Google Scholar] [CrossRef]
- Wang, Q.; Sui, G.; Wu, X.; Teng, D.; Zhu, L.; Guan, S.; Ran, H.; Wang, Z.; Wang, H. A sequential targeting nanoplatform for anaplastic thyroid carcinoma theranostics. Acta Biomater. 2020, 102, 367–383. [Google Scholar] [CrossRef]
- Wang, S.; Qi, G.; Zhang, Z.; Yin, Q.; Li, N.; Li, Z.; Shi, G.; Hu, H.; Hao, L. cRGD-Conjugated GdIO Nanoclusters for the Theranostics of Pancreatic Cancer through the Combination of T1–T2 Dual-Modal MRI and DTX Delivery. Molecules 2023, 28, 6134. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, K.; Li, X.; Zhang, J.; Ma, Z.; Foda, M.F.; Mu, Y.; Dai, X.; Han, H. Au Hollow Nanorods-Chimeric Peptide Nanocarrier for NIR-II Photothermal Therapy and Real-time Apoptosis Imaging for Tumor Theranostics. Theranostics 2019, 9, 4971–4981. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, C.; Gao, Y.; Long, J.; Zhang, W.; Chen, Y.; Yang, Y.; Luo, Y.; Lai, Y.; Zhang, H.; et al. NIR-II Emissive Persistent Neutral π-Radical with Rapid Doublet Internal Conversion for Efficient Cancer Photothermal Theranostics. Adv. Sci. 2025, 12, e2411733. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhao, Q.; Qing, W.; Li, S.; Liu, Z.; Li, Q.; Huang, Y. Carrier-Free Delivery of Ultrasmall π-Conjugated Oligomer Nanoparticles with Photothermal Conversion over 80% for Cancer Theranostics. Small 2021, 18, e2104521. [Google Scholar] [CrossRef]
- Yang, M.; Chen, D.; Zhang, L.; Ye, M.; Song, Y.; Xu, J.; Cao, Y.; Liu, Z. Porphyrin-Based Organic Nanoparticles with NIR-IIa Fluorescence for Orthotopic Glioblastoma Theranostics. ACS Appl. Mater. Interfaces 2024, 16, 35925–35935. [Google Scholar] [CrossRef]
- Mohammadzadeh, P.; Cohan, R.A.; Ghoreishi, S.M.; Bitarafan-Rajabi, A.; Ardestani, M.S. AS1411 Aptamer-Anionic Linear Globular Dendrimer G2-Iohexol Selective Nano-Theranostics. Sci. Rep. 2017, 7, 11832. [Google Scholar] [CrossRef]
- Dreifuss, T.; Betzer, O.; Shilo, M.; Popovtzer, A.; Motiei, M.; Popovtzer, R. A challenge for theranostics: Is the optimal particle for therapy also optimal for diagnostics ? Nanoscale 2015, 7, 15175–15184. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, K.; Zhang, B.; Zhao, Y. Superparamagnetic iron oxide nanoparticles conjugated with folic acid for dual target-specific drug delivery and MRI in cancer theranostics. Mater. Sci. Eng. C 2017, 70, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, D.; Sagayaraj, P.M.A.; Dasgupta, T.; GR, T.; Ramasamy, T.; Sundaramoorthy, A.; Nandigam, N.; Udayakumar, K.; Kumar, V.S.; Karthikeyan, S.; et al. Doxorubicin-Conjugated Terbium-Doped Carbon Dots for Site-Specific Colon Cancer Theranostics. ACS Appl. Nano Mater. 2025, 8, 6274–6287. [Google Scholar] [CrossRef]
- Rajkumar, S.; Prabaharan, M. Multi-functional nanocarriers based on iron oxide nanoparticles conjugated with doxorubicin, poly(ethylene glycol) and folic acid as theranostics for cancer therapy. Colloids Surfaces B Biointerfaces 2018, 170, 529–537. [Google Scholar] [CrossRef]
- Hung, B.-Y.; Kuthati, Y.; Kankala, R.K.; Kankala, S.; Deng, J.-P.; Liu, C.-L.; Lee, C.-H. Utilization of Enzyme-Immobilized Mesoporous Silica Nanocontainers (IBN-4) in Prodrug-Activated Cancer Theranostics. Nanomaterials 2015, 5, 2169–2191. [Google Scholar] [CrossRef]
- Kang, X.; Sun, T.; Zhang, L.; Zhou, C.; Xu, Z.; Du, M.; Xiao, S.; Liu, Y.; Gong, M.; Zhang, D. Synergistic Theranostics of Magnetic Resonance Imaging and Photothermal Therapy of Breast Cancer Based on the Janus Nanostructures Fe3O4-Aushell-PEG. Int. J. Nanomed. 2021, 16, 6383–6394. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.L.; Moonshi, S.S.; Wu, Y.; Cowin, G.; Prada, K.X.V.; Tran, H.D.; Bulmer, A.C.; Ta, H.T. A methotrexate labelled dual metal oxide nanocomposite for long-lasting anti-cancer theranostics. Mater. Today Bio 2024, 30, 101377. [Google Scholar] [CrossRef]
- Zahraei, M.; Marciello, M.; Lazaro-Carrillo, A.; Villanueva, A.; Herranz, F.; Talelli, M.; Costo, R.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; et al. Versatile theranostics agents designed by coating ferrite nanoparticles with biocompatible polymers. Nanotechnology 2016, 27, 255702–255714. [Google Scholar] [CrossRef]
- Entract, G.M.; Bryden, F.; Domarkas, J.; Savoie, H.; Allott, L.; Archibald, S.J.; Cawthorne, C.; Boyle, R.W. Development of PDT/PET Theranostics: Synthesis and Biological Evaluation of an 18F-Radiolabeled Water-Soluble Porphyrin. Mol. Pharm. 2015, 12, 4414–4423. [Google Scholar] [CrossRef]
- Chen, Q.T.; Shi, X.D.; Liang, W.W.; Jiang, L.Y.; Fang, S.M.; Chen, F.H. Synthesis of Fluorescence/MRI Dual Targeted Imaging Theranostics Reagent. Chin. J. Inorg. Chem. 2021, 37, 1555–1562. [Google Scholar] [CrossRef]
- Chen, Z.; Liao, T.; Wan, L.; Kuang, Y.; Liu, C.; Duan, J.; Xu, X.; Xu, Z.; Jiang, B.; Li, C. Dual-stimuli responsive near-infrared emissive carbon dots/hollow mesoporous silica-based integrated theranostics platform for real-time visualized drug delivery. Nano Res. 2021, 14, 4264–4273. [Google Scholar] [CrossRef]
- Tong, H.; Chen, Y.; Li, Z.; Li, H.; Chen, T.; Jin, Q.; Ji, J. Glutathione Activatable Photosensitizer-Conjugated Pseudopolyrotaxane Nanocarriers for Photodynamic Theranostics. Small 2016, 12, 6223–6232. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Yang, F.; He, W.; Song, L.; Qiu, F.; Xu, N.; Xu, L.; Zhang, Y.; Hua, Z.; Gu, N. A Multi-Gradient Targeting Drug Delivery System Based on RGD-l-TRAIL-Labeled Magnetic Microbubbles for Cancer Theranostics. Adv. Funct. Mater. 2016, 26, 8313–8324. [Google Scholar] [CrossRef]
- Deng, G.; Peng, X.; Sun, Z.; Zheng, W.; Yu, J.; Du, L.; Chen, H.; Gong, P.; Zhang, P.; Cai, L.; et al. Natural-Killer-Cell-Inspired Nanorobots with Aggregation-Induced Emission Characteristics for Near-Infrared-II Fluorescence-Guided Glioma Theranostics. ACS Nano 2020, 14, 11452–11462. [Google Scholar] [CrossRef]
- Yu, B.; Goel, S.; Ni, D.; Ellison, P.A.; Siamof, C.M.; Jiang, D.; Cheng, L.; Kang, L.; Yu, F.; Liu, Z.; et al. Reassembly of 89Zr-Labeled Cancer Cell Membranes into Multicompartment Membrane-Derived Liposomes for PET-Trackable Tumor-Targeted Theranostics. Adv. Mater. 2018, 30, e1704934. [Google Scholar] [CrossRef]
- Ghorbani, M.; Bigdeli, B.; Jalili-Baleh, L.; Baharifar, H.; Akrami, M.; Dehghani, S.; Goliaei, B.; Amani, A.; Lotfabadi, A.; Rashedi, H.; et al. Curcumin-lipoic acid conjugate as a promising anticancer agent on the surface of gold-iron oxide nanocomposites: A pH-sensitive targeted drug delivery system for brain cancer theranostics. Eur. J. Pharm. Sci. 2018, 114, 175–188. [Google Scholar] [CrossRef]
- Omidian, H.; Chowdhury, S.D. Advances in Photothermal and Photodynamic Nanotheranostics for Precision Cancer Treatment. J. Nanotheranostics 2024, 5, 228–252. [Google Scholar] [CrossRef]
- Wen, K.; Xu, X.; Chen, J.; Lv, L.; Wu, L.; Hu, Y.; Wu, X.; Liu, G.; Peng, A.; Huang, H. Triplet Tellurophene-Based Semiconducting Polymer Nanoparticles for Near-Infrared-Mediated Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 17884–17893. [Google Scholar] [CrossRef]
- Qi, G.; Liu, X.; Shi, L.; Wu, M.; Liu, J.; Liu, B. Enzyme-Mediated Intracellular Polymerization of AIEgens for Light-Up Tumor Localization and Theranostics. Adv. Mater. 2021, 34, 2106885. [Google Scholar] [CrossRef]
- Yan, S.; Chen, J.; Cai, L.; Xu, P.; Zhang, Y.; Li, S.; Hu, P.; Chen, X.; Huang, M.; Chen, Z. Phthalocyanine-based photosensitizer with tumor-pH-responsive properties for cancer theranostics. J. Mater. Chem. B 2018, 6, 6080–6088. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Jin, R.; Cai, B.; Liu, S.; Bai, Y.; Chen, X. Multifunctional hierarchical nanohybrids perform triple antitumor theranostics in a cascaded manner for effective tumor treatment. Acta Biomater. 2021, 128, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Wang, R.; Zhang, H.; Zhang, Q.; Qin, Y.; Du, C.; Zhang, X.; Ye, J.; Shi, C.; Shen, H.; et al. Preclinical and First-in-Human Study of a Compact Radionuclide Labeled Self-Assembly Nanomedicine for Chemo-Radio-Theranostics of Cancer. ACS Nano 2025, 19, 3953–3965. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Feng, W.; Chen, Z.; Huang, Z.; Mu, X.; Lu, Y.; Zhou, X. Engineering thio-/seleno-ether linkers into zwitterionic small molecule nano-prodrugs for traceable cancer theranostics. Sensors Actuators B Chem. 2023, 387, 133771. [Google Scholar] [CrossRef]
- Yan, X.; Su, M.; Liu, Y.; Zhang, Y.; Zhang, H.; Li, C. Molecularly Engineered Hierarchical Nanodisc from Antiparallel J-stacked BODIPY Conjugates: Application to Theranostics with Mutually Beneficial Properties. Adv. Funct. Mater. 2020, 31, 2008406. [Google Scholar] [CrossRef]
- Yang, Y.; Jing, L.; Li, X.; Lin, L.; Yue, X.; Dai, Z. Hyaluronic Acid Conjugated Magnetic Prussian Blue@Quantum Dot Nanoparticles for Cancer Theranostics. Theranostics 2017, 7, 466–481. [Google Scholar] [CrossRef]
- Yi, X.; Xu, M.; Zhou, H.; Xiong, S.; Qian, R.; Chai, Z.; Zhao, L.; Yang, K. Ultrasmall Hyperbranched Semiconducting Polymer Nanoparticles with Different Radioisotopes Labeling for Cancer Theranostics. ACS Nano 2018, 12, 9142–9151. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Ma, D.; Yang, R.; Xu, F.; Wu, H.; He, C.; Liu, L.; Dong, J.; Shao, Y. Nanoassembly and Multiscale Computation of Multifunctional Optical-Magnetic Nanoprobes for Tumor-Targeted Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 41069–41081. [Google Scholar] [CrossRef]
- Fang, S.; Li, C.; Lin, J.; Zhu, H.; Cui, D.; Xu, Y.; Li, Z. Gold Nanorods-Based Theranostics for Simultaneous Fluorescence/Two-Photon Luminescence Imaging and Synergistic Phototherapies. J. Nanomater. 2016, 2016, 1082746. [Google Scholar] [CrossRef]
- Praseetha, P.K.; Litany, R.I.J.; Alharbi, H.M.; Khojah, A.A.; Akash, S.; Bourhia, M.; Mengistie, A.A.; Shazly, G.A. Green synthesis of highly fluorescent carbon quantum dots from almond resin for advanced theranostics in biomedical applications. Sci. Rep. 2024, 14, 24435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, T.; Tao, J.; Wan, G.; Zhao, H. Co-delivery of paclitaxel and indocyanine green by PEGylated graphene oxide: A potential integrated nanoplatform for tumor theranostics. RSC Adv. 2016, 6, 15460–15468. [Google Scholar] [CrossRef]
- Jia, X.; Pei, M.; Zhao, X.; Tian, K.; Zhou, T.; Liu, P. PEGylated Oxidized Alginate-DOX Prodrug Conjugate Nanoparticles Cross-Linked with Fluorescent Carbon Dots for Tumor Theranostics. ACS Biomater. Sci. Eng. 2016, 2, 1641–1648. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Lee, J.S.; Choi, J.H.; Park, K.M.; Lee, Y.; Park, K.D. Hierarchical self-assembly of magnetic nanoclusters for theranostics: Tunable size, enhanced magnetic resonance imagability, and controlled and targeted drug delivery. Acta Biomater. 2016, 35, 109–117. [Google Scholar] [CrossRef]
- Dada, S.N.; Babanyinah, G.K.; Tetteh, M.T.; Palau, V.E.; Walls, Z.F.; Krishnan, K.; Croft, Z.; Khan, A.U.; Liu, G.; Wiese, T.E.; et al. Covalent and Noncovalent Loading of Doxorubicin by Folic Acid-Carbon Dot Nanoparticles for Cancer Theranostics. ACS Omega 2022, 7, 23322–23331. [Google Scholar] [CrossRef]
- Setiawan, H.; Yuba, E.; Harada, A.; Aoki, I.; Kono, K. Fabrication of gold nanohybrids modified with antibody and functional dendrimers for targeted photothermal theranostics. Nano Sel. 2020, 2, 779–790. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, G.; Teng, D.; Wang, Q.; Qu, J.; Zhu, L.; Ran, H.; Wang, Z.; Jin, C.; Wang, H. Low intensity focused ultrasound (LIFU) triggered drug release from cetuximab-conjugated phase-changeable nanoparticles for precision theranostics against anaplastic thyroid carcinoma. Biomater. Sci. 2018, 7, 196–210. [Google Scholar] [CrossRef]
- Wen, R.; Lv, X.; Yang, T.; Li, Y.; Tang, Y.; Bai, X.; Ke, H.; Shen, J.; Chen, H. Albumin nanoreactor-templated synthesis of Gd2O3/CuS hybrid nanodots for cancer theranostics. Sci. China Mater. 2017, 60, 554–562. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Patil, S.I.; Tofail, S.A.M.; Bauer, J.; Thorat, N.D. MRI Guided Magneto-chemotherapy with High-Magnetic-Moment Iron Oxide Nanoparticles for Cancer Theranostics. ACS Appl. Bio Mater. 2020, 3, 2305–2313. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Z.; Yang, B.; Wang, J.; Li, Y. Synthesis of dual-functional targeting probes for cancer theranostics based on iron oxide nanoparticles coated by centipede-like polymer connected with pH-responsive anticancer drug. J. Biomater. Sci. Polym. Ed. 2015, 26, 1178–1189. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, P.; Huang, K.; Deng, G.; Zhou, Z.; Wang, J.; Wang, M.; Zhang, Y.; Yang, H.; Yang, S. Amplifying Apoptosis Homing Nanoplatform for Tumor Theranostics. Adv. Heal. Mater. 2018, 7, e1800296. [Google Scholar] [CrossRef]
- Grabowska-Jadach, I.; Kalinowska, D.; Drozd, M.; Pietrzak, M. Synthesis, characterization and application of plasmonic hollow gold nanoshells in a photothermal therapy—New particles for theranostics. Biomed. Pharmacother. 2019, 111, 1147–1155. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, F.; Xiong, Z.; Shen, M.; Shi, X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surfaces B Biointerfaces 2015, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhou, J.; Yu, J.; Li, Q.; Liu, X.; Sun, L.; Peng, T.; Wang, J.; Zhu, J.; Sun, J.; et al. A Novel Gd-DTPA-conjugated Poly(L-γ-glutamyl-glutamine)-paclitaxel Polymeric Delivery System for Tumor Theranostics. Sci. Rep. 2017, 7, 3799. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Liu, T.; Yang, Z.; Zhou, Z.; Tang, W.; Fan, W.; Liu, Y.; Mu, J.; Li, L.; Bregadze, V.I.; et al. Small-sized gadolinium oxide based nanoparticles for high-efficiency theranostics of orthotopic glioblastoma. Biomaterials 2020, 235, 119783. [Google Scholar] [CrossRef]
- Tedla, G.; Plotkin, J.; Dellinger, A.; Kepley, C. Design and Testing of Dual-Targeted Gd3N@C80-Containing Glioblastoma Theranostics. J. Nanomater. 2019, 2019, 1242930. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, X.; Chau, H.-F.; Thor, W.; Jiang, L.; Zha, S.; Fok, W.-Y.; Mak, H.-N.; Zhang, J.; Cai, J.; et al. Lanthanide–Cyclen–Camptothecin Nanocomposites for Cancer Theranostics Guided by Near-Infrared and Magnetic Resonance Imaging. ACS Appl. Nano Mater. 2021, 4, 271–278. [Google Scholar] [CrossRef]
- Zhu, J.; Xiong, Z.; Shen, M.; Shi, X. Encapsulation of doxorubicin within multifunctional gadolinium-loaded dendrimer nanocomplexes for targeted theranostics of cancer cells. RSC Adv. 2015, 5, 30286–30296. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.S.; Park, S.-J.; Na, K. Vitamin Bc-Bearing Hydrophilic Photosensitizer Conjugate for Photodynamic Cancer Theranostics. Macromol. Biosci. 2015, 15, 1081–1090. [Google Scholar] [CrossRef]
- Mansoorianfar, M.; Hussain, Z.; Simchi, A.; Cao, Y.; Ullah, I.; Ullah, S. Target-responsive DNA aptamer-conjugated superparamagnetic Ag/CuS nanoparticles as near-infrared light-triggered theranostics and dual-modal imaging. Appl. Mater. Today 2023, 34, 101913. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mukherjee, S.; Das, S.; Patra, C.R.; Iyer, P.K. Multifunctional (3-in-1) cancer theranostics applications of hydroxyquinoline-appended polyfluorene nanoparticles. Chem. Sci. 2017, 8, 7566–7575. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Shende, P. Anti-CD64 Antibody-Conjugated PLGA Nanoparticles Containing Methotrexate and Gold for Theranostics Application in Rheumatoid Arthritis. Aaps Pharmscitech 2024, 25, 22. [Google Scholar] [CrossRef]
- Huang, C.; Chen, F.; Zhang, L.; Yang, Y.; Yang, X.; Pan, W. 99mTc Radiolabeled HA/TPGS-Based Curcumin-Loaded Nanoparticle for Breast Cancer Synergistic Theranostics: Design, in vitro and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 2987–2998. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Kuan, C.-H.; Wang, L.-W.; Wu, H.-C.; Chen, Y.; Chang, C.-W.; Huang, R.-Y.; Wang, T.-W. Integrated self-assembling drug delivery system possessing dual responsive and active targeting for orthotopic ovarian cancer theranostics. Biomaterials 2016, 90, 12–26. [Google Scholar] [CrossRef]
- Pan, G.-Y.; Jia, H.-R.; Zhu, Y.-X.; Sun, W.; Cheng, X.-T.; Wu, F.-G. Cyanine-Containing Polymeric Nanoparticles with Imaging/Therapy-Switchable Capability for Mitochondria-Targeted Cancer Theranostics. ACS Appl. Nano Mater. 2018, 1, 2885–2897. [Google Scholar] [CrossRef]
- Mansur, A.A.; Amaral-Júnior, J.C.; Carvalho, S.M.; Carvalho, I.C.; Mansur, H.S. Cu-In-S/ZnS@carboxymethylcellulose supramolecular structures: Fluorescent nanoarchitectures for targeted-theranostics of cancer cells. Carbohydr. Polym. 2020, 247, 116703. [Google Scholar] [CrossRef]
- Singh, H.; Dhar, D.; Das, S.; Halder, M. Methotrexate-loaded manganese nitrogen dual-doped carbon quantum dots as targeted nano drug-delivery system for potential use in cancer theranostics. J. Photochem. Photobiol. A Chem. 2024, 455, 115692. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, F.; Zhao, Y.; Zhang, J.; Dawulieti, J.; Pan, Y.; Cui, L.; Sun, M.; Shao, D.; Li, M.; et al. Self-assembled dual fluorescence nanoparticles for CD44-targeted delivery of anti-miR-27a in liver cancer theranostics. Theranostics 2018, 8, 3808–3823. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Li, A.; Xu, S.; Manghnani, P.N.; Liu, C.; Guo, L.; Zheng, H.; Liu, B. Molecular Engineering of Conjugated Polymers for Biocompatible Organic Nanoparticles with Highly Efficient Photoacoustic and Photothermal Performance in Cancer Theranostics. ACS Nano 2017, 11, 10124–10134. [Google Scholar] [CrossRef]
- Guo, B.; Wu, M.; Shi, Q.; Dai, T.; Xu, S.; Jiang, J.; Liu, B. All-in-One Molecular Aggregation-Induced Emission Theranostics: Fluorescence Image Guided and Mitochondria Targeted Chemo- and Photodynamic Cancer Cell Ablation. Chem. Mater. 2020, 32, 4681–4691. [Google Scholar] [CrossRef]
- Ma, X.; Wang, P.; Wu, Q.; Zhou, J.; Wang, D.; Yadav, D.; Zhang, H.; Zhang, Y. Porphyrin Centered Paclitaxel Tetrameric Prodrug Nanoassemblies as Tumor-Selective Theranostics for Synergized Breast Cancer Therapy. Adv. Healthc. Mater. 2022, 12, e2202024. [Google Scholar] [CrossRef]
- Yang, X.; Luo, Y.; Li, S.; Xu, X.; Bao, Y.; Yang, J.; Ouyang, D.; Fan, X.; Gong, P.; Cai, L. Small Molecular Prodrug Amphiphile Self-Assembled AIE Dots for Cancer Theranostics. Front. Bioeng. Biotechnol. 2020, 8, 903. [Google Scholar] [CrossRef] [PubMed]
- Pal, J.; Khan, A.; Samanta, P.; Khamrai, M.; Mallick, A.I.; Dhara, D. Raspberry-like gold nano-conjugates of block copolymer prodrug based bicontinuous nanoparticles for cancer theranostics. J. Colloid Interface Sci. 2025, 687, 817–829. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, L.; Shang, W.; Liu, Z.; Xie, W.; Qiang, C.; Xiong, Z.; Zhang, R.; Li, B.; Sun, X.; et al. General synthesis of high-performing magneto-conjugated polymer core–shell nanoparticles for multifunctional theranostics. Nano Res. 2016, 10, 704–717. [Google Scholar] [CrossRef]
- Choi, M.J.; Lee, Y.K.; Choi, K.C.; Lee, D.H.; Jeong, H.Y.; Kang, S.J.; Kim, M.W.; You, Y.M.; Im, C.S.; Lee, T.S.; et al. Tumor-Targeted Erythrocyte Membrane Nanoparticles for Theranostics of Triple-Negative Breast Cancer. Pharmaceutics 2023, 15, 350. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, Z.; Neubi, G.M.N.; Cheng, H.; Zhang, C.; Zhang, H.; Wang, R.; Zhou, J.; Ding, Y. Lipoprotein-inspired penetrating nanoparticles for deep tumor-targeted shuttling of indocyanine green and enhanced photo-theranostics. Biomater. Sci. 2019, 7, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhou, H.; Zhang, Z.; Xiong, S.; Yang, K. X-rays-optimized delivery of radiolabeled albumin for cancer theranostics. Biomaterials 2020, 233, 119764. [Google Scholar] [CrossRef]
- He, C.; Guo, Y.; Zhou, N.; Wang, Z.; Liu, T.; Xu, X.; Wang, F.; Zhu, H.; Yang, Z.; Yang, X.; et al. Construction and Application of a PD-L1-Targeted Multimodal Diagnostic and Dual-Functional Theranostics Nanoprobe. Int. J. Nanomed. 2024, 19, 5479–5492. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Alajangi, H.K.; Sharma, A.; Kaur, H.; Sharma, P.; Negi, S.; Kumari, L.; Trivedi, M.; Yadav, A.K.; Kumar, R.; et al. Theranostics: Aptamer-assisted carbon nanotubes as MRI contrast and photothermal agent for breast cancer therapy. Nanoscale Res. Lett. 2024, 19, 145. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, M.; Cao, J.; Zhang, Y.; Yuan, Z.; Wu, Q.; Wang, W. Multivalent nanoparticles for personalized theranostics based on tumor receptor distribution behavior. Nanoscale 2019, 11, 5005–5013. [Google Scholar] [CrossRef]
- Chatap, V.; Vanjari, P.; Bhilare, N.V. Curcumin loaded magnetic nanocellulose fiber composites with con-a cap for theranostics application in breast cancer. Cellulose 2025, 32, 3855–3876. [Google Scholar] [CrossRef]
- Ma, B.; Zhuang, W.; Xu, H.; Li, G.; Wang, Y. Hierarchical Responsive Nanoplatform with Two-Photon Aggregation-Induced Emission Imaging for Efficient Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 47259–47269. [Google Scholar] [CrossRef]
- Khan, A.; Tripathi, A.; Gandhi, M.; Bellare, J.; Srivastava, R. Development of injectable upconversion nanoparticle-conjugated doxorubicin theranostics electrospun nanostructure for targeted photochemotherapy in breast cancer. J. Biomed. Mater. Res. Part A 2024, 112, 1612–1626. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Fu, Z.; Guo, H.; Liu, K.; Ren, J.; Huang, Z.; Yang, F.; Mao, H. TRPC6-targeted dexamethasone nanobubbles with ultrasound-guided theranostics for adriamycin-induced nephropathy. J. Nanobiotechnology 2025, 23, 398. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Qiao, Z.; Tang, J.; He, X.; Shi, H.; Ye, X.; Yan, L.; He, D.; Wang, K. DNA nanotriangle-scaffolded activatable aptamer probe with ultralow background and robust stability for cancer theranostics. Theranostics 2018, 8, 4062–4071. [Google Scholar] [CrossRef] [PubMed]
- Raskolupova, V.I.; Wang, M.; Dymova, M.A.; Petrov, G.O.; Shchudlo, I.M.; Taskaev, S.Y.; Abramova, T.V.; Godovikova, T.S.; Silnikov, V.N.; Popova, T.V. Design of the New Closo-Dodecarborate-Containing Gemcitabine Analogue for the Albumin-Based Theranostics Composition. Molecules 2023, 28, 2672. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.; Pathiriparambath, M.S.R.; Thomas, V.; Tharayil, H.; Jayasree, R.S.; Nair, L.V. Cysteine-stabilized platinum nanocluster self-assembly for targeted theranostics in vitro. Mater. Today Chem. 2025, 45, 102669. [Google Scholar] [CrossRef]
- Li, Q.; Hou, M.; Ren, J.; Lu, S.; Xu, Z.; Li, C.M.; Kang, Y.; Xue, P. Co-delivery of Chlorin e6 and Doxorubicin Using PEGylated Hollow Nanocapsules for ‘all-in-one’ Tumor Theranostics. Nanomedicine 2019, 14, 2273–2292. [Google Scholar] [CrossRef]
- Bakshi, S.; Zakharchenko, A.; Minko, S.; Kolpashchikov, D.M.; Katz, E. Towards Nanomaterials for Cancer Theranostics: A System of DNA-Modified Magnetic Nanoparticles for Detection and Suppression of RNA Marker in Cancer Cells. Magnetochemistry 2019, 5, 24. [Google Scholar] [CrossRef]
- Dong, H.; Gao, Y.; Huang, X.; Wu, X. Synthesis of sialic acid conjugates of the clinical near-infrared dye as next-generation theranostics for cancer phototherapy. J. Mater. Chem. B 2022, 10, 927–934. [Google Scholar] [CrossRef]
- Guo, L.; Liu, W.; Niu, G.; Zhang, P.; Zheng, X.; Jia, Q.; Zhang, H.; Ge, J.; Wang, P. Polymer nanoparticles with high photothermal conversion efficiency as robust photoacoustic and thermal theranostics. J. Mater. Chem. B 2017, 5, 2832–2839. [Google Scholar] [CrossRef]
- Devendiran, R.M.; Chinnaiyan, S.K.; Yadav, N.K.; Moorthy, G.K.; Ramanathan, G.; Singaravelu, S.; Sivagnanam, U.T.; Perumal, P.T. Green synthesis of folic acid-conjugated gold nanoparticles with pectin as reducing/stabilizing agent for cancer theranostics. RSC Adv. 2016, 6, 29757–29768. [Google Scholar] [CrossRef]
- Jin, G.; He, R.; Liu, Q.; Dong, Y.; Lin, M.; Li, W.; Xu, F. Theranostics of Triple-Negative Breast Cancer Based on Conjugated Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 10634–10646. [Google Scholar] [CrossRef]
- Jin, X.; Zeng, Q.; Zheng, J.; Xing, D.; Zhang, T. Aptamer-Functionalized Upconverting Nanoformulations for Light-Switching Cancer-Specific Recognition and In Situ Photodynamic–Chemo Sequential Theranostics. ACS Appl. Mater. Interfaces 2020, 13, 9316–9328. [Google Scholar] [CrossRef]
- Sonali; Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L.; et al. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ma, X.; Feng, S.; Liang, X.; Dai, Z.; Tian, J.; Yue, X. Hyaluronic Acid Modified Tantalum Oxide Nanoparticles Conjugating Doxorubicin for Targeted Cancer Theranostics. Bioconjugate Chem. 2015, 26, 2530–2541. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, Y.; Cao, F.; Chen, J.; Chen, C.; Chang, J.; Hou, L.; Zhang, Z. In Situ Autophagy Disruption Generator for Cancer Theranostics. ACS Appl. Mater. Interfaces 2019, 11, 29641–29654. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.-T.; Baek, M.-J.; Lee, S.M.; Kim, D.; Yoo, S.-Y.; Lee, J.-Y.; Kim, D.-D. Photobleaching-mediated charge-convertible cyclodextrin nanoparticles achieve deep tumour penetration for rectal cancer theranostics. Nat. Nanotechnol. 2024, 19, 1723–1734. [Google Scholar] [CrossRef]
- Devi, L.S.; Casadidio, C.; Gigliobianco, M.R.; Di Martino, P.; Censi, R. Multifunctionality of cyclodextrin-based polymeric nanoparticulate delivery systems for chemotherapeutics, combination therapy, and theranostics. Int. J. Pharm. 2024, 654, 123976. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, R.; Esmaeili, A.; Nematollahzadeh, A. Preparation of Fe3O4/Ag3VO4/Au nanocomposite coated with Caerophyllum macropodum extract modified with oleic acid for theranostics agent in medical imaging. J. Photochem. Photobiol. A Chem. 2022, 425, 113724. [Google Scholar] [CrossRef]
- Thorat, N.D.; Bohara, R.A.; Tofail, S.A.M.; Alothman, Z.A.; Shiddiky, M.J.A.; Hossain, S.A.; Yamauchi, Y.; Wu, K.C. Superparamagnetic Gadolinium Ferrite Nanoparticles with Controllable Curie Temperature–Cancer Theranostics for MR-Imaging-Guided Magneto-Chemotherapy. Eur. J. Inorg. Chem. 2016, 2016, 4586–4597. [Google Scholar] [CrossRef]
- Thorat, N.D.; Bohara, R.A.; Noor, M.R.; Dhamecha, D.; Soulimane, T.; Tofail, S.A.M. Effective Cancer Theranostics with Polymer Encapsulated Superparamagnetic Nanoparticles: Combined Effects of Magnetic Hyperthermia and Controlled Drug Release. ACS Biomater. Sci. Eng. 2016, 3, 1332–1340. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Hao, Y.; Zhang, P.; Zhao, Y.; Meng, D.; Li, D.; Chang, J.; Zhang, Z. Magnetic Multi-Walled Carbon Nanotubes for Tumor Theranostics. J. Biomed. Nanotechnol. 2015, 11, 1653–1661. [Google Scholar] [CrossRef]
- Yao, J.; Yang, Z.; Huang, L.; Yang, C.; Wang, J.; Cao, Y.; Hao, L.; Zhang, L.; Zhang, J.; Li, P.; et al. Low-Intensity Focused Ultrasound-Responsive Ferrite-Encapsulated Nanoparticles for Atherosclerotic Plaque Neovascularization Theranostics. Adv. Sci. 2021, 8, 2100850. [Google Scholar] [CrossRef]
- Wang, F.; Men, X.; Chen, H.; Mi, F.; Xu, M.; Men, X.; Yuan, Z.; Lo, P.K. Second near-infrared photoactivatable biocompatible polymer nanoparticles for effective in vitro and in vivo cancer theranostics. Nanoscale 2021, 13, 13410–13420. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shao, C.; Liu, T.; Chao, Z.; Chen, H.; Xiao, F.; He, H.; Wei, Z.; Zhu, Y.; Wang, H.; et al. An NIR-II-Emissive Photosensitizer for Hypoxia-Tolerant Photodynamic Theranostics. Adv. Mater. 2020, 32, e2003471. [Google Scholar] [CrossRef]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.-K. A hyaluronic acid nanogel for photo–chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Goel, S.; Ehlerding, E.B.; Rosenkrans, Z.T.; Jiang, D.; Sun, T.; Aluicio-Sarduy, E.; Engle, J.W.; Ni, D.; Cai, W. Ultrasmall Porous Silica Nanoparticles with Enhanced Pharmacokinetics for Cancer Theranostics. Nano Lett. 2021, 21, 4692–4699. [Google Scholar] [CrossRef]
- Shamsel-Din, H.A.; Swidan, M.M.; Ibrahim, A.B.; Motaleb, M.A.; Sakr, T.M. Novel pyranopyridine derivatives and their radiolabeled nanoconjugates: An augmented approach for targeted cancer theranostics. J. Drug Deliv. Sci. Technol. 2025, 107, 106802. [Google Scholar] [CrossRef]
- Swidan, M.M.; Essa, B.M.; Sakr, T.M. Pristine/folate-functionalized graphene oxide as two intrinsically radioiodinated nano-theranostics: Self/dual in vivo targeting comparative study. Cancer Nanotechnol. 2023, 14, 6. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.; Liu, L.; Jiang, A.; Mao, F.; Liu, D.; Wang, L.; Zhou, J. Simultaneous Activation of Short-Wave Infrared (SWIR) Light and Paramagnetism by a Functionalized Shell for High Penetration and Spatial Resolution Theranostics. Adv. Funct. Mater. 2017, 28, 1705057. [Google Scholar] [CrossRef]
- Lyu, Y.; Fang, Y.; Miao, Q.; Zhen, X.; Ding, D.; Pu, K. Intraparticle Molecular Orbital Engineering of Semiconducting Polymer Nanoparticles as Amplified Theranostics for in Vivo Photoacoustic Imaging and Photothermal Therapy. ACS Nano 2016, 10, 4472–4481. [Google Scholar] [CrossRef]
- Li, J.; Kang, M.; Zhang, Z.; Li, X.; Xu, W.; Wang, D.; Gao, X.; Tang, B.Z. Synchronously Manipulating Absorption and Extinction Coefficient of Semiconducting Polymers via Precise Dual-Acceptor Engineering for NIR-II Excited Photothermal Theranostics. Angew. Chem. Int. Ed. Engl. 2023, 62, e202301617. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhou, X.; Zhang, H.; Ou, H.; Lam, J.W.Y.; Liu, Y.; Shi, L.; Ding, D.; Tang, B.Z. Molecular Motion in Aggregates: Manipulating TICT for Boosting Photothermal Theranostics. J. Am. Chem. Soc. 2019, 141, 5359–5368. [Google Scholar] [CrossRef]
- Kim, I.; Han, E.H.; Ryu, J.; Min, J.-Y.; Ahn, H.; Chung, Y.-H.; Lee, E. One-Dimensional Supramolecular Nanoplatforms for Theranostics Based on Co-Assembly of Peptide Amphiphiles. Biomacromolecules 2016, 17, 3234–3243. [Google Scholar] [CrossRef]
- Yang, L.; Li, W.; Huang, Y.; Zhou, Y.; Chen, T. Rational Design of Cancer-Targeted Benzoselenadiazole by RGD Peptide Functionalization for Cancer Theranostics. Macromol. Rapid Commun. 2015, 36, 1559–1565. [Google Scholar] [CrossRef]
- Panchal, H.; Panjwani, D.; Patel, S.; Ahlawat, P.; Patel, L.D.; Dharamsi, A.; Patel, A. Quantum Dots Functionalized Polymeric Nanoparticles as Cancer Theranostics: An Advanced Nanomedicine Strategy. Curr. Cancer Drug Targets 2025, 25, 1083–1107. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhu, M.; Yuan, P.; Liu, T.; Tian, R.; Bai, Y.; Zhang, Y.; Chen, X. The facile formation of hierarchical mesoporous silica nanocarriers for tumor-selective multimodal theranostics. Biomater. Sci. 2021, 9, 5237–5246. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, D.; Kuzmin, A.; Pliss, A.; Shao, W.; Xia, J.; Qu, J.; Prasad, P.N. ICG-Sensitized NaYF4:Er Nanostructure for Theranostics. Adv. Opt. Mater. 2018, 6, 1701142. [Google Scholar] [CrossRef]
- Li, S.; Deng, Q.; Zhang, Y.; Li, X.; Wen, G.; Cui, X.; Wan, Y.; Huang, Y.; Chen, J.; Liu, Z.; et al. Rational Design of Conjugated Small Molecules for Superior Photothermal Theranostics in the NIR-II Biowindow. Adv. Mater. 2020, 32, e2001146. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Shi, X.; Dong, L.; Yang, Y.; Zhang, Y.; Wu, G.; Zhu, R. Radionuclide 188Re-Loaded Photothermal Hydrogel for Cancer Theranostics. Part. Part. Syst. Charact. 2020, 37, 1900421. [Google Scholar] [CrossRef]
- Hsu, S.P.; Dhawan, U.; Tseng, Y.-Y.; Lin, C.-P.; Kuo, C.-Y.; Wang, L.-F.; Chung, R.-J. Glioma-sensitive delivery of Angiopep-2 conjugated iron gold alloy nanoparticles ensuring simultaneous tumor imaging and hyperthermia mediated cancer theranostics. Appl. Mater. Today 2020, 18, 100510. [Google Scholar] [CrossRef]
- Autio, K.A.; Garcia, J.A.; Alva, A.S.; Hart, L.L.; Milowsky, M.I.; Posadas, E.M.; Ryan, C.J.; Summa, J.M.; Youssoufian, H.; Scher, H.I.; et al. A phase 2 study of BIND-014 (PSMA-targeted docetaxel nanoparticle) administered to patients with chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2016, 34, 233. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Mita, M.M.; Ramanathan, R.K.; Weiss, G.J.; Mita, A.C.; Lorusso, P.M.; Burris, H.A., 3rd; Hart, L.L.; Low, S.C.; Parsons, D.M.; et al. Phase I Study of PSMA-Targeted Docetaxel-Containing Nanoparticle BIND-014 in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016, 22, 3157–3163. [Google Scholar] [CrossRef] [PubMed]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and Pharmacokinetic Studies of CYT-6091, a Novel PEGylated Colloidal Gold-rhTNF Nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [PubMed]

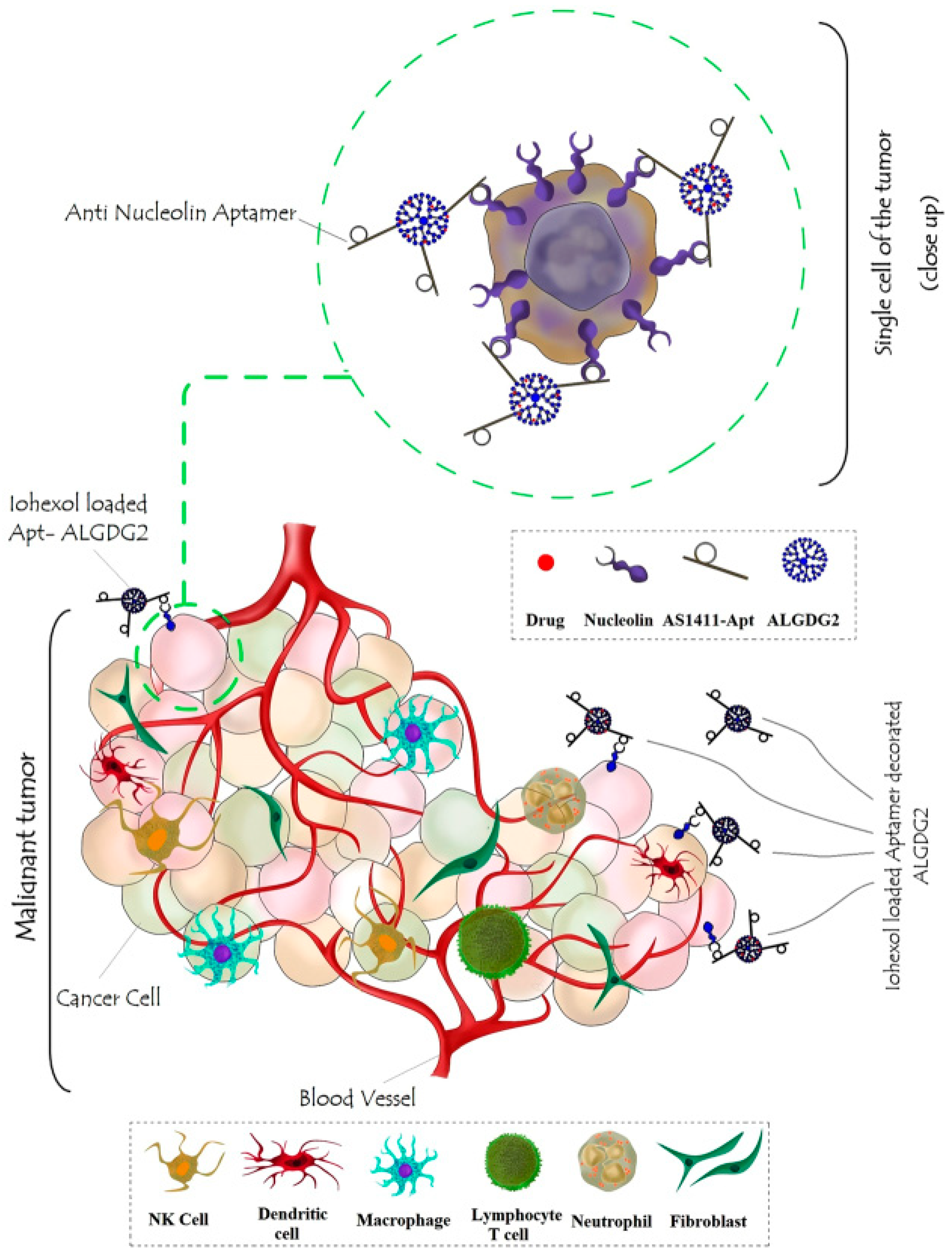

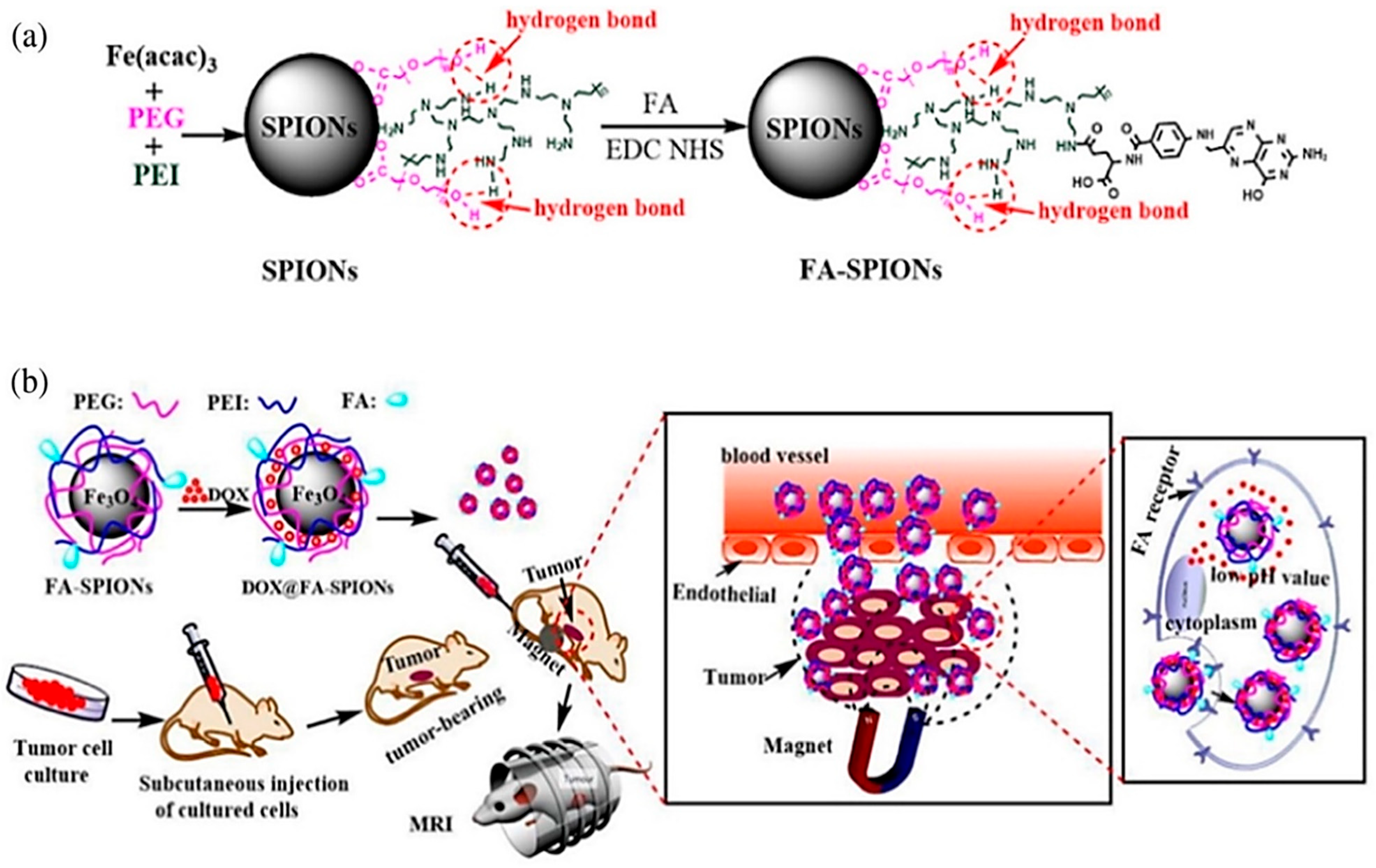
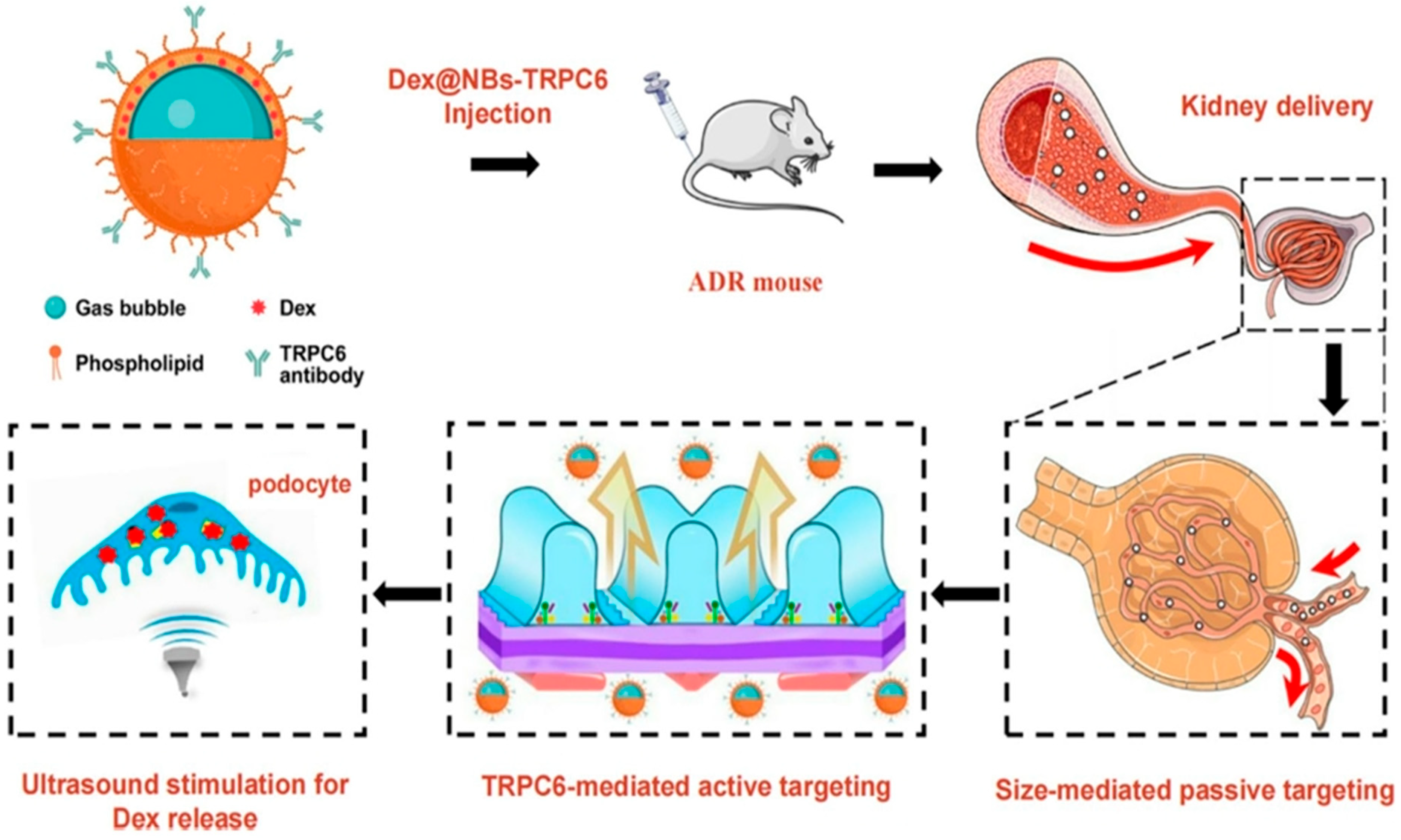


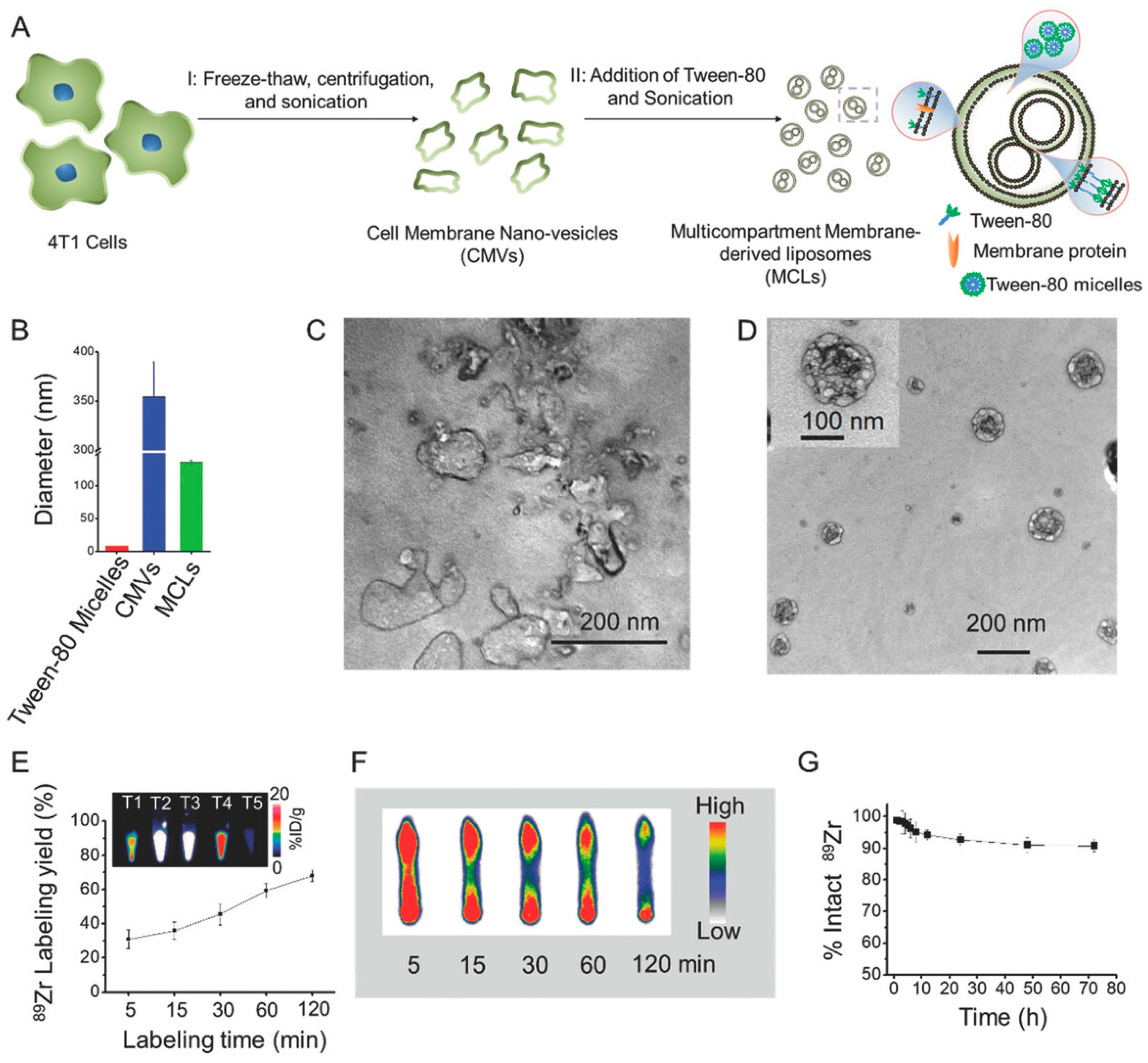
| Nanoplatform Class | Structural Features and Surface Engineering | Functional Role in Theranostics | Clinical Status/Challenges/Limitations | Supporting Studies |
|---|---|---|---|---|
| Magnetic Iron-Based Nanoparticles | Magnetic cores (Fe3O4, γ-Fe2O3, MnFe2O4, CoFe2O4, Fe/Fe3O4) combined with PEG, PDA, or antibody/ligand coatings (e.g., RGD, FA) enable tunable magnetism, colloidal stability, and targeted delivery. | MRI contrast agents, magnetothermal therapy, pH-sensitive drug release | Preclinical; effective imaging and delivery. Challenges: toxicity (e.g., Co), clearance, scale-up, and in vivo consistency. | [2,5,15,17,45,50,51,52] |
| Gold-Based Nanomaterials | Au cores (solid, hollow, Janus) functionalized with PEG, antibodies, or photo agents (e.g., ICG, Ce6) allow surface plasmon resonance-based imaging and photothermal conversion. | NIR-triggered phototherapy, photoacoustic imaging, theranostics | Preclinical; strong photothermal effects. Issues: size optimization, organ accumulation, gold persistence, and biocompatibility. | [4,9,14,47,53,54] |
| Gadolinium & Lanthanide MRI Agents | Gd3+ or Yb3+ cores encapsulated in polymers or conjugated to dendrimers, PEG, or targeting ligands (FA, RGD) enable high relaxivity, biocompatibility, and dual-modality imaging. | MRI contrast, tumor tracking, multimodal diagnosis | Preclinical; high relaxivity and targeting. Limits: Gd3+ toxicity, complex synthesis, and long-term safety unknown. | [23,55,56,57,58,59] |
| Targeting Ligands (RGD, FA, Antibodies, Aptamers) | Diverse nanocores (metallic, polymeric, QDs) are functionalized with specific ligands (RGD, FA, EGFR, aptamers) to match biological receptors, boosting cell-type specificity. | Receptor-mediated uptake, blood–brain barrier (BBB) penetration, cancer targeting | Preclinical; improved uptake and specificity. Hurdles: ligand stability, immune responses, receptor heterogeneity. | [2,14,15,23,53,54,60,61] |
| Polymer & Micelle Nanocarriers | Amphiphilic polymers (PLGA, PEG, PAA, TPGS) self-assemble around hydrophobic cores or drugs, with surface modification (antibodies, peptides) enhancing stability and controlled release. | Stimuli-responsive chemotherapy, combinational drug delivery | Preclinical; controlled release and targeting shown. Barriers: synthesis reproducibility, degradation, and scale-up. | [7,39,62,63,64,65,66] |
| Quantum Dots (Carbon, Graphene, Cu-In-S/ZnS) | Fluorescent cores (CQDs, GQDs, CuInS/ZnS) are surface engineered with PEG, FA, or aptamers, providing water solubility, charge balance, and targeted imaging properties. | Bioimaging, targeted drug delivery, light-activated therapy | Preclinical; strong imaging and delivery. Challenges: metal toxicity, photostability, biosafety, and synthesis control. | [1,23,46,67,68,69] |
| Photosensitizers & Photothermal Agents | Organic/inorganic cores (ICG, Ce6, porphyrins, BODIPY, π-conjugates) functionalized via covalent or cleavable bonds enhance solubility, targeting, and light-triggered activation. | Photothermal therapy, PDT, NIRII diagnostics | Preclinical; potent ablation and imaging. Issues: light penetration, ROS side effects, retention, and targeting control. | [9,10,25,37,41,60,70] |
| Self-Assembled & Carrier-Free Nanodrugs | Drugs or conjugates (e.g., PTX, CPT, IDIC-4F) self-assemble into nanostructures via π–π or H-bond interactions; targeting or responsive units (e.g., disulfides) enhance delivery precision. | High drug loading, redox-sensitive delivery, structure-defined release | Some early clinical data (e.g., EB-CPT). Generally preclinical. Key issues: safety, synthesis, and release kinetics. | [11,35,36,71,72,73] |
| Hybrid/Core–Shell Nanostructures | Multi-core architectures (e.g., Fe3O4@SiO2@Au, QDs@HA) functionalized with polymers or biomolecules for combining magnetic, optical, and biochemical responses. | Dual-mode imaging, on-demand drug release, spatiotemporal control | Preclinical; multimodal functions. Obstacles: complex fabrication, stability, clearance, and multi-material regulations. | [4,29,34,40,52,74,75] |
| Biologically Derived/Biomimetic Systems | Natural or cell membrane-based nanocarriers (RBC, NK, cancer cell, HDL) function with therapeutic agents or imaging dyes retain surface proteins to enhance targeting and immune evasion. | Biomimetic delivery, prolonged circulation, immune escape | Preclinical; strong targeting and circulation. Challenges: membrane source consistency, immune response, scalability. | [27,28,76,77,78] |
| Radiolabeled & Multimodal Theranostics | Nanocores (liposomes, polymers, AuNPs) radiolabeled with isotopes (99mTc, 89Zr, 131I) and functionalized with ligands (FA, PEG, antibody) for concurrent imaging and therapy. | PET/SPECT-guided delivery, radiotherapy, image-based treatment planning | Some clinical translation (e.g., EB-CPT). Most are preclinical. Key concerns: radiolabel stability, handling, regulation. | [22,28,35,39,79] |
| Stimulus Type | Mode of Action | Implementation Considerations | Selected Literature |
|---|---|---|---|
| pH-Responsive Systems | Acidic tumor or endosomal pH triggers drug release (e.g., DOX, PTX); in some systems, pH also promotes endosomal escape or conformation change for cellular uptake. | Requires careful pKa tuning to avoid premature leakage; endosomal escape mechanisms (e.g., proton sponge effect) can be integrated for enhanced cytosolic delivery. Preclinical (in vitro/in vivo, no human trials). | [2,5,24,43,51,68,69,88] |
| Redox-Responsive Systems (GSH-Sensitive) | High intracellular GSH cleaves disulfide or selenoether bonds, enabling drug release inside tumor cells; redox environment may also enable structural transformation for targeting or cell entry. | Ideal for cytosolic drug delivery; linker length and steric affect cleavage efficiency; combination with other stimuli can improve selectivity. Preclinical only. | [24,25,36,58] |
| Light/NIR-Triggered Systems | Light (especially NIR) is used to trigger drug release or photoactivation (PDT/PTT); in some designs, it also activates targeting via conformation switch or cleavage of a masking group. | Allows precise control of activation site and timing; requires device integration and tumor accessibility; suitable for superficial tumors or guided fiber delivery. Evaluated only in mice. | [7,9,84,89] |
| Enzyme-Responsive Systems | Tumor-specific enzymes (e.g., MMPs, cathepsins) cleave linkers to trigger drug release or activate targeting ligands (e.g., PEG shedding exposes targeting domain). | Enzyme specificity can reduce off-target effects; co-expression variability across tumor types requires careful biomarker selection and validation. Preclinical efficacy demonstrated. | [32] |
| MicroRNA or mRNA-Activated Systems | Tumor-overexpressed mRNA/miRNA activates DNA zymes or opens nanostructures for targeted release; in some cases, this also triggers exposure of targeting moieties. | Allows personalized therapy; requires sequence specificity and intracellular delivery to the cytosol; may be combined with nanocarriers enabling endosomal escape. Tested in vitro/in vivo. | [34,90] |
| Photothermal/Photodynamic Activation | Photothermal or photodynamic agents generate heat or ROS to induce cytotoxicity or rupture nanocarriers, releasing payload; can also aid membrane permeability or nuclear/mitochondrial targeting. | Good for drug-resistant tumors; ROS/heat must be tightly controlled to avoid off-target effects; photothermal conversion efficiency and stability are key design factors. All preclinical. | [3,22,28,31,66,91,92] |
| Tumor Microenvironment-Driven Passive Systems | Leverage endogenous features (e.g., low pH, high GSH, EPR effect) for passive targeting and drug release, often without external ligands. | Simpler synthesis and scalable; works better in highly vascularized tumors; limited control over precise targeting or timing, often combined with active or external triggers. In vivo only. | [24,33,36,58] |
| Biomimetic Activation (e.g., Cell Membrane Cloaking) | Biomimetic coatings can unmask targeting ligands or release drugs in response to tumor microenvironment cues, offering immune evasion and responsive targeting. | High biocompatibility and circulation time; batch variability and reproducibility are manufacturing challenges; responsive uncoating or degradation improves tumor homing. Shown effective in glioma models only. | [27] |
| Imaging-Therapy Integration | Design Features and Representative Nanoplatforms | Therapeutic Applications and Translational Relevance | Supporting Literature |
|---|---|---|---|
| Chemotherapy + Imaging | DOX, PTX paired with MRI, FL using pH-sensitive or receptor-targeted nanocarriers. | Foundational for drug delivery tracking and image-guided dosing | [2,17,23,59,88] |
| PTT + Imaging | NIR/NIR-II laser-triggered ablation with MRI, FL, or PAI using biocompatible nanoplatforms. | Used in non-invasive thermal therapy and real-time image-guided monitoring. | [3,12,31,106] |
| PDT + Imaging | Photosensitizers (AIEgen, porphyrins, Ce6) activated by light, paired with PET or FL for theranostics. | Enables ROS-mediated tumor ablation with real-time imaging of activation or localization. | [22,28,71,107] |
| Chemo + PTT/PDT + Imaging | DOX-based systems integrating NIR-triggered PTT or PDT with FL or optical imaging for combination therapy. | Enables synergistic cancer therapy and real-time image-guided delivery with reduced resistance. | [89,108] |
| Gene Therapy + Imaging | siRNA/miRNA or DNAzyme-loaded nanoplatforms integrated with FL or MRI for image-guided gene silencing. | Enables targeted genetic modulation and fluorescence/MRI-tracked therapeutic monitoring. | [69,90,104] |
| Magneto-Therapy + Imaging | Magnetic nanoplatforms (e.g., SPIONs, GdIO, MNP hybrids) enabling MRI-guided hyperthermia or chemotherapeutic delivery. | Useful in deep tumor targeting, magnetically triggered therapy, and non-contact thermal ablation with MRI feedback. | [15,26,50,75,102] |
| Radiotherapy + Imaging | Nanoplatforms radiolabeled with I-131, Lu-177, or Y-90 for combined radionuclide therapy and PET/SPECT imaging, enabling real-time dosimetry and tumor tracking. | Enables precise systemic radiotherapy with companion diagnostics and quantitative biodistribution. | [35,39,109,110,111] |
| FL Imaging + Multiple Therapies | Nanoplatforms incorporating visible/NIR/NIR-II fluorescence for subcellular tracking of drug release, photothermal/photodynamic response, or gene delivery. | Enables preclinical real-time therapy-response monitoring, improving therapeutic scheduling and tumor specificity. | [1,9,25,46,74,112] |
| MRI + multi-Therapies | T1/T2 MRI-guided nanoplatforms combining chemotherapy or photothermal therapy, often via pH-sensitive or ligand-targeted drug release. | Enables real-time, clinical-grade tumor visualization, drug tracking, and therapy response monitoring. | [2,8,17,19,55,81] |
| PAI + PTT/PDT/Drug Tracking | Photoacoustic contrast agents integrated with NIR-triggered photothermal or photodynamic therapies, often using semiconducting polymers or dye-loaded carriers. | Enables real-time, deep-tissue imaging of thermal or oxidative stress with simultaneous tumor ablation or drug response monitoring. | [11,37,66,70,113] |
| PET/SPECT/CT + Therapy | Nanocarriers labeled with PET/SPECT/CT isotopes co-delivering chemotherapeutic, PDT, or radiotherapeutic agents. | Enables full-body biodistribution tracking, dose planning, and image-guided therapy in preclinical or clinical theranostic frameworks. | [28,35,64,78,110] |
| Multimodal Imaging + Therapy | Hybrid nanoplatforms integrating two or more imaging modalities (e.g., MRI/FL, MRI/PAI, US/FL) with PTT, chemotherapy, or PDT. | Supports pre-treatment planning, real-time intra-treatment monitoring, and post-treatment evaluation in preclinical models. | [7,23,37,38,112] |
| pH-Responsive Therapy + Imaging | Smart nanocarriers triggered by tumor acidity for controlled drug release or imaging contrast activation, often using MRI or fluorescence. | Enables tumor-specific drug release and imaging, minimizing systemic toxicity in preclinical models. | [17,24,29,51,88] |
| Redox-Responsive Therapy + Imaging | Nanoparticles that activate drug release or imaging signals via tumor-associated GSH or ROS levels, using disulfide linkers, ROS-sensitive structures, or fluorescence switching. | Enables selective drug delivery and imaging in reductive or oxidative tumor environments, improving specificity and reducing systemic effects. | [36,46,54,58,74] |
| Light-Activated Theranostics | NIR-triggered (808–1064 nm) nanoplatforms integrating PTT or imaging agents (NIR-II FL, PAI) with tumor-targeting elements (e.g., BBB-crossing, cell membrane camouflage, charge switching). | Enables high-resolution imaging and spatially confined therapy, suitable for brain tumors, surgical guidance, or minimally invasive treatment. | [12,27,106,114,115] |
| Ultrasound-Triggered Theranostics | Acoustic droplet vaporization or LIFU-triggered drug release using nanobubbles or polymer nanocarriers with ultrasound/MRI/photoacoustic imaging. | Enables non-invasive, real-time therapy in deep tissues (e.g., kidney, vascular plaques, brain); useful in oncology and organ-specific disease. | [48,85,105] |
| Ligand-Targeted Systems + Imaging | Nanoparticles functionalized with ligands (e.g., RGD, folate, HA, octreotide) for receptor-mediated targeting and visualized via MRI or fluorescence. | Improves therapeutic index and imaging precision; valuable for receptor-overexpressing tumors and personalized cancer therapy. | [8,23,59,69,116,117] |
| Theranostic Design Focus | Representative Approaches | Implications for Design and Translation | Supporting Literature |
|---|---|---|---|
| Tumor-Specific Targeting and Selective Cytotoxicity | Receptor-mediated uptake or tumor microenvironment (TME)-responsive activation to preferentially accumulate in tumors and minimize off-target effects. | Emphasize tumor-specific delivery to enhance therapeutic efficacy and reduce systemic toxicity in solid tumors. | [1,8,33,38,68,69,84,119] |
| Ligand/Peptide/Antibody-Mediated Targeting | Functionalization with ligands such as folate, transferrin, RGD, CD44 antibodies to increase tumor selectivity and receptor-specific uptake. | Match ligand design to receptor expression profiles for patient-specific and tumor-type-specific targeting strategies. | [8,38,57,59,69,93,96,117] |
| Stimuli-Responsive Drug Release | Triggered therapeutic release based on tumor-specific cues (e.g., acidic pH, high GSH, ROS, NIR light) to achieve spatiotemporal control. | Engineer adaptable nanocarriers to match intratumoral heterogeneity and optimize on-site activation. | [9,24,25,36,83,108] |
| Biocompatibility and Low Systemic Toxicity | Evaluated through biodistribution studies, histology, blood chemistry, and weight monitoring; confirmed safety in vitro and in vivo across nanoplatforms. | Incorporate biosafety screening early in development to meet preclinical safety and regulatory standards. | [3,11,28,39,43,86] |
| Imaging-Guided Theranostics | Multimodal platforms (MRI, PET, PA, NIRF, SWIR) enable tumor visualization, real-time monitoring, and guided therapy with high spatial and temporal resolution. | Embed imaging functionalities during nanoparticle design to facilitate precision therapy and noninvasive tracking. | [4,11,28,49,56,112,120] |
| Photothermal and Photodynamic Therapy (PTT/PDT) | Light-triggered nanotherapeutics utilizing NIR/NIR-II wavelengths (e.g., 1064 nm) for tumor ablation via thermal (PTT) or oxidative (PDT) mechanisms. Some systems combine both modalities for synergistic effects. | Focus on clinically relevant NIR-II wavelengths to improve tissue penetration, treatment depth, and translational applicability. | [9,11,41,77,106,114,121] |
| Multimodal or Synergistic Therapy | Co-delivery of chemo, gene therapy, PTT, PDT, or radiotherapy in programmable, stimulus-responsive platforms for enhanced tumor killing and reduced resistance. | Optimize combinatorial regimens, treatment sequencing, and nanocarrier design to maximize synergy and therapeutic outcome. | [34,35,95,99,122] |
| BBB Penetration/CNS Targeting | BBB-crossing ligands (RGD, Angiopep-2, lactoferrin) and biomimetic membranes (e.g., NK cells) enable delivery across the BBB and accumulation in gliomas. | Confirm therapeutic efficacy and imaging performance in orthotopic glioblastoma models to validate CNS-targeted nanoplatforms. | [12,27,56,123] |
| Real-World & Translational Models | Use of orthotopic, patient-derived xenografts (PDX), and first-in-human data to evaluate nanotheranostics in clinically relevant settings. | Bridge preclinical and clinical gaps by incorporating advanced in vivo models and early-phase translational studies. | [35,39,65,99] |
| Disease Applications Beyond Cancer | Adaptation of nanoplatforms for non-cancer conditions including nephropathy, atherosclerosis, and lymphatic diseases; platforms provide imaging and targeted therapy. | Expand theranostic applications to inflammatory and chronic metabolic diseases by leveraging versatile nanocarrier designs. | [85,105] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Gill, E.J.; Cubeddu, L.X. Conjugate Nanoparticles in Cancer Theranostics. J. Nanotheranostics 2025, 6, 24. https://doi.org/10.3390/jnt6030024
Omidian H, Gill EJ, Cubeddu LX. Conjugate Nanoparticles in Cancer Theranostics. Journal of Nanotheranostics. 2025; 6(3):24. https://doi.org/10.3390/jnt6030024
Chicago/Turabian StyleOmidian, Hossein, Erma J. Gill, and Luigi X. Cubeddu. 2025. "Conjugate Nanoparticles in Cancer Theranostics" Journal of Nanotheranostics 6, no. 3: 24. https://doi.org/10.3390/jnt6030024
APA StyleOmidian, H., Gill, E. J., & Cubeddu, L. X. (2025). Conjugate Nanoparticles in Cancer Theranostics. Journal of Nanotheranostics, 6(3), 24. https://doi.org/10.3390/jnt6030024






