Innovation in Lung Cancer Management from Herbal Nanomedicine to Artificial Intelligence
Abstract
1. Introduction
2. Biomarkers Targeted for Lung Cancer Treatment
2.1. Signalling Pathways Targeting Tumour Immunity
2.1.1. CTLA-4
2.1.2. PD-L1
2.2. Signalling Pathways Targeting Angiogenesis
2.2.1. PDGF
2.2.2. VEGF
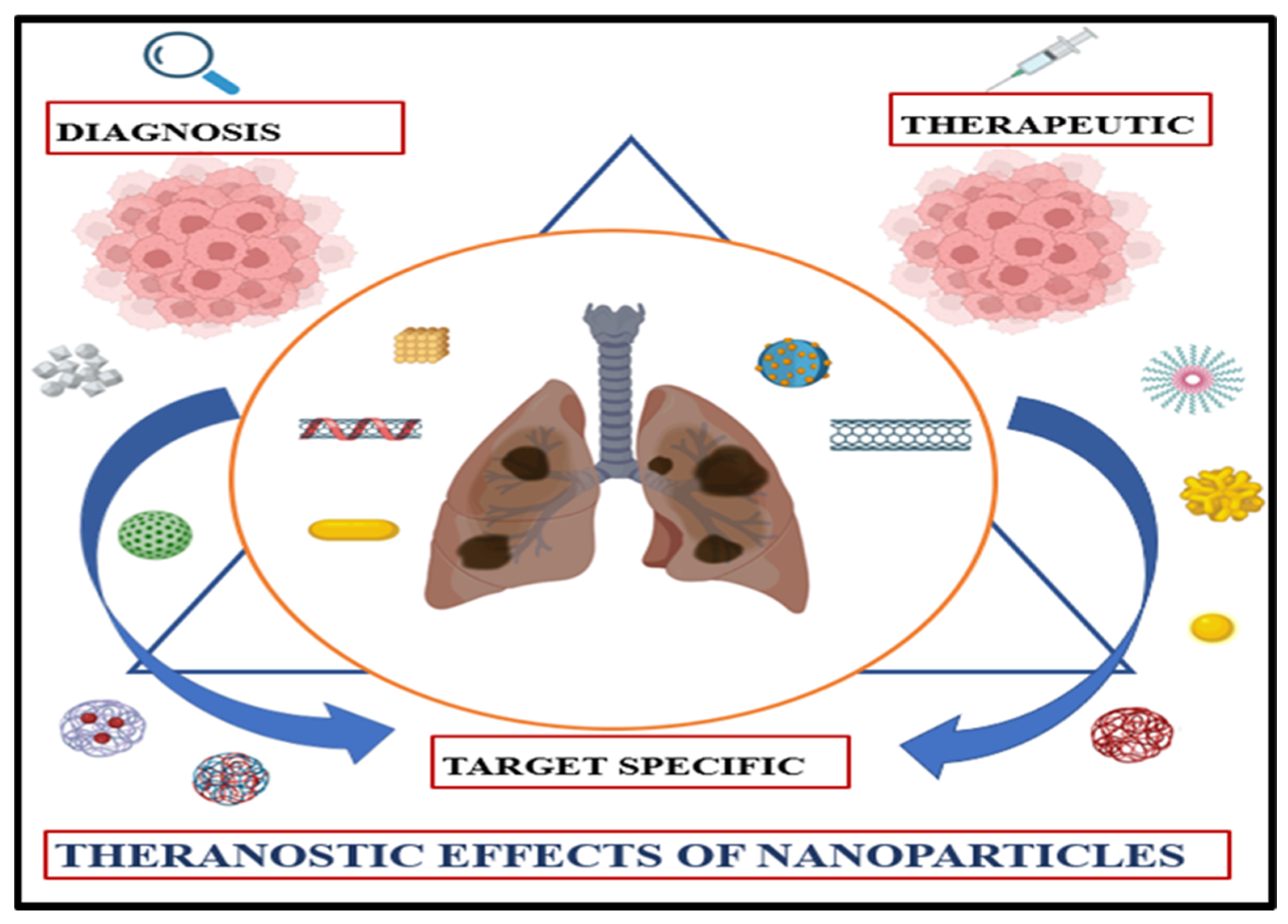
3. Nanoparticles as Drug Delivery Vehicles
3.1. Barriers to Pulmonary Drug Targeting
3.2. Passive and Active Targeting
3.3. Lymphatic Drug Delivery: An Alternative Route for Improved Pulmonary Targeting
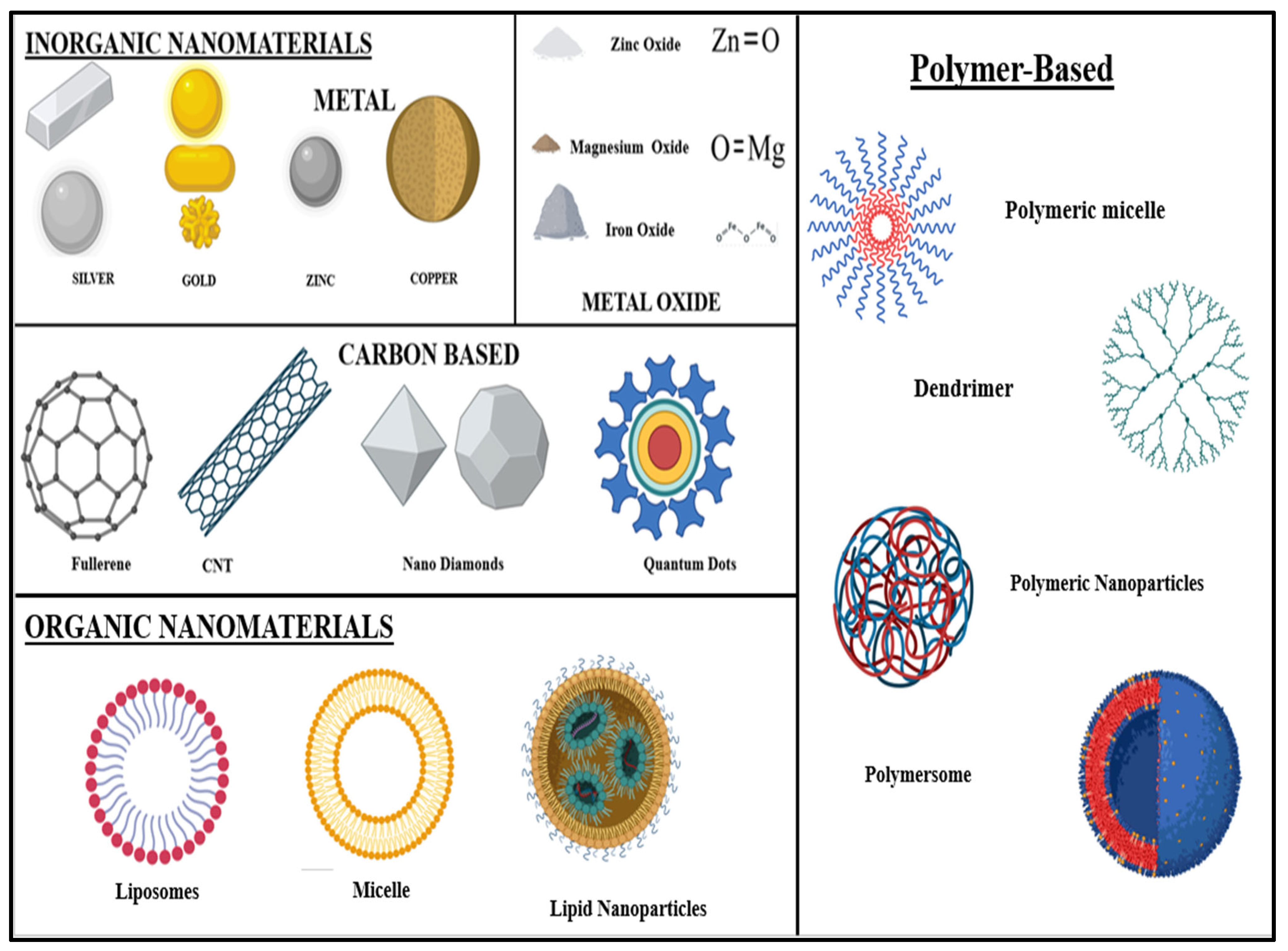
3.4. Types of Nanoparticles Used for Lung Cancer Therapy
- (A)
- Inorganic Nanomaterials: Various metals like gold (Au), silver (Ag), aluminium (Al), zinc (Zn), copper (Cu), iron (Fe), cadmium (Cd), and lead (Pb), as well as metal oxides like zinc oxide (ZnO), iron oxide (Fe2O3 or Fe3O4), copper oxide (CuO), titanium dioxide (TiO2), magnesium aluminium oxide (MgAl2O4), cerium oxide (CeO2), silica (SiO2), etc., are used to synthesise nanoparticles [63].
- (B)
- Organic Nanomaterials: Nanomaterials are made of organic matter—liposomes, dendrimers, micelles, cyclodextrin, nanogels, and compact polymers. Some organic nanoparticles, like liposomes and micelles, contain a hollow sphere and are biodegradable and non-toxic. This is beneficial for drug delivery purposes [64].
3.5. Gold-Based Lung Cancer Therapy
3.6. Silver-Based Lung Cancer Therapy
3.7. Zinc-Based Lung Cancer Therapy
3.8. Diamond-Based Lung Cancer Therapy
3.9. Two-Dimensional-Based Lung Cancer Therapy
3.10. Three-Dimensional-Based Lung Cancer Therapy
4. Herbal Nanomedicine in Lung Cancer Therapy
| S. No. | Nanostructure | Herbs | Bioactive Compound | Biomarkers | Ref. |
|---|---|---|---|---|---|
| 1. | Gold and silver NPs | Platycodi Radix (Platycodon grandiflorum) | Platycodin D (PLD) | PD-L1 | [110] |
| 2. | Silver NPs | Morus alba (white mulberry) | Prenylated flavonoids like morusin | STAT3, NF-κB, caspases, EGFR, PDGFR, ROS, etc. | [111,112,113,114] |
| 3. | Gold nanoclusters | Most fruits and vegetables | Kaempferol (flavonoid) | TNF-α, IL, NF-κB, Akt, VEGF, AP-1, PIK3R1, AKT1, EGFR, and IGF1R | [115,116] |
| 4. | Lipid NPs | Found in many essential oils of plants | Geraniol (GOH) | Inhibit HMGCR | [117,118] |
| 5. | Biocompatible lipid NPs | Lippia alba and Clinopodium nepeta essential oils | Monoterpenes (limonene in L. alba and pulegone in C. nepeta) | Increase ROS generation | [119] |
| 6. | Solid lipid NPs | Myrica rubra tree bark | Myricetin (MYC) | RIPK3 and MLKL upregulation | [120] |
| 7. | Core–shell lipid–polymer hybrid NPs (LPNs) | Skin of grapes, raspberries, mulberries | Resveratrol (RSV) | VEGF, caspase-3 | [121,122] |
| 8. | Liquid crystalline NPs (LCNs) | Rhizoma coptidis | Berberine | VEGF, TF AP-1, NF-κB | [123] |
| 9. | Poly (lactic-co-glycolic acid)-based AE NPs (nanoAE) | Aloe vera and Rheum officinale | Aloe-emodin (AE) | Caspase-3, poly (ADP-ribose) polymerase (PARP), caspase-8, and caspase-9 | [124] |
| 10. | Poly (lactic acid)–poly (ethylene glycol) (PLA-PEG) NPs | Green vegetables like broccoli, cabbage, celery, spinach | Luteolin (flavonoid) | PD-L1 | [125,126] |
| 11. | Oil nanoemulsions | Piper nigrum (black pepper) | Piperine | Wnt, NF-κB, cAMP response element-BP, TF-2, PPAR- γ, human G-quadruplex DNA, | [127,128] |
| 12. | Nanoemulsions | Green tea | Epigallocatechin-3-gallate (EGCG) | ROS, NF-κB, Akt, VEGF, PPAR, Bcl-2, MAP kinases | [129] |
| Nanocarrier/Delivery System | Herbal Compound | Cancer Model/Type | Key Outcomes | Ref. |
|---|---|---|---|---|
| Baicalein/Paclitaxel | A549/PTX lung cancer | Highest tumour regression with dual targeting ligands | [130] |
| Berberine/Etoposide | A549/Lung-cancer-bearing mice | Improved cellular internalisation Elevated caspase-3 and downregulated VEGF | [131] |
| Resveratrol/Pemetrexed | A549/Lung-cancer-bearing mice | Inhibition of angiogenesis Induction of apoptosis | [132] |
| Doxorubicin/Ellagic acid | A549/lung-cancer-bearing mice | Improved cellular internalisation Improved the anticancer efficacy of DOX | |
| Curcumin/Berberine | A549 cell line | Inhibited MDR1 Activity Improved cellular internalisation | [133] |
| Berberine/Rapamycin | A549/Lung-cancer-bearing mice | Improved internalisation |
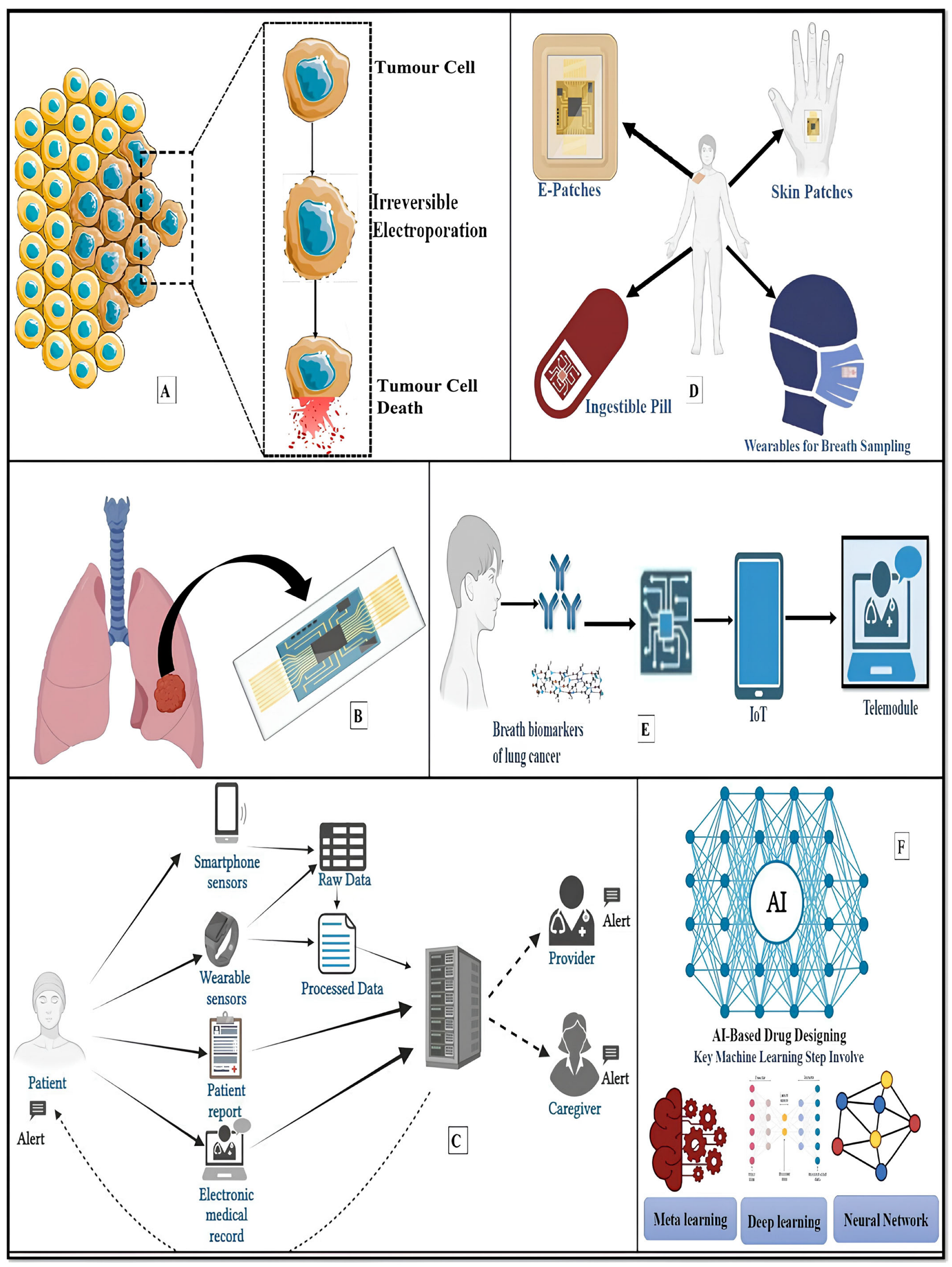
5. Innovative Lung Cancer Theranostics
5.1. Electrical LCT
5.2. Lung-on-a-Chip
5.3. Smartphone-Enabled LCT/Telemedicine/mHealth Applications
5.4. Robotics/Drone-Based Lung Cancer Treatment
5.4.1. Robotics in Lung Cancer Diagnosis and Treatment
5.4.2. Nanorobots in Targeted Drug Delivery
5.4.3. Drone Technology in Healthcare Delivery
5.5. Wearable/Lung e-Patch/Nose-on-a-Chip
5.6. Artificial Intelligence (AI)-Based LCT
6. Conclusions
Funding
Conflicts of Interest
References
- Ji, Y.; Zhang, Y.; Liu, S.; Li, J.; Jin, Q.; Wu, J.; Duan, H.; Liu, X.; Yang, L.; Huang, Y. The epidemiological landscape of lung cancer: Current status, temporal trend and future projections based on the latest estimates from GLOBOCAN 2022. J. Natl. Cancer Cent. 2025, 5, 278–286. [Google Scholar] [CrossRef]
- Jiang, P.; Liang, B.; Zhang, Z.; Fan, B.; Zeng, L.; Zhou, Z.; Mao, Z.; Xu, Q.; Yao, W.; Shen, Q. New insights into nanosystems for non-small-cell lung cancer: Diagnosis and treatment. RSC Adv. 2023, 13, 19540–19564. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The effect of advances in lung-cancer treatment on population mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef]
- Nooreldeen, R.; Bach, H. Current and future development in lung cancer diagnosis. Int. J. Mol. Sci. 2021, 22, 8661. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, J.-X.; Ma, L.-R.; Xu, D.-H.; Wang, P.; Li, L.-Q.; Yu, L.-L.; Li, Y.; Li, R.-Z.; Zhang, H.; et al. Traditional herbal medicine: A potential therapeutic approach for adjuvant treatment of non-small cell lung cancer in the future. Integr. Cancer Ther. 2022, 21, 15347354221144312. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Non-small cell lung cancer, Version 2.2021 featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Abdulridha, M.K.; Al-Marzoqi, A.H.; Al-Awsi, G.R.L.; Mubarak, S.M.; Heidarifard, M.; Ghasemian, A. Anticancer effects of herbal medicine compounds and novel formulations: A literature review. J. Gastrointest. Cancer 2020, 51, 765–773. [Google Scholar] [CrossRef]
- Guo, M.; Qin, S.; Wang, S.; Sun, M.; Yang, H.; Wang, X.; Fan, P.; Jin, Z. Herbal medicine nanocrystals: A potential novel therapeutic strategy. Molecules 2023, 28, 6370. [Google Scholar] [CrossRef]
- Barkat, M.A.; Goyal, A.; Barkat, H.A.; Salauddin, M.; Pottoo, F.H.; Anwer, E.T. Herbal medicine: Clinical perspective and regulatory status. Comb. Chem. High Throughput Screen. 2021, 24, 1573–1582. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef]
- Mason, M.D.; Sydes, M.R.; Glaholm, J.; Langley, R.E.; Huddart, R.A.; Sokal, M.; Stott, M.; Robinson, A.C.; James, N.D.; Parmar, M.K.; et al. Oral sodium clodronate for nonmetastatic prostate cancer—Results of a randomized double-blind placebo-controlled trial: Medical Research Council PR04 (ISRCTN61384873). J. Natl. Cancer Inst. 2007, 99, 765–776. [Google Scholar] [CrossRef]
- Chavda, V.P.; Patel, A.B.; Mistry, K.J.; Suthar, S.F.; Wu, Z.X.; Chen, Z.S.; Hou, K. Nano-drug delivery systems entrapping natural bioactive compounds for cancer: Recent progress and future challenges. Front. Oncol. 2022, 12, 867655. [Google Scholar] [CrossRef]
- Taioli, E.; Flores, R.M.; Abdelhamid, A.; Untalan, M.; Ivic-Pavlicic, T.; Tuminello, S. Antibiotic use and survival in late-stage non-small cell lung cancer patients treated with chemo-immunotherapy. JTO Clin. Res. Rep. 2024, 5, 100710. [Google Scholar]
- Tinetti, A. Novel CAR design dually targets HER2+ breast cancer and MDSCs to improve efficacy in solid tumors. Preprint 2023. [Google Scholar] [CrossRef]
- Onoi, K.; Chihara, Y.; Uchino, J.; Shimamoto, T.; Morimoto, Y.; Iwasaku, M.; Kaneko, Y.; Yamada, T.; Takayama, K. Immune checkpoint inhibitors for lung cancer treatment: A review. J. Clin. Med. 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- King, H.A.; Lewin, S.R. Immune checkpoint inhibitors in infectious disease. Immunol. Rev. 2024, 328, 350–371. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995, 182, 459–465. [Google Scholar] [CrossRef]
- Zhang, Q.; Tang, L.; Zhou, Y.; He, W.; Li, W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: Current understanding in characteristics, diagnosis, and management. Front. Immunol. 2021, 12, 663986. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From mechanism to autoimmune therapy. Int. Immunopharmacol. 2020, 80, 106221. [Google Scholar] [CrossRef]
- De Silva, P.; Aiello, M.; Gu-Trantien, C.; Migliori, E.; Willard-Gallo, K.; Solinas, C. Targeting CTLA-4 in cancer: Is it the ideal companion for PD-1 blockade immunotherapy combinations? Int. J. Cancer 2021, 149, 31–41. [Google Scholar] [CrossRef]
- Yuan, M.; Zhao, Y.; Arkenau, H.T.; Lao, T.; Chu, L.; Xu, Q. Signal pathways and precision therapy of small-cell lung cancer. Signal Transduct. Target. Ther. 2022, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood J. Am. Soc. Hematol. 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Sansom, D.M. CD28, CTLA-4 and their ligands: Who does what and to whom? Immunology 2000, 101, 169. [Google Scholar] [CrossRef]
- McLachlan, J. Immune checkpoint inhibitors and their side effects. Pathology 2019, 51, S17. [Google Scholar] [CrossRef]
- Ramagopal, U.A.; Liu, W.; Garrett-Thomson, S.C.; Bonanno, J.B.; Yan, Q.; Srinivasan, M.; Wong, S.C.; Bell, A.; Mankikar, S.; Rangan, V.S.; et al. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc. Natl. Acad. Sci. USA 2017, 114, E4223–E4232. [Google Scholar] [CrossRef]
- Malas, S.; Harrasser, M.; Lacy, K.E.; Karagiannis, S.N. Antibody therapies for melanoma: New and emerging opportunities to activate immunity. Oncol. Rep. 2014, 32, 875–886. [Google Scholar] [CrossRef]
- Kennedy, L.B.; Salama, A.K. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020, 70, 86–104. [Google Scholar] [CrossRef]
- Parvez, A.; Choudhary, F.; Mudgal, P.; Khan, R.; Qureshi, K.A.; Farooqi, H.; Aspatwar, A. PD-1 and PD-L1: Architects of immune symphony and immunotherapy breakthroughs in cancer treatment. Front. Immunol. 2023, 14, 1296341. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coyne, G.O.S.; Madan, R.A.; Gulley, J.L. Nivolumab: Promising survival signal coupled with limited toxicity raises expectations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 986. [Google Scholar] [CrossRef]
- Wilkinson, E. Nivolumab success in untreated metastatic melanoma. Lancet Oncol. 2015, 16, e9. [Google Scholar] [CrossRef]
- Mathieu, L.N.; Larkins, E.; Sinha, A.K.; Mishra-Kalyani, P.S.; Jafri, S.; Kalavar, S.; Ghosh, S.; Goldberg, K.B.; Pazdur, R.; Beaver, J.A.; et al. FDA approval summary: Atezolizumab as adjuvant treatment following surgical resection and platinum-based chemotherapy for stage II to IIIA NSCLC. Clin. Cancer Res. 2023, 29, 2973–2978. [Google Scholar] [CrossRef]
- Lin, X.; Lu, X.; Luo, G.; Xiang, H. Progress in PD-1/PD-L1 pathway inhibitors: From biomacromolecules to small molecules. Eur. J. Med. Chem. 2020, 186, 111876. [Google Scholar] [CrossRef]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Combinational therapeutic strategies to overcome resistance to immune checkpoint inhibitors. Front. Immunol. 2025, 16, 1546717. [Google Scholar] [CrossRef]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.Y.; Elserwy, W.S.; El-Mansy, M.F.; Serry, A.M.; Salem, A.M.; Abdou, A.M.; Abdelrahman, B.A.; Elsayed, K.H.; Elaziz, M.R.A. Angiokinase inhibition of VEGFR-2, PDGFR and FGFR and cell growth inhibition in lung cancer: Design, synthesis, biological evaluation and molecular docking of novel azaheterocyclic coumarin derivatives. Bioorganic Med. Chem. Lett. 2021, 48, 128258. [Google Scholar] [CrossRef] [PubMed]
- Homsi, J.; Daud, A.I. Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 2007, 14, 285–294. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Upadhyay, T.K.; Seungjoon, M.; Park, M.N.; Kim, B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed. Pharmacother. 2023, 161, 114491. [Google Scholar] [CrossRef]
- Farooqi, A.A.; Siddik, Z.H. Platelet-derived growth factor (PDGF) signalling in cancer: Rapidly emerging signalling landscape. Cell Biochem. Funct. 2015, 33, 257–265. [Google Scholar] [CrossRef]
- Heldin, C.H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 1–18. [Google Scholar] [CrossRef]
- Wang, P.; Song, L.; Ge, H.; Jin, P.; Jiang, Y.; Hu, W.; Geng, N. Crenolanib, a PDGFR inhibitor, suppresses lung cancer cell proliferation and inhibits tumor growth in vivo. OncoTargets Ther. 2014, 7, 1761–1768. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z. Non-small cell lung cancer targeted therapy: Drugs and mechanisms of drug resistance. Int. J. Mol. Sci. 2022, 23, 15056. [Google Scholar] [CrossRef] [PubMed]
- Frezzetti, D.; Gallo, M.; Maiello, M.R.; D’Alessio, A.; Esposito, C.; Chicchinelli, N.; Normanno, N.; De Luca, A. VEGF as a potential target in lung cancer. Expert Opin. Ther. Targets 2017, 21, 959–966. [Google Scholar] [CrossRef]
- Niu, Z.; Jin, R.; Zhang, Y.; Li, H. Signaling pathways and targeted therapies in lung squamous cell carcinoma: Mechanisms and clinical trials. Signal Transduct. Target. Ther. 2022, 7, 353. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X.; et al. VEGF/VEGFR-targeted therapy and immunotherapy in non-small cell lung cancer: Targeting the tumor microenvironment. Int. J. Biol. Sci. 2022, 18, 3845. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mathy, N.W.; Lu, H. The role of VEGF in the diagnosis and treatment of malignant pleural effusion in patients with non-small cell lung cancer. Mol. Med. Rep. 2018, 17, 8019–8030. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [Google Scholar] [CrossRef]
- Dev, M.; Chandel, P. Nanostructured Lipid Carriers in Pulmonary Drug Delivery: Progress and Prospects. Curr. Pharm. Res. 2025, 1, 131–143. [Google Scholar]
- Ge, D.; Ma, S.; Sun, T.; Li, Y.; Wei, J.; Wang, C.; Chen, X.; Liao, Y. Pulmonary delivery of dual-targeted nanoparticles improves tumor accumulation and cancer cell targeting by restricting macrophage interception in orthotopic lung tumors. Biomaterials 2025, 315, 122955. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhou, Q.; Pan, F.; Wang, R. Utilizing nanoparticles to overcome anti-PD-1/PD-L1 immunotherapy resistance in non-small cell lung cancer: A potential strategy. Int. J. Nanomed. 2025, 20, 2371–2394. [Google Scholar] [CrossRef] [PubMed]
- Carita, A.C.; Eloy, J.O.; Chorilli, M.; Lee, R.J.; Leonardi, G.R. Recent advances and perspectives in liposomes for cutaneous drug delivery. Curr. Med. Chem. 2018, 25, 606–635. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci 2020, 7, 193. [Google Scholar] [CrossRef]
- McCright, J.; Naiknavare, R.; Yarmovsky, J.; Maisel, K. Targeting lymphatics for nanoparticle drug delivery. Front. Pharmacol. 2022, 13, 887402. [Google Scholar] [CrossRef]
- Huynh, K.H.; Lee, K.Y.; Chang, H.; Lee, S.H.; Kim, J.; Pham, X.H.; Lee, Y.S.; Rho, W.Y.; Jun, B.H. Bioapplications of Nanomaterials. In Nanotechnology for Bioapplications; Springer: Singapore, 2021; pp. 235–255. [Google Scholar]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Baimyrza, A. Bismuth Telluride Nanoparticles for Thermoelectrical Application, Synthesis and Incorporation into Polymer Hybrid Composites. Doctoral Dissertation, Normandie Université, Caen, France, 2022. [Google Scholar]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Gautam, B. Tuning the Thermal Conductivity of Lignin@ Fe3O4 Colloidal Suspension Through External Magnetic Field. Master’s Thesis, University of Dayton, Dayton, OH, USA, 2022. [Google Scholar]
- Altammar, K.A. A review on nanoparticles: Characteristics, synthesis, applications, and challenges. Front. Microbiol. 2023, 14, 1155622. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Alshammari, B.H.; Lashin, M.M.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and inorganic nanomaterials: Fabrication, properties and applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef] [PubMed]
- Paramasivam, G.; Palem, V.V.; Sundaram, T.; Sundaram, V.; Kishore, S.C.; Bellucci, S. Nanomaterials: Synthesis and applications in theranostics. Nanomaterials 2021, 11, 3228. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, L.; Huang, H.; He, W.; Ming, W. Progress in laser ablation and biological synthesis processes:“Top-Down” and “Bottom-Up” approaches for the green synthesis of Au/Ag nanoparticles. Int. J. Mol. Sci. 2022, 23, 14658. [Google Scholar] [CrossRef] [PubMed]
- Giraldez-Perez, R.M.; Grueso, E.; Dominguez, I.; Pastor, N.; Kuliszewska, E.; Prado-Gotor, R.; Requena-Domenech, F. Biocompatible DNA/5-fluorouracil-gemini surfactant-functionalized gold nanoparticles as promising vectors in lung cancer therapy. Pharmaceutics 2021, 13, 423. [Google Scholar] [CrossRef]
- Woodman, C.; Vundu, G.; George, A.; Wilson, C.M. Applications and strategies in nanodiagnosis and nanotherapy in lung cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2021; Volume 69, pp. 349–364. [Google Scholar]
- Jindal, M.; Nagpal, M.; Singh, M.; Aggarwal, G.; Dhingra, G.A. Gold nanoparticles-boon in cancer theranostics. Curr. Pharm. Des. 2020, 26, 5134–5151. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Xin, H.; Liu, R.; Wang, Q.; Cai, W.; Peng, X.; Yang, F.; Xin, H. Nanoparticles advanced from preclinical studies to clinical trials for lung cancer therapy. Cancer Nanotechnol. 2023, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, J.Q.; Ashby Jr, C.R.; Zeng, L.; Fan, Y.F.; Chen, Z.S. Gold nanoparticles: Synthesis, physiochemical properties and therapeutic applications in cancer. Drug Discov. Today 2021, 26, 1284–1292. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef]
- Mueller, R.; Yasmin-Karim, S.; DeCosmo, K.; Vazquez-Pagan, A.; Sridhar, S.; Kozono, D.; Hesser, J.; Ngwa, W. Increased carcinoembryonic antigen expression on the surface of lung cancer cells using gold nanoparticles during radiotherapy. Phys. Medica 2020, 76, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Crous, A.; Abrahamse, H. Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int. J. Mol. Sci. 2020, 21, 3742. [Google Scholar] [CrossRef]
- Peng, J.; Wang, R.; Sun, W.; Huang, M.; Wang, R.; Li, Y.; Wang, P.; Sun, G.; Xie, S. Delivery of miR-320a-3p by gold nanoparticles combined with photothermal therapy for directly targeting Sp1 in lung cancer. Biomater. Sci. 2021, 9, 6528–6541. [Google Scholar] [CrossRef]
- Kanipandian, N.; Li, D.; Kannan, S. Induction of intrinsic apoptotic signaling pathway in A549 lung cancer cells using silver nanoparticles from Gossypium hirsutum and evaluation of in vivo toxicity. Biotechnol. Rep. 2019, 23, e00339. [Google Scholar] [CrossRef] [PubMed]
- Jakic, K.; Selc, M.; Macova, R.; Kurillova, A.; Kvitek, L.; Panacek, A.; Babelova, A. Effects of different-sized silver nanoparticles on morphological and functional alterations in lung cancer and non-cancer lung cells. Neoplasma 2023, 70, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer therapy by silver nanoparticles: Fiction or reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef]
- Raj, A.; Thomas, R.K.; Vidya, L.; Aparna, V.M.; Neelima, S.; Sudarsanakumar, C. Exploring the cytotoxicity on human lung cancer cells and DNA binding stratagem of camptothecin functionalised silver nanoparticles through multi-spectroscopic, and calorimetric approach. Sci. Rep. 2023, 13, 9045. [Google Scholar] [CrossRef]
- Singh, S.; Goel, T.; Singh, A.; Chugh, H.; Chakraborty, N.; Roy, I.; Tiwari, M.; Chandra, R. Synthesis and characterization of Fe3O4@ SiO2@ PDA@ Ag core–shell nanoparticles and biological application on human lung cancer cell line and antibacterial strains. Artif. Cells Nanomed. Biotechnol. 2024, 52, 46–58. [Google Scholar] [CrossRef]
- Tanino, R.; Amano, Y.; Tong, X.; Sun, R.; Tsubata, Y.; Harada, M.; Fujita, Y.; Isobe, T. Anticancer activity of ZnO nanoparticles against human small-cell lung cancer in an orthotopic mouse model. Mol. Cancer Ther. 2020, 19, 502–512. [Google Scholar] [CrossRef]
- Mishra, P.; Ahmad, A.; Al-Keridis, L.A.; Alshammari, N.; Alabdallah, N.M.; Muzammil, K.; Saeed, M.; Ansari, I.A. Doxorubicin-conjugated zinc oxide nanoparticles, biogenically synthesised using a fungus Aspergillus niger, exhibit high therapeutic efficacy against lung cancer cells. Molecules 2022, 27, 2590. [Google Scholar] [CrossRef]
- Mishra, P.; Ahmad, M.F.A.; Al-Keridis, L.A.; Saeed, M.; Alshammari, N.; Alabdallah, N.M.; Tiwari, R.K.; Ahmad, A.; Verma, M.; Fatima, S.; et al. Methotrexate-conjugated zinc oxide nanoparticles exert a substantially improved cytotoxic effect on lung cancer cells by inducing apoptosis. Front. Pharmacol. 2023, 14, 1194578. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liang, S.; Liu, D.; Ma, K.; Yun, K.; Yao, J.; Peng, Y.; Hai, L.; Zhang, Q.; Wang, Z. Manganese-Enriched Zinc Peroxide Functional Nanoparticles for Potentiating Cancer Immunotherapy. Nano Lett. 2023, 23, 10350–10359. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pierstorff, E.; Osawa, E.; Ho, D. Active nanodiamond hydrogels for chemotherapeutic delivery. Nano Lett. 2007, 7, 3305–3314. [Google Scholar] [CrossRef]
- Ali, M.S.; Elhabak, M.; Osman, R.; Nasr, M. Towards more efficient inhalable chemotherapy: Fabrication of nanodiamonds-releasing microspheres. Int. J. Pharm. 2022, 626, 122169. [Google Scholar] [CrossRef]
- Grudinkin, S.A.; Bogdanov, K.V.; Tolmachev, V.A.; Baranov, M.A.; Kaliya, I.E.; Golubev, V.G.; Baranov, A.V. Multifunctional Core/Shell Diamond Nanoparticles Combining Unique Thermal and Light Properties for Future Biological Applications. Nanomaterials 2023, 13, 3124. [Google Scholar] [CrossRef] [PubMed]
- Padya, B.S.; Pandey, A.; NIkam, A.; Kulkarni, S.; Fernandes, G.; Mutalik, S. Two-dimensional materials-based nanoplatforms for lung cancer management: Synthesis, properties, and targeted therapy. In Advanced Drug Delivery Systems in the Management of Cancer; Academic Press: Cambridge, MA, USA, 2021; pp. 415–429. [Google Scholar]
- Zhu, W.; Li, H.; Luo, P. Emerging 2D nanomaterials for multimodel theranostics of cancer. Front. Bioeng. Biotechnol. 2021, 9, 769178. [Google Scholar] [CrossRef]
- Wen, W.; Song, Y.; Yan, X.; Zhu, C.; Du, D.; Wang, S.; Asiri, A.M.; Lin, Y. Recent advances in emerging 2D nanomaterials for biosensing and bioimaging applications. Mater. Today 2018, 21, 164–177. [Google Scholar] [CrossRef]
- Sharker, S.M. Hexagonal boron nitrides (white graphene): A promising method for cancer drug delivery. Int. J. Nanomed. 2019, 14, 9983–9993. [Google Scholar] [CrossRef]
- Bhatt, H.N.; Pena-Zacarias, J.; Beaven, E.; Zahid, M.I.; Ahmad, S.S.; Diwan, R.; Nurunnabi, M. Potential and progress of 2D materials in photomedicine for cancer treatment. ACS Appl. Bio Mater. 2023, 6, 365–383. [Google Scholar] [CrossRef]
- Sadiq, M.; Pang, L.; Johnson, M.; Sathish, V.; Zhang, Q.; Wang, D. 2D Nanomaterial, Ti3C2 MXene-based sensor to guide lung cancer therapy and management. Biosensors 2021, 11, 40. [Google Scholar] [CrossRef]
- Gholami, A.; Hashemi, S.A.; Yousefi, K.; Mousavi, S.M.; Chiang, W.H.; Ramakrishna, S.; Mazraedoost, S.; Alizadeh, A.; Omidifar, N.; Behbudi, G.; et al. 3D nanostructures for tissue engineering, cancer therapy, and gene delivery. J. Nanomater. 2020, 2020, 1852946. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Böker, A. Self-Assembly of Nanoparticles in 2D and 3D: Recent Advances and Future Trends. Macromol. Chem. Phys. 2019, 220, 1900196. [Google Scholar] [CrossRef]
- Shahriar, S.M.S.; Andrabi, S.M.; Islam, F.; An, J.M.; Schindler, S.J.; Matis, M.P.; Lee, D.Y.; Lee, Y.-K. Next-generation 3D scaffolds for nano-based chemotherapeutics delivery and cancer treatment. Pharmaceutics 2022, 14, 2712. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.; Tan, M.; Ou, L.; Kong, D.; Yang, Z. Conjugation of two complementary anti-cancer drugs confers molecular hydrogels as a co-delivery system. Chem. Commun. 2012, 48, 395–397. [Google Scholar] [CrossRef]
- Rincón-Pérez, J.; Rodríguez-Hernández, L.; Ruíz-Valdiviezo, V.M.; Abud-Archila, M.; Luján-Hidalgo, M.C.; Ruiz-Lau, N.; González-Mendoza, D.; Gutiérrez-Miceli, F.A. Fatty Acids Profile, Phenolic Compounds and Antioxidant Capacity in Elicited Callus ofThevetia peruviana (Pers.) K. Schum. J. Oleo Sci. 2016, 65, 311–318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, C.; Su, L.; Zhang, F.; Zhu, X.; Zhu, Y.; Wei, L.; Jiao, X.; Hou, Y.; Chen, X.; Wang, W.; et al. Thevebioside, the active ingredient of traditional Chinese medicine, promotes ubiquitin-mediated SRC-3 degradation to induce NSCLC cells apoptosis. Cancer Lett. 2020, 493, 167–177. [Google Scholar] [CrossRef]
- Balan, D.J.; Rajavel, T.; Das, M.; Sathya, S.; Jeyakumar, M.; Devi, K.P. Thymol induces mitochondrial pathway-mediated apoptosis via ROS generation, macromolecular damage and SOD diminution in A549 cells. Pharmacol. Rep. 2021, 73, 240–254. [Google Scholar] [CrossRef]
- Bonifácio, B.V.; da Silva, P.B.; Ramos, M.A.D.S.; Negri, K.M.S.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Ansari, S.H.; Islam, F.; Sameem, M. Influence of nanotechnology on herbal drugs: A Review. J. Adv. Pharm. Technol. Res. 2012, 3, 142–146. [Google Scholar] [CrossRef]
- Türk, G.; Ateşşahin, A.; Sönmez, M.; Çeribaşi, A.O.; Yüce, A. Improvement of cisplatin-induced injuries to sperm quality, the oxidant-antioxidant system, and the histologic structure of the rat testis by ellagic acid. Fertil. Steril. 2008, 89, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Abd Elwakil, M.M.; Mabrouk, M.T.; Helmy, M.W.; Abdelfattah, E.Z.A.; Khiste, S.K.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable lactoferrin–chondroitin nanocomposites for combined delivery of doxorubicin and ellagic acid to lung carcinoma. Nanomedicine 2018, 13, 2015–2035. [Google Scholar] [CrossRef]
- Mardani, R.; Hamblin, M.R.; Taghizadeh, M.; Banafshe, H.R.; Nejati, M.; Mokhtari, M.; Borran, S.; Davoodvandi, A.; Khan, H.; Jaafari, M.R.; et al. Nanomicellar-curcumin exerts its therapeutic effects via affecting angiogenesis, apoptosis, and T cells in a mouse model of melanoma lung metastasis. Pathol.-Res. Pract. 2020, 216, 153082. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, M.M.; Helmy, M.W.; Mortada, S.M.; Elzoghby, A.O. Dual-targeted nano-in-nano albumin carriers enhance the efficacy of combined chemo/herbal therapy of lung cancer. Nanomedicine 2018, 13, 2221–2224. [Google Scholar] [CrossRef]
- Kumkoon, T.; Srisaisap, M.; Boonserm, P. Biosynthesized silver nanoparticles using Morus alba (white mulberry) leaf extract as potential antibacterial and anticancer agents. Molecules 2023, 28, 1213. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Son, K.H.; Kwon, C.S.; Kwon, G.S.; Kang, S.S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Panek-Krzyśko, A.; Stompor-Gorący, M. The pro-health benefits of morusin administration—An update review. Nutrients 2021, 13, 3043. [Google Scholar] [CrossRef]
- Park, H.J.; Min, T.R.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018, 505, 194–200. [Google Scholar] [CrossRef]
- Govindaraju, S.; Roshini, A.; Lee, M.H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Almatroudi, A.; Allemailem, K.S.; Alwanian, W.M.; Alharbi, B.F.; Alrumaihi, F.; Khan, A.A.; Almatroodi, S.A.; Rahmani, A.H. Effects and mechanisms of kaempferol in the management of cancers through modulation of inflammation and signal transduction pathways. Int. J. Mol. Sci. 2023, 24, 8630. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhang, G.; Wu, J.; Liu, Z.; Liu, C.; Wang, H.; Miao, S.; Deng, L.; Cao, K.; et al. To explore the effect of kaempferol on non-small cell lung cancer based on network pharmacology and molecular docking. Front. Pharmacol. 2023, 14, 1148171. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Gambaro, R.; Cisneros, J.S.; Huck-Iriart, C.; Padula, G.; Castro, G.R.; Chain, C.Y.; Islan, G.A. Enhanced anticancer activity of encapsulated geraniol into biocompatible lipid nanoparticles against A549 human lung cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104159. [Google Scholar] [CrossRef]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One hundred faces of geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef] [PubMed]
- Rodenak-Kladniew, B.; Castro, M.A.; Gambaro, R.C.; Girotti, J.; Cisneros, J.S.; Viña, S.; Padula, G.; Crespo, R.; Castro, G.R.; Gehring, S.; et al. Cytotoxic screening and enhanced anticancer activity of lippia alba and clinopodium nepeta essential oils-loaded biocompatible lipid nanoparticles against lung and colon cancer cells. Pharmaceutics 2023, 15, 2045. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Rocha-González, H.I.; Ambriz-Tututi, M.; Granados-Soto, V. Resveratrol: A natural compound with pharmacological potential in neurodegenerative diseases. CNS Neurosci. Ther. 2008, 14, 234–247. [Google Scholar] [CrossRef]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef]
- Paudel, K.R.; Mehta, M.; Yin, G.H.S.; Yen, L.L.; Malyla, V.; Patel, V.K.; Panneerselvam, J.; Madheswaran, T.; MacLoughlin, R.; Jha, N.K.; et al. Berberine-loaded liquid crystalline nanoparticles inhibit non-small cell lung cancer proliferation and migration in vitro. Environ. Sci. Pollut. Res. 2022, 29, 46830–46847. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhang, J.H.; Gao, J.H.; Li, Y.S. Aloe-emodin (AE) nanoparticles suppresses proliferation and induces apoptosis in human lung squamous carcinoma via ROS generation in vitro and in vivo. Biochem. Biophys. Res. Commun. 2017, 490, 601–607. [Google Scholar] [CrossRef]
- Majumdar, D.; Jung, K.-H.; Zhang, H.; Nannapaneni, S.; Wang, X.; Amin, A.R.; Chen, Z.; Chen, Z.; Shin, D.M. Luteolin nanoparticle in chemoprevention: In vitro and in vivo anticancer activity. Cancer Prev. Res. 2014, 7, 65–73. [Google Scholar] [CrossRef]
- Rauf, A.; Wilairatana, P.; Joshi, P.B.; Ahmad, Z.; Olatunde, A.; Hafeez, N.; Hemeg, H.A.; Mubarak, M.S. Revisiting luteolin: An updated review on its anticancer potential. Heliyon 2024, 10, e26701. [Google Scholar] [CrossRef]
- Alshehri, S.; Bukhari, S.I.; Imam, S.S.; Hussain, A.; Alghaith, A.F.; Altamimi, M.A.; AlAbdulkarim, A.S.; Almurshedi, A. Formulation of Piperine-Loaded Nanoemulsion: In Vitro Characterization, Ex Vivo Evaluation, and Cell Viability Assessment. ACS Omega 2023, 8, 22406–22413. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Ray, A.K.; Mishra, S.K. Molecular and pharmacological aspects of piperine as a potential molecule for disease prevention and management: Evidence from clinical trials. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 16. [Google Scholar] [CrossRef]
- Chen, B.H.; Hsieh, C.H.; Tsai, S.Y.; Wang, C.Y.; Wang, C.C. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci. Rep. 2020, 10, 5163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Wong, H.L.; Xue, H.Y.; Eoh, J.Y.; Wu, X.Y. Nanomedicine of synergistic drug combinations for cancer therapy–Strategies and perspectives. J. Control. Release 2016, 240, 489–503. [Google Scholar] [CrossRef]
- Elgohary, M.M.; Helmy, M.W.; Abdelfattah, E.Z.A.; Ragab, D.M.; Mortada, S.M.; Fang, J.Y.; Elzoghby, A.O. Targeting sialic acid residues on lung cancer cells by inhalable boronic acid-decorated albumin nanocomposites for combined chemo/herbal therapy. J. Control Release 2018, 285, 230–243. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, H.M.; Elzoghby, A.O.; Helmy, M.W.; Samaha, M.W.; Fang, J.Y.; Freag, M.S. Liquid crystalline assembly for potential combinatorial chemo–herbal drug delivery to lung cancer cells. Int. J. Nanomed. 2019, 14, 499–517. [Google Scholar] [CrossRef]
- Alemi, A.; Karamallah, M.H.; Sabaghan, M.; Hosseini, S.A.; Veisi, A.; Karamallah, S.H.; Farokhifar, M. Combination drug therapy by herbal nanomedicine prevent multidrug resistance protein 1: Promote apoptosis in Lung Carcinoma. J. Appl. Biomater. Funct. Mater. 2024, 22, 22808000241235442. [Google Scholar] [CrossRef]
- Zhong, S.; Yao, S.; Zhao, Q.; Wang, Z.; Liu, Z.; Li, L.; Wang, Z.L. Electricity-Assisted Cancer Therapy: From Traditional Clinic Applications to Emerging Methods Integrated with Nanotechnologies. Adv. NanoBiomed Res. 2023, 3, 2200143. [Google Scholar] [CrossRef]
- Ahmad, U.; Machuzak, M. Electric shock therapy for lung cancer: Taking palliation to the next level. J. Thorac. Cardiovasc. Surg. 2018, 155, 2160–2161. [Google Scholar] [CrossRef]
- Kodama, H.; Vroomen, L.G.; Ueshima, E.; Reilly, J.; Brandt, W.; Paluch, L.R.; Monette, S.; Jones, D.; Solomon, S.B.; Srimathveeravalli, G. Catheter-based endobronchial electroporation is feasible for the focal treatment of peribronchial tumors. J. Thorac. Cardiovasc. Surg. 2018, 155, 2150–2159. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Venugopal, A.; Chandrasekhar, M.; Suriyanarayanan, A.; Balasubramani, K.; Ilangovan, A.S.; Kamalakannan, S.; Gunaseelan, R.; Ayyadurai, N.; Gopalakrishnan, A.V.; et al. Electrical based cancer therapy for solid tumours-Theranostics approach. Biosens. Bioelectron. X 2022, 11, 100214. [Google Scholar] [CrossRef]
- Alshahat, M.A.; Elgenedy, M.A.; Aboushady, A.A.; Williams, M.T. Cancer Treatment: An Overview of Pulsed Electric Field Utilization and Generation. Appl. Sci. 2023, 13, 10029. [Google Scholar] [CrossRef]
- Xin, Y.L.; Xue, F.Z.; Ge, B.S.; Zhao, F.R.; Shi, B.; Zhang, W. Electrochemical treatment of lung cancer. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1997, 18, 8–13. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Guo, Q.; Kuang, J.; Li, D.; Wu, M.; Mo, Y.; Zhang, T.; Gao, X.; Tan, J. Advanced lung organoids and lung-on-a-chip for cancer research and drug evaluation: A review. Front. Bioeng. Biotechnol. 2023, 11, 1299033. [Google Scholar] [CrossRef]
- Li, Y.; Gao, X.; Ni, C.; Zhao, B.; Cheng, X. The application of patient-derived organoid in the research of lung cancer. Cell. Oncol. 2023, 46, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, J.; Razavi Bazaz, S.; Aboulkheyr Es, H.; Yaghobian Azari, D.; Thierry, B.; Ebrahimi Warkiani, M.; Ghadiri, M. Lung-on-a-chip: The future of respiratory disease models and pharmacological studies. Crit. Rev. Biotechnol. 2020, 40, 213–230. [Google Scholar] [CrossRef]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human organ chip models recapitulate orthotopic lung cancer growth, therapeutic responses, and tumor dormancy in vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab A Chip 2018, 18, 486–495. [Google Scholar] [CrossRef]
- Arathi, A.; Joseph, X.; Akhil, V.; Mohanan, P.V. L-Cysteine capped zinc oxide nanoparticles induced cellular response on adenocarcinomic human alveolar basal epithelial cells using a conventional and organ-on-a-chip approach. Colloids Surf. B Biointerfaces 2022, 211, 112300. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, P.C. Promise of smartphone-enabled teleconsultation for global cancer prevention. JCO Glob. Oncol. 2020, 6, GO.20.00424. [Google Scholar] [CrossRef] [PubMed]
- Pardolesi, A.; Gherzi, L.; Pastorino, U. Telemedicine for management of patients with lung cancer during COVID-19 in an Italian cancer institute: SmartDoc Project. Tumori J. 2022, 108, 357–363. [Google Scholar] [CrossRef]
- Dhawale, T.M.; Bhat, R.S.; Johnson, P.C.; Srikonda, S.; Lau-Min, K.S.; Boateng, K.; Lee, H.; Amonoo, H.L.; Nipp, R.; Lindvall, C.; et al. Telemedicine-based serious illness conversations, healthcare utilization, and end of life care among patients with advanced lung cancer. The Oncologist 2024, 29, e1762–e1769. [Google Scholar] [CrossRef] [PubMed]
- Magarinos, J.; Lutzow, L.; Dass, C.; Ma, G.X.; Erkmen, C.P. Feasibility of single-encounter telemedicine lung cancer screening: A retrospective cohort study in an underserved population. Cancer Control 2023, 30, 10732748221121391. [Google Scholar] [CrossRef]
- Steinhubl, S.R.; Muse, E.D.; Topol, E.J. The emerging field of mobile health. Sci. Transl. Med. 2015, 7, rv3–rv283. [Google Scholar] [CrossRef]
- Vercell, A.; Gasteiger, N.; Yorke, J.; Dowding, D. Patient-facing cancer mobile apps that enable patient reported outcome data to be collected: A systematic review of content, functionality, quality, and ability to integrate with electronic health records. Int. J. Med. Inform. 2023, 170, 104931. [Google Scholar] [CrossRef]
- Ciani, O.; Cucciniello, M.; Petracca, F.; Apolone, G.; Merlini, G.; Novello, S.; Pedrazzoli, P.; Zilembo, N.; Broglia, C.; Capelletto, E.; et al. Lung Cancer App (LuCApp) study protocol: A randomised controlled trial to evaluate a mobile supportive care app for patients with metastatic lung cancer. BMJ Open 2019, 9, e025483. [Google Scholar] [CrossRef]
- Ji, W.; Kwon, H.; Lee, S.; Kim, S.; Hong, J.S.; Park, Y.R.; Kim, H.R.; Lee, J.C.; Jung, E.J.; Kim, D.; et al. Mobile health management platform–based pulmonary rehabilitation for patients with non–small cell lung cancer: Prospective clinical trial. JMIR Mhealth Uhealth 2019, 7, e12645. [Google Scholar] [CrossRef]
- Henshall, C.; Davey, Z. Development of an app for lung cancer survivors (iEXHALE) to increase exercise activity and improve symptoms of fatigue, breathlessness and depression. Psycho-Oncol. 2020, 29, 139–147. [Google Scholar] [CrossRef]
- Ni, X.; Lou, Y.; Hu, W.; Wang, H.; Xu, H.; Li, S.; Zhou, Y.; Ni, Y. Development of mobile health–based self-management support for patients with lung cancer: A stepwise approach. Nurs. Open 2022, 9, 1612–1624. [Google Scholar] [CrossRef]
- Berzenji, L.; Yogeswaran, K.; Van Schil, P.; Lauwers, P.; Hendriks, J.M. Use of robotics in surgical treatment of non-small cell lung cancer. Curr. Treat. Options Oncol. 2020, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, G. Robotic surgery for the treatment of early-stage lung cancer. Curr. Opin. Oncol. 2013, 25, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Siu, I.C.; Chang, A.T.; Li, M.S.; Lau, R.W.; Mok, T.S.; Ng, C.S. Transbronchial techniques for lung cancer treatment: Where are we now? Cancers 2023, 15, 1068. [Google Scholar] [CrossRef]
- Chen, A.C.; Gillespie, C.T. Robotic endoscopic airway challenge: REACH assessment. Ann. Thorac. Surg. 2018, 106, 293–297. [Google Scholar] [CrossRef]
- da Silva, L.G.V.; Barros, K.V.G.; de Araújo, F.V.C.; da Silva, G.B.; da Silva, P.A.F.; Condori, R.C.I.; Mattos, L. Nanorobotics in drug delivery systems for treatment of cancer: A review. J. Mat. Sci. Eng. A 2016, 6, 167–180. [Google Scholar]
- Muskan, A.; Kumar, S. The Use of Nanorobotics in the Treatment Therapy of Cancer and Its Future Aspects: A Review. Cureus 2022, 14, e29366. [Google Scholar]
- Oigbochie, A.E.; Odigie, E.B.; Adejumo, B.I.G. Importance of drones in healthcare delivery amid a pandemic: Current and generation next application. Open J. Med. Res. (ISSN: 2734-2093) 2021, 2, 1–13. [Google Scholar] [CrossRef]
- Abeygunawaradana, P.; Gamage, N.; De Alwis, L.; Ashan, S.; Nilanka, C.; Godamune, P. E-Medic–Autonomous Drone for Healthcare System. In 2021 International Conference on Computing, Communication, and Intelligent Systems (ICCCIS); IEEE: Greater Noida, India, 2021; pp. 994–999. [Google Scholar]
- Qiu, C.; Wu, F.; Han, W.; Yuce, M.R. A wearable bioimpedance chest patch for real-time ambulatory respiratory monitoring. IEEE Trans. Biomed. Eng. 2022, 69, 2970–2981. [Google Scholar] [CrossRef]
- John, M.; Van Der Westen, R.G.; Yordanova, G.; Groenendaal, W.; Agell, C. Validation of a novel wearable monitoring patch for continuous respiratory monitoring. Eur. Respir. J. 2022, 60, 4391. [Google Scholar]
- Moon, K.S.; Lee, S.Q. A Wearable Multimodal Wireless Sensing System for Respiratory Monitoring and Analysis. Sensors 2023, 23, 6790. [Google Scholar] [CrossRef] [PubMed]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 15, 1–23. [Google Scholar] [CrossRef]
- Guntner, A.T.; Abegg, S.; Konigstein, K.; Gerber, P.A.; Schmidt-Trucksass, A.; Pratsinis, S.E. Breath sensors for health monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef]
- Lin, L.P.; Tan, M.T.T. Biosensors for the detection of lung cancer biomarkers: A review on biomarkers, transducing techniques and recent graphene-based implementations. Biosens. Bioelectron. 2023, 237, 115492. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Hrinczenko, B.; Swain, G.M.; Peteu, S.F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef]
- Schmidt, F.; Kohlbrenner, D.; Malesevic, S.; Huang, A.; Klein, S.D.; Puhan, M.A.; Kohler, M. Mapping the landscape of lung cancer breath analysis: A scoping review (ELCABA). Lung Cancer 2023, 175, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, Q. Ru-Doped MoS2 Monolayer for Exhaled Breath Detection on Early Lung Cancer Diagnosis: A First-Principles Investigation. ACS Omega 2024, 9, 13951–13959. [Google Scholar] [CrossRef]
- Lu, G.; Ji, T.; He, S.; Ai, F.; Yan, L.; Hu, J. Recent progress of exhaled gas-based diagnosis based on field effect transistor sensors. Adv. Funct. Mater. 2024, 35, 2309111. [Google Scholar] [CrossRef]
- Amalraj, J.J.; Banumathi, S.; John, J.J. IOT sensors and applications: A survey. Int. J. Sci. Technol. Res. 2019, 8, 998–1003. [Google Scholar]
- Chaudhary, V.; Khanna, V.; Awan, H.T.A.; Singh, K.; Khalid, M.; Mishra, Y.K.; Bhansali, S.; Li, C.-Z.; Kaushik, A. Towards hospital-on-chip supported by 2D MXenes-based 5th generation intelligent biosensors. Biosens. Bioelectron. 2023, 220, 114847. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaushik, A.; Furukawa, H.; Khosla, A. Towards 5th generation ai and iot driven sustainable intelligent sensors based on 2d mxenes and borophene. ECS Sens. Plus 2022, 1, 013601. [Google Scholar] [CrossRef]
- Taha, B.A.; Chaudhary, V.; Rustagi, S.; Singh, P. Fate of Sniff-the-Diseases Through Nanomaterials-Supported Optical Biochip Sensors. ECS J. Solid State Sci. Technol. 2024, 13, 047004. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gaur, P.; Rustagi, S. Sensors, society, and sustainability. Sustain. Mater. Technol. 2024, 40, e00952. [Google Scholar] [CrossRef]
- Rong, R.; Sheng, H.; Jin, K.W.; Wu, F.; Luo, D.; Wen, Z.; Tang, C.; Yang, D.M.; Jia, L.; Amgad, M.; et al. A deep learning approach for histology-based nucleus segmentation and tumor microenvironment characterization. Mod. Pathol. 2023, 36, 100196. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Jiang, T.; Yang, C.; Li, N. Artificial intelligence in lung cancer: Current applications, future perspectives, and challenges. Front. Oncol. 2024, 14, 1486310. [Google Scholar] [CrossRef] [PubMed]
- Kehl, K.L.; Xu, W.; Gusev, A.; Bakouny, Z.; Choueiri, T.K.; Bin Riaz, I.; Elmarakeby, H.; Van Allen, E.M.; Schrag, D. Artificial intelligence-aided clinical annotation of a large multi-cancer genomic dataset. Nat. Commun. 2021, 12, 7304. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.; Bonfante, M.; Zurek, E.; Cherezov, D.; Goldgof, D.; Hall, L.; Schabath, M. A radiogenomics ensemble to predict EGFR and KRAS mutations in NSCLC. Tomography 2021, 7, 154–168. [Google Scholar] [CrossRef]
- Duch, W.; Swaminathan, K.; Meller, J. Artificial intelligence approaches for rational drug design and discovery. Curr. Pharm. Des. 2007, 13, 1497–1508. [Google Scholar] [CrossRef]
- Zhong, F.; Xing, J.; Li, X.; Liu, X.; Fu, Z.; Xiong, Z.; Lu, D.; Wu, X.; Zhao, J.; Tan, X.; et al. Artificial intelligence in drug design. Sci. China Life Sci. 2018, 61, 1191–1204. [Google Scholar] [CrossRef]
- Murphy, A.; Skalski, M.; Gaillard, F. The utilisation of convolutional neural networks in detecting pulmonary nodules: A review. Br. J. Radiol. 2018, 91, 20180028. [Google Scholar] [CrossRef]
- Cellina, M.; Cacioppa, L.M.; Cè, M.; Chiarpenello, V.; Costa, M.; Vincenzo, Z.; Pais, D.; Bausano, M.V.; Rossini, N.; Bruno, A.; et al. Artificial intelligence in lung cancer screening: The future is now. Cancers 2023, 15, 4344. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Rong, R.; Zhou, Q.; Yang, D.M.; Zhang, X.; Zhan, X.; Bishop, J.; Chi, Z.; Wilhelm, C.J.; Zhang, S.; et al. Deep learning of cell spatial organizations identifies clinically relevant insights in tissue images. Nat. Commun. 2023, 14, 7872. [Google Scholar] [CrossRef] [PubMed]
- Quiros, A.C.; Coudray, N.; Yeaton, A.; Yang, X.; Liu, B.; Le, H.; Chiriboga, L.; Karimkhan, A.; Narula, N.; Moore, D.A.; et al. Mapping the landscape of histomorphological cancer phenotypes using self-supervised learning on unannotated pathology slides. Nat. Commun. 2024, 15, 4596. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Chen, B.; Williamson, D.F.K.; Chen, R.J.; Zhao, M.; Chow, A.K.; Ikemura, K.; Kim, A.; Pouli, D.; Patel, A.; et al. A multimodal generative AI copilot for human pathology. Nature 2024, 634, 466–473. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choudhary, F.; Naikoo, U.M.; Rizwan, A.; Kaur, J.; Abdin, M.Z.; Farooqi, H. Innovation in Lung Cancer Management from Herbal Nanomedicine to Artificial Intelligence. J. Nanotheranostics 2025, 6, 19. https://doi.org/10.3390/jnt6030019
Choudhary F, Naikoo UM, Rizwan A, Kaur J, Abdin MZ, Farooqi H. Innovation in Lung Cancer Management from Herbal Nanomedicine to Artificial Intelligence. Journal of Nanotheranostics. 2025; 6(3):19. https://doi.org/10.3390/jnt6030019
Chicago/Turabian StyleChoudhary, Furqan, Ubaid Mushtaq Naikoo, Amber Rizwan, Jasmeet Kaur, Malik Z. Abdin, and Humaira Farooqi. 2025. "Innovation in Lung Cancer Management from Herbal Nanomedicine to Artificial Intelligence" Journal of Nanotheranostics 6, no. 3: 19. https://doi.org/10.3390/jnt6030019
APA StyleChoudhary, F., Naikoo, U. M., Rizwan, A., Kaur, J., Abdin, M. Z., & Farooqi, H. (2025). Innovation in Lung Cancer Management from Herbal Nanomedicine to Artificial Intelligence. Journal of Nanotheranostics, 6(3), 19. https://doi.org/10.3390/jnt6030019








