Nanomedicine: Transforming the Management of Ocular Neuroinflammatory and Neurodegenerative Diseases
Abstract
:1. Introduction
2. Different Neuro-Ophthalmological Manifestations and the Contribution of Nanomedicine
2.1. Primary Headache Disorders
2.1.1. Migraines
2.1.2. Migraine Diagnosis
2.1.3. Nanotechnology-Based Treatment of Migraine
2.2. Neuroimmune Diseases
2.2.1. Ocular Manifestations in Multiple Sclerosis (MS)
2.2.2. MS Immunopathophysiology of MS
2.2.3. Nanomedicine-Based Diagnosis and Therapy of MS
2.3. Ocular Myasthenia Gravis (OMG)
2.3.1. MG Pathophysiology
2.3.2. Ophthalmological Features of MG
2.3.3. Current Diagnosis and Treatment of OMG
2.3.4. OMG and Nanomedicine
2.4. Neurodegenerative Diseases
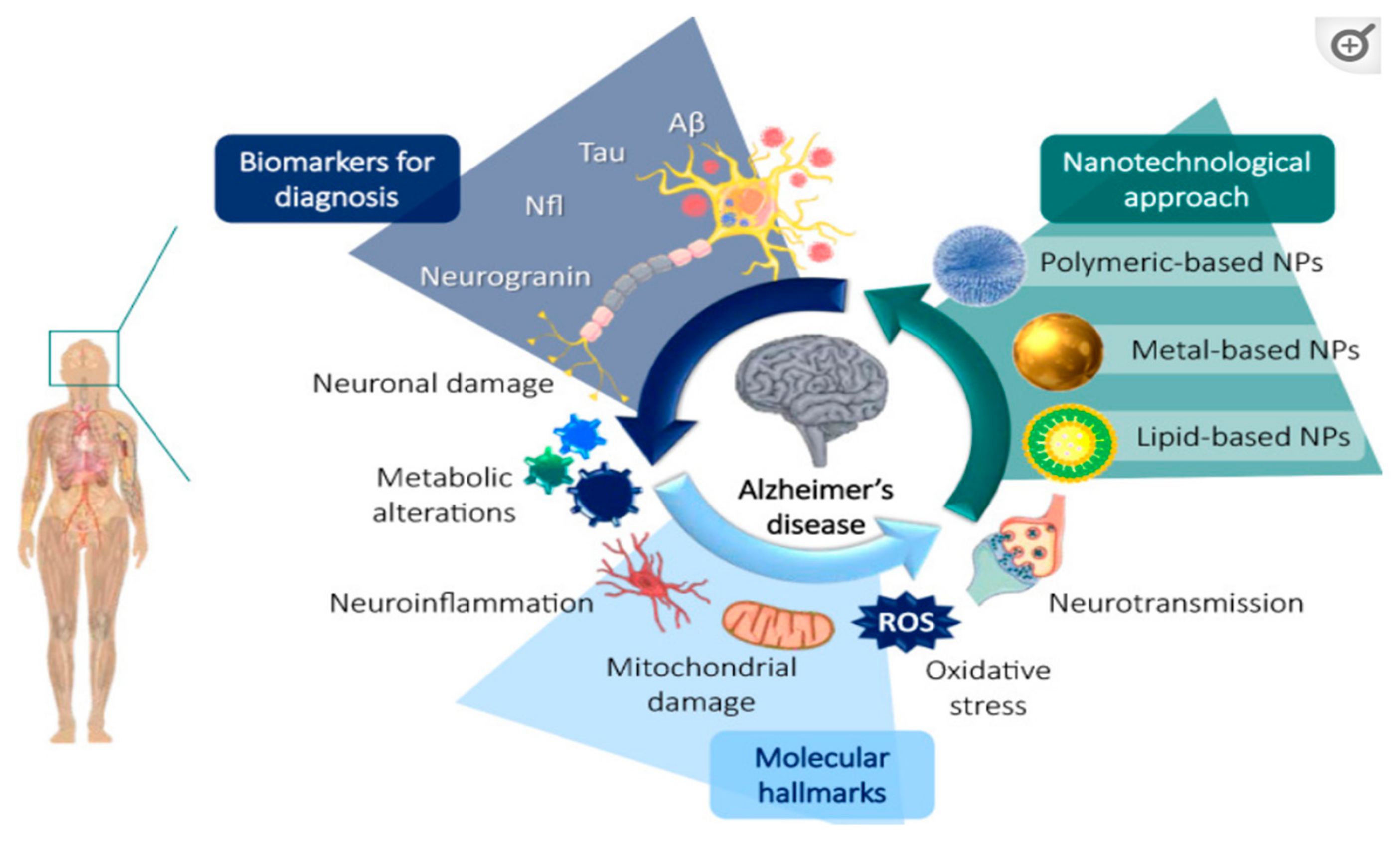
2.4.1. Alzheimer’s Disease (AD)
Nanomedicine and AD
2.4.2. Parkinson’s Disease (PD)
Nanomedicine and PD
3. Insight into Ophthalmic Symptoms in Neurodegenerative and Neuroimmune Diseases
Safety and Toxicity Issues
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.; Chen, L.; Fu, Y. Nanotechnology-based ocular drug delivery systems: Recent advances and future prospects. J. Nanobiotechnol. 2023, 21, 232. [Google Scholar] [CrossRef] [PubMed]
- Zarbin, M.A.; Montemagno, C.; Leary, J.F.; Ritch, R. Nanomedicine for the treatment of retinal and optic nerve diseases. Curr. Opin. Pharmacol. 2013, 13, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Pande, S.; Sagathia, V.; Ranch, K.; Beladiya, J.; Boddu, S.H.S.; Jacob, S.; Al-Tabakha, M.M.; Hassan, N.; Shahwan, M. Nanocarriers for the Delivery of Neuroprotective Agents in the Treatment of Ocular Neurodegenerative Diseases. Pharmaceutics 2023, 15, 837. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.V. Neuro-Ophthalmic Symptoms of Primary Headache Disorders: Why the Patient with Headache May Present to Neuro-Ophthalmology. J. Neuro-Ophthalmol. 2019, 39, 200–207. [Google Scholar] [CrossRef]
- Benoliel, R.; Eliav, E. Primary Headache Disorders. Dent. Clin. N. Am. 2013, 57, 513–539. [Google Scholar] [CrossRef]
- Demircan, S.; Ataş, M.; Yüksel, S.A.; Ulusoy, M.D.; Yuvacı, İ.; Arifoğlu, H.B.; Başkan, B.; Zararsız, G. The Impact of Migraine on Posterior Ocular Structures. J. Ophthalmol. 2015, 2015, 868967. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Migraine pathophysiology: Anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013, 154 (Suppl. 1), S44–S53. [Google Scholar] [CrossRef]
- Lucas, C. Migraine with aura. Rev. Neurol. 2021, 177, 779–784. [Google Scholar] [CrossRef]
- Istrate, B.M.; Vîlciu, C.; Răgan, C. Retinal migraine. Rom. J. Ophthalmol. 2020, 64, 96–99. [Google Scholar] [CrossRef]
- Schoenen, J.; Vandersmissen, B.; Jeangette, S.; Herroelen, L.; Vandenheede, M.; Gérard, P.; Magis, D. Migraine prevention with a supraorbital transcutaneous stimulator. Neurology 2013, 80, 697–704. [Google Scholar] [CrossRef]
- Bhaskar, S.; Saeidi, K.; Borhani, P.; Amiri, H. Recent progress in migraine pathophysiology: Role of cortical spreading depression and magnetic resonance imaging. Eur. J. Neurosci. 2013, 38, 3540–3551. [Google Scholar] [CrossRef] [PubMed]
- Hansraj, G.P.; Singh, S.K.; Kumar, P. Sumatriptan succinate loaded chitosan solid lipid nanoparticles for enhanced anti-migraine potential. Int. J. Biol. Macromol. 2015, 81, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Girotra, P.; Singh, S.K.; Kumar, G. Development of zolmitriptan loaded PLGA/poloxamer nanoparticles for migraine using quality by design approach. Int. J. Biol. Macromol. 2016, 85, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, D.A.E.; Khalifa, M.K.A.; Gad, S.S. Zolmitriptan brain targeting via intranasal route using solid lipid nanoparticles for migraine therapy: Formulation, characterization, in-vitro and in-vivo assessment. Int. J. Appl. Pharm. 2020, 12, 86–93. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Vincent, M. Neuroimaging clues of migraine aura. J. Headache Pain 2019, 20, 32. [Google Scholar] [CrossRef]
- Almenara-Fuentes, L.; Rodriguez-Fernandez, S.; Rosell-Mases, E.; Kachler, K.; You, A.; Salvado, M.; Andreev, D.; Steffen, U.; Bang, H.; Bozec, A.; et al. A new platform for autoimmune diseases. Inducing tolerance with liposomes encapsulating autoantigens. Nanomed. Nanotechnol. Biol. Med. 2023, 48, 102635. [Google Scholar] [CrossRef]
- Kondiah, P.P.; Choonara, Y.E.; Kondiah, P.J.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Modi, G.; Pillay, V. Nanocomposites for therapeutic application in multiple sclerosis. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 391–408. [Google Scholar]
- Caballero-Villarraso, J.; Sawas, J.; Escribano, B.M.; Martín-Hersog, F.A.; Valverde-Martínez, A.; Túnez, I. Gene and cell therapy and nanomedicine for the treatment of multiple sclerosis: Bibliometric analysis and systematic review of clinical outcomes. Expert Rev. Neurother. 2021, 21, 431–441. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Demetzos, C. Promising Nanotechnology Approaches in Treatment of Autoimmune Diseases of Central Nervous System. Brain Sci. 2020, 10, 338. [Google Scholar] [CrossRef]
- Green, A.J.; McQuaid, S.; Hauser, S.L.; Allen, I.V.; Lyness, R. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain 2010, 133, 1591–1601. [Google Scholar] [CrossRef]
- Graham, S.L.; Klistorner, A. Afferent visual pathways in multiple sclerosis: A review. Clin. Exp. Ophthalmol. 2017, 45, 62–72. [Google Scholar] [CrossRef]
- Casselman, P.; Cassiman, C.; Casteels, I.; Schauwvlieghe, P. Insights into multiple sclerosis-associated uveitis: A scoping review. Acta Ophthalmol. 2021, 99, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Graham, E.M.; A Francis, D.; Sanders, M.D.; Rudge, P. Ocular inflammatory changes in established multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Britze, J.; Frederiksen, J.L. Optical coherence tomography in multiple sclerosis. Eye 2018, 32, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, D.; Picone, P. Multiple sclerosis: Focus on extracellular and artificial vesicles, nanoparticles as potential therapeutic approaches. Int. J. Mol. Sci. 2021, 22, 8866. [Google Scholar] [CrossRef]
- van Schaik, P.E.M.; Zuhorn, I.S.; Baron, W. Targeting Fibronectin to Overcome Remyelination Failure in Multiple Sclerosis: The Need for Brain- and Lesion-Targeted Drug Delivery. Int. J. Mol. Sci. 2022, 23, 8418. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Owe, J.F.; Hoff, J.M.; Romi, F.; Skeie, G.O.; Aarli, J.A. Myasthenia gravis: A review of available treatment approaches. Autoimmune Dis. 2011, 2011, 847393. [Google Scholar] [CrossRef]
- Patil-Chhablani, P.; Nair, A.; Venkatramani, D.; Gandhi, R. Ocular myasthenia gravis: A review. Indian J. Ophthalmol. 2014, 62, 985–991. [Google Scholar] [CrossRef]
- Naqvi, S.; Panghal, A.; Flora, S.J.S. Nanotechnology: A Promising Approach for Delivery of Neuroprotective Drugs. Front. Neurosci. 2020, 14, 494. [Google Scholar] [CrossRef]
- Tan, A.; Fraser, C.; Khoo, P.; Watson, S.; Ooi, K. Statins in Neuro-ophthalmology. Neuro-Ophthalmology 2021, 45, 219–237. [Google Scholar] [CrossRef]
- Katsimpris, A.; Karamaounas, A.; Sideri, A.M.; Katsimpris, J.; Georgalas, I.; Petrou, P. Optical coherence tomography angiography in Alzheimer’s disease: A systematic review and meta-analysis. Eye 2022, 36, 1419–1426. [Google Scholar] [CrossRef]
- Molitor, R.J.; Ko, P.C.; Ally, B.A. Eye movements in Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Koronyo, Y.; Rentsendorj, A.; Mirzaei, N.; Regis, G.C.; Sheyn, J.; Shi, H.; Barron, E.; Cook-Wiens, G.; Rodriguez, A.R.; Medeiros, R.; et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol. 2023, 145, 409–438. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Turowski, P.; Ettcheto, M.; Duskey, J.T.; Tosi, G.; Sánchez-López, E.; García, M.L.; Camins, A.; Souto, E.B.; Ruiz, A.; et al. Nanomedicine-based technologies and novel biomarkers for the diagnosis and treatment of Alzheimer’s disease: From current to future challenges. J. Nanobiotechnol. 2021, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-P.; Sun, J.-G.; Yao, J.; Shan, K.; Liu, B.-H.; Yao, M.-D.; Ge, H.-M.; Jiang, Q.; Zhao, C.; Yan, B. Effect of nanoencapsulation using poly (lactide-co-glycolide) (PLGA) on anti-angiogenic activity of bevacizumab for ocular angiogenesis therapy. Biomed. Pharmacother. 2018, 107, 1056–1063. [Google Scholar] [CrossRef]
- Callahan, C.M.; Apostolova, L.G.; Gao, S.; Risacher, S.L.; Case, J.; Saykin, A.J.; Lane, K.A.; Swinford, C.G.; Yoder, M.C. Novel Markers of Angiogenesis in the Setting of Cognitive Impairment and Dementia. J. Alzheimer’s Dis. 2020, 75, 959–969. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Zhang, P.-L.; Chen, Y.; Zhang, C.-H.; Wang, Y.-X.; Fernandez-Funez, P. Genetics of Parkinson’s disease and related disorders. J. Med. Genet. 2017, 55, 73–80. [Google Scholar] [CrossRef]
- Kwan, S.C.K.; Kwan, S.C.K.; Atiya, A.; Atiya, A.; Hussaindeen, J.R.; Hussaindeen, J.R.; Praveen, S.; Praveen, S.; Ambika, S.; Ambika, S. Ocular features of patients with Parkinson’s disease examined at a Neuro-Optometry Clinic in a tertiary eye care center. Indian J. Ophthalmol. 2022, 70, 958–961. [Google Scholar] [CrossRef]
- Armstrong, R.A. Visual symptoms in Parkinson’s disease. Park. Dis. 2011, 2011, 908306. [Google Scholar] [CrossRef]
- Borm, C.D.; Smilowska, K.; de Vries, N.M.; Bloem, B.R.; Theelen, T. The Neuro-Ophthalmological Assessment in Parkinson’s Disease. J. Park. Dis. 2019, 9, 427–435. [Google Scholar] [CrossRef]
- Jagaran, K.; Singh, M. Lipid Nanoparticles: Promising Treatment Approach for Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 9361. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Godínez, F.J.; Ruiz-Ortega, L.I.; Guerra-Crespo, M. Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes. Cells 2022, 11, 3445. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Gaikwad, S.; Padovani, F.H.; Alves, M. The role of nanotechnology in control of human diseases: Perspectives in ocular surface diseases. Crit. Rev. Biotechnol. 2016, 36, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Mathew, G.E.; Uddin, S.; Kim, H.; Mathew, B. Advancements in nanotherapeutics for Alzheimer’s disease: Current perspectives. J. Pharm. Pharmacol. 2019, 71, 1370–1383. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Dewey, R.B.; Rynders, A.; Evan, J.; Evan, J.; Ligozio, S.; Ho, K.S.; Sguigna, P.V.; Glanzman, R.; Hotchkin, M.T.; et al. Evidence of brain target engagement in Parkinson’s disease and multiple sclerosis by the investigational nanomedicine, CNM-Au8, in the REPAIR phase 2 clinical trials. J. Nanobiotechnol. 2023, 21, 478. [Google Scholar] [CrossRef]
- Tandon, A.; Singh, S.J.; Chaturvedi, R.K. Nanomedicine against Alzheimer’s and Parkinson’s Disease. Curr. Pharm. Des. 2021, 27, 1507–1545. [Google Scholar] [CrossRef]
- Kamaleddin, M.A. Nano-ophthalmology: Applications and considerations. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1459–1472. [Google Scholar] [CrossRef]
- Yang, C.; Yang, J.; Lu, A.; Gong, J.; Yang, Y.; Lin, X.; Li, M.; Xu, H. Nanoparticles in ocular applications and their potential toxicity. Front. Mol. Biosci. 2022, 9, 931759. [Google Scholar] [CrossRef]
- Meng, T.; Kulkarni, V.; Simmers, R.; Brar, V.; Xu, Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov. Today 2019, 24, 1524–1538. [Google Scholar] [CrossRef]
- Shah, N. Nanocarriers: Drug Delivery System: An Evidence Based Approach; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Kesler, A. Neuro-ophthalmology: The eye as a window to the brain. Harefuah 2013, 152, 66–68, 124. [Google Scholar]
- Wei, J.; Mu, J.; Tang, Y.; Qin, D.; Duan, J.; Wu, A. Next-generation nanomaterials: Advancing ocular anti-inflammatory drug therapy. J. Nanobiotechnol. 2023, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Mahaling, B.; Baruah, N.; Dinabandhu, A. Nanomedicine in Ophthalmology: From Bench to Bedside. J. Clin. Med. 2024, 13, 7651. [Google Scholar] [CrossRef] [PubMed]
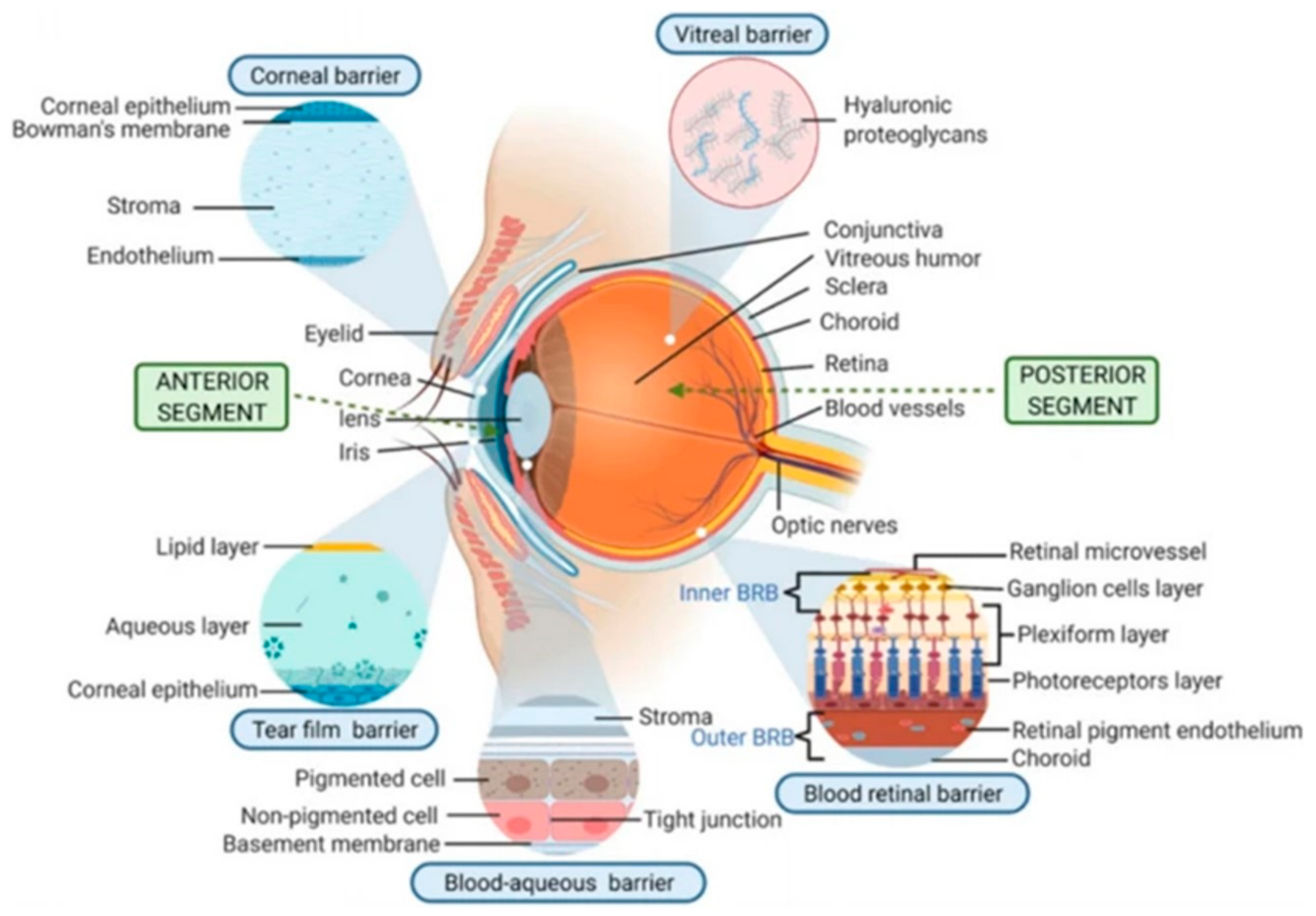

| Disease Category | Disease Name | Ocular Symptoms |
|---|---|---|
| Primary Headache Disorders | Migraines | Eye flashes, “foggy” vision, bright zig-zag lines or dots, scotoma, and aura. |
| Neuroimmune diseases | Multiple Sclerosis | Uveitis, retinal atrophy, inflammatory-mediated optic nerve injuries, retinal peri phlebitis, optic disc atrophy, and slits in the retinal nerve fiber. |
| Neuroimmune diseases | Ocular Myasthenia Gravis | Cranial nerve palsies, gaze palsies, internuclear ophthalmoplegia, blepharospasm, and stroke. |
| Neurodegenerative Diseases | Alzheimer’s Disease | Problems in the visual field, decreased contrast sensitivity, color defects, eye flashes, ocular movement abnormalities, disorientation, and visual hallucinations. |
| Neurodegenerative Diseases | Parkinson’s disease | Visual field disturbances, dry eye symptoms, color vision abnormalities, altered contrast sensitivity, visual hallucinations, vision loss, asthenopia. |
| Test Name | Test Type | General Details |
|---|---|---|
| Sleep Test | Test in the clinic | Observing the re-appearance of MG signs like ptosis (within 5 min), after a 30 min sleep. |
| Ice Test | Test in the clinic | Application of an icepack on the patient’s eyelid, and measurement of the change in ocular motility and ptosis. |
| Electromyogram (EMG) | Test in the clinic | A nerve is electrically stimulated, and the muscle responses are measured through EMG. |
| Edrophonium Test | Pharmacological Test | Blocks acetylcholinesterase function in the NMJs, preventing ACh breakdown. |
| Neostigmine Test | Pharmacological Test | An alternative to the edrophonium testing, with a better duration of action. |
| AChR antibodies | Pharmacological Test | Diagnostic “Gold Standard”, high levels of AChR Abs confirm MG diagnosis. |
| Anti-MuSK antibodies | Immunological Testing | Mainly used for seronegative MG-patients. |
| Brain MRI | Immunological Testing | Identification of structural brain stem lesions related to MG. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvidou, G.; Spyratou, E.; Zachou, M.-E.; Efstathopoulos, E.P. Nanomedicine: Transforming the Management of Ocular Neuroinflammatory and Neurodegenerative Diseases. J. Nanotheranostics 2025, 6, 6. https://doi.org/10.3390/jnt6010006
Savvidou G, Spyratou E, Zachou M-E, Efstathopoulos EP. Nanomedicine: Transforming the Management of Ocular Neuroinflammatory and Neurodegenerative Diseases. Journal of Nanotheranostics. 2025; 6(1):6. https://doi.org/10.3390/jnt6010006
Chicago/Turabian StyleSavvidou, Georgia, Ellas Spyratou, Maria-Eleni Zachou, and Efstathios P. Efstathopoulos. 2025. "Nanomedicine: Transforming the Management of Ocular Neuroinflammatory and Neurodegenerative Diseases" Journal of Nanotheranostics 6, no. 1: 6. https://doi.org/10.3390/jnt6010006
APA StyleSavvidou, G., Spyratou, E., Zachou, M.-E., & Efstathopoulos, E. P. (2025). Nanomedicine: Transforming the Management of Ocular Neuroinflammatory and Neurodegenerative Diseases. Journal of Nanotheranostics, 6(1), 6. https://doi.org/10.3390/jnt6010006








