Aptamers as Theranostics in Cardiovascular Diseases
Abstract
:1. Introduction
2. Cardiovascular Diseases
- Strokes and transient ischemic attacks occur when the blood supply to the brain is temporarily reduced (in the case of a stroke) or wholly cut off [8].
- Coronary heart disease is caused by an imbalance between the oxygen supply and demand in the cardiac muscle.
- Peripheral arterial disease is caused by plaque buildup or decreased blood flow in the peripheral arteries (limbs).
- Aortic disease occurs when the blood supply to the aorta is interrupted or significantly reduced, resulting in potential side effects like aneurysms or aortic dissection [9].
- A blood clot blocking one of the pulmonary arteries results in a pulmonary embolism, which prevents blood from reaching the lungs [10].
- Another condition brought on by blood clots is deep vein thrombosis, which typically develops in the deep veins of the legs and may cause life-threatening complications if it spreads to the lungs [11].
- Coronary artery disease is characterized by the restriction or obstruction of the coronary arteries, which deliver oxygen-rich blood to the heart muscle and frequently cause chest pain or heart attacks.
- High blood pressure, or hypertension, can strain and harm blood vessels all over the body, raising the risk of developing several cardiovascular diseases.
- Heart valve abnormalities that affect proper blood flow and may result in heart failure or other complications, which are known as valvular heart disease.
- Congenital heart defects are structural flaws that can interfere with normal blood flow and are present at birth [12].
3. Nanotheranostics and Their Role in CVDs
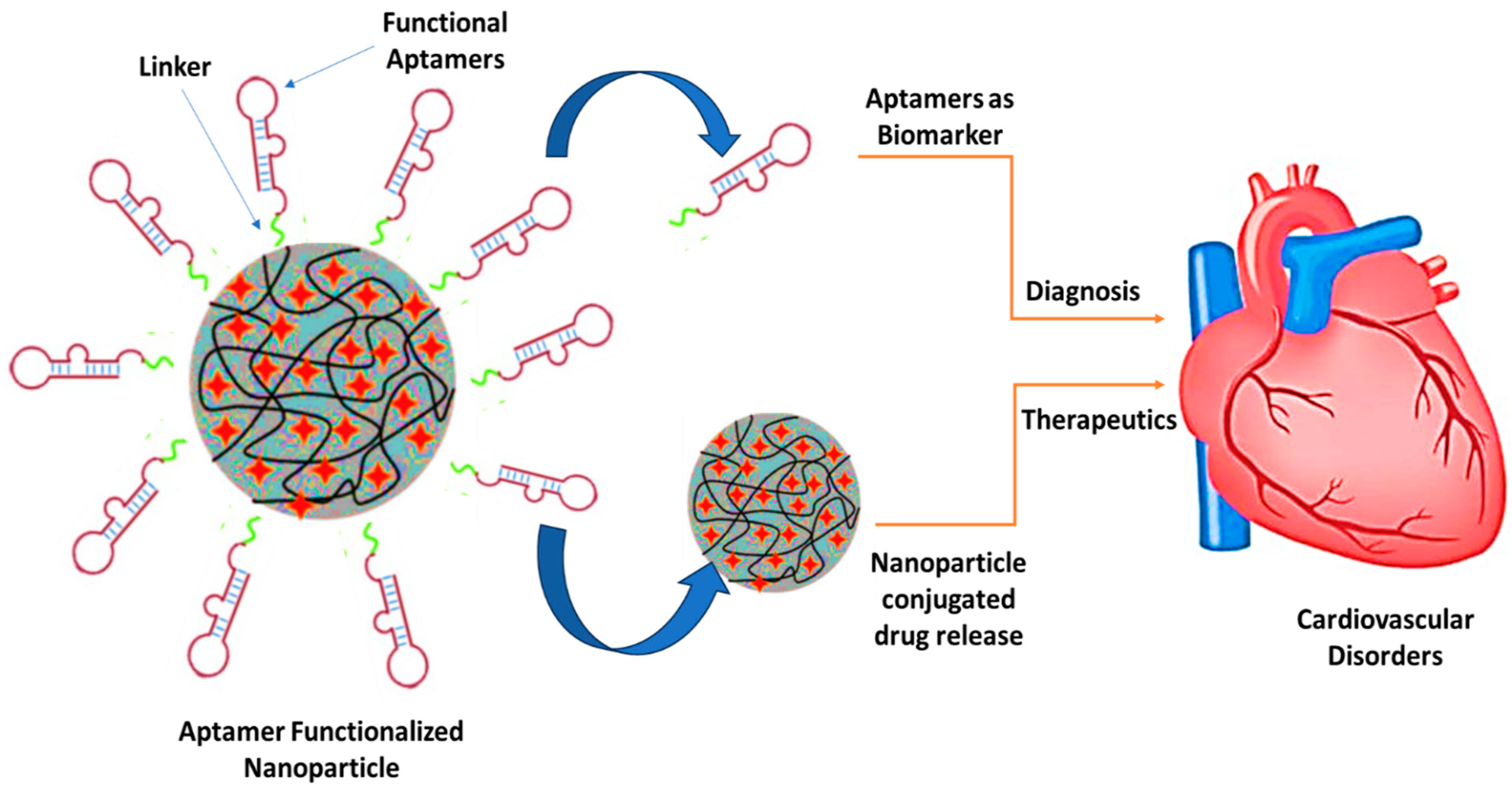
4. Aptamer Nanoconjugates for Theranostic Applications in Cardiovascular Diseases
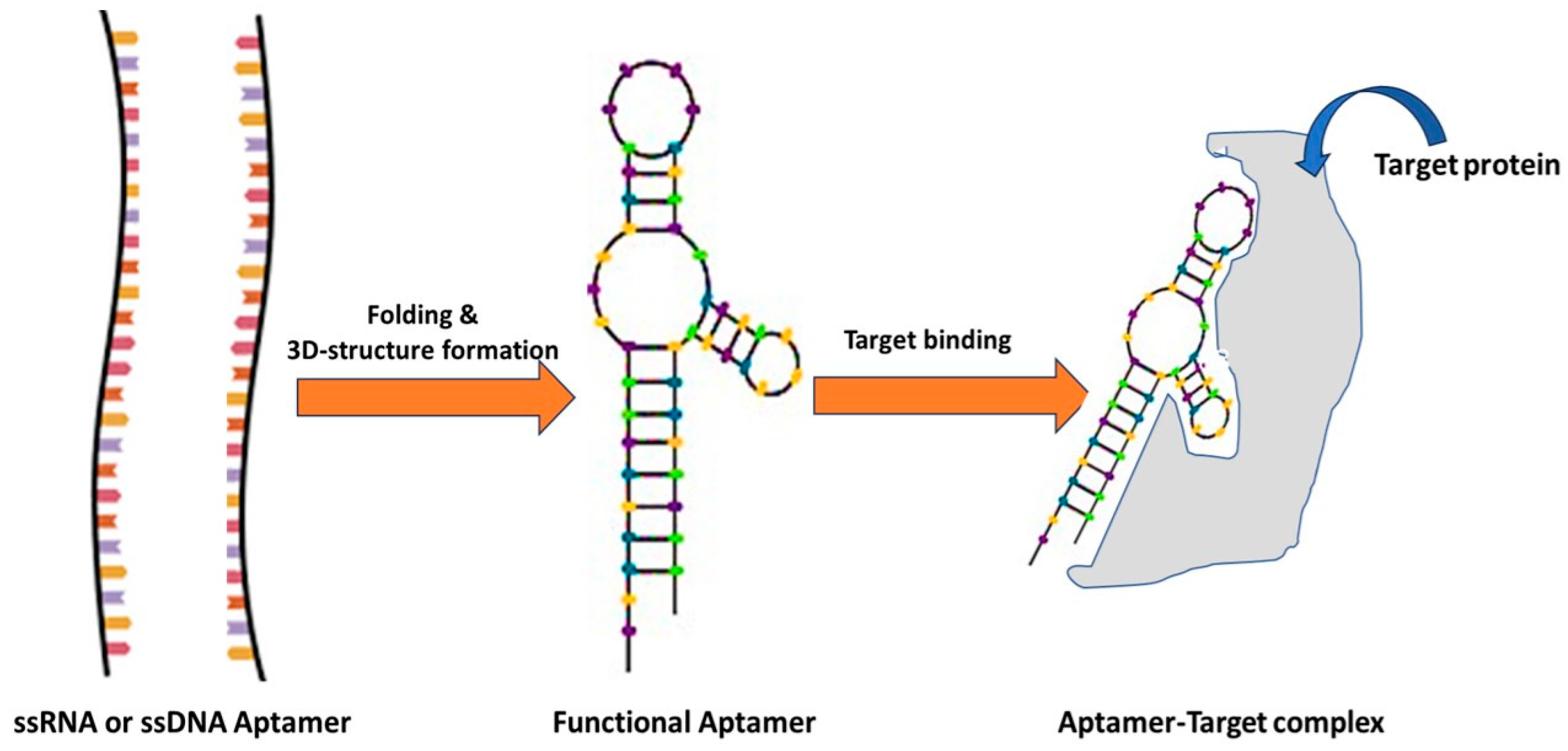
4.1. Aptamers
4.2. Aptamers for Diagnosis of Cardiovascular Diseases
4.2.1. Troponin I
4.2.2. Myoglobin
4.2.3. Creatine Kinase
4.2.4. Cardiac-Related Fatty Acid Membrane-Bound Protein
4.2.5. C-Reactive Protein
4.2.6. Other Diagnostic Aptasensors
4.3. Aptamers for the Treatment of Cardiovascular Diseases with Clinical Trial Pipelines
4.3.1. Aptamers Binding von Willebrand Factor (vWF)
4.3.2. Aptamers Binding Thrombin
4.3.3. Aptamers Binding to Factor IX
5. Challenges and Future Aspects of Aptamers as Theranosticsin CVDs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Li, G.; Yang, L.; Luo, R.; Guo, G. Development of Innovative Biomaterials and Devices for the Treatment of Cardiovascular Diseases. Adv. Mater. 2022, 34, 2201971. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Mishra, A.K.; Tirth, V.; Yerramsetty, S.V.; Murali, S.V.; Ahmad, S.U.; Mohanta, Y.K.; Attia, M.S.; Algahtani, A.; et al. Nanomaterials: A Promising Therapeutic Approach for Cardiovascular Diseases. J. Nanomater. 2022, 2022, 4155729. [Google Scholar] [CrossRef]
- Rhee, J.W.; Wu, J.C. Advances in nanotechnology for the management of coronary artery disease. Trends Cardiovasc. Med. 2013, 23, 39–45. [Google Scholar] [CrossRef]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Cherry, S.R.; Louie, A.Y.; Jacobs, R.E. The Integration of Positron Emission Tomography with Magnetic Resonance Imaging. Proc. IEEE 2008, 96, 416–438. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Hong, H.; Zhang, Y.; Cai, W.; Fang, D. Aptamers as Therapeutics in Cardiovascular Diseases. Curr. Med. Chem. 2011, 18, 4169–4174. [Google Scholar] [CrossRef]
- Nawaz, M.S.; Shoaib, B.; Ashraf, M.A. Intelligent Cardiovascular Disease Prediction Empowered with Gradient Descent Optimization. Heliyon 2021, 7, e06948. [Google Scholar] [CrossRef]
- Easton, J.D.; Saver, J.L.; Albers, G.W.; Alberts, M.J.; Chaturvedi, S.; Feldmann, E.; Hatsukami, T.S.; Higashida, R.T.; Johnston, S.C.; Kidwell, C.S.; et al. Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American heart association/American stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardio. Stroke 2009, 40, 2276–2293. [Google Scholar] [CrossRef]
- Choi, D.; Hwang, K.C.; Lee, K.Y.; Kim, Y.H. Ischemic heart diseases: Current treatments and future. J. Control Release 2009, 140, 194–202. [Google Scholar] [CrossRef]
- Amorim, B.J.; Rigolon, M.Y.; Ramos, C.D. Diagnosis of pulmonary embolism. Nucl. Cardiol. Basic. Adv. Concepts Clin. Pract. 2021, 168, 723–739. [Google Scholar]
- Esmon, C.T. Inflammation and thrombosis. J. Thromb. Haemost. 2003, 1, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Ghofrani, H.A.; Grünig, E.; Klose, H.; Olschewski, H.; Rosenkranz, S. Pulmonary hypertension. Dtsch. Arztebl. Int. 2017, 114, 73–84. [Google Scholar] [CrossRef]
- Choy, G.; Khalilzadeh, O.; Michalski, M.; Do, S.; Samir, A.E.; Pianykh, O.S.; Geis, J.R.; Pandharipande, P.V.; Brink, J.A.; Dreyer, K.J. Current Applications and Future Impact of Machine Learning in Radiology. Radiology 2018, 288, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, G.J.; Han, L.N.; Bai, Y.Y.; He, M.; Liu, H.B. Novel biomarkers for cardiovascular risk prediction. J. Geriatr. Cardiol. 2017, 14, 135–150. [Google Scholar]
- Counseller, Q.; Aboelkassem, Y. Recent technologies in cardiac imaging. Front. Med. Technol. 2023, 4, 984492. [Google Scholar] [CrossRef] [PubMed]
- Tobis, J.M.; Abudayyeh, I. New devices and technology in interventional cardiology. J. Cardiol. 2015, 65, 5–16. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, L.; Gao, F.; He, S.J.; Zhao, H.J.; Fang, Y.; Yang, J.M.; An, Y.; Ye, Z.W.; Dong, Z. Integration of Artificial Intelligence, Blockchain, and Wearable Technology for Chronic Disease Management: A New Paradigm in Smart Healthcare. Curr. Med. Sci. 2021, 41, 1123–1133. [Google Scholar] [CrossRef]
- Zaiou, M.; El Amri, H. Cardiovascular pharmacogenetics: A promise for genomically-guided therapy and personalized medicine. Clin. Genet. 2017, 91, 355–370. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jain, S.; Kumar, D.; Soni, S.L.; Sharma, M. A Review on Theranostics: An Approach to Targeted Diagnosis and Therapy. Asian J. Pharm. Res. Dev. 2019, 7, 63–69. [Google Scholar] [CrossRef]
- Mura, S.; Couvreur, P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416. [Google Scholar] [CrossRef]
- Hahn, M.A.; Singh, A.K.; Sharma, P.; Brown, S.C.; Moudgil, B.M. Nanoparticles as contrast agents for in-vivo bioimaging: CNanoparticles as contrast agents for in-vivo bioimaging: Current status and future perspectivesCardioprotective medications have disadvantages, such as poor bioavailability, nonspecific action, les. Anal. Bioanal. Chem. 2011, 399, 3–27. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Liu, Y.; Huang, H.; Roy, S.; Song, Z.; Guo, B. Recent advances in nanomedicines for imaging and therapy of myocardial ischemia-reperfusion injury. J. Control Release 2023, 353, 563–590. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Okur, N.Ü.; Karantas, I.D.; Okur, M.E.; Gündoğdu, E.A. Current update on nanoplatforms as therapeutic and diagnostic tools: A review for the materials used as nanotheranostics and imaging modalities. Asian J. Pharm. Sci. 2021, 16, 24–46. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, R.; Tran, H.D.N.; Kurniawan, N.D.; Moonshi, S.S.; Whittaker, A.K.; Ta, H.T. Chitosan Nanococktails Containing Both Ceria and Superparamagnetic Iron Oxide Nanoparticles for Reactive Oxygen Species-Related Theranostics. ACS Appl. Nano Mater. 2021, 4, 3604–3618. [Google Scholar] [CrossRef]
- Ma, B.; Xu, H.; Wang, Y.; Yang, L.; Zhuang, W.; Li, G.; Wang, Y. Biomimetic-Coated Nanoplatform with Lipid-Specific Imaging and ROS Responsiveness for Atherosclerosis-Targeted Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 35410–35421. [Google Scholar] [CrossRef]
- Kang, C.; Gwon, S.; Song, C.; Kang, P.M.; Park, S.C.; Jeon, J.; Hwang, D.W.; Lee, D. Fibrin-Targeted and H2O2-Responsive Nanoparticles as a Theranostics for Thrombosed Vessels. ACS Nano 2017, 11, 6194–6203. [Google Scholar] [CrossRef]
- Lu, K.Y.; Lin, P.Y.; Chuang, E.Y.; Shih, C.M.; Cheng, T.M.; Lin, T.Y.; Sung, H.W.; Mi, F.L. H2O2-Depleting and O2-Generating Selenium Nanoparticles for Fluorescence Imaging and Photodynamic Treatment of Proinflammatory-Activated Macrophages. ACS Appl. Mater. Interfaces 2017, 9, 5158–5172. [Google Scholar] [CrossRef]
- Somasuntharam, I.; Yehl, K.; Carroll, S.L.; Maxwell, J.T.; Martinez, M.D.; Che, P.L.; Brown, M.E.; Salaita, K.; Davis, M.E. Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials 2016, 83, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Oumzil, K.; Ramin, M.A.; Lorenzato, C.; Hémadou, A.; Laroche, J.; Jacobin-Valat, M.J.; Mornet, S.; Roy, C.-E.; Kauss, T.; Gaudin, K.; et al. Solid Lipid Nanoparticles for Image-Guided Therapy of Atherosclerosis. Bioconjug Chem. 2016, 27, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Cowan, D.B.; Yao, R.; Akurathi, V.; Snay, E.R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Ericsson, M.; Friehs, I.; Wu, Y.; Levitsky, S.; et al. Intracoronary delivery of mitochondria to the ischemic heart for cardioprotection. PLoS ONE 2016, 11, e0160889. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, A.; Jia, Y.; Yang, L.; Ning, Y.; Xu, L.; Zhong, Y.; Zhuang, Z.; Guan, J.; Zhang, X.; et al. PH-Responsive Multifunctional Theranostic Rapamycin-Loaded Nanoparticles for Imaging and Treatment of Acute Ischemic Stroke. ACS Appl. Mater. Interfaces 2021, 13, 56909–56922. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Chen, J.; Liu, T.; Gu, Z.; Zhang, J.; Gu, X.; Teng, G.; Yang, F.; Gu, N. Platelet bio-nanobubbles as microvascular recanalization nanoformulation for acute ischemic stroke lesion theranostics. Theranostics 2018, 8, 4870–4883. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Liu, R.; Qiao, C.; Lu, Z.; Shi, Y.; Fan, Z.; Zhang, Z.; Zhang, X. Dual-targeting theranostic system with mimicking apoptosis to promote myocardial infarction repair via modulation of macrophages. Theranostics 2017, 7, 4149. [Google Scholar] [CrossRef]
- Vazquez-Prada, K.X.; Moonshi, S.S.; Wu, Y.; Akther, F.; Tse, B.W.C.; Sokolowski, K.A.; Peter, K.; Wang, X.; Xu, G.; Ta, H.T. A Spiky Silver-Iron Oxide Nanoparticle for Highly Efficient Targeted Photothermal Therapy and Multimodal Imaging of Thrombosis. Small 2023, 19, 2205744. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Bao, Q.; Miao, Y.; Cheng, Q.; Chen, Y.; Yang, S.; Mao, C.; Yang, M. Peptide-Enabled Thrombus-Targeting Nanoparticles for Highly Effective Targeted CT Imaging and Eradication of Thrombi. Adv. Funct. Mater. 2023, 2303331, early view. [Google Scholar] [CrossRef]
- Jin, H. Perspectives of Aptamers for Medical Applications. In Aptamers for Medical Applications From Diagnosis to Therapeutics; Dong, Y., Ed.; Springer: Singapore, 2021; pp. 405–462. [Google Scholar]
- Aljohani, M.M.; Chinnappan, R.; Eissa, S.; Alsager, O.A.; Weber, K.; Cialla-May, D.; Popp, J.; Zourob, M. In Vitro Selection of Specific DNA Aptamers Against the Anti-Coagulant Dabigatran Etexilate. Sci. Rep. 2018, 8, 13290. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-Based Detection of Circulating Targets for Precision Medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Lo, Y.; Shiu, S.C.C.; Kinghorn, A.B.; Tanner, J.A. Aptamer-Enabled Nanomaterials for Therapeutics, Drug Targeting and Imaging. Cells 2022, 11, 159. [Google Scholar] [CrossRef]
- Hong, K.L.; Sooter, L.J. Single-Stranded DNA Aptamers against Pathogens and Toxins: Identification and Biosensing Applications. Biomed. Res. Int. 2015, 2015, 419318. [Google Scholar] [CrossRef]
- Wang, K.; Wang, M.; Ma, T.; Li, W.; Zhang, H. Review on the Selection of Aptamers and Application in Paper-Based Sensors. Biosensors 2023, 13, 39. [Google Scholar] [CrossRef]
- MacRitchie, N.; Di Francesco, V.; Ferreira, M.F.M.M.; Guzik, T.J.; Decuzzi, P.; Maffia, P. Nanoparticle theranostics in cardiovascular inflammation. Semin. Immunol. 2021, 56, 101536. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, J.; Wu, M.; Zhao, J.X. Aptamers: Active targeting ligands for cancer diagnosis and therapy. Theranostics 2015, 5, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Babuin, L.; Jaffe, A.S. Troponin: The biomarker of choice for the detection of cardiac injury. Can. Med. Assoc. J. 2005, 173, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Korff, S.; Katus, H.A.; Giannitsis, E. Differential diagnosis of elevated troponins. Heart 2006, 92, 987–993. [Google Scholar] [CrossRef]
- Teekakirikul, P.; Ho, C.Y.; Seidman, C.E. Chapter 47—Inherited Cardiomyopathies. In Korf BBTE and RP and P of MG; Rimoin, D., Pyeritz, R., Sixth, E., Eds.; Academic Press: Oxford, UK, 2013; pp. 1–38. [Google Scholar]
- Malik, P.; Gupta, R.; Malik, V.; Ameta, R.K. Emerging nanomaterials for improved biosensing. Meas. Sensors 2021, 16, 100050. [Google Scholar] [CrossRef]
- Nooranian, S.; Mohammadinejad, A.; Mohajeri, T.; Aleyaghoob, G.; Kazemi Oskuee, R. Biosensors based on aptamer-conjugated gold nanoparticles: A review. Biotechnol. Appl. Biochem. 2022, 69, 1517–1534. [Google Scholar] [CrossRef]
- Jo, H.; Her, J.; Lee, H.; Shim, Y.B.; Ban, C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017, 165, 442–448. [Google Scholar] [CrossRef]
- Schlapak, R.; Danzberger, J.; Armitage, D.; Morgan, D.; Ebner, A.; Hinterdorfer, P.; Pollheimer, P.; Gruber, H.J.; Schäffler, F.; Howorka, S. Nanoscale DNA tetrahedra improve biomolecular recognition on patterned surfaces. Small 2012, 8, 89–97. [Google Scholar] [CrossRef]
- Sun, D.; Luo, Z.; Lu, J.; Zhang, S.; Che, T.; Chen, Z.; Zhang, L. Electrochemical dual-aptamer-based biosensor for nonenzymatic detection of cardiac troponin I by nanohybrid electrocatalysts labeling combined with DNA nanotetrahedron structure. Biosens. Bioelectron. 2019, 134, 49–56. [Google Scholar] [CrossRef]
- Villalonga, A.; Estabiel, I.; Pérez-Calabuig, A.M.; Mayol, B.; Parrado, C.; Villalonga, R. Amperometric aptasensor with sandwich-type architecture for troponin I based on carboxyethylsilanetriol-modified graphene oxide coated electrodes. Biosens. Bioelectron. 2021, 183, 113203. [Google Scholar] [CrossRef]
- Yan, P.; Shu, S.; Zou, L.; Liu, Y.; Li, J.; Wei, F. Density functional theory study of active sites on nitrogen-doped graphene for oxygen reduction reaction. R. Soc. Open Sci. 2021, 8, 210272. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Khajehsharifi, H.; Hashemnia, S.; Solati, Z.; Azimpanah, R.; Shahrokhian, S. Evaluation of molecular imprinted polymerized methylene blue/aptamer as a novel hybrid receptor for Cardiac Troponin I (cTnI) detection at glassy carbon electrodes modified with new biosynthesized ZnONPs. Sens. Actuators B Chem. 2020, 320, 128316. [Google Scholar] [CrossRef]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in acute myocardial infarction: Current perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H. Traditional and novel diagnostic biomarkers for acute myocardial infarction. Egypt. J. Intern. Med. 2022, 34, 87. [Google Scholar] [CrossRef]
- Rabai, S.; Teniou, A.; Catanante, G.; Benounis, M.; Marty, J.L.; Rhouati, A. Fabrication of AuNPs/MWCNTS/chitosan nanocomposite for the electrochemical aptasensing of cadmium in water. Sensors 2022, 22, 105. [Google Scholar] [CrossRef]
- Gomes, E.S.; Leite, F.R.F.; Ferraz, B.R.L.; Mourão, H.A.J.L.; Malagutti, A.R. Voltammetric sensor based on cobalt-poly(methionine)-modified glassy carbon electrode for determination of estriol hormone in pharmaceuticals and urine. J. Pharm. Anal. 2019, 9, 347–357. [Google Scholar] [CrossRef]
- Bagheri, H.; Talemi, R.P.; Afkhami, A. Gold nanoparticles deposited on fluorine-doped tin oxide surface as an effective platform for fabricating a highly sensitive and specific digoxin aptasensor. RSC Adv. 2015, 5, 58491–58498. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, J.; Jang, J. Label-Free, Highly Sensitive Electrochemical Aptasensors Using Polymer-Modified Reduced Graphene Oxide for Cardiac Biomarker Detection. ACS Omega 2020, 5, 3924–3931. [Google Scholar] [CrossRef]
- Radi, A.E.; Abd-Ellatief, M.R. Electrochemical aptasensors: Current status and future perspectives. Diagnostics 2021, 11, 104. [Google Scholar] [CrossRef]
- Lou, X.; Oh, S.S.; Zhang, Y.; Xiao, Y.; Soh, H.T.; Qian, J. Generation of Highly Specific Aptamers via Micromagnetic Selection. Anal. Chem. 2011, 83, 1866. [Google Scholar]
- Wang, Q.; Liu, F.; Yang, X.; Wang, K.; Wang, H.; Deng, X. Sensitive point-of-care monitoring of cardiac biomarker myoglobin using aptamer and ubiquitous personal glucose meter. Biosens. Bioelectron. 2015, 64, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, T.T.; Huang, Z.F.; Hu, S.W.; Zhao, W.; Xu, J.J.; Chen, H.-Y. An exploration of nucleic acid liquid biopsy using a glucose meter. Chem. Sci. 2018, 9, 3517–3522. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Yasuhara, T.; Gomi, K. Creatine kinase and its isozymes. Rinsho Byori. 2001, 185, 1–8. [Google Scholar]
- Zhang, J.; Lv, X.; Feng, W.; Li, X.; Li, K.; Deng, Y. Aptamer-based fluorometric lateral flow assay for creatine kinase MB. Microchim. Acta 2018, 185, 364. [Google Scholar] [CrossRef]
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-type fatty acid-binding protein (H-FABP) and its role as a biomarker in heart failure: What do we know so far? J. Clin. Med. 2020, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Sanders, L.; Lawler, O.; Riley, T.; Maki, K. Hyperlipidemia. In Caballero BBTE of HN; Fourth, E., Ed.; Academic Press: Oxford, UK, 2023; pp. 361–379. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Taghdisi, S.M.; Yazdian-Robati, R.; Mansouri, A.; Abnous, K.; Ahmad Mohajeri, S. Selection of DNA aptamers for tramadol through the systematic evolution of ligands by exponential enrichment method for fabrication of a sensitive fluorescent aptasensor based on graphene oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 259, 119840. [Google Scholar] [CrossRef]
- Grabowska, I.; Sharma, N.; Vasilescu, A.; Iancu, M.; Badea, G.; Boukherroub, R.; Ogale, S.; Szunerits, S. Electrochemical Aptamer-Based Biosensors for the Detection of Cardiac Biomarkers. ACS Omega 2018, 3, 12010–12018. [Google Scholar] [CrossRef]
- Omage, J.I.; Easterday, E.; Rumph, J.T.; Brula, I.; Hill, B.; Kristensen, J.; Ha, D.T.; Galindo, C.L.; Danquah, M.K.; Sims, N.; et al. Cancer Diagnostics and Early Detection Using Electrochemical Aptasensors. Micromachines 2022, 13, 522. [Google Scholar] [CrossRef]
- Shanmugam, N.R.; Muthukumar, S.; Tanak, A.S.; Prasad, S. Multiplexed electrochemical detection of three cardiac biomarkers cTnI, cTnT and BNP using nanostructured ZnO-sensing platform. Future Cardiol. 2018, 14, 131–141. [Google Scholar] [CrossRef]
- Qiao, X.; Li, K.; Xu, J.; Cheng, N.; Sheng, Q.; Cao, W.; Yue, T.; Zheng, J. Novel electrochemical sensing platform for ultrasensitive detection of cardiac troponin I based on aptamer-MoS2 nanoconjugates. Biosens. Bioelectron. 2018, 113, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Dorraj, G.S.; Rassaee, M.J.; Latifi, A.M.; Pishgoo, B.; Tavallaei, M. Selection of DNA aptamers against Human Cardiac Troponin I for colorimetric sensor based dot blot application. J. Biotechnol. 2015, 208, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Krasitskaya, V.V.; Goncharova, N.S.; Biriukov, V.V.; Bashmakova, E.E.; Kabilov, M.R.; Baykov, I.K.; Sokolov, A.E.; Frank, L.A. The Ca2+-Regulated Photoprotein Obelin as a Tool for SELEX Monitoring and DNA Aptamer Affinity Evaluation. Photochem. Photobiol. 2020, 96, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Torrini, F.; Palladino, P.; Brittoli, A.; Baldoneschi, V.; Minunni, M.; Scarano, S. Characterization of troponin T binding aptamers for an innovative enzyme-linked oligonucleotide assay (ELONA). Anal. Bioanal. Chem. 2019, 411, 7709–7716. [Google Scholar] [CrossRef]
- Ara, M.N.; Hyodo, M.; Ohga, N.; Hida, K.; Harashima, H. Development of a novel DNA aptamer ligand targeting to primary cultured tumor endothelial cells by a cell-based SELEX method. PLoS ONE 2012, 7, e50174. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Xing, Y.; Yang, X.; Wang, K.; Jiang, R.; Wang, P.; Zhao, Q. Screening of DNA aptamers against myoglobin using a positive and negative selection units integrated microfluidic chip and its biosensing application. Anal. Chem. 2014, 86, 6572–6579. [Google Scholar] [CrossRef]
- Sharma, A.; Jang, J. Flexible electrical aptasensor using dielectrophoretic assembly of graphene oxide and its subsequent reduction for cardiac biomarker detection. Sci Rep. 2019, 9, 5970. [Google Scholar] [CrossRef]
- Lai, X.H.; Liang, R.L.; Liu, T.C.; Dong, Z.N.; Wu, Y.S.; Li, L.H. A fluorescence immunochromatographic assay using europium (III) chelate microparticles for rapid, quantitative and sensitive detection of creatine kinase MB. J. Fluoresc. 2016, 26, 987–996. [Google Scholar] [CrossRef]
- Kakoti, A.; Goswami, P. Multifaceted analyses of the interactions between human heart type fatty acid binding protein and its specific aptamers. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 3289–3299. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Wang, K.; Wang, Q.; Wang, P.; Lin, M.; Chen, N.; Tan, Y. DNA aptamer-based surface plasmon resonance sensing of human C-reactive protein. RSC Adv. 2014, 4, 30934–30937. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, R.; Wang, Q.; Huang, J.; Yang, X.; Wang, K.; Li, W.; Chen, N.; Li, Q. Detection of C-reactive protein using nanoparticle-enhanced surface plasmon resonance using an aptamer-antibody sandwich assay. Chem. Commun. 2016, 52, 3568–3571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Chen, Y.; Xue, F.; Teng, J.; Cao, J.; Lu, C.; Chen, W. Magnetic microparticle-based SELEX process for the identification of highly specific aptamers of heart marker--brain natriuretic peptide. Microchim. Acta 2015, 182, 331–339. [Google Scholar] [CrossRef]
- Huang, R.H.; Fremont, D.H.; Diener, J.L.; Schaub, R.G.; Sadler, J.E. A Structural Explanation for the Antithrombotic Activity of ARC1172, a DNA Aptamer that Binds von Willebrand Factor Domain A1. Structure 2009, 17, 1476–1484. [Google Scholar] [CrossRef]
- Liu, M.; Zaman, K.; Fortenberry, Y.M. Overview of the therapeutic potential of aptamers targeting coagulation factors. Int. J. Mol. Sci. 2021, 22, 3897. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, K.; Zhao, M.; Yang, X.; Chen, M.; Lan, X. Development of aptamer oligonucleotides as anticoagulants and antithrombotics for cardiovascular diseases: Current status. Thromb. Res. 2014, 134, 769–773. [Google Scholar] [CrossRef]
- Mittal, R.; Jhaveri, V.M.; McMurry, H.S.; Kay, S.I.S.; Sutherland, K.J.; Nicole, L.; Mittal, J.; Jayant, R.D. Recent treatment modalities for cardiovascular diseases with a focus on stem cells, aptamers, exosomes and nanomedicine. Artif. CellsNanomed. Biotechnol. 2018, 46, 831–840. [Google Scholar] [CrossRef]
- Diener, J.L.; Daniel Lagassé, H.A.; Duerschmied, D.; Merhi, Y.; Tanguay, J.F.; Hutabarat, R.; Gilbert, J.; Wagner, D.D.; Schaub, R. Inhibition of von Willebrand factor-mediated platelet activation and thrombosis by the anti-von Willebrand factor A1-domain aptamer ARC1779. J. Thromb. Haemost. 2009, 7, 1155–1162. [Google Scholar]
- Sakai, K.; Someya, T.; Harada, K.; Yagi, H.; Matsui, T.; Matsumoto, M. Novel aptamer to Von Willebrand factor A1 domain (TAGX-0004) shows total inhibition of thrombus formation superior to ARC1779 and comparable to caplacizumab. Haematologica 2020, 105, 2631–2638. [Google Scholar] [CrossRef]
- Siller-Matula, J.M.; Merhi, Y.; Tanguay, J.F.; Duerschmied, D.; Wagner, D.D.; McGinness, K.E.; Pendergrast, P.S.; Chung, J.-K.; Tian, X.; Schaub, R.G.; et al. ARC15105 is a potent antagonist of von Willebrand factor mediated platelet activation and adhesion. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 902–909. [Google Scholar] [CrossRef]
- Zhu, S.; Gilbert, J.C.; Liang, Z.; Kang, D.; Li, M.; Tarantino, P.M.; Jilma, B. Potent and rapid reversal of the von Willebrand factor inhibitor aptamer BT200. J. Thromb. Haemost. 2020, 18, 1695–1704. [Google Scholar] [CrossRef]
- Kovacevic, K.D.; Greisenegger, S.; Langer, A.; Gelbenegger, G.; Buchtele, N.; Pabinger, I.; Petroczi, K.; Zhu, S.; Gilbert, J.C.; Jilma, B. The aptamer BT200 blocks von Willebrand factor and platelet function in blood of stroke patients. Sci. Rep. 2021, 11, 3092. [Google Scholar] [CrossRef]
- Nimjee, S.M.; Dornbos, D.; Pitoc, G.A.; Wheeler, D.G.; Layzer, J.M.; Venetos, N.; Huttinger, A.; Talentino, S.E.; Musgrave, N.J.; Moody, H.; et al. Preclinical Development of a vWF Aptamer to Limit Thrombosis and Engender Arterial Recanalization of Occluded Vessels. Mol. Ther. 2019, 27, 1228–1241. [Google Scholar] [CrossRef] [PubMed]
- Eikelboom, J.W.; Hirsh, J.; Spencer, F.A.; Baglin, T.P.; Weitz, J.I. Antiplatelet drugs—Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012, 141, e89S–e119S. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ma, Y.; Xie, Y.; Pu, J. Aptamer-based applications for cardiovascular disease. Front. Bioeng. Biotechnol. 2022, 10, 1002285. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Rossi, J.; Biology, C.; Rd, E.D. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 8, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Sattari, R.; Palizban, A.; Khanahmad, H. Single-Strand DNA-Like Oligonucleotide Aptamer Against Proprotein Convertase Subtilisin/Kexin 9 Using CE-SELEX: PCSK9 Targeting Selection. Cardiovasc. Drugs Ther. 2020, 34, 475–485. [Google Scholar] [CrossRef]
- Yu, H.; Frederiksen, J.; Sullenger, B.A. Applications and future of aptamers that achieve rapid-onset anticoagulation. RNA 2023, 29, 455–462. [Google Scholar] [CrossRef]
- Ni, X.; Castanares, M.; Mukherjee, A.; Lupold, S.E. Nucleic Acid Aptamers: Clinical Applications and Promising New Horizons. Curr. Med. Chem. 2011, 18, 4206–4214. [Google Scholar] [CrossRef]
- Riccardi, C.; Meyer, A.; Vasseur, J.J.; Cavasso, D.; Krauss, I.R.; Paduano, L.; Morvan, F.; Montesarchio, D. Design, synthesis and characterization of cyclic nu172 analogues: A biophysical and biological insight. Int. J. Mol. Sci. 2020, 21, 3860. [Google Scholar] [CrossRef]
- Pasternak, A.; Hernandez, F.J.; Rasmussen, L.M.; Vester, B.; Wengel, J. Improved thrombin binding aptamer by incorporation of a single unlocked nucleic acid monomer. Nucleic Acids Res. 2011, 39, 1155–1164. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian. J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Rossi, J.J. RNA-based therapeutics: Current progress and future prospects. Chem. Biol. 2012, 19, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.N.; Olthoff, J.T.; Matthews, A.J.; Houston, D.W. Use of fully modified 2′-O-methyl antisense oligos for loss-of-function studies in vertebrate embryos. Genesis 2012, 49, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Hahn, U.; Rentmeister, A. Cell-specific aptamers as emerging therapeutics. J. Nucleic Acids 2011, 2011, 904750. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Zhang, X.; Giangrande, P.H.; McNamara, J.O.; Nimjee, S.M.; Sarraf-Yazdi, S.; Sullenger, B.A.; Clary, B.M. Targeted inhibition of αvβ3 integrin with an RNA aptamer impairs endothelial cell growth and survival. Biochem. Biophys. Res. Commun. 2005, 338, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Kara, N.; Ayoub, N.; Ilgu, H.; Fotiadis, D.; Ilgu, M. Aptamers Targeting Membrane Proteins for Sensor and Diagnostic Applications. Molecules 2023, 28, 3728. [Google Scholar] [CrossRef]
- Vater, A.; Klussmann, S. Turning mirror-image oligonucleotides into drugs: The evolution of Spiegelmer® therapeutics. Drug Discov. Today 2015, 20, 147–155. [Google Scholar] [CrossRef]
- Torrado, J.; Buckley, L.; Durán, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzmán, L.A. Restenosis, Stent Thrombosis, and Bleeding Complications: Navigating Between Scylla and Charybdis. J. Am. Coll. Cardiol. 2018, 71, 1676–1695. [Google Scholar] [CrossRef]
- Fathi-Karkan, S.; Mirinejad, S.; Ulucan-Karnak, F.; Mukhtar, M.; Ghahramani Almanghadim, H.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Biomedical applications of aptamer-modified chitosan nanomaterials: An updated review. Int. J. Biol. Macromol. 2023, 238, 124103. [Google Scholar] [CrossRef]
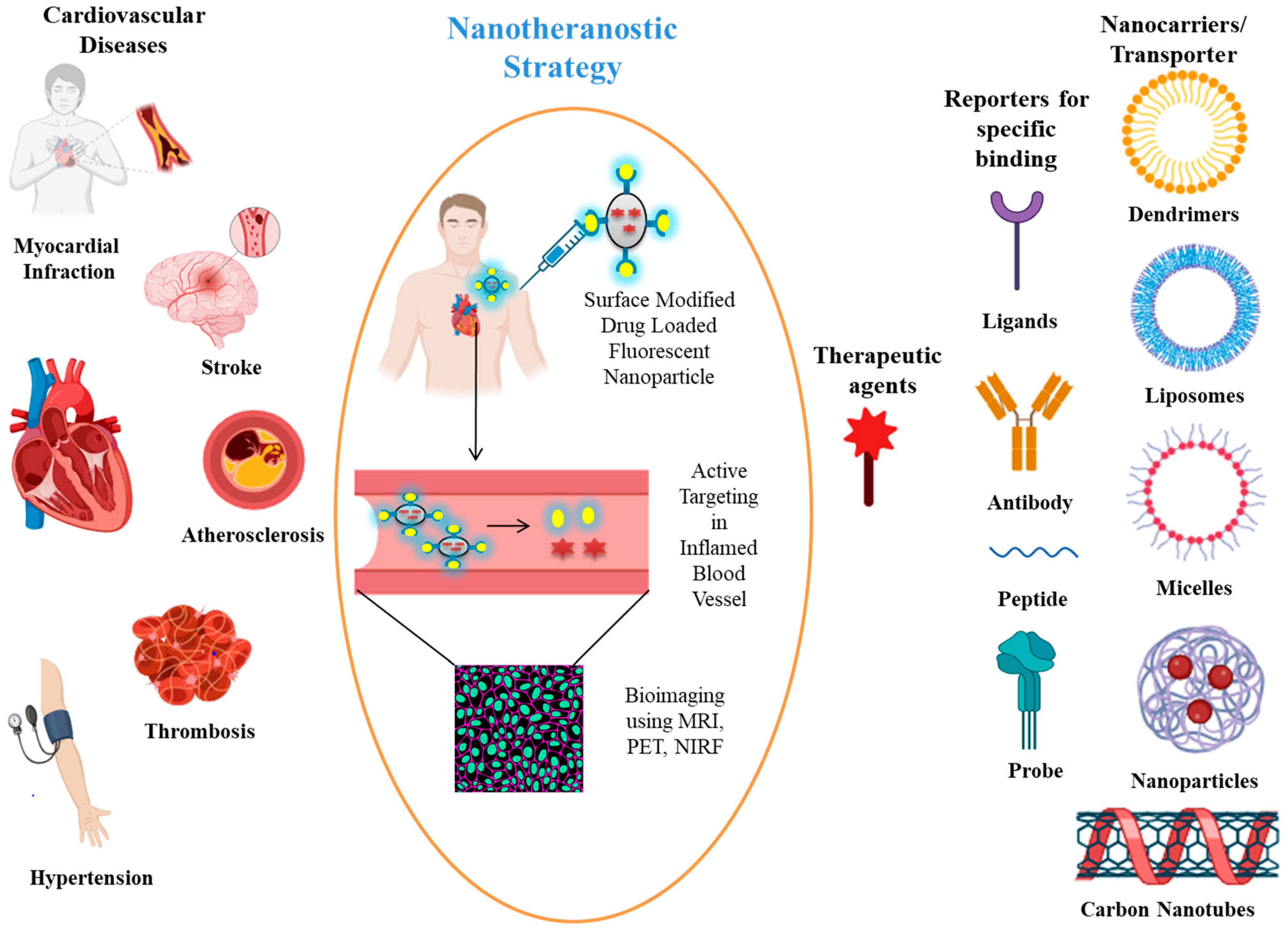
| Carrier | Bioimaging Mode | Therapeutic Agent | Target | Disease | References |
|---|---|---|---|---|---|
| Mesoporous silica-coated conversion nanoparticles | NIR fluorescence | Chlorin e6 (Photosensitiser) | THP-1 Macrophage apoptosis | Atherosclerosis | [29] |
| Nanoparticles | NIR fluorescence | Cathepsin-B activated (L-SR15) | Cathepsin-B plaque destabilizing by eliminating macrophage | Atheroma | [27] |
| Single-walled carbon nanotubes | NIR fluorescence | Carbon nanotube | Macrophage-rich atherosclerotic lesions | Atherosclerosis | [28] |
| Gold nanorods | Micro-computed tomography (CT) imaging | Gold nanorods | Inflammatory macrophage | Atherosclerosis | [30] |
| Magnetic nanostructure | MRI | High-density lipoprotein | Cholesterol-rich macrophage | Atherosclerotic vascular lesions | [31] |
| Solid lipid NPs | MRI | α-tocopherol or prostacyclin PGI2 | Platelet activation and aggregation | Atherosclerosis | [32] |
| Iron NPs | Micro-CT, PET, MRI | Mitochondria loaded with 18F-R6G | Mitochondria | Ischemic heart disease | [32] |
| Nanoparticles | MRI, NIRF | Rapamycin | Ischemic Lesions | Acute ischemic stroke | [33] |
| BiomemticNanobubbles | NIRF | Platelets | Platelet aggreagation | Acute ischemic stroke | [34] |
| Magnetic iron oxide nanocubes | MRI | Poly(lactide)-polycarboxybetaine coated iron oxide nanocubes | Phosphatidylserine receptor | Myocardial infarction | [34] |
| Silver oxide NPs | MRI, NIRF | Silver oxide NPs | Activated platelets | Thrombosis | [34] |
| GK coated macroporus silica-Bi core shell NPs | CT | Urokinase | Enriched thrombus | Thrombotic disease | [35] |
| Biomarker | Aptamer | Disease Diagnosis | Binding Affinity(KD) | Method for Affinity | Reference |
|---|---|---|---|---|---|
| cTnT | TnIApt23 | Acute coronary syndrome (ACS), AMI, acute chest pain | 2.7 nM | Fluorescence | [75] |
| TnIApt19 | 6.3 nM | Fluorescence | [75] | ||
| TnIApt18 | 9 nM | Fluorescence | [75] | ||
| TnIApt11 | 10.25 nM | Fluorescence | [75] | ||
| Troponin | TnApt.1 | Acute myocardial infarction | 61.51 nM | Bioluminescence | [76] |
| TnApt.2 | 42.01 nM | Bioluminescence | [76] | ||
| TnApt.4 | 167.1 nM | Bioluminescence | [76] | ||
| TnApt.5 | 255.7 nM | Bioluminescence | [76] | ||
| TnApt.10 | 121.4 nM | Bioluminescence | [76] | ||
| TnApt.12 | 24.16 nM | Bioluminescence | [76] | ||
| TnApt.14 | 79.04 nM | Bioluminescence | [76] | ||
| TnAp2t1 | 39.06 nM | Bioluminescence | [76] | ||
| TnAp2t2 | 24.93 nM | Bioluminescence | [76] | ||
| TnAp2t3 | 80.6 nM | Bioluminescence | [76] | ||
| cTnI | Apt.1 | Acute coronary syndromes, venous thrombosis | 122 nM | SPR | [77] |
| Apt.2 | 190 nM | SPR | [77] | ||
| AraHH001 | 43 nM | Flowcytometry | [78] | ||
| Myoglobin | Myo40-7-27 | Acute myocardial infarction | 4.93 nM | SPR | [79] |
| Myo40-7-69 | 6.38 nM | SPR | [79] | ||
| Myo40-7-34 | 5.58 nM | SPR | [79] | ||
| Mb 089 | 65 pM | SPR | [80] | ||
| Creatine Kinase | C.Apt.21 | Chronic heart failure, hypertension | 255.7 nM | Bioluminescence | [81] |
| C.Apt.30 | 121.4 nM | Bioluminescence | [81] | ||
| HFABP | N13 | Heart failure, myocardial infarction | 24.16 nM | Bioluminescence | [82] |
| N53 | 79.04 nM | Bioluminescence | [82] | ||
| C-Reactive Protein | CRP-80-17 | Atherothrombotic, cardiac death, ischemic stroke, myocardial infarction | 3.9 nM | Bioluminescence | [83] |
| CRP-40-17 | 16.2 nM | Bioluminescence | [83] | ||
| CRP-62-40 | 16.2 nM | Bioluminescence | [84] | ||
| BNP | A10 | von Willebrand disease, atherosclerosis, stroke, arterial thrombosis | 12 nM | Fluorescence | [85] |
| A8 | 139.4 nM | Fluorescence | [85] | ||
| A11 | 28 nM | Fluorescence | [85] | ||
| A14-1 | 22.4 nM | Fluorescence | [85] | ||
| A14-5 | 104.6 nM | Fluorescence | [85] |
| Drug | Composition | Target | Condition | Current Phase | Reference |
|---|---|---|---|---|---|
| ARC1779 | DNA and 2′-O-methyl, coupled to a 20 kDa PEG, 3′ inverted dT | Willebrand factor (vWF) | Carotid artery disease and thrombotic microangiopathies | Phase II | [91,92] |
| ARC1172 | Biotin-41-mer DNA aptamer complex | Willebrand factor (vWF) | Vascular injury | Phase I | [86] |
| ARC183 | 15-nucleotide single-stranded DNA aptamer without modifications | Thrombin | Coronary artery disease and cardiac surgery | Phase I | [108] |
| REG1 | 2′-O-methyl antidote in addition to 2′-ribo purine/2′-fluoro pyrimidine (RB006)/40 kDa PEG | Coagulation factor IXa | Percutaneous coronary interventions | Phase I, IIa, and IIb | [105] |
| NU172 | Unmodified-DNA aptamer | Thrombin or coagulation factor II | Cardiopulmonary bypass | Phase II | [101,102] |
| Rondoraptivon pegol (BT 200) | PEGylated RNA aptamer | von Willebrand factor | Arterial thrombosis, atherosclerosis, stroke, hemophilia A, and von Willebrand disease | Phase II, Phase I, and Preclinical | [94,95] |
| Anivamersenpegnivacogin | RNA aptamer made of 31 nucleotides and PEGylated | Factor IXa | Vascular thrombosis and acute coronary syndromes | Phase I | [98] |
| Egaptivon pegol | RNA aptamer linked with polyethylene glycol | von Willebrand factor | Thrombosis, hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and von Willebrand disease | Phase I | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramchandani, M.; Kumari, P.; Goyal, A.K. Aptamers as Theranostics in Cardiovascular Diseases. J. Nanotheranostics 2023, 4, 408-428. https://doi.org/10.3390/jnt4030018
Ramchandani M, Kumari P, Goyal AK. Aptamers as Theranostics in Cardiovascular Diseases. Journal of Nanotheranostics. 2023; 4(3):408-428. https://doi.org/10.3390/jnt4030018
Chicago/Turabian StyleRamchandani, Manish, Priyanka Kumari, and Amit K. Goyal. 2023. "Aptamers as Theranostics in Cardiovascular Diseases" Journal of Nanotheranostics 4, no. 3: 408-428. https://doi.org/10.3390/jnt4030018
APA StyleRamchandani, M., Kumari, P., & Goyal, A. K. (2023). Aptamers as Theranostics in Cardiovascular Diseases. Journal of Nanotheranostics, 4(3), 408-428. https://doi.org/10.3390/jnt4030018





