Consolidation of Gold and Gadolinium Nanoparticles: An Extra Step towards Improving Cancer Imaging and Therapy
Abstract
1. Introduction
2. Gold Nanoparticles
2.1. Gold Nanoparticles in Medical Imaging
2.2. Gold Nanoparticles in Therapy
3. Gadolinium Nanoparticles
3.1. Gadolinium Nanoparticles in Medical Imaging
3.2. Gadolinium Nanoparticles in Therapy
Gadolinium Nanoparticles in Imaging-Guided Neutron Capture Therapy
4. Consolidation of Gold and Gadolinium Nanoparticles
4.1. Applications in MRI
4.2. Applications in Multimodal Imaging
4.3. Theranostic Agents
4.4. Applications in Cancer Treatment
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer invasion and metastasis: Molecular and cellular perspective. In Madame Curie Bioscience Database; Landes Bioscience: Austin, TX, USA, 2013. [Google Scholar]
- Siddique, S.; Chow, J.C.L. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Ojha, A.; Jaiswal, S.; Bharti, P.; Mishra, S.K. Nanoparticles and Nanomaterials-Based Recent Approaches in Upgraded Targeting and Management of Cancer: A Review. Cancers 2023, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.; Moreau, M.; Yasmin-Karim, S.; Protti, A.; Tillement, O.; Berbeco, R.; Hesser, J.; Ngwa, W. Imaging and Characterization of Sustained Gadolinium Nanoparticle Release from Next Generation Radiotherapy Biomaterial. Nanomaterials 2020, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Salehiabar, M.; Charmi, J.; Yaray, K.; Ghaffarlou, M.; Balcioglu, E.; Ertas, Y.N. Enhanced In Vivo Radiotherapy of Breast Cancer Using Gadolinium Oxide and Gold Hybrid Nanoparticles. ACS Appl. Bio Mater. 2023, 6, 784–792. [Google Scholar] [CrossRef]
- Wu, C.; Cai, R.; Zhao, T.; Wu, L.; Zhang, L.; Jin, J.; Xu, L.; Li, P.; Li, T.; Zhang, M.; et al. Hyaluronic Acid-Functionalized Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging-Guided Radiotherapy of Tumors. Nanoscale Res. Lett. 2020, 15, 94. [Google Scholar] [CrossRef]
- Ertas, Y.N.; Jarenwattananon, N.N.; Bouchard, L.-S. Oxide-Free Gadolinium Nanocrystals with Large Magnetic Moments. Chem. Mater. 2015, 27, 5371–5376. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Kudo, S.; Nagasaki, Y. Gd@C82 metallofullerenes for neutron capture therapy—Fullerene solubilization by poly (ethylene glycol)-block-poly (2-(N, N-diethylamino) ethyl methacrylate) and resultant efficacy in vitro. Sci. Technol. Adv. Mater. 2011, 12, 44607. [Google Scholar] [CrossRef]
- You, Q.; Sun, Q.; Yu, M.; Wang, J.; Wang, S.; Liu, L.; Cheng, Y.; Wang, Y.; Song, Y.; Tan, F.; et al. BSA–Bioinspired Gadolinium Hybrid-Functionalized Hollow Gold Nanoshells for NIRF/PA/CT/MR Quadmodal Diagnostic Imaging-Guided Photothermal/Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 40017–40030. [Google Scholar] [CrossRef]
- Li, D.; Wen, S.; Sun, W.; Zhang, J.; Jin, D.; Peng, C.; Shen, M.; Shi, X. One-Step Loading of Gold and Gd2O3 Nanoparticles within PEGylated Polyethylenimine for Dual Mode Computed Tomography/Magnetic Resonance Imaging of Tumors. ACS Appl. Bio Mater. 2018, 1, 221–225. [Google Scholar] [CrossRef]
- Roy, A.; Pandit, C.; Gacem, A.; Alqahtani, M.S.; Bilal, M.; Islam, S.; Hossain, J.; Jameel, M. Biologically Derived Gold Nanoparticles and Their Applications. Bioinorg. Chem. Appl. 2022, 2022, 8184217. [Google Scholar] [CrossRef]
- Gehan, H.; Fillaud, L.; Felidj, N.; Aubard, J.; Lang, P.; Chehimi, M.M.; Mangeney, C. A general approach combining diazonium salts and click chemistries for gold surface functionalization by nanoparticle assemblies. Langmuir 2010, 26, 3975–3980. [Google Scholar] [CrossRef]
- Ackerson, C.J.; Jadzinsky, P.D.; Jensen, G.J.; Kornberg, R.D. Rigid, Specific, and Discrete Gold Nanoparticle/Antibody Conjugates. J. Am. Chem. Soc. 2006, 128, 2635–2640. [Google Scholar] [CrossRef]
- Pinter, B.; Broeckaert, L.; Turek, J.; Růžička, A.; De Proft, F. Dimers of N-Heterocyclic Carbene Copper, Silver, and Gold Halides: Probing Metallophilic Interactions through Electron Density Based Concepts. Chem.—A Eur. J. 2014, 20, 734–744. [Google Scholar] [CrossRef]
- Faraday, M.X. The Bakerian Lecture.—Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar]
- Edwards, P.P.; Thomas, J.M. Gold in a Metallic Divided State—From Faraday to Present-Day Nanoscience. Angew. Chem. Int. Ed. 2007, 46, 5480–5486. [Google Scholar] [CrossRef]
- Cheheltani, R.; Ezzibdeh, R.M.; Chhour, P.; Pulaparthi, K.; Kim, J.; Jurcova, M.; Hsu, J.C.; Blundell, C.; Litt, H.I.; Ferrari, V.A.; et al. Tunable, biodegradable gold nanoparticles as contrast agents for computed tomography and photoacoustic imaging. Biomaterials 2016, 102, 87–97. [Google Scholar] [CrossRef]
- Yu, S.-B.; Watson, A.D. Metal-Based X-ray Contrast Media. Chem. Rev. 1999, 99, 2353–2378. [Google Scholar] [CrossRef]
- Bernstein, A.L.; Dhanantwari, A.; Jurcova, M.; Cheheltani, R.; Naha, P.C.; Ivanc, T.; Shefer, E.; Cormode, D.P. Improved sensitivity of computed tomography towards iodine and gold nanoparticle contrast agents via iterative reconstruction methods. Sci. Rep. 2016, 6, 26177. [Google Scholar] [CrossRef]
- Nadolski, G.J.; Stavropoulos, S.W. Contrast alternatives for iodinated contrast allergy and renal dysfunction: Options and limitations. J. Vasc. Surg. 2013, 57, 593–598. [Google Scholar] [CrossRef]
- Shilo, M.; Reuveni, T.; Motiei, M.; Popovtzer, R. Nanoparticles as computed tomography contrast agents: Current status and future perspectives. Nanomedicine 2012, 7, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, B.; Li, J.; Rajh, T.; Chaudhary, A.; Chmura, S.J.; Pelizzari, C.; Wietholt, C.; Kurtoglu, M.; Redmond, P. AuNP-DG: Deoxyglucose-labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol. Imaging Biol. 2010, 12, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tung, G.A.; Sun, S. Size and Concentration Effect of Gold Nanoparticles on X-ray Attenuation As Measured on Computed Tomography. Chem. Mater. 2008, 20, 4167–4169. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Hajfathalian, M.; Maidment, P.S.N.; Hsu, J.C.; Naha, P.C.; Si-Mohamed, S.; Breuilly, M.; Kim, J.; Chhour, P.; Douek, P.; et al. Effect of Gold Nanoparticle Size on Their Properties as Contrast Agents for Computed Tomography. Sci. Rep. 2019, 9, 14912. [Google Scholar] [CrossRef]
- Popovtzer, R.; Agrawal, A.; Kotov, N.; Popovtzer, A.; Balter, J.; Carey, T.; Kopelman, R. Targeted Gold Nanoparticles Enable Molecular CT Imaging of Cancer. Nano Lett. 2008, 8, 4593–4596. [Google Scholar] [CrossRef]
- Sun, I.-C.; Eun, D.-K.; Na, J.H.; Lee, S.; Kim, I.-J.; Youn, I.-C.; Ko, C.-Y.; Kim, H.-S.; Lim, D.; Choi, K.; et al. Heparin-Coated Gold Nanoparticles for Liver-Specific CT Imaging. Chem.—A Eur. J. 2009, 15, 13341–13347. [Google Scholar] [CrossRef]
- Sun, I.-C.; Na, J.H.; Jeong, S.Y.; Kim, D.-E.; Kwon, I.C.; Choi, K.; Ahn, C.-H.; Kim, K. Biocompatible Glycol Chitosan-Coated Gold Nanoparticles for Tumor-Targeting CT Imaging. Pharm. Res. 2014, 31, 1418–1425. [Google Scholar] [CrossRef]
- Ashton, J.R.; Gottlin, E.B.; Patz, E.F., Jr.; West, J.L.; Badea, C.T. A comparative analysis of EGFR-targeting antibodies for gold nanoparticle CT imaging of lung cancer. PLoS ONE 2018, 13, e0206950. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Jing, L.; Liang, X.; Deng, Z.; Feng, S.; Li, X.; Huang, M.; Li, C.; Dai, Z. Prussian blue coated gold nanoparticles for simultaneous photoacoustic/CT bimodal imaging and photothermal ablation of cancer. Biomaterials 2014, 35, 5814–5821. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Her, S.; Jaffray, D.A.; Allen, C. Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Adv. Drug Deliv. Rev. 2017, 109, 84–101. [Google Scholar] [CrossRef]
- Arnold, H. Basic Clinical Radiobiology, 4th ed.; CRC Press: London, UK, 2009. [Google Scholar]
- Ahn, G.-O.; Brown, J.M. Matrix Metalloproteinase-9 Is Required for Tumor Vasculogenesis but Not for Angiogenesis: Role of Bone Marrow-Derived Myelomonocytic Cells. Cancer Cell 2008, 13, 193–205. [Google Scholar] [CrossRef]
- Garcia-Barros, M.; Paris, F.; Cordon-Cardo, C.; Lyden, D.; Rafii, S.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Tumor Response to Radiotherapy Regulated by Endothelial Cell Apoptosis. Science 2003, 300, 1155–1159. [Google Scholar] [CrossRef]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with Anti–CTLA-4 antibodyfractionated radiation synergizes with immunotherapy. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. On the Primary Water Radicals’ Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study. Nanomaterials 2020, 10, 2478. [Google Scholar] [CrossRef]
- Howard, D.; Sebastian, S.; Le, Q.V.-C.; Thierry, B.; Kempson, I. Chemical Mechanisms of Nanoparticle Radiosensitization and Radioprotection: A Review of Structure-Function Relationships Influencing Reactive Oxygen Species. Int. J. Mol. Sci. 2020, 21, 579. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Yelin, D. High levels of reactive oxygen species in gold nanoparticle-targeted cancer cells following femtosecond pulse irradiation. Sci. Rep. 2013, 3, srep02146. [Google Scholar] [CrossRef]
- Jawaid, P.; Rehman, M.U.; Zhao, Q.-L.; Misawa, M.; Ishikawa, K.; Hori, M.; Shimizu, T.; Saitoh, J.-I.; Noguchi, K.; Kondo, T. Small size gold nanoparticles enhance apoptosis-induced by cold atmospheric plasma via depletion of intracellular GSH and modification of oxidative stress. Cell Death Discov. 2020, 6, 83. [Google Scholar] [CrossRef]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. Selective Oxidation of Transient Organic Radicals in the Presence of Gold Nanoparticles. Nanomaterials 2021, 11, 727. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Zeng, S.; Ramamurthy, G.; Burda, C.; Basilion, J.P. Prostate-specific membrane antigen targeted gold nanoparticles for prostate cancer radiotherapy: Does size matter for targeted particles? Chem. Sci. 2019, 10, 8119–8128. [Google Scholar] [CrossRef] [PubMed]
- Farahani, S.; Alam, N.R.; Haghgoo, S.; Khoobi, M.; Geraily, G.H.; Gorji, E. Dosimetry and radioenhancement comparison of gold nanoparticles in kilovoltage and megavoltage radiotherapy using MAGAT polymer gel dosimeter. J. Biomed. Phys. Eng. 2019, 9, 199. [Google Scholar] [CrossRef]
- Konefał, A.; Lniak, W.; Rostocka, J.; Orlef, A.; Sokół, M.; Kasperczyk, J.; Jarząbek, P.; Wrońska, A.; Rusiecka, K. Influence of a shape of gold nanoparticles on the dose enhancement in the wide range of gold mass concentration for high-energy X-ray beams from a medical linac. Rep. Pract. Oncol. Radiother. 2020, 25, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Tudda, A.; Donzelli, E.; Nicolini, G.; Semperboni, S.; Bossi, M.; Cavaletti, G.; Castriconi, R.; Mangili, P.; del Vecchio, A.; Sarno, A.; et al. Breast radiotherapy with kilovoltage photons and gold nanoparticles as radiosensitizer: An in vitro study. Med. Phys. 2022, 49, 568–578. [Google Scholar] [CrossRef]
- Yogo, K.; Misawa, M.; Shimizu, H.; Kitagawa, T.; Hirayama, R.; Ishiyama, H.; Yasuda, H.; Kametaka, S.; Takami, S. Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays. Nanomaterials 2022, 12, 771. [Google Scholar] [CrossRef]
- Abdollahi, B.B.; Malekzadeh, R.; Azar, F.P.; Salehnia, F.; Naseri, A.R.; Ghorbani, M.; Hamishehkar, H.; Farajollahi, A.R. Main Approaches to Enhance Radiosensitization in Cancer Cells by Nanoparticles: A Systematic Review. Adv. Pharm. Bull. 2020, 11, 212–223. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Rahman, W.N.; Bishara, N.; Ackerly, T.; He, C.F.; Jackson, P.; Wong, C.; Davidson, R.; Geso, M. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 136–142. [Google Scholar] [CrossRef]
- Guerra, D.B.; Oliveira, E.M.N.; Sonntag, A.R.; Sbaraine, P.; Fay, A.P.; Morrone, F.B.; Papaléo, R.M. Intercomparison of radiosensitization induced by gold and iron oxide nanoparticles in human glioblastoma cells irradiated by 6 MV photons. Sci. Rep. 2022, 12, 9602. [Google Scholar] [CrossRef]
- Gregersen, E. Gadolinium chemical element. In Britannica; The Editors of Encyclopaedia, Ed.; Encyclopedia Britannica: Chicago, IL, USA, 2022. [Google Scholar]
- American Elements. Gadolinium Nanoparticles. Available online: https://www.americanelements.com/gadolinium-nanoparticles-7440-54-2 (accessed on 15 April 2023).
- Cao, M.; Wang, P.; Kou, Y.; Wang, J.; Liu, J.; Li, Y.-H.; Li, J.; Wang, L.; Chen, C. Gadolinium(III)-Chelated Silica Nanospheres Integrating Chemotherapy and Photothermal Therapy for Cancer Treatment and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2015, 7, 25014–25023. [Google Scholar] [CrossRef]
- Ahmad, M.Y.; Ahmad, W.; Yue, H.; Ho, S.L.; Park, J.A.; Jung, K.-H.; Cha, H.; Marasini, S.; Ghazanfari, A.; Liu, S.; et al. In Vivo Positive Magnetic Resonance Imaging Applications of Poly(methyl vinyl ether-alt-maleic acid)-coated Ultra-small Paramagnetic Gadolinium Oxide Nanoparticles. Molecules 2020, 25, 1159. [Google Scholar] [CrossRef]
- Mortezazadeh, T.; Gholibegloo, E.; Alam, N.R.; Dehghani, S.; Haghgoo, S.; Ghanaati, H.; Khoobi, M. Gadolinium (III) oxide nanoparticles coated with folic acid-functionalized poly(β-cyclodextrin-co-pentetic acid) as a biocompatible targeted nano-contrast agent for cancer diagnostic: In vitro and in vivo studies. Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 487–500. [Google Scholar] [CrossRef]
- Toth, E.; Helm, L.; Merbach, A. Relaxivity of gadolinium (III) complexes: Theory and mechanism. In The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging; Wiley: Hoboken, NJ, USA, 2013; pp. 25–81. [Google Scholar]
- Yue, H.; Park, J.Y.; Chang, Y.; Lee, G.H. Ultrasmall Europium, Gadolinium, and Dysprosium Oxide Nanoparticles: Polyol Synthesis, Properties, and Biomedical Imaging Applications. Mini-Reviews Med. Chem. 2020, 20, 1767–1780. [Google Scholar] [CrossRef]
- Kochebina, O.; Halty, A.; Taleb, J.; Kryza, D.; Janier, M.; Sadr, A.B.; Baudier, T.; Rit, S.; Sarrut, D. In vivo gadolinium nanoparticle quantification with SPECT/CT. EJNMMI Phys. 2019, 6, 9. [Google Scholar] [CrossRef]
- Cho, M.; Sethi, R.; Narayanan, J.S.A.; Lee, S.S.; Benoit, D.N.; Taheri, N.; Decuzzi, P.; Colvin, V.L. Gadolinium oxide nanoplates with high longitudinal relaxivity for magnetic resonance imaging. Nanoscale 2014, 6, 13637–13645. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, C.; Wang, S.; Li, Q.; Zhang, M.; Li, J.; Xu, K. Comparative study on in vivo behavior of PEGylated gadolinium oxide nanoparticles and Magnevist as MRI contrast agent. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 547–555. [Google Scholar] [CrossRef]
- Catanzaro, V.; Digilio, G.; Capuana, F.; Padovan, S.; Cutrin, J.C.; Carniato, F.; Porta, S.; Grange, C.; Filipović, N.; Stevanović, M. Gadolinium-Labelled Cell Scaffolds to Follow-up Cell Transplantation by Magnetic Resonance Imaging. J. Funct. Biomater. 2019, 10, 28. [Google Scholar] [CrossRef]
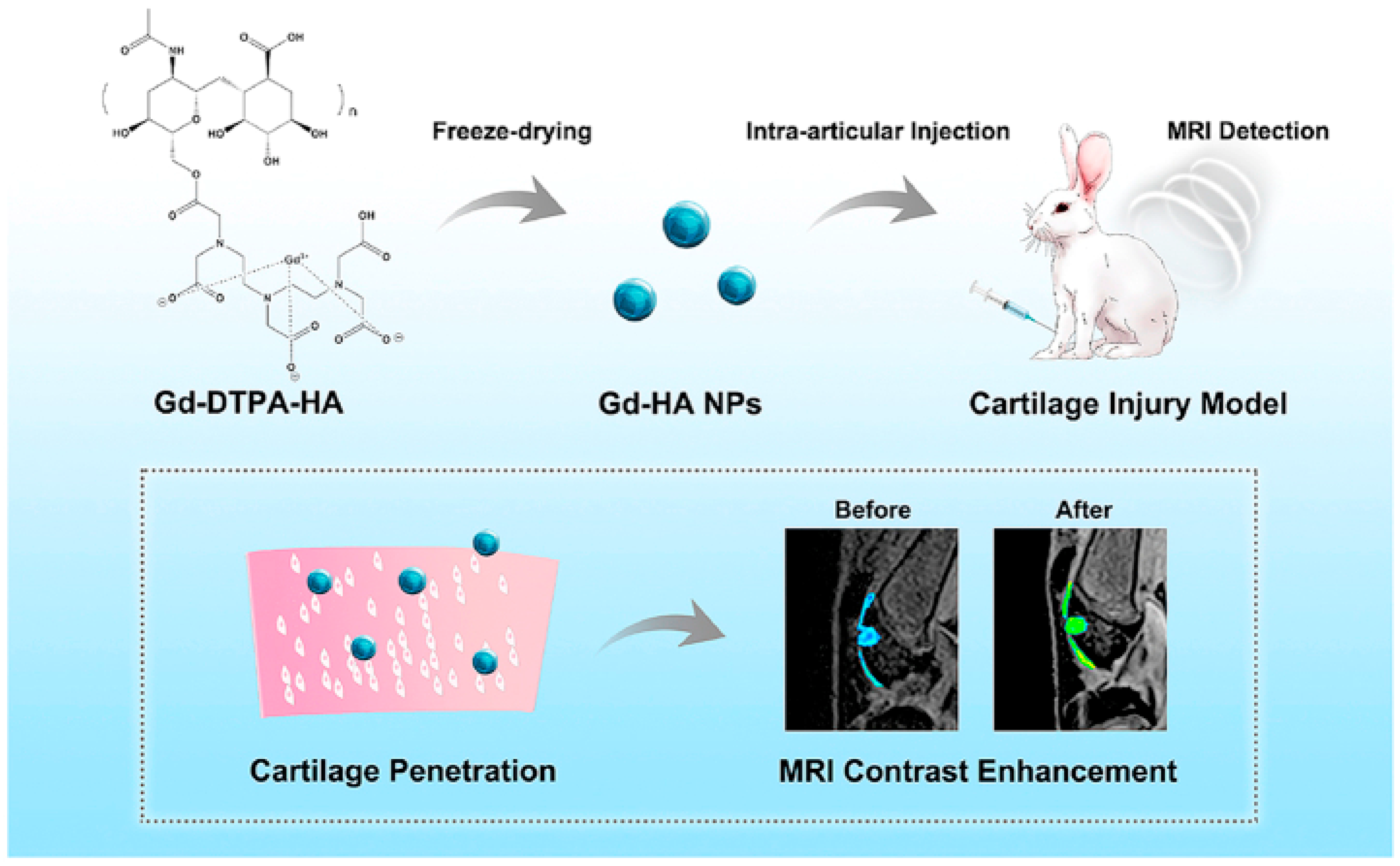
- Lu, R.; Zhang, Y.; Tao, H.; Zhou, L.; Li, H.; Chen, T.; Zhang, P.; Lu, Y.; Chen, S. Gadolinium-hyaluronic acid nanoparticles as an efficient and safe magnetic resonance imaging contrast agent for articular cartilage injury detection. Bioact. Mater. 2020, 5, 758–767. [Google Scholar] [CrossRef]
- Schulte-Altedorneburg, G.; Gebhard, M.; Wohlgemuth, W.; Fischer, W.; Zentner, J.; Wegener, R.; Balzer, T.; Bohndorf, K. MR arthrography: Pharmacology, efficacy and safety in clinical trials. Skelet. Radiol. 2003, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Lu, S.-T.; Yu, H.; Liao, R.-F.; Li, H.; Lucie Zafitatsimo, B.V.; Li, Y.-S.; Zhang, Y.; Zhu, X.-L.; Liu, H.-G.; et al. Gadolinium-chelate functionalized bismuth nanotheranostic agent for in vivo MRI/CT/PAI imaging-guided photothermal cancer therapy. Biomaterials 2018, 159, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Aime, S.; Caravan, P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2009, 30, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Li, X.-Q.; Huang, T.; Lu, S.-T.; Wan, B.; Liao, R.-F.; Li, Y.-S.; Baidya, A.; Long, Q.-Y.; Xu, H.-B. MRI-guided tumor chemo-photodynamic therapy with Gd/Pt bifunctionalized porphyrin. Biomater. Sci. 2017, 5, 1746–1750. [Google Scholar] [CrossRef] [PubMed]
- Jahanbin, T.; Sauriat-Dorizon, H.; Spearman, P.; Benderbous, S.; Korri-Youssoufi, H. Development of Gd(III) porphyrin-conjugated chitosan nanoparticles as contrast agents for magnetic resonance imaging. Mater. Sci. Eng. C 2015, 52, 325–332. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef]
- Tamiaki, H.; Unno, S.; Takeuchi, E.; Tameshige, N.; Shinoda, S.; Tsukube, H. Induced circular dichroism by complexation of gadolinium(III) porphyrinates with chiral amino acids and dipeptides: Effects of axial β-diketonate ligands on chirality sensing and recognition. Tetrahedron 2003, 59, 10477–10483. [Google Scholar] [CrossRef]
- Heitmann, G.; Schütt, C.; Gröbner, J.; Huber, L.; Herges, R. Azoimidazole functionalized Ni-porphyrins for molecular spin switching and light responsive MRI contrast agents. Dalton Trans. 2016, 45, 11407–11412. [Google Scholar] [CrossRef]
- Winter, M.B.; Klemm, P.J.; Phillips-Piro, C.M.; Raymond, K.N.; Marletta, M.A. Porphyrin-Substituted H-NOX Proteins as High-Relaxivity MRI Contrast Agents. Inorg. Chem. 2013, 52, 2277–2279. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, X.; Dai, Z. Porphyrin-loaded nanoparticles for cancer theranostics. Nanoscale 2016, 8, 12394–12405. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Y.; Xu, L.; Liu, L.; Zhang, W. Redox-responsive supramolecular amphiphiles constructed via host–guest interactions for photodynamic therapy. Biomater. Sci. 2015, 3, 1218–1227. [Google Scholar] [CrossRef]
- Malina, L.; Tomankova, K.B.; Malohlava, J.; Jiravova, J.; Manisova, B.; Zapletalova, J.; Kolarova, H. The in vitro cytotoxicity of metal-complexes of porphyrin sensitizer intended for photodynamic therapy. Toxicol. In Vitro 2016, 34, 246–256. [Google Scholar] [CrossRef]
- Li, B.; Sun, L.; Li, T.; Zhang, Y.; Niu, X.; Xie, M.; You, Z. Ultra-small gold nanoparticles self-assembled by gadolinium ions for enhanced photothermal/photodynamic liver cancer therapy. J. Mater. Chem. B 2021, 9, 1138–1150. [Google Scholar] [CrossRef]
- Coughlin, A.J.; Ananta, J.S.; Deng, N.; Larina, I.V.; Decuzzi, P.; West, J.L. Gadolinium-Conjugated Gold Nanoshells for Multimodal Diagnostic Imaging and Photothermal Cancer Therapy. Small 2014, 10, 556–565. [Google Scholar] [CrossRef]
- Verry, C.; Sancey, L.; Dufort, S.; Le Duc, G.; Mendoza, C.; Lux, F.; Grand, S.; Arnaud, J.; Quesada, J.L.; Villa, J.; et al. Treatment of multiple brain metastases using gadolinium nanoparticles and radiotherapy: NANO-RAD, a phase I study protocol. BMJ Open 2019, 9, e023591. [Google Scholar] [CrossRef]
- Verry, C.; Dufort, S.; Villa, J.; Gavard, M.; Iriart, C.; Grand, S.; Charles, J.; Chovelon, B.; Cracowski, J.-L.; Quesada, J.-L.; et al. Theranostic AGuIX nanoparticles as radiosensitizer: A phase I, dose-escalation study in patients with multiple brain metastases (NANO-RAD trial). Radiother. Oncol. 2021, 160, 159–165. [Google Scholar] [CrossRef]
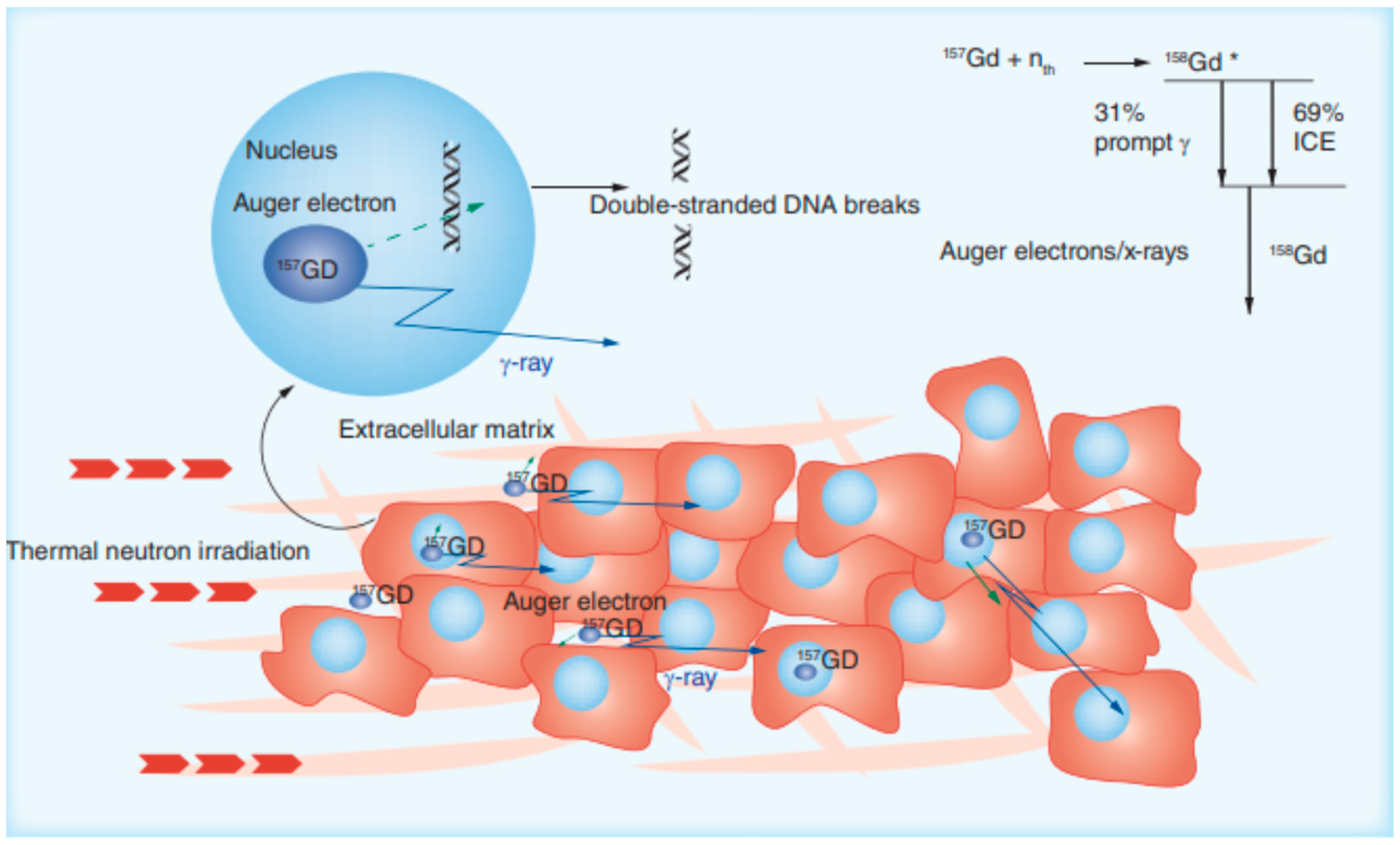
- Salt, C.; Lennox, A.J.; Takagaki, M.; Maguire, J.A.; Hosmane, N.S. Boron and gadolinium neutron capture therapy. Russ. Chem. Bull. 2004, 53, 1871–1888. [Google Scholar] [CrossRef]
- Brugger, R.M.; Shih, J.A. Evaluation of gadolinium-157 as a neutron capture therapy agent. Strahlenther. Onkol. 1989, 165, 153–156. [Google Scholar]
- Akine, Y.; Tokita, N.; Tokuuye, K.; Satoh, M.; Churei, H.; Le Pechoux, C.; Kobayashi, T.; Kanda, K. Suppression of Rabbit VX-2 Subcutaneous Tumor Growth by Gadolinium Neutron Capture Therapy. Jpn. J. Cancer Res. 1993, 84, 841–843. [Google Scholar] [CrossRef]
- Deagostino, A.; Protti, N.; Alberti, D.; Boggio, P.; Bortolussi, S.; Altieri, S.; Crich, S.G. Insights into the use of gadolinium and gadolinium/boron-based agents in imaging-guided neutron capture therapy applications. Futur. Med. Chem. 2016, 8, 899–917. [Google Scholar] [CrossRef]
- Perry, H.L.; Botnar, R.M.; Wilton-Ely, J.D.E.T. Gold nanomaterials functionalised with gadolinium chelates and their application in multimodal imaging and therapy. Chem. Commun. 2020, 56, 4037–4046. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, E.; Desché, P. Gadolinium retention as a safety signal: Experience of a manufacturer. Investig. Radiol. 2020, 55, 20. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A.; Ullah, H. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Sharma, J.; Chhabra, R.; Yan, H.; Liu, Y. A facile in situ generation of dithiocarbamate ligands for stable gold nanoparticle–oligonucleotide conjugates. Chem. Commun. 2008, 18, 2140–2142. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.L.; Yoon, I.-C.; Chabloz, N.G.; Molisso, S.; Stasiuk, G.J.; Botnar, R.; Wilton-Ely, J.D.E.T. Metallostar Assemblies Based on Dithiocarbamates for Use as MRI Contrast Agents. Inorg. Chem. 2020, 59, 10813–10823. [Google Scholar] [CrossRef]
- Lohrke, J.; Frenzel, T.; Endrikat, J.; Alves, F.C.; Grist, T.M.; Law, M.; Lee, J.M.; Leiner, T.; Li, K.-C.; Nikolaou, K.; et al. 25 Years of Contrast-Enhanced MRI: Developments, Current Challenges and Future Perspectives. Adv. Ther. 2016, 33, 1–28. [Google Scholar] [CrossRef]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2018, 119, 957–1057. [Google Scholar] [CrossRef]
- Holbrook, R.J.; Rammohan, N.; Rotz, M.W.; MacRenaris, K.W.; Preslar, A.T.; Meade, T.J. Gd(III)-Dithiolane Gold Nanoparticles for T1-Weighted Magnetic Resonance Imaging of the Pancreas. Nano Lett. 2016, 16, 3202–3209. [Google Scholar] [CrossRef]
- Fatehbasharzad, P.; Stefania, R.; Carrera, C.; Hawala, I.; Castelli, D.D.; Baroni, S.; Colombo, M.; Prosperi, D.; Aime, S. Relaxometric studies of gd-chelate conjugated on the surface of differently shaped gold nanoparticles. Nanomaterials 2020, 10, 1115. [Google Scholar] [CrossRef]
- Chabloz, N.G.; Wenzel, M.N.; Perry, H.L.; Yoon, I.; Molisso, S.; Stasiuk, G.J.; Elson, D.; Cass, A.E.G.; Wilton-Ely, J.D.E.T. Polyfunctionalised Nanoparticles Bearing Robust Gadolinium Surface Units for High Relaxivity Performance in MRI. Chem.—A Eur. J. 2019, 25, 10895–10906. [Google Scholar] [CrossRef]
- Aouidat, F.; Boumati, S.; Khan, M.; Tielens, F.; Doan, B.T.; Spadavecchia, J. Design and synthesis of gold-gadolinium-core-shell nanoparticles as contrast agent: A smart way to future nanomaterials for nanomedicine applications. Int. J. Nanomed. 2019, 14, 9309. [Google Scholar] [CrossRef]
- Samadian, H.; Hosseini-Nami, S.; Kamrava, S.K.; Ghaznavi, H.; Shakeri-Zadeh, A. Folate-conjugated gold nanoparticle as a new nanoplatform for targeted cancer therapy. J. Cancer Res. Clin. Oncol. 2016, 142, 2217–2229. [Google Scholar] [CrossRef]
- Lee, D.-E.; Koo, H.; Sun, I.-C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, T.; Yang, S.; Chen, Q.; Xing, D. Gadolinium(III)-gold nanorods for MRI and photoacoustic imaging dual-modality detection of macrophages in atherosclerotic inflammation. Nanomedicine 2013, 8, 1611–1624. [Google Scholar] [CrossRef]
- Cherukula, K.; Manickavasagam Lekshmi, K.; Uthaman, S.; Cho, K.; Cho, C.-S.; Park, I.-K. Multifunctional Inorganic Nanoparticles: Recent Progress in Thermal Therapy and Imaging. Nanomaterials 2016, 6, 76. [Google Scholar] [CrossRef]
- Degrauwe, N.; Hocquelet, A.; Digklia, A.; Schaefer, N.; Denys, A.; Duran, R. Theranostics in Interventional Oncology: Versatile Carriers for Diagnosis and Targeted Image-Guided Minimally Invasive Procedures. Front. Pharmacol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Hou, W.; Xia, F.; Alfranca, G.; Yan, H.; Zhi, X.; Liu, Y.; Peng, C.; Zhang, C.; de la Fuente, J.M.; Cui, D. Nanoparticles for multi-modality cancer diagnosis: Simple protocol for self-assembly of gold nanoclusters mediated by gadolinium ions. Biomaterials 2017, 120, 103–114. [Google Scholar] [CrossRef]
- Zhou, B.; Xiong, Z.; Wang, P.; Peng, C.; Shen, M.; Mignani, S.; Majoral, J.-P.; Shi, X. Targeted tumor dual mode CT/MR imaging using multifunctional polyethylenimine-entrapped gold nanoparticles loaded with gadolinium. Drug Deliv. 2018, 25, 178–186. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Z.; Di, H.; Zeng, E.; Jiang, Y.; Liu, D. Gadolinium-doped Au@prussian blue nanoparticles as MR/SERS bimodal agents for dendritic cell activating and tracking. Theranostics 2020, 10, 6061–6071. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen TD, T.; Maurmann, L.; Key, J.; Bossmann, S.H.; Aryal, S. Gd3+ tethered gold nanorods for combined magnetic resonance imaging and photo-thermal therapy. J. Biomed. Nanotechnol. 2017, 13, 417–426. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Chen, J.; Zhu, S.; Liu, X.; Zhou, L.; Shi, P.; Niu, D.; Gu, J.; Shi, J. Multifunctional gold nanostar-based nanocomposite: Synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation. Biomaterials 2015, 60, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meyers, J.D.; Broome, A.-M.; Kenney, M.E.; Basilion, J.P.; Burda, C. Deep Penetration of a PDT Drug into Tumors by Noncovalent Drug-Gold Nanoparticle Conjugates. J. Am. Chem. Soc. 2011, 133, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, D.; Wang, G. Magnetic-gold theranostic nanoagent used for targeting quad modalities T 1 & T 2-MRI/CT/PA imaging and photothermal therapy of tumours. RSC Adv. 2021, 11, 18440–18447. [Google Scholar] [PubMed]
- Caravan, P.; Esteban-Gómez, D.; Rodríguez-Rodríguez, A.; Platas-Iglesias, C. Water exchange in lanthanide complexes for MRI applications. Lessons learned over the last 25 years. Dalton Trans. 2019, 48, 11161–11180. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Boumati, S.; Arib, C.; Diallo, A.T.; Djaker, N.; Doan, B.-T.; Spadavecchia, J. Doxorubicin (DOX) Gadolinium–Gold-Complex: A New Way to Tune Hybrid Nanorods as Theranostic Agent. Int. J. Nanomed. 2021, 16, 2219–2236. [Google Scholar] [CrossRef]
- Usman, M.S.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J.; Saad, F.F.A. Gadolinium-doped gallic acid-zinc/aluminium-layered double hydroxide/gold theranostic nanoparticles for a bimodal magnetic resonance imaging and drug delivery system. Nanomaterials 2017, 7, 244. [Google Scholar] [CrossRef]
- Usman, M.S.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J.; Saad, F.F.A. A bimodal theranostic nanodelivery system based on [graphene oxide-chlorogenic acid-gadolinium/gold] nanoparticles. PLoS ONE 2018, 13, e0200760. [Google Scholar] [CrossRef]
- Han, L.; Xia, J.-M.; Hai, X.; Shu, Y.; Chen, X.-W.; Wang, J.-H. Protein-Stabilized Gadolinium Oxide-Gold Nanoclusters Hybrid for Multimodal Imaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 6941–6949. [Google Scholar] [CrossRef]
- Miladi, I.; Alric, C.; Dufort, S.; Mowat, P.; Dutour, A.; Mandon, C.; Laurent, G.; Bräuer-Krisch, E.; Herath, N.; Coll, J.-L.; et al. The In Vivo Radiosensitizing Effect of Gold Nanoparticles Based MRI Contrast Agents. Small 2014, 10, 1116–1124. [Google Scholar] [CrossRef]
- Durand, M.; Lelievre, E.; Chateau, A.; Berquand, A.; Laurent, G.; Carl, P.; Roux, S.; Chazee, L.; Bazzi, R.; Eghiaian, F.; et al. The detrimental invasiveness of glioma cells controlled by gadolinium chelate-coated gold nanoparticles. Nanoscale 2021, 13, 9236–9251. [Google Scholar] [CrossRef]
- Debouttière, P.-J.; Roux, S.; Vocanson, F.; Billotey, C.; Beuf, O.; Favre-Reguillon, A.; Lin, Y.; Pellet-Rostaing, S.; Lamartine, R.; Perriat, P.; et al. Design of Gold Nanoparticles for Magnetic Resonance Imaging. Adv. Funct. Mater. 2006, 16, 2330–2339. [Google Scholar] [CrossRef]
- Tomić, S.; Đokić, J.; Vasilijić, S.; Ogrinc, N.; Rudolf, R.; Pelicon, P.; Vučević, D.; Milosavljević, P.; Janković, S.; Anžel, I.; et al. Size-Dependent Effects of Gold Nanoparticles Uptake on Maturation and Antitumor Functions of Human Dendritic Cells In Vitro. PLoS ONE 2014, 9, e96584. [Google Scholar] [CrossRef]
- Qiu, T.A.; Bozich, J.S.; Lohse, S.E.; Vartanian, A.M.; Jacob, L.M.; Meyer, B.M.; Gunsolus, I.L.; Niemuth, N.J.; Murphy, C.J.; Haynes, C.L.; et al. Gene expression as an indicator of the molecular response and toxicity in the bacterium Shewanella oneidensis and the water flea Daphnia magna exposed to functionalized gold nanoparticles. Environ. Sci. Nano 2015, 2, 615–629. [Google Scholar] [CrossRef]
- Hühn, D.; Kantner, K.; Geidel, C.; Brandholt, S.; De Cock, I.; Soenen, S.J.H.; Rivera_Gil, P.; Montenegro, J.-M.; Braeckmans, K.; Müllen, K.; et al. Polymer-Coated Nanoparticles Interacting with Proteins and Cells: Focusing on the Sign of the Net Charge. ACS Nano 2013, 7, 3253–3263. [Google Scholar] [CrossRef]
- Deng, J.; Yao, M.; Gao, C. Cytotoxicity of gold nanoparticles with different structures and surface-anchored chiral polymers. Acta Biomater. 2017, 53, 610–618. [Google Scholar] [CrossRef]
- Hou, H.; Chen, L.; He, H.; Chen, L.; Zhao, Z.; Jin, Y. Fine-tuning the LSPR response of gold nanorod–polyaniline core–shell nanoparticles with high photothermal efficiency for cancer cell ablation. J. Mater. Chem. B 2015, 3, 5189–5196. [Google Scholar] [CrossRef]
- Thierry, B.; Ng, J.; Krieg, T.; Griesser, H.J. A robust procedure for the functionalization of gold nanorods and noble metal nanoparticles. Chem. Commun. 2009, 13, 1724–1726. [Google Scholar] [CrossRef]
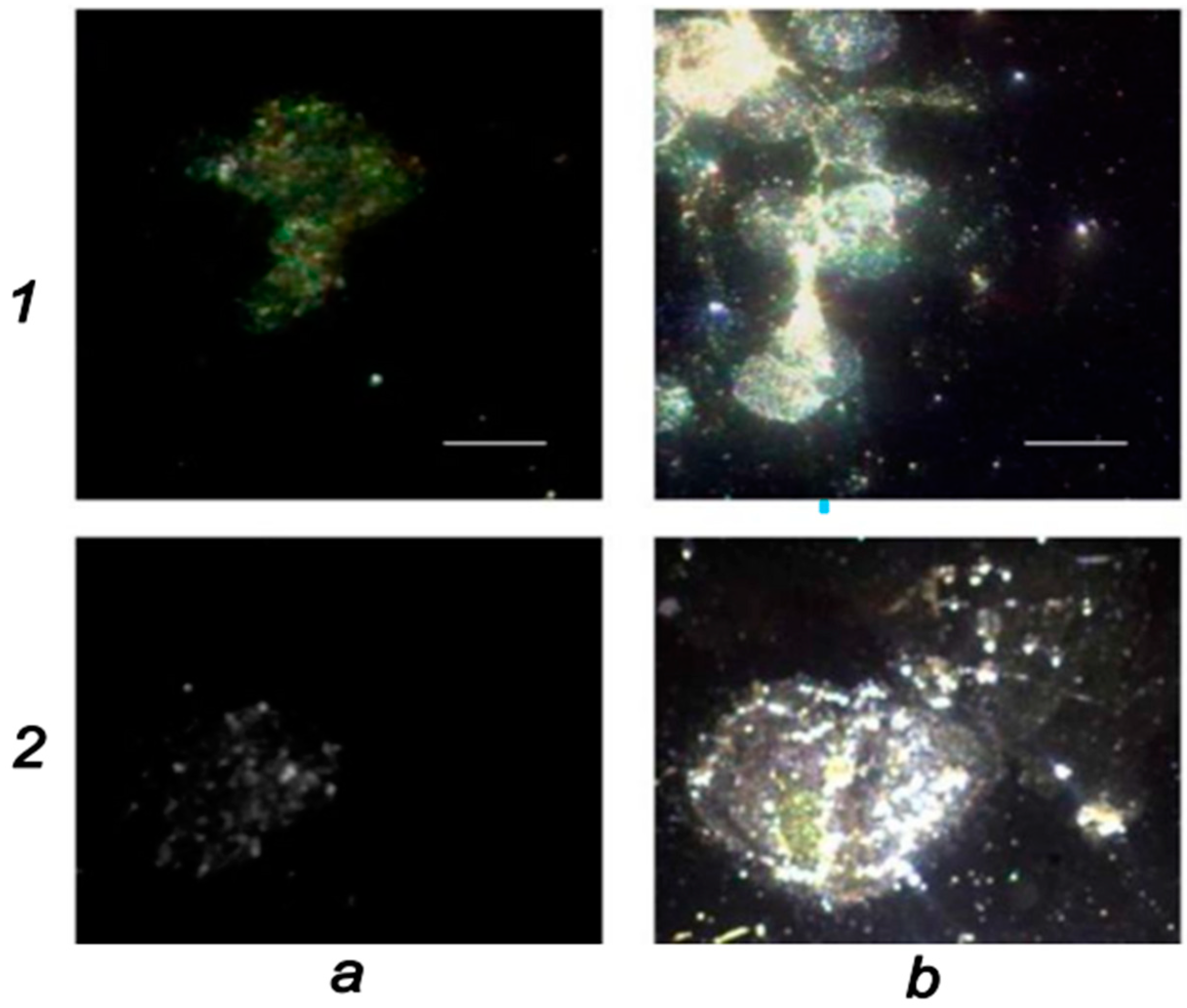
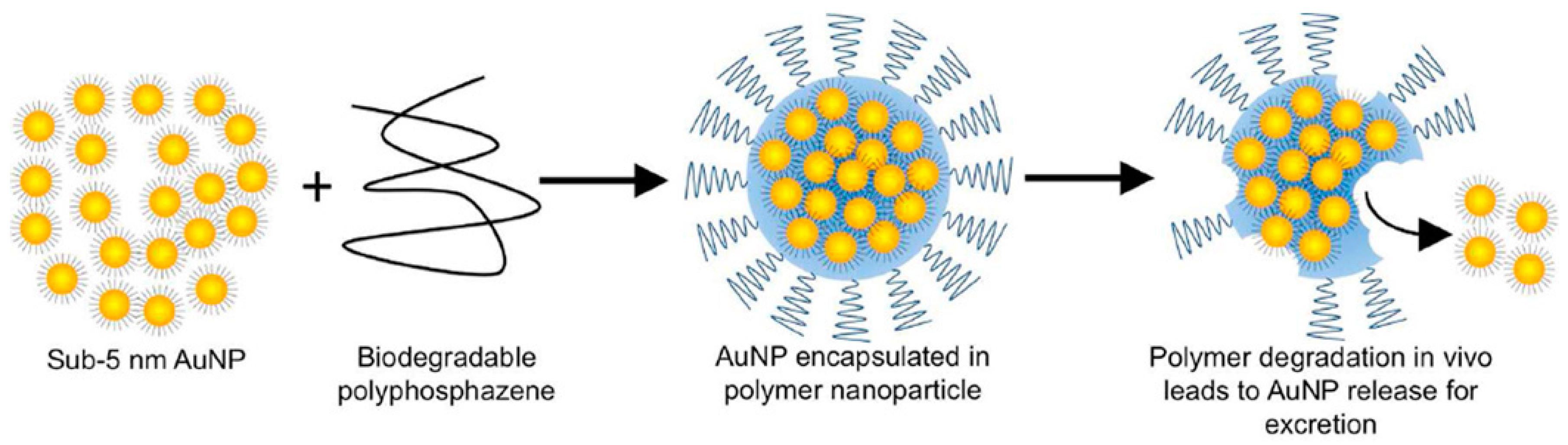
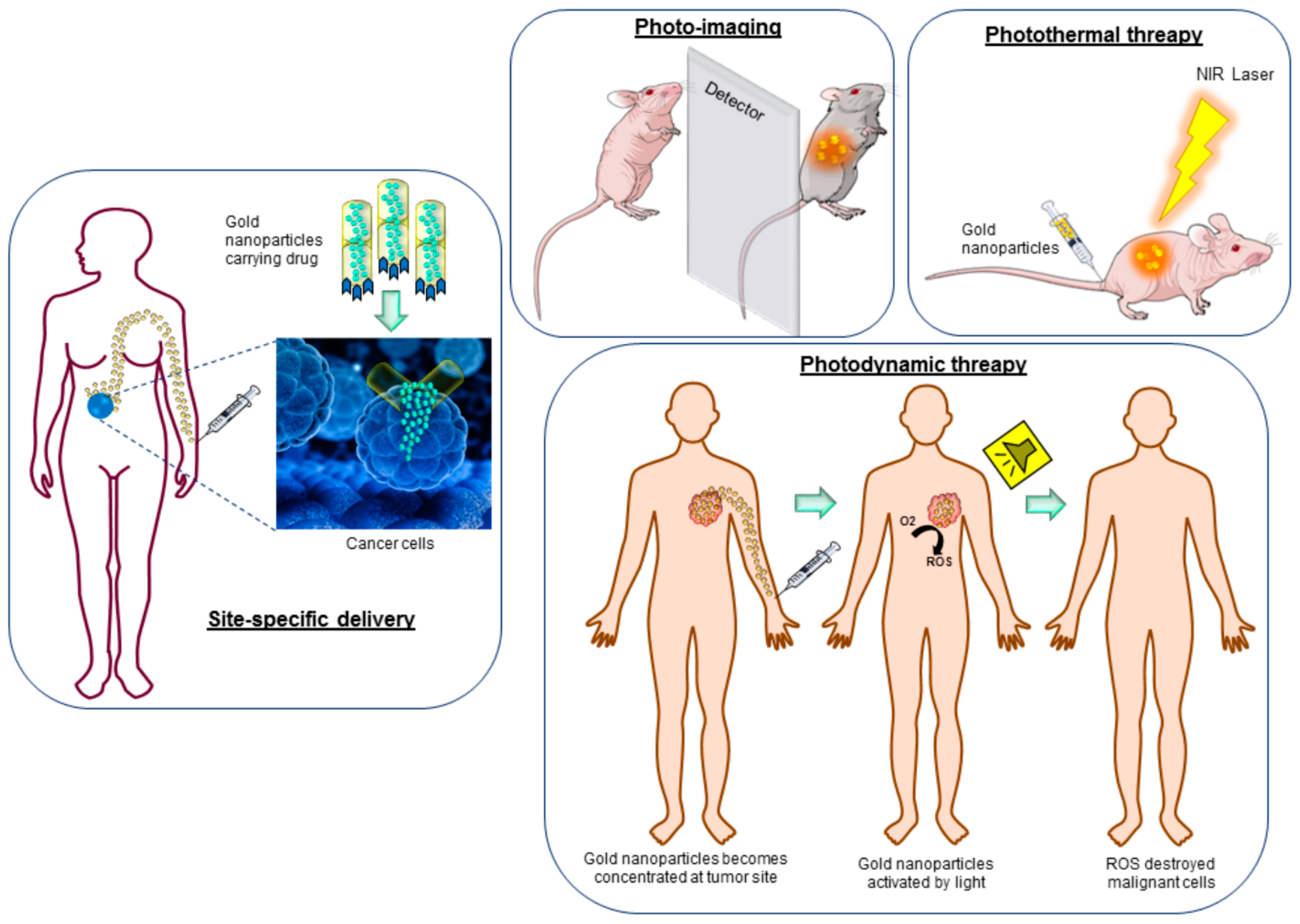
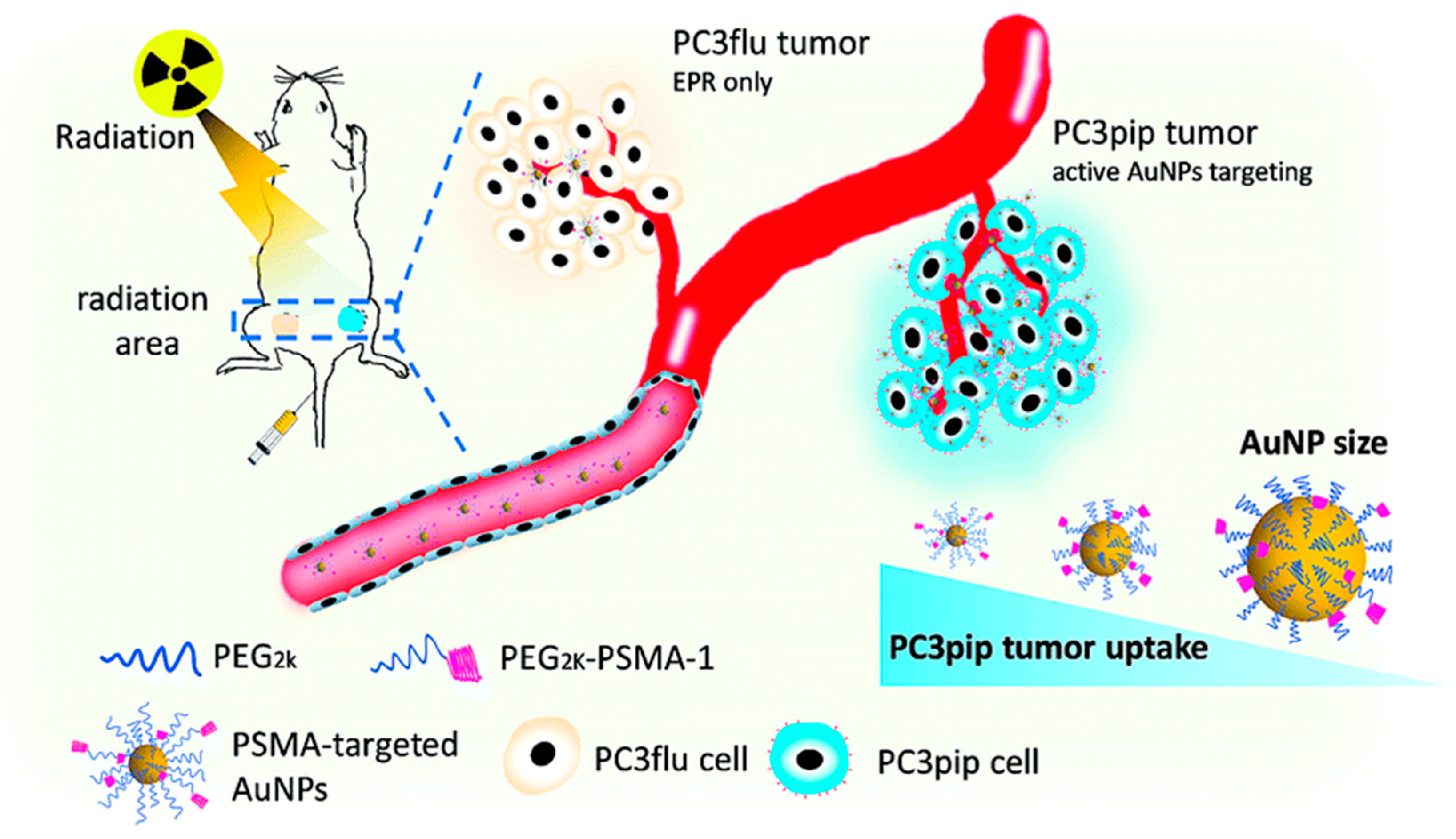
| Nanoparticle | Medical Application | Research Team |
|---|---|---|
| PEGylated gold nanopheres–gadolinium NPs (PEG-Gd@SPhGNPs) | MRI contrast agent | Fatehbasharzad et al. [94] |
| PEGylated gold nano concave cubes–gadolinium NPs (PEG-Gd@CCNPs) | MRI contrast agent | Fatehbasharzad et al. [94] |
| Gadolinium–biopolymer–gold bimetallic NP system | MRI contrast agent | Aouidat et al. [96] |
| Octadentate gadolinium unit based on DO3A with a dithiocarbamate tether, attached to the surface of gold NPs | MRI contrast agent | Chabloz et al. [95] |
| Gold nanorod with surface-bound gadolinium chelates | MRI/photoacoustic imaging agent | Qin et al. [99] |
| Gadolinium–gold nanocluster NPs | NIR/CT/MR imaging agent for A549 human non-small cell lung cancer cell imaging in vitro | Hou et al. [102] |
| Gadolinium chelated gold NPs-acetylated PEI surface amines (FA-Gd-Au PENPs) | CT/MR imaging agent in vivo | Zhou et al. [103] |
| Gold–Prussian blue–gadolinium ovalbumin nanoparticles (APG@OVA NPs) | MRI/surface-enhanced Raman scattering agent | Zhang et al. [104] |
| Doxorubicin (DOX) gadolinium–gold complexes (DOX ON-Gd-AuNRs) (DOX IN-Gd-AuNRs) | Photothermal therapy application/MRI agent | Khan et al. [110] |
| Double-layered Zn/Al-gallic acid (GA)–gadolinium (NO3)3–gold nanoparticles (GAGZAu) | Theranostic nanodelivery system (drug delivery system/MRI agent) | Sani Usman et al. [111,112] |
| Graphene oxide–chlorogenic acid–gadolinium–gold nanoparticles (GOGCA) | Theranostic nanodelivery system (drug delivery system/MRI agent) | Usman et al. [112] |
| Gadolinium oxide–gold nanoclusters hybrid (Gd2O3-AuNCs) | Theranostic nanoplatform (PDT application/drug delivery system/NIRF/MR/CT imaging agent) | Han et al. [113] |
| Gold core dithiolated polyaminocarboxylate shell doped with gadolinium ions (Au@DTDTPA(Gd)) | MRI agent for guided radiation therapy of brain tumors | Debouttière et al. [116] |
| Spherical self-assembly of gold NPs–gadolinium ions–metalloproteinase-2-IR820 (Gd–AuNPS@IR820) | PDT/PTT application on liver cancer | Li et al. [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kouri, M.A.; Polychronidou, K.; Loukas, G.; Megapanou, A.; Vagena, I.-A.; Gerardos, A.M.; Spyratou, E.; Eftsathopoulos, E.P. Consolidation of Gold and Gadolinium Nanoparticles: An Extra Step towards Improving Cancer Imaging and Therapy. J. Nanotheranostics 2023, 4, 127-149. https://doi.org/10.3390/jnt4020007
Kouri MA, Polychronidou K, Loukas G, Megapanou A, Vagena I-A, Gerardos AM, Spyratou E, Eftsathopoulos EP. Consolidation of Gold and Gadolinium Nanoparticles: An Extra Step towards Improving Cancer Imaging and Therapy. Journal of Nanotheranostics. 2023; 4(2):127-149. https://doi.org/10.3390/jnt4020007
Chicago/Turabian StyleKouri, Maria Anthi, Konstantina Polychronidou, Grigorios Loukas, Aikaterini Megapanou, Ioanna-Aglaia Vagena, Angelica M. Gerardos, Ellas Spyratou, and Eftstathios P. Eftsathopoulos. 2023. "Consolidation of Gold and Gadolinium Nanoparticles: An Extra Step towards Improving Cancer Imaging and Therapy" Journal of Nanotheranostics 4, no. 2: 127-149. https://doi.org/10.3390/jnt4020007
APA StyleKouri, M. A., Polychronidou, K., Loukas, G., Megapanou, A., Vagena, I.-A., Gerardos, A. M., Spyratou, E., & Eftsathopoulos, E. P. (2023). Consolidation of Gold and Gadolinium Nanoparticles: An Extra Step towards Improving Cancer Imaging and Therapy. Journal of Nanotheranostics, 4(2), 127-149. https://doi.org/10.3390/jnt4020007










