Abstract
In this work, we have developed a method for synthesizing new 8-substituted triazolo[5,1-b]purines using diacetoxyiodobenzene as an oxidizing agent with good yields (59–67%). The advantages of this approach include mild reaction conditions and removing the need to use transition metals. Based on the results obtained, a plausible reaction pathway was proposed. The developed approach opens new possibilities for the preparation of previously inaccessible condensed purine derivatives, which are of interest for the development of biomolecules with a variety of pharmacological applications. The structures of the compounds were confirmed by the data of 1H, 13C NMR spectroscopy, IR spectroscopy, and an elemental analysis.
1. Introduction
Natural purines are among the most known and well-studied representatives of nitrogen heterocycles due to their involvement in the regulation of essential biological processes in many living organisms [1,2,3]. The presence of these heterocyclic fragments in the structure of nucleic acids has inspired active research aimed at creating modified nucleosides and peptide nucleic acids, which, in turn, has led to the development of new drugs based on purines [4,5,6,7,8,9,10,11] (Figure 1).

Figure 1.
Examples of purine-based drugs.
Indeed, drugs based on natural purines are used to treat different types of cancer [12,13,14,15], including leukemia, with medications that have different mechanisms of action [16,17]; they are also included in antiretroviral therapy, one of the few ways to treat HIV [18,19,20]. Originally, such drugs were guanine- and adenine-based nucleosides [21,22], but subsequently, self-modified aglycones were also used. Some of these modified systems include 2-aminopurine derivatives that are not direct analogs of guanine [23,24]. Not only do 2-aminopurines have a wide range of biological activities [25,26,27,28] but they also exhibit fluorescent properties [29,30], which provide possibilities for their use in analyzing the geometry and dynamics of nucleic acids [31,32]. Based on this, it is essential to develop new polycyclic structures based on 2-aminopurine to obtain compounds for the use as potential drugs or organic luminescent materials.
By polycyclic structures, we primarily refer to azoloannelated purines, namely triazolo[5,1-b]purines. In the literature, there are few methods for obtaining such heterocyclic systems, and all involve the sequential annelation of the pyrimidine cycle to the 1,2,4-triazole moiety, followed by the formation of the imidazole ring (Scheme 1). For example, Tenor and Kröger use 2-acetamidocyanoacetic ester for this purpose [33], while in more recent work, the construction is based on the corresponding nitro derivatives [34,35]. Previously, we developed a method for the preparation of 2-aminopurine starting from aminotetrazole [36], and a preparation of 6,7-diaminoazolo [1,5-a]pyrimidines was proposed in Gazizov’s publication [37,38]. Combining the ideas of these works, the present study, proposes a new approach to obtain C-8 modified triazolo[5,1-b]purines, starting from 6,7-diamino-2-thienyl-1,2,4-triazolo[1,5-a]pyrimidine and involving oxidation of the corresponding Schiff bases.

Scheme 1.
State-of-the-art and current work [33,34,35,36,38].
2. Materials and Methods
Commercial reagents were obtained from Sigma-Aldrich (Burlington, MA, USA), Acros Organics (Antwerpen, Belgium), or Alfa Aesar (Ward Hill, MA, USA), and were used without preprocessing. All workup and purification procedures were performed using analytical-grade solvents. The spectra were acquired using a Bruker DRX-400 (Karlsruhe, Germany) spectrometer at 400 MHz (1H) and 101 MHz (13C), respectively, or a Bruker Avance NEO 600 instrument at 151 MHz (13C), using DMSO-d6 and CF3COOD as solvents and an external reference, respectively. Chemical shifts are expressed in δ (parts per million, ppm) values and coupling constants are expressed in hertz (Hz). The following abbreviations are used for the multiplicity of NMR signals: s, singlet; d, doublet; t, triplet; dd, doublet of doublet; m, multiplet; and AN, anthracene. IR spectra were recorded on a Bruker α spectrometer equipped with a ZnSe ATR accessory. Elemental analysis was performed on a PerkinElmer PE 2400 elemental analyzer (Waltham, MA, USA). Melting points were determined on a Stuart SMP3 (Staffordshire, UK) and are uncorrected. Monitoring the reaction progress was completed using TLC on Sorbfil plates (Imid Ltd., Krasnodar, Russia) (the eluent is EtOAc, visualizing with UV light).
General procedure (1) for the synthesis of 4-(aryl)-amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3a–g).
To a suspension of 2.32 g (0.01 mol) 2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-6,7-diamine 1 in 20 mL DMF, the 0.01 mol of corresponding aldehyde 3a–g and 10% mol (0.06 mL) of MeSO3H were added and the mixture was heated at 120 °C on an oil bath for 12 h. The reaction mixture was cooled to room temperature and the precipitate was filtered. The precipitate was then washed with MeOH and Et2O.
6-((4-(Dimethylamino)benzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3a). The reaction was performed according to the general procedure (1) employing 1.49 g (0.01 mol, 1 equiv.) of 4-(dimethylamino)benzaldehyde 2a. Yellow powder. Yield 86% (3.12 g). mp. 283–285 °C. IR ν, cm−1: 3270, 3233, 1276, 1237. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 8.73 (1H, s, -CH=N), 8.55 (1H, s, H-4), 7.91 (4H, m, H-2″, H-3″, H-5″, H-6″), 7.84 (1H, d, J = 3.6 Hz, H-3′), 7.74 (1H, d, J = 5.0 Hz, H-5′), 7.25 (1H, t, J = 3.5 Hz, H-4′), 6.79 (2H, d, J = 8.5 Hz, NH2), 3.03 (6H, s, (CH3)2). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 160.61, 157.70, 155.01, 152.92, 145.25, 142.55, 134.46 (2C), 131.01 (2C), 129.19, 128.57, 128.11, 124.82, 118.91, 118.87 (2C). Calculated for C18H17N7S: C 59.49, H 4.71, N, 26.98; found: C 59.41, H 4.76, N 27.04.
6-((4-Methoxybenzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3b). The reaction was performed according to the general procedure (1) employing 1.36 g (0.01 mol, 1 equiv.) of 4-methoxybenzaldehyde 2b. Yellow powder. Yield 81% (2.83 g). mp. >300 °C. IR ν, cm−1: 3265, 3235, 1218, 1178. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 8.86 (1H, s, -CH=N), 8.62 (1H, s, H-4), 8.21 (2H, s, NH2), 8.06 (2H, d, J = 8.7, H-2″, H-6″), 7.85 (1H, dd, J = 3.7, 1.3 Hz, H-3′), 7.76 (1H, dd, J = 5.0, 1.3 Hz, H-5′), 7.25 (1H, dd, J = 5.0, 3.6 Hz, H-4′), 7.06 (d, J = 8.7 Hz, 2H, H-3″, H-5″), 3.85 (3H, s, CH3). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 161.77, 160.09, 156.38, 154.75, 145.12, 142.30, 133.79, 130.72 (2C), 129.40, 128.91, 128.20, 127.76, 117.52, 114.06 (2C), 55.40. Calculated for C17H14N6OS: C 58.27, H 4.03, N, 23.98; found: C 58.21, H 4.14, N 23.94.
6-((Anthracen-9-ylmethylene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3c). The reaction was performed according to the general procedure (1) employing 2.06 g (0.01 mol, 1 equiv.) of 9-anthracenecarboxaldehyde 2c. Orange powder. Yield 82% (3.44 g). mp. 270–272 °C. IR ν, cm−1: 3404, 3143, 1278, 1217. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 10.08 (1H, s, -CH=N), 8.62 (1H, s, H-4), (3H, m, 3x HAN), 8.80 (1H, s, H-4), 8.19 (2H, d, J = 8.2 Hz, 2x HAN), 8.15 (2H, s, NH2), 7.89 (1H, dd, J = 3.7, 1.2 Hz, H-3′), 7.79 (1H, dd, J = 5.0, 1.2 Hz, H-5′), 7.63 (4H, m, 4x HAN), 7.27 (1H, dd, J = 5.0, 3.6 Hz, H-4′). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 160.73, 158.08, 155.52, 145.46, 143.85, 134.25, 131.35 (4C), 130.98, 130.70 (4C), 129.50, 129.34, 128.72, 128.36, 127.99, 127.84, 126.09, 125.82, 119.58. Calculated for C24H16N6S: C 68.55, H 3.84, N, 19.99; found: C 68.50, H 3.89, N 19.27.
2-(Thiophen-2-yl)-6-((thiophen-3-ylmethylene)amino)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3d). The reaction was performed according to the general procedure (1) employing 1.12 g (0.01 mol, 1 equiv.) of thiophene-2-carboxaldehyde 2d. Yellow powder. Yield 68% (2.22 g). mp. >300 °C. IR ν, cm−1: 3407, 3231, 1258. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 9.04 (1H, s, -CH=N), 8.56 (1H, s, H-4), 7.84 (1H, d, J = 3.6 Hz, Hthpen), 7.81 (2H, s, NH2), 7.68 (1H, dd, J = 15.9, 4.3 Hz, Hthpen), 7.66 (1H, dd, J = 13.1, 3.6 Hz, H-5thpen), 7.62 (1H, dd, J = 5.1, 1.6 Hz, Hthpen), 7.19 (q, J = 4.5 Hz, 2H, Hthpen). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 160.64, 155.27, 151.62, 145.23, 143.35, 143.19, 134.20, 133.24, 131.80, 129.46, 128.70, 128.60, 128.30, 117.88. Calculated for C14H10N6S2: C 51.52, H 3.09, N, 25.75; found: C 51.43, H 3.18, N 25.79.
6-((4-Bromobenzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3e). The reaction was performed according to the general procedure (1) employing 1.85 g (0.01 mol, 1 equiv.) of 4-bromobenzaldehyde 2e. Light green crystalline powder. Yield 70% (2.79 g). mp. >300 °C. IR ν, cm−1: 3238, 1258. 1H NMR (600 MHz, DMSO-d6) δ, ppm (J, Hz): 8.99 (1H, s, -CH=N), 8.83 (1H, s, H-4), 8.77 (1H, dd, J = 4.5, 1.3 Hz, Har), 8.62 (2H, s, NH2), 8.57 (1H, dd, J = 8.0, 1.1 Hz, Har), 8.12 (1H, m, H-3′), 7.86 (1H, dd, J = 3.6, 1.3 Hz, Har), 7.78 (1H, dd, J = 4.9, 1.2 Hz, Har), 7.63 (1H, ddd, J = 7.4, 5.0, 1.3 Hz, H-5′), 7.25 (1H, m, H-4′). 13C NMR (151 MHz, DMSO-d6) δ, ppm (J, Hz): 160.30, 155.04, 153.16, 152.74, 147.93, 146.19, 142.75, 133.39 (2C), 129.29, 128.33 (2C), 128.15, 125.77, 123.26, 115.58. Calculated for C16H11BrN6S: C 48.13, H 2.78, N, 21.05; found: C 48.18, H 2.75, N 21.03.
6-((Pyridin-2-ylmethylene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3f). The reaction was performed according to the general procedure (1) employing 1.07 g (0.01 mol, 1 equiv.) of 3-pyridinecarboxaldehyde 2f. Dark yellow powder. Yield 65% (2.08 g). mp. 183–185 °C. IR ν, cm−1: 3449, 3083, 1186. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 8.93 (1H, s, -CH=N), 8.74 (1H, s, H-4), 8.69 (1H, d, J = 4.0 Hz H-3′), 8.60 (1H, d, J = 8.0 Hz, H-6″), 8.30 (2H, s, NH2), 7.93 (1H, td, J = 7.7, 1.8 Hz, H-4″), 7.85 (1H, dd, J = 3.6, 1.2 Hz, H-5′), 7.65 (1H, dd, J = 5.0, 1.2 Hz, H-4′), 7.47 (1H, dd, J = 7.4, 4.8 Hz, H-5″), 7.21 (1H, dd, J = 5.0, 3.6 Hz, H-3″). 13C NMR (151 MHz, DMSO-d6) δ, ppm (J, Hz): 160.29, 155.07, 153.60, 153.09, 148.17, 146.11, 142.80, 138.72, 133.43, 129.25, 128.31, 128.11, 125.66, 123.01, 115.64. Calculated for C15H11N7S: C 56.06, H 3.45, N, 30.51; found: C 56.11, H 3.44, N 30.57.
6-((4-(Diphenylamino)benzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine (3g). The reaction was performed according to the general procedure (1) employing 2.73 g (0.01 mol, 1 equiv.) of 4-(diphenylamino)benzaldehyde 2g. Yellow powder. Yield 61% (2.97 g). mp. 265–267 °C. IR ν, cm−1: 3444, 3057, 1274. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 8.81 (1H, s, -CH=N), 8.60 (1H, s, H-4), 8.16 (2H, s, NH2), 7.95 (2H, d, J = 8.4 Hz, HC6H4), 7.83 (H, m, HPh), 7.75 (H, m, HPh), 7.37 (4H, t, J = 7.7 Hz, HPh), 7.23 (H, dd, J = 5.0, 3.6 Hz, HPh), 7.13 (6H, m, HPh), 6.96 (2H, d, J = 8.3 Hz, HC6H4). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 160.07, 156.06, 154.71, 149.93, 146.38 (2C), 145.05, 142.24, 133.79, 130.22 (2C), 129.74 (4C), 128.88, 128.17, 127.72, 125.19 (4C), 124.25 (2C), 120.57 (2C), 117.65 (2C). Calculated for C28H21N7S: C 68.97, H 4.34, N, 20.11; found: C 69.04, H 4.29, N 20.17.
General procedure (2) for the synthesis of aryl-4-(2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purin-7-yl)aniline (4a–g) is as follows:
To a solution of the corresponding Schiff base (0.001 mol), 3a–g in 5 mL CF3COOH and 0.40 g (0.00125 mol, 1.25 equiv.) of PhI(OAc)2 were added. The reaction mixture was stirred at room temperature for 4 h. Then, 5 mL MeOH was added to the reaction mixture. The resulting solution was stirred for another 15 min and then evaporated. The residue was purified by flash chromatography; eluent—CHCl3/MeOH (9/1).
N,N-Dimethyl-4-(2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purin-7-yl)aniline (4a). The reaction was performed according to the general procedure (2) employing 0.45 g (0.00125 mol, 1 equiv.) of ((4-(dimethylamino)benzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3a. Yellow powder. Yield 60% (0.27 g). mp. 275-277 °C. IR ν, cm−1: 3088, 3059, 1133, 1094. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 13.71 (1H, s, NH), 8.94 (1H, s, H-4), 8.10 (2H, d, J = 8.8 Hz, H-2″, H-6″), 7.87 (1H, dd, J = 3.6, 1.2 Hz, H-3′), 7.61 (1H, dd, J = 5.0, 1.2 Hz, H-5′), 7.21 (1H, dd, J = 5.0, 3.6 Hz, H-4′), 6.83 (2H, d, J = 8.8 Hz, H-3″, H-5″), 3.10 (6H, s, (CH3)2). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 159.24, 154.93, 153.95, 152.14, 144.88, 139.03, 133.84, 128.45 (2C), 128.31, 127.99, 127.18, 120.37, 114.47, 111.62 (2C), 40.3 (2C). Calculated for C18H15N7S: C 59.82, H 4.18, N, 27.13; found: C 59.78, H 4.23, N 27.04.
7-(4-Methoxyphenyl)-2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (4b). The reaction was performed according to the general procedure (2) employing 0.44 g (0.00125 mol, 1 equiv.) of ((4-methoxybenzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3b. White powder. Yield 66% (0.28 g). mp. >300 °C. IR ν, cm−1: 3061, 2839, 1438, 1177. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 13.99 (1H, s, NH), 9.03 (1H, s, H-4), 8.24 (2H, d, J = 8.8 Hz, H-2″, H-6″), 7.88 (1H, dd, J = 3.6, 1.2 Hz, 1H, H-3′), 7.62 (1H, d, J = 5.0 Hz, 1H, H-5′), 7.21 (1H, dd, J = 5.0, 3.6 Hz, H-4′), 7.12 (2H, d, J = 8.6 Hz, H-3″, H-5″), 4.04 (3H, s, CH3). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 161.92, 159.46, 154.14, 153.78, 144.57, 140.30, 133.84, 129.05 (2C), 128.69, 128.29, 127.48, 120.65, 120.49, 114.77 (2C), 55.52. Calculated for C17H12N7OS: C 58.61, H 3.47, N, 24.12; found: C 58.52, H 3.55, N 24.08.
7-(Anthracen-9-yl)-2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (4c). The reaction was performed according to the general procedure (2) employing 0.52 g (0.00125 mol, 1 equiv.) of (anthracen-9-ylmethylene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3c. Orange powder. Yield 59% (0.31 g). mp. >300 °C °C. IR ν, cm−1: 3378, 3363, 1057. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 14.59 (1H, s, NH), 9.29 (1H, s, H-4), 8.95 (1H, s, HAN), 8.27 (2H, d, J = 8.3 Hz, 2H, HAN), 7.93 (1H, dd, J = 3.7, 1.2 Hz, H-3′), 7.84 (2H, d, J = 8.6 Hz, HAN), 7.78 (1H, d, J = 5.0, H-5′), 7.60 (5H, m, H-5′, HAN), 7.22 (1H, dd, J = 5.0, 3.6 Hz, H-4′). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 159.56, 154.22, 152.17, 144.46, 133.78 (2C), 130.61 (2C), 130.55 (2C), 130.18, 128.75 (2C), 128.66, 128.33 (2C), 127.55, 127.48, 125.87 (2C), 125.27 (2C), 123.16, 120.64. Calculated for C24H14N6S: C 68.88, H 3.37, N, 20.08; found: C 68.80, H 3.35, N 20.03.
2,7-Di(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (4d). The reaction was performed according to the general procedure (2) employing 0.41 g (0.00125 mol, 1 equiv.) of 2-(thiophen-2-yl)-6-((thiophen-2-ylmethylene)amino)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3d. Gray powder. Yield 62% (0.25 g). mp. 254–256 °C. IR ν, cm−1: 3082, 1281, 1189. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 14.46 (1H, s, NH), 9.10 (1H, s, H-4), 8.06 (1H, d, J = 3.8 Hz, H-3″), 7.95 (1H, d, J = 5.0 Hz, H-5″), 7.90 (1H, dd, J = 3.6, 1.2 Hz, H-3′), 7.77 (1H, d, J = 5.0 Hz, H-5′), 7.33 (1H, dd, J = 5.0, 3.7 Hz, H-4″), 7.25 (1H, dd, J = 5.1, 3.6 Hz, H-4′). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 159.56, 154.18, 149.12, 133.73 (2C), 131.73 (2C), 131.32 (2C), 129.38, 128.97, 128.78, 128.31, 127.54. Calculated for C14H8N6S2: C 51.84, H 2.49, N, 25.91; found: C 51.79, H 2.45, N 25.90.
7-(4-Bromophenyl)-2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (4e). The reaction was performed according to the general procedure (2) employing 0.49 g (0.00125 mol, 1 equiv.) of ((4-bromobenzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3e. Yellow powder. Yield 66% (0.33 g). mp. >300 °C. IR ν, cm−1: 3081, 1087, 1044. 1H NMR (600 MHz, DMSO-d6) δ, ppm (J, Hz): 14.52 (1H, s, NH), 9.16 (1H, s, H-4), 8.24 (2H, m, H-2″, H-6″), 7.90 (1H, dd, J = 3.5, 1.2 Hz, H-3′), 7.86 (2H, m, H-3″, H-5″), 7.77 (1H, dd, J = 5.0, 1.2 Hz, H-5′), 7.25 (1H, dd, J = 5.0, 3.6 Hz, H-4′). 13C NMR (151 MHz, DMSO-d6 + CF3COOD) δ, ppm (J, Hz): 159.56, 154.22, 152.41 (2C), 144.23, 141.17, 133.71, 132.43 (2C), 129.13 (2C), 128.82, 128.35, 127.58, 127.46, 125.23. Calculated for C16H9BrN6S: C 48.38, H 2.28, N, 21.16; found: C 48.43, H 2.26, N 21.21.
7-(Pyridin-2-yl)-2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (4f). The reaction was performed according to the general procedure (2) employing 0.40 g (0.00125 mol, 1 equiv.) of ((pyridin-2-ylmethylene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3f. Beige powder. Yield 64% (0.25 g). mp. >300 °C. IR ν, cm−1: 3084, 3029, 1140. 1H NMR (600 MHz, DMSO-d6+CF3COOD) δ, ppm (J, Hz): 9.03 (1H, s, H-4), 8.75 (1H, d, J = 4.8 Hz, H-2″), 8.41 (1H, d, J = 7.8 Hz, H-5″), 8.04 (1H, td, J = 7.7, 1.7 Hz, H-4″), 7.88 (1H, dd, J = 3.5, 1.2 Hz, H-3′), 7.68 (1H, d, J = 5.0 Hz, H-5′), 7.57 (1H, dd, J = 7.6, 4.8 Hz, H-3″), 7.19 (1H, dd, J = 5.0, 3.5 Hz, H-4′). 13C NMR (151 MHz, DMSO-d6+CF3COOD) δ, ppm (J, Hz): 159.36, 154.12, 152.82, 150.08, 146.73, 144.97, 142.44, 138.96, 133.56, 129.50, 128.82, 128.60, 126.72, 123.52, 121.66. Calculated for C15H9N7S: C 56.42, H 2.84, N, 30.70; found: C 56.51, H 2.91, N 30.65.
N,N-Diphenyl-4-(2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purin-7-yl)aniline (4g). The reaction was performed according to the general procedure (2) employing 0.61 g (0.00125 mol, 1 equiv.) of ((4-(diphenylamino)benzylidene)amino)-2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine 3g. Yellow powder. Yield 67% (0.41 g). mp. >300 °C. IR ν, cm−1: 1453, 1315, 1272. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 14.13 (1H, s, NH), 9.06 (1H, s, H-4), 8.16 (2H, d, J = 8.5 Hz, HC6H4), 7.91 (1H, d, J = 4.0 Hz, H-3′), 7.76 (1H, d, J = 5.0 Hz, H-5′), 7.42 (4H, t, J = 7.7 Hz, HPh), 7.26 (1H, t, J = 4.3 Hz, H-4′), 7.20 (6H, m, HPh), 7.06 (2H, d, J = 8.5 Hz, HC6H4).13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 160.07, 154.75, 154.28, 150.87, 146.66 (4C), 134.44 (2C), 130.36 (4C), 129.12 (2C), 128.69, 127.91, 126.18 (4C), 125.20 (3C), 120.77 (3C). Calculated for C28H19N7S: C 69.26, H 3.94, N, 20.19; found: C 69.34, H 3.88, N 20.22.
The procedure for the synthesis of 7-(thiophen-2-yl)-3H-[1,2,3]triazolo[4,5-e][1,2,4]triazolo[1,5-a]pyrimidine (5) is as follows:
To a mixture of 5.0 mL acetic acid and 0.350 mL of isoamyl nitrite (0.0026 mol), 0.3 g (0.0013 mol) of 2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidine-6,7-diamine was added, 1 and the mixture was heated at 60 °C on an oil bath for 5 h. The resulting suspension was cooled to room temperature and the precipitate was filtered out to give the pure product.
7-(Thiophen-2-yl)-3H-[1,2,3]triazolo[4,5-e][1,2,4]triazolo[1,5-a]pyrimidine (5). Yellow powder. Yield 58% (0.18 g). mp. >300 °C. IR ν, cm−1: 3082, 1513. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 9.66 (1H, s, H-4), 7.90 (1H, dd, J = 3.6, 1.3 Hz, H-3′), 7.79 (1H, dd, J = 5.0, 1.3 Hz, H-5′), 7.26 (1H, dd, J = 5.0, 3.6 Hz, H-4′). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 159.86, 154.79, 149.00, 143.15, 132.90, 129.42, 129.16, 128.42, 127.94. Calculated for C9H5N7S: C 44.44, H 2.07, N, 40.31; found: C 44.37, H 2.12, N 40.33.
The procedure for the synthesis of 7-methyl-2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (6) is as follows:
To a mixture of 3.0 mL (0.018 mol) of triethylorthoacetate and 1.0 mL (0.01 mol) of acetic anhydride, 0.3 g (0.0013 mol) of 2-(thiophen-2-yl)-[1,2,4]triazolo[1,5-a]pyrimidine-6,7-diamine was added 1 and the mixture was heated at 145 °C on an oil bath for 4 h. The resulting solution was evaporated and 5.0 mL acetone was added. The precipitate was filtered out to provide the pure product.
7-Methyl-2-(thiophen-2-yl)-6H-[1,2,4]triazolo[5,1-b]purine (6). Brown powder. Yield 62% (0.21 g). mp. >300 °C. IR ν, cm−1: 3334, 1284. 1H NMR (400 MHz, DMSO-d6) δ, ppm (J, Hz): 13.69 (1H, s, NH), 9.03 (1H, s, H-4), 7.85 (1H, dd, J = 3.7, 1.2 Hz, H-3′), 7.74 (1H, dd, J = 5.0, 1.2 Hz, H-5′), 7.24 (1H, dd, J = 5.0, 3.6 Hz, H-4′), 2.65 (3H, s, CH3). 13C NMR (101 MHz, DMSO-d6) δ, ppm (J, Hz): 159.37, 155.36, 153.90, 144.15, 140.33, 133.84, 128.63, 128.25, 127.39, 119.61, 14.97. Calculated for C11H8N6S: C 51.55, H 3.15, N, 32.79; found: C 51.50, H 3.19, N 32.70.
3. Results and Discussion
To obtain 8-substituted triazolo[5,1-b]purines, we selected 2-(thiophen-2-yl)-6,7-diamino-1,2,4-triazolo[1,5-a]pyrimidine 1 as an initial substrate since it is fairly easy to synthesize according to the method [14] and it has an additional conjugated heterocyclic ring, allowing us to obtain a polycyclic purine structure. First, we optimized the reaction of diamine 1 with 4-(N,N-dimethylamino)benzaldehyde 2a (Scheme 2) under various conditions as shown in Table 1.

Scheme 2.
Preparation of the Schiff base 3a.

Table 1.
Optimization of condensation reaction 1.
It was found that boiling the starting reagents in MeOH, EtOH, MeCN, and DMF without a catalyst for 12 h allowed us to obtain the desired product 3a with low yields (Table 1, entry 1-4). At the same time, boiling compounds 1 and 2a in DMF in the presence of methanesulfonic acid led to a significant increase in the conversion of the reaction and easier purification of the product.
With the optimized condition in hand, we carried out the reaction of triazolo[1,5-a]pyrimidine-6,7-diamine 1 with various aromatic aldehydes (Scheme 3). Moreover, products 3a–g have been isolated as pure solids by simple filtration of the reaction mixture, which is a significant advantage of the synthetic procedure.

Scheme 3.
Preparation of the Schiff bases 3a–g.
All synthesized compounds were fully characterized using 1H-NMR, 13C-NMR, IR-spectroscopy, and elemental analysis. In addition, based on the 1H-13C HMBC correlation data of compound 3b, it was established that the condensation reaction involves exclusively the amino group in position 6 of the azolopyrimidine system (Figure 2), since the -CH=N group proton (8.85 ppm) has cross-peaks with C-5 (142.77 ppm) C-2′, C-6′ (131.15 ppm) and C-1′ (129.39 ppm) carbon atoms.

Figure 2.
Key interactions of the proton of the methylene fragment in compound 3b.
The experimental data obtained correlate with the results from the literature [39,40,41]. It can be assumed that the increased nucleophilicity of the amino group at position 6 of 6,7-diamino-1,2,4-triazolo[1,5-a]pyrimidine 1 is caused by the rapid establishment of equilibrium between amine and imine forms [42], as shown in Scheme 4.

Scheme 4.
Amine–Imine equilibrium.
The next step was the oxidation of the obtained imines 3a–g into the corresponding azolopurines. As in the previous step, we conducted an optimization of oxidation reaction conditions using different oxidizing agents. The results are summarized in Table 2.

Table 2.
Optimization of oxidation reaction 1.
Interestingly, the oxidation of Schiff base 3a turned out to be a non-trivial process. We determined that literature methods for similar processes were not applicable to this compound. For example, the reaction did not proceed with red lead (Table 2, entry 1) and DDQ (Table 2, entry 2), which are often used in the formation of heterocyclic C-N bonds [43]. Copper (II) salts (Table 2, entry 3, 4) [44] as well as such inorganic oxidizing agents, such as hydrogen peroxide (Table 2, entry 5) and manganese (IV) oxide (Table 2, entry 6), did not show any effect [35]. Interestingly, oxidation occurred with a low yield when using PCC in dimethylformamide upon heating (Table 2, entry 8), while it did not occur when heated in acetic acid (Table 2, entry 7). Finally, using the derivative of hypervalent iodine, namely (diacetoxyiodo)benzene, was successful. It was found that oxidation proceeded by heating in formic acid, but the conversion was low (Table 2, entry 8), however, carrying out the reaction in trifluoroacetic acid at room temperature resulted in product 4a with 60% yield (Table 2, entry 9).
Thus, using the optimized reaction conditions we performed the oxidation of the obtained Schiff bases 3a–g into the corresponding azolopurines 4a–g with yields from 59 to 67%. It should be noted that all derivatives 4a–g were obtained with comparable yields, which indicates an insignificant influence of the nature of the substituents on the oxidation process (Scheme 5). All the synthesized compounds were fully characterized using 1H-NMR, 13C-NMR, IR-spectroscopy, and elemental analysis (Supplementary Materials).
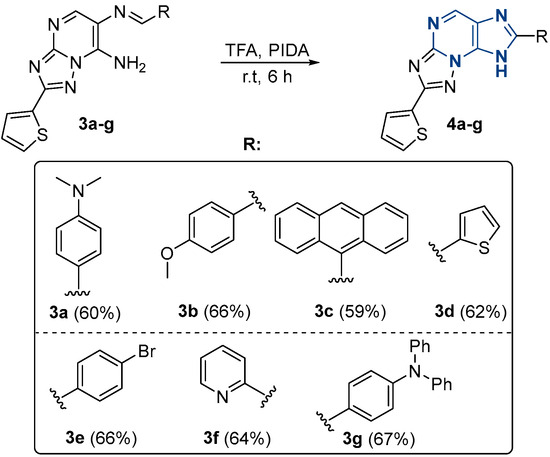
Scheme 5.
Preparation of Schiff 4a–g bases.
In addition, we studied the possibility of obtaining purines based on 2-(thiophen-2-yl)-6,7-diamino-1,2,4-triazolo[1,5-a]pyrimidine 1 by other methods. Thus, with the action of isoamyl nitrite on diamine 1 in acetic acid upon heating, 7-(thiophen-2-yl)-1H-[1,2,3]triazolo[4,5-e][1,2,4]triazolo[1,5-a]pyrimidine 6 was obtained with a 58% yield, and boiling 1 with triethylorthoacetate in acetic anhydride created 7-methyl-2-(thiophen-2-yl)-8H-[1,2,4]triazolo[5,1-b]purine in 62% yield (Scheme 6).

Scheme 6.
Preparation of azolopurines 5,6.
Based on the experimental data obtained and previously published studies on the use of (diacetoxyiodo)benzene as an oxidizing agent for the synthesis of condensed systems [45,46], a plausible reaction pathway was proposed (Scheme 7). The first step involves nucleophilic substitution of the acetoxy group of (diacetoxyiodo)benzene to form an intermediate 8. In the next step, the amino cation is formed by eliminating iodobenzene. Subsequent intramolecular cyclization and aromatization lead to the formation of triazolo[5,1-b]purine.
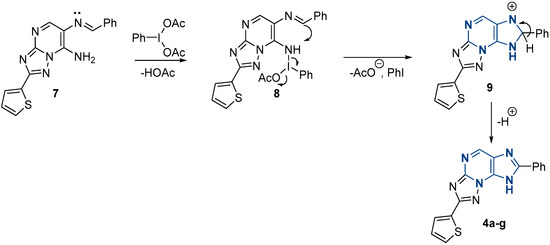
Scheme 7.
Plausible pathway of oxidation by PIDA.
4. Conclusions
Therefore, we have developed a method for the synthesis of novel 8-substituted triazolo[5,1-b]purines using (diacetoxyiodo)benzene as an oxidizing agent by intramolecular C-N bond formation with good yields (59–67%). The advantages of this approach include mild cyclization conditions and good yields of the reaction products. In addition, alternative methods for cyclization of the selected diamine have been proposed to obtain other azolopurine derivatives. The developed approach opens the possibility of obtaining previously inaccessible condensed purine derivatives, which are of interest for creating molecules with varied useful biological and photophysical properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/reactions5040058/s1, Figures S1–S33: 1H-, 13C-NMR and IR spectra of compounds 3a–g, 4a–g, 5, 6.
Author Contributions
Synthesis, A.O.N. and V.V.F.; methodology, V.V.F., E.N.U., D.N.L. and V.L.R.; writing—original draft preparation, A.O.N., V.V.F. and D.N.L.; writing—review and editing, A.O.N., V.V.F., S.V.A. and D.N.L.; visualization, V.V.F. and D.N.L.; supervision, E.N.U., V.V.F. and V.L.R.; project administration, V.L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, Project № 23-23-00642, https://rscf.ru/project/23-23-00642/. Accessed on 1 January 2023.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Acknowledgments
The team of authors would like to thank the Laboratory for Comprehensive Research and Expert Evaluation of Organic Materials under the direction of O.S. Eltsov.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Burnstock, G. Purine and Pyrimidine Receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Legraverend, M. Recent Advances in the Synthesis of Purine Derivatives and Their Precursors. Tetrahedron 2008, 64, 8585–8603. [Google Scholar] [CrossRef]
- Meijer, L.; Raymond, E. Roscovitine and Other Purines as Kinase Inhibitors. From Starfish Oocytes to Clinical Trials. Acc. Chem. Res. 2003, 36, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Weller, S.; Pakes, G.E. A Review of the Pharmacokinetics of Abacavir. Clin. Pharmacokinet. 2008, 47, 351–371. [Google Scholar] [CrossRef]
- Iwasa, T.; Kishi, T.; Matsuura, K.; Wakae, O. Streptomyces novoguineensis sp. nov., an Amipurimycin Producer, and Antimicrobial Activity of Amipurimycin. J. Antibiot. 1977, 30, 1–10. [Google Scholar] [CrossRef]
- Dolečková, I.; Česnek, M.; Dračinský, M.; Brynda, J.; Voller, J.; Janeba, Z.; Kryštof, V. Synthesis and Biological Evaluation of Guanidino Analogues of Roscovitine. Eur. J. Med. Chem. 2013, 62, 443–452. [Google Scholar] [CrossRef]
- Cicenas, J.; Kalyan, K.; Sorokinas, A.; Stankunas, E.; Levy, J.; Meskinyte, I.; Stankevicius, V.; Kaupinis, A.; Valius, M. Roscovitine in Cancer and Other Diseases. Ann. Transl. Med. 2015, 3, 135. [Google Scholar] [CrossRef]
- Perry, C.M.; Wagstaff, A.J. Famciclovir: A Review of Its Pharmacological Properties and Therapeutic Efficacy in Herpesvirus Infections. Drugs 1995, 50, 396–415. [Google Scholar] [CrossRef]
- Cirelli, R.; Herne, K.; McCrary, M.; Lee, P.; Tyring, S.K. Famciclovir: Review of Clinical Efficacy and Safety. Antivir. Res. 1996, 29, 141–151. [Google Scholar] [CrossRef]
- Faulds, D.; Heel, R.C. Ganciclovir: A Review of Its Antiviral Activity, Pharmacokinetic Properties and Therapeutic Efficacy in Cytomegalovirus Infections. Drugs 1990, 39, 597–638. [Google Scholar] [CrossRef]
- Matthews, T.; Boehme, R. Antiviral Activity and Mechanism of Action of Ganciclovir. Clin. Infect. Dis. 1988, 10, S490–S494. [Google Scholar] [CrossRef] [PubMed]
- Parker, W.B. Enzymology of Purine and Pyrimidine Antimetabolites Used in the Treatment of Cancer. Chem. Rev. 2009, 109, 2880–2893. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Hatse, S.; De Clercq, E.; Balzarini, J. Role of Antimetabolites of Purine and Pyrimidine Nucleotide Metabolism in Tumor Cell Differentiation. Biochem. Pharmacol. 1999, 58, 539–555. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Adinolfi, E. Extracellular Purines, Purinergic Receptors and Tumor Growth. Oncogene 2017, 36, 293–303. [Google Scholar] [CrossRef]
- Bonate, P.L.; Arthaud, L.; Cantrell, W.R.; Stephenson, K.; Secrist, J.A.; Weitman, S. Discovery and Development of Clofarabine: A Nucleoside Analogue for Treating Cancer. Nat. Rev. Drug Discov. 2006, 5, 855–863. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Rida, S.M.; Ashour, F.A.; El-Hawash, S.A.M.; El-Semary, M.M.; Badr, M.H. Synthesis of Some Novel Substituted Purine Derivatives As Potential Anticancer, Anti-HIV-1 and Antimicrobial Agents. Arch. Pharm. 2007, 340, 185–194. [Google Scholar] [CrossRef]
- Kang, D.; Fang, Z.; Huang, B.; Zhang, L.; Liu, H.; Pannecouque, C.; Naesens, L.; De Clercq, E.; Zhan, P.; Liu, X. Synthesis and Preliminary Antiviral Activities of Piperidine-substituted Purines against HIV and Influenza A/H1N1 Infections. Chem. Biol. Drug Des. 2015, 86, 568–577. [Google Scholar] [CrossRef]
- Ashour, F.A.; Rida, S.M.; El-Hawash, S.A.M.; ElSemary, M.M.; Badr, M.H. Synthesis, Anticancer, Anti-HIV-1, and Antimicrobial Activity of Some Tricyclic Triazino and Triazolo [4,3-e]Purine Derivatives. Med. Chem. Res. 2012, 21, 1107–1119. [Google Scholar] [CrossRef]
- Kataev, V.E.; Garifullin, B.F. Antiviral Nucleoside Analogs. Chem. Heterocycl. Comp. 2021, 57, 326–341. [Google Scholar] [CrossRef]
- Wong, X.K.; Yeong, K.Y. From Nucleic Acids to Drug Discovery: Nucleobases as Emerging Templates for Drug Candidates. CMC 2021, 28, 7076–7121. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, D.A.; Musiyak, V.V.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Purine Derivatives with Antituberculosis Activity. Russ. Chem. Rev. 2018, 87, 604–618. [Google Scholar] [CrossRef]
- Conejo-Garcia, A.; Cruz-Lopez, O.; Gomez-Perez, V.; Morales, F.; Garcia-Rubino, M.E.; Kimatrai, M.; Nunez, M.C.; Campos, J.M. ChemInform Abstract: Synthesis of Purine Derivatives as Scaffolds for a Diversity of Biological Activities. ChemInform 2011, 42, chin.201113247. [Google Scholar] [CrossRef]
- Peacock, H.; Maydanovych, O.; Beal, P.A. N2-Modified 2-Aminopurine Ribonucleosides as Minor-Groove-Modulating Adenosine Replacements in Duplex RNA. Org. Lett. 2010, 12, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhu, Y.; Zhao, Z.; Du, J.; Yang, X.; Fang, H.; Hou, X. Structure-Based Design of 2-Aminopurine Derivatives as CDK2 Inhibitors for Triple-Negative Breast Cancer. Front. Pharmacol. 2022, 13, 864342. [Google Scholar] [CrossRef]
- Fernández-Cureses, G.; de Castro, S.; Jimeno, M.; Balzarini, J.; Camarasa, M. Design, Synthesis, and Biological Evaluation of Unconventional Aminopyrimidine, Aminopurine, and Amino-1,3,5-triazine Methyloxynucleosides. Chem. Med. Chem. 2015, 10, 321–335. [Google Scholar] [CrossRef]
- Griffin, R.J.; Henderson, A.; Curtin, N.J.; Echalier, A.; Endicott, J.A.; Hardcastle, I.R.; Newell, D.R.; Noble, M.E.M.; Wang, L.-Z.; Golding, B.T. Searching for Cyclin-Dependent Kinase Inhibitors Using a New Variant of the Cope Elimination. J. Am. Chem. Soc. 2006, 128, 6012–6013. [Google Scholar] [CrossRef]
- Sebris, A.; Traskovskis, K.; Novosjolova, I.; Turks, M. Synthesis and Photophysical Properties of 2-Azolyl-6-Piperidinylpurines. Chem. Heterocycl. Comp. 2021, 57, 560–567. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Gonçalves, J.N.D.; Pêra, A.S.; Senhorães, N.R.; Rodrigues, A.R.O.; Oliveira, R.; Coutinho, P.J.G.; Castanheira, E.M.S.; Dias, A.M. Highly Fluorescent 2-Aminopurine Derivatives: Synthesis, Photo-physical Characterization, and Preliminary Cytotoxicity Evaluation. Eur. J. Org. Chem. 2023, 26, e202300176. [Google Scholar] [CrossRef]
- Jean, J.M.; Hall, K.B. 2-Aminopurine Electronic Structure and Fluorescence Properties in DNA. Biochemistry 2002, 41, 13152–13161. [Google Scholar] [CrossRef] [PubMed]
- Rachofsky, E.L.; Osman, R.; Ross, J.B.A. Probing Structure and Dynamics of DNA with 2-Aminopurine: Effects of Local Environment on Fluorescence. Biochemistry 2001, 40, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Tenor, E.; Kröger, C. Über 1.2.4-Triazole, VII. Synthese Und Reaktivität von 7-Amino-s-triazolo [1.5-a]Pyrimidonen-(5). Chem. Ber. 1964, 97, 1373–1382. [Google Scholar] [CrossRef]
- Savateev, K.V.; Ulomsky, E.N.; Borisov, S.S.; Voinkov, E.K.; Fedotov, V.V.; Rusinov, V.L. 8-Alkyl[1,2,4]Triazolo [5,1-b]Purines. Chem. Heterocycl. Comp. 2014, 50, 880–887. [Google Scholar] [CrossRef]
- Gazizov, D.A.; Gorbunov, E.B.; Rusinov, G.L.; Ulomsky, E.N.; Charushin, V.N. A New Family of Fused Azolo [1,5-a]Pteridines and Azolo [5,1-b]Purines. ACS Omega 2020, 5, 18226–18233. [Google Scholar] [CrossRef]
- Neymash, A.O.; Ulomsky, E.N.; Fedotov, V.V.; Aminov, S.V.; Lyapustin, D.N.; Gorbunov, E.B.; Ishimnikov, V.A.; Slepukhin, P.A.; Rusinov, V.L. Reconstructive Methodology in the Synthesis of 2-Aminopurine. Molecules 2023, 29, 134. [Google Scholar] [CrossRef]
- Gazizov, D.A.; Fedotov, V.V.; Gorbunov, E.B.; Ulomskiy, E.N.; Yeltsov, O.S.; Rusinov, G.L.; Rusinov, V.L. Effective Method for the Synthesis of Azolo [1,5-a]Pyrimidin-7-Amines. Chem. Heterocycl. Comp. 2019, 55, 573–577. [Google Scholar] [CrossRef]
- Gazizov, D.A.; Fedotov, V.V.; Chistyakov, K.A.; Gorbunov, E.B.; Rusinov, G.L.; Charushin, V.N. Access to Azolopyrimidine-6,7-Diamines as a Valuable “Building-Blocks” to Develop New Fused Heteroaromatic Systems. Tetrahedron 2021, 89, 132172. [Google Scholar] [CrossRef]
- Taher, A.; Smith, V.J. N.-(4-Aminopyrimidin-5-Yl)-4-Methyl-N.-(4-Methylphenylsulfonyl)Benzenesulfonamide. Acta Crystallogr. E Struct. Rep. Online 2012, 68, o3362. [Google Scholar] [CrossRef]
- Ratsep, P.C.; Pless, R.C. Hydrolysis of 2-Aminopurine Deoxyribonucleoside in Neutral Solution. J. Org. Chem. 1988, 53, 3241–3246. [Google Scholar] [CrossRef]
- Undheim, K.; Benneche, T. Pyrimidines and Their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry II; Elsevier: Cambridge, MA, USA, 1996; pp. 93–231. ISBN 9780080965185. [Google Scholar]
- Das, S.; Sahana, A.; Banerjee, A.; Lohar, S.; Safin, D.A.; Babashkina, M.G.; Bolte, M.; Garcia, Y.; Hauli, I.; Mukhopadhyay, S.K.; et al. Ratiometric Fluorescence Sensing and Intracellular Imaging of Al3+ Ions Driven by an Intramolecular Excimer Formation of a Pyrimidine–Pyrene Scaffold. Dalton Trans. 2013, 42, 4757. [Google Scholar] [CrossRef] [PubMed]
- Batista, V.S.; Crabtree, R.H.; Konezny, S.J.; Luca, O.R.; Praetorius, J.M. Oxidative Functionalization of Benzylic C–H Bonds by DDQ. New J. Chem. 2012, 36, 1141. [Google Scholar] [CrossRef]
- Fedotov, V.V.; Ulomsky, E.N.; Belskaya, N.P.; Eltyshev, A.K.; Savateev, K.V.; Voinkov, E.K.; Lyapustin, D.N.; Rusinov, V.L. Benzimidazoazapurines: Design, Synthesis, and Photophysical Study. J. Org. Chem. 2021, 86, 8319–8332. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Zhdankin, V.V. Recent Progress in Synthetic Applications of Hypervalent Iodine(III) Reagents. Chem. Rev. 2024, 124, 11108–11186. [Google Scholar] [CrossRef]
- Sun, X.; Yu, M.; Mu, X.; Zhou, Z.; Wang, L.; Liu, J.; Liu, X. A Facile Approach to [1,2,4]Triazolo [3,4-i]Purine via PIDA Oxidation Ring-closing Reaction. J. Heterocycl. Chem. 2021, 58, 2270–2279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).