Regioselective Bond-Forming and Hydrolysis Reactions of Doubly Charged Vanadium Oxide Anions in the Gas Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mass Spectrometry
3. Results and Discussion
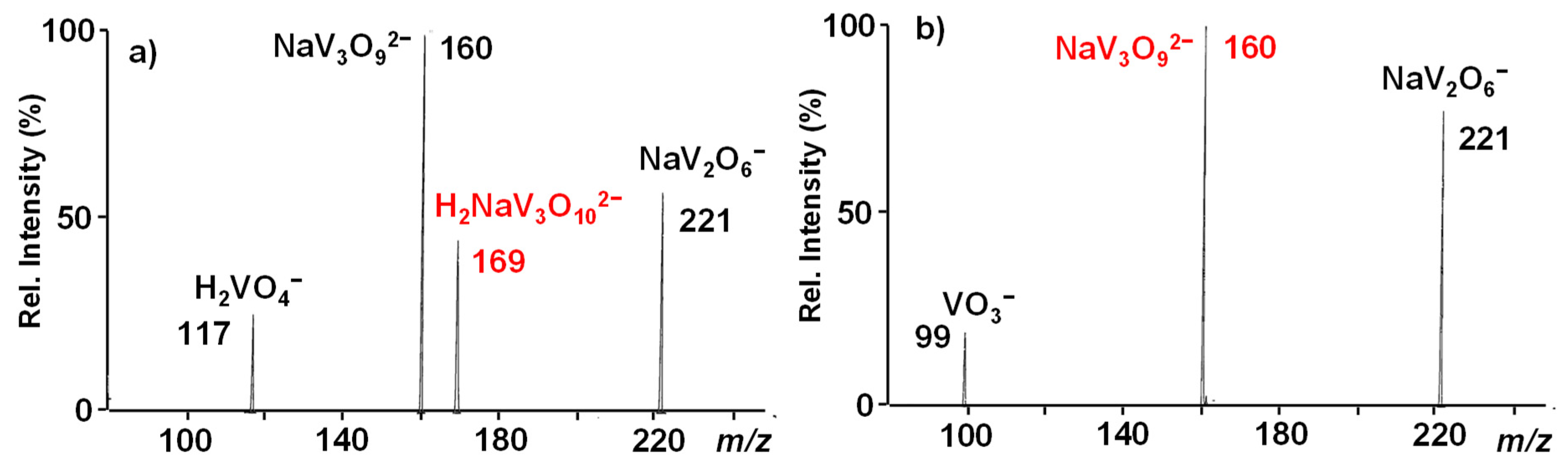
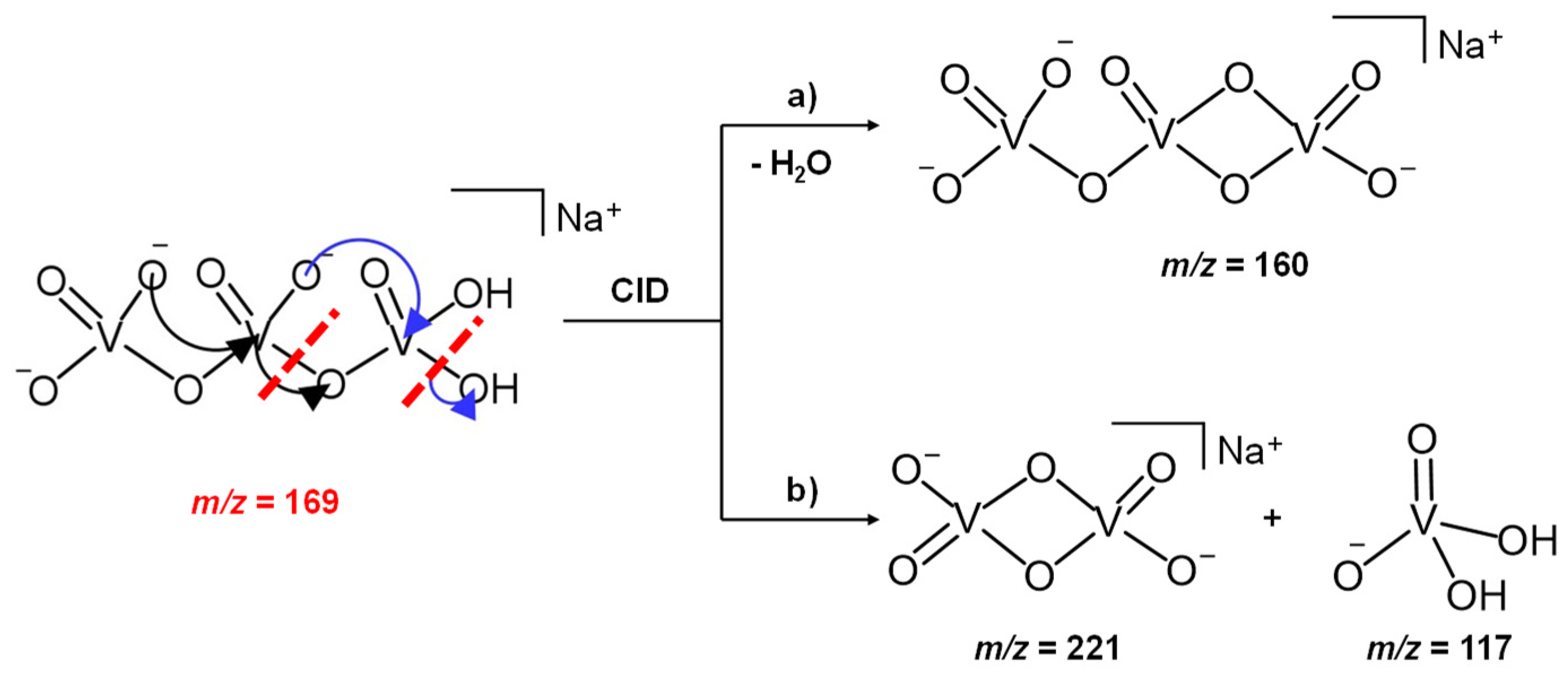
3.1. The NaV3O92− and H2NaV3O102− Reactant Dianions
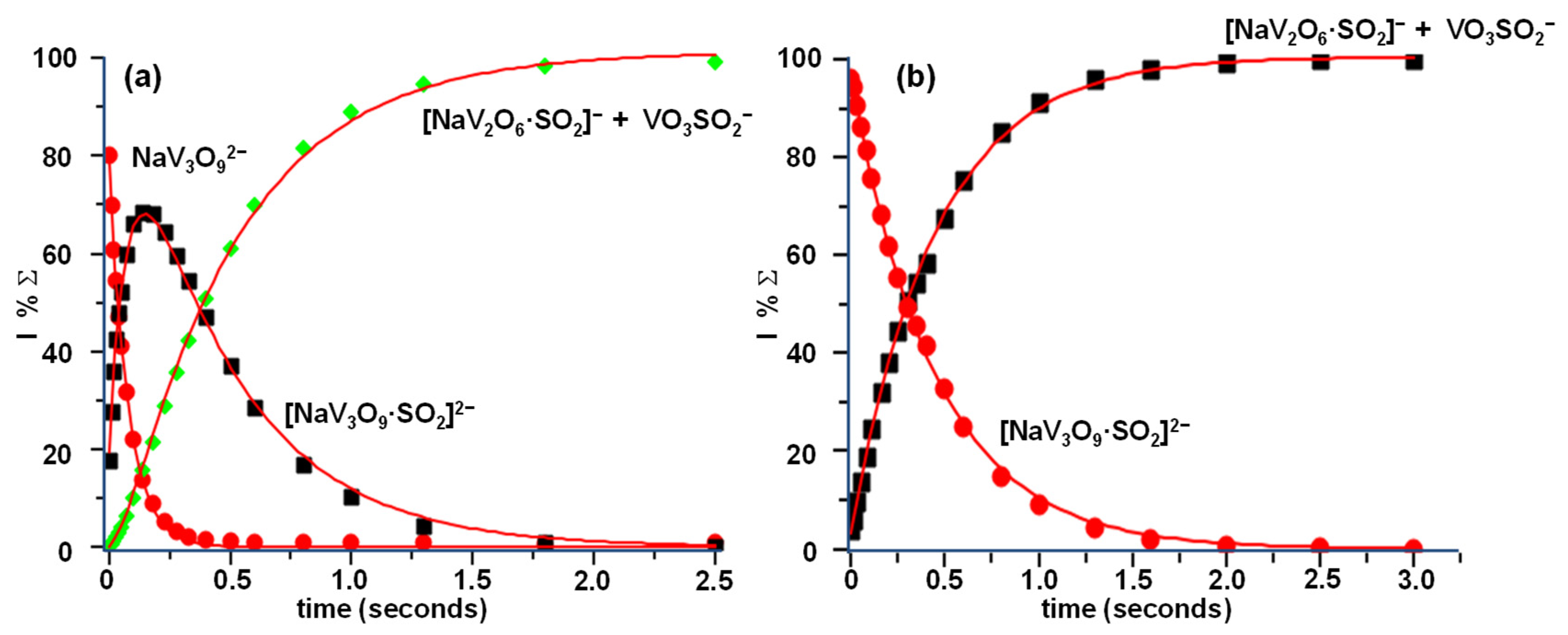
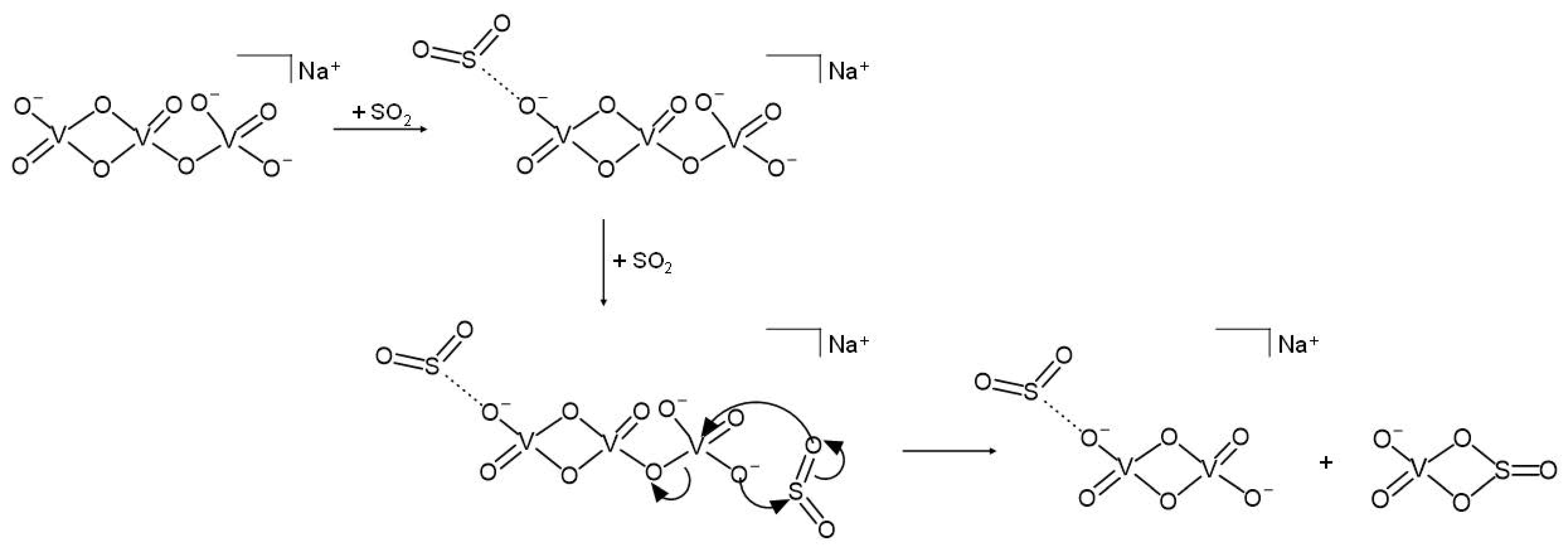
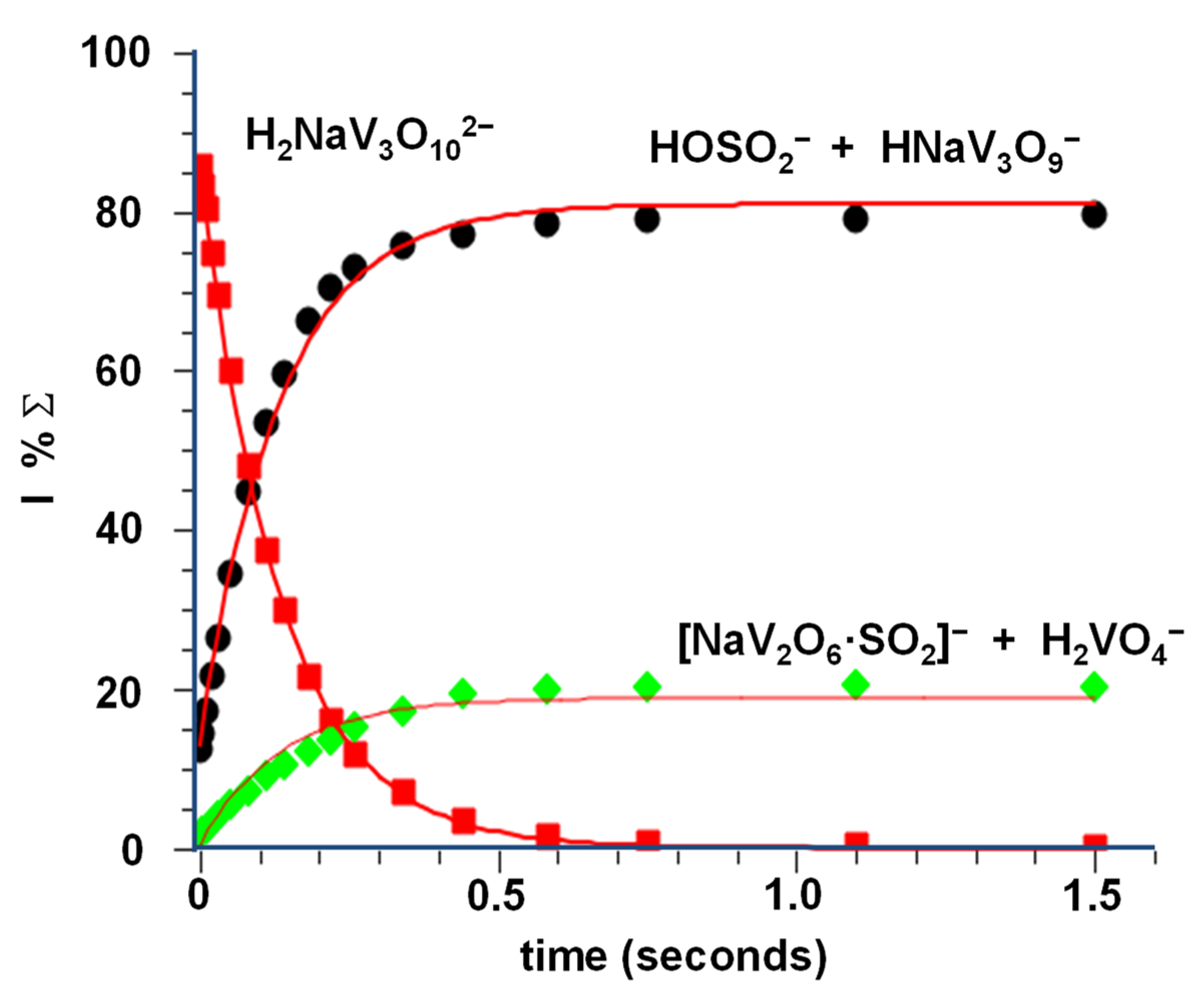
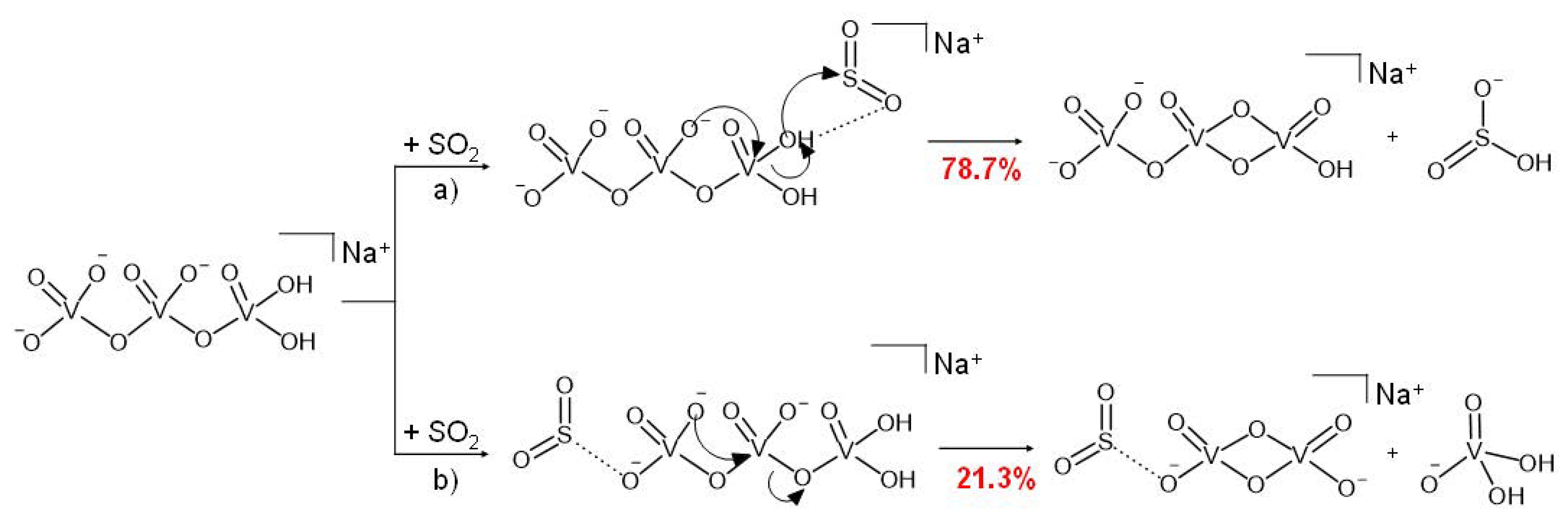
3.2. Reactivity of NaV3O92− and H2NaV3O102− Dianions towards SO2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Weckhuysen, B.M.; Keller, D.E. Chemistry, Spectroscopy and the role of supported vanadium oxides. Catal. Today 2003, 78, 25–46. [Google Scholar] [CrossRef]
- Chenier, J.P. Survey of Industrial Chemistry; John Wiley & Sons: New York, NY, USA, 1987; pp. 45–57. [Google Scholar]
- Whittingham, M.S. Lithium batteries and cathode materials. Chem. Rev. 2004, 104, 4271–4302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Song, H.; Zhang, C.; Liu, C.; Liu, Y.; Cao, G. Interface reduction synthesis of H2V2O3 nanobelts-graphene for high-rate Li-ion batteries. J. Phys. Chem. C 2015, 119, 11399. [Google Scholar]
- Xu, X.; Yan, M.; Tian, X.; Yang, C.; Shi, M.; Wei, Q.; Xu, L.; Mai, L. In situ investigation of Li and Na transport with single nanowire electrochemical devices. Nano Lett. 2015, 15, 3879–3884. [Google Scholar] [CrossRef]
- Anjass, M.; Lowe, G.A.; Streb, C. Molecular vanadium oxides for energy conversion and energy storage: Current trends and emerging opportunities. Angew. Chem. Int. Ed. 2021, 60, 7522–7532. [Google Scholar] [CrossRef]
- Johnson, G.; Tyo, C.C.; Castleman, A.W. Cluster reactivity experiments: Employing mass spectrometry to investigate the molecular level details of catalytic oxidation reactions. Proc. Natl. Acad. Sci. USA 2008, 105, 18108–18113. [Google Scholar] [CrossRef]
- Johnson, G.E.; Mitrić, R.; Bonačić-Koutecký, V.; Castleman, A. Clusters as model systems for investigating nanoscale oxidation catalysis. Chem. Phys. Lett. 2009, 475, 1–9. [Google Scholar] [CrossRef]
- Schlangen, M.; Schwarz, H. Effects of ligands, cluster size, and charge state in gas-phase catalysis: A happy marriage of experimental and computational studies. Catal. Lett. 2012, 142, 1265–1278. [Google Scholar] [CrossRef]
- Dietl, D.-C.N.; Troiani, A.; Schlangen, M.; Ursini, O.; Angelini, G.; Apeloig, Y.; de Petris, G.; Schwarz, H. Mechanistic aspects of gas-phase hydrogen-atom transfer from methane to [CO]+ and [SiO]+: Why do they differ? Chem. Eur. J. 2013, 19, 6662–6669. [Google Scholar] [CrossRef]
- Schlangen, M.; Schwarz, H. Probing elementary steps of nickel-mediated bond activation in gas-phase reactions: Ligand- and cluster-size effects. J. Catal. 2011, 284, 126–137. [Google Scholar] [CrossRef]
- Vikse, K.L.; McIndoe, J.S. Mechanistic insights from mass spectrometry: Examination of the elementary steps of catalytic reactions in the gas phase. Pure Appl. Chem. 2015, 87, 361–377. [Google Scholar] [CrossRef]
- Vikse, K.L.; Ahmadi, Z.; McIndoe, J.S. The application of electrospray ionization mass spectrometry to homogeneous catalysis. Coord. Chem. Rev. 2014, 279, 96–114. [Google Scholar] [CrossRef]
- Bell, R.C.; Zemski, K.A.; Kerns, K.P.; Deng, H.T.; Castleman, A.W. Reactivities and collision-induced dissociation of Vanadium oxide clusters cations. J. Phys. Chem. A 1998, 102, 1733–1742. [Google Scholar] [CrossRef]
- Zemski, K.A.; Justes, D.R.; Castleman, A.W. Reactions of group V transition metal oxide cluster ions with ethane and ethylene. J. Phys. Chem. A 2001, 105, 10237–10245. [Google Scholar] [CrossRef]
- Feyel, S.; Schröder, D.; Rozanska, X.; Sauer, J.; Schwarz, H. Gas-phase oxidation of propane and 1-butene with [V3O7]+: Experiment and theory in concert. Angew. Chem. Int. Ed. 2006, 45, 4677–4681. [Google Scholar] [CrossRef] [PubMed]
- Feyel, S.; Döbler, J.; Schröder, D.; Sauer, J.; Schwarz, H. Thermal activation of methane by tetranuclear [V4O10]+. Angew. Chem. Int. Ed. 2006, 45, 4681–4685. [Google Scholar] [CrossRef] [PubMed]
- Dietl, N.; Engeser, M.; Schwarz, H. Competitive hydrogen-atom abstraction versus oxygen-atom and electron transfers in gas-phase reactions of [X4O10]+ (X = P, V) with C2H4. Chem. Eur. J. 2010, 16, 4452–4456. [Google Scholar] [CrossRef]
- Zhou, S.; Li, J.; Schlangen, M.; Schwarz, H. Differences and commonalities in the gas-phase reactions of closed-shell metal dioxide clusters [MO2]+ (M = V, Nb, and Ta) with methane. Chem. Eur. J. 2016, 22, 7225–7228. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Wu, X.N.; Zhao, Y.X.; Ma, J.B.; Ding, X.L.; He, S.G. Room-temperature methane activation by a bimetallic oxide cluster AlVO4+. Chem. Phys. Lett. 2010, 489, 25–29. [Google Scholar] [CrossRef]
- Dietl, N.; Hockendorf, R.F.; Schlangen, M.; Lerch, M.; Beyer, M.K.; Schwarz, H. Generation, reactivity towards hydrocarbons, and electronic structure of heteronuclear vanadium phosphorous oxygen cluster ions. Angew. Chem. Int. Ed. 2011, 50, 1430–1434. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Liu, J.-W.; Schlangen, M.; Weiske, T.; Schröder, D.; Sauer, J.; Schwarz, H. Thermal methane activation by a binary V-Nb transition-metal oxide cluster cation: A further example for the crucial role of oxygen-centered radical. Chem. Eur. J. 2013, 19, 11496–11501. [Google Scholar] [CrossRef] [PubMed]
- Dietl, N.; Wende, T.; Chen, K.; Jiang, L.; Schlangen, M.; Zhang, X.; Asmis, K.R.; Schwarz, H. Structure and chemistry of the heteronuclear oxo-cluster [VPO4]·+: A model system for the gas-phase oxidation of small hydrocarbons. J. Am. Chem. Soc. 2013, 135, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Y.; Li, H.F.; Zhao, Y.X.; He, S.G. Gold(III) mediated activation and transformation of methane on Au1-doped vanadium oxide cluster cations AuV2O6+. J. Am. Chem. Soc. 2016, 138, 9437–9443. [Google Scholar] [CrossRef]
- Bell, R.C.; Castleman, A.W. Reactions of vanadium oxide cluster ions with 1,3-butadiene and isomers of butene. J. Phys. Chem. A 2002, 106, 9893–9899. [Google Scholar] [CrossRef]
- Li, X.-N.; Xu, B.; Ding, X.-L.; He, S.-G. Interaction of vanadium oxide cluster anions with water: An experimental and theoretical study on the reactivity and mechanism. Dalton Trans. 2012, 41, 5562–5570. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mirabal, A.; Demuth, J.; Wöste, L.; Siebert, T. A complete reactant-product analysis of the oxygen transfer reaction in [V4O11·C3H6]−: A cluster complex for modelling surface activation and reactivity. J. Am. Chem. Soc. 2008, 130, 16832–16833. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Wu, X.-N.; Ma, J.-B.; He, S.-G.; Ding, X.-L. Experimental and theoretical study of the reactions between vanadium-silicon heteronuclear oxide cluster anions with n-butane. J. Phys. Chem. C 2010, 114, 12271–12279. [Google Scholar] [CrossRef]
- Janssens, E.; Lang, S.M.; Brümmer, M.; Niedziela, A.; Santambrogio, G.; Asmis, K.R.; Sauer, J. Kinetic study of the reaction of vanadium and vanadium-titanium oxide cluster anions with SO2. Phys. Chem. Chem. Phys. 2012, 14, 14344–14353. [Google Scholar] [CrossRef]
- Dinca, A.; Davis, T.P.; Fisher, K.J.; Smith, D.R.; Willett, G.D. Vanadium oxide anion cluster reactions with methyl isobutyrate and methyl methacrylate monomer and dimer: A study by FT/ICR mass spectrometry. Int. J. Mass Spectrom. 1999, 182–183, 73–84. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Z.-Y.; Zhou, Z.-X.; Liu, Q.-Y.; He, S.-G. Thermal reactions of (V2O5)nO− (n = 1–3) cluster anions with ethylene and propylene: Oxygen atom transfer versus molecular association. J. Phys. Chem. C 2014, 118, 14967–14976. [Google Scholar] [CrossRef]
- Jakubikova, E.; Bernestein, E.R. Reactions of sulfur dioxide with neutral vanadium oxide clusters in the gas phase. I. Density functional theory study. J. Phys. Chem. A 2007, 111, 13339–13346. [Google Scholar] [CrossRef] [PubMed]
- He, S.-G.; Xie, Y.; Dong, F.; Heinbuch, S.; Jakubikova, E.; Rocca, J.J.; Bernstein, E.R. Reactions of sulfur dioxide with neutral vanadium oxide clusters in the gas phase. II. Experimental study employing single-photon ionization. J. Phys. Chem. A 2008, 112, 11067–11077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coelho, F.; Eberlin, M.N. The bridge connecting gas-phase and solution chemistries. Angew. Chem. Int. Ed. 2011, 50, 5261–5263. [Google Scholar] [CrossRef] [PubMed]
- Schröder, D.; Engeser, M.; Schwarz, H.; Harvey, J.N. Energetics of the ligated vanadium dications VO2+, VOH2+, and [V,O,H2]2+. ChemPhysChem 2002, 3, 584–591. [Google Scholar] [CrossRef]
- Warzok, U.; Mahnke, L.K.; Bensch, W. Soluble hetero-polyoxovanadates and their solution chemistry analysed by electrospray ionization mass spectrometry. Chem. Eur. J. 2019, 25, 1405–1419. [Google Scholar] [CrossRef]
- Ranasinghe, Y.A.; MacMahon, T.J.; Freiser, B.S. Gas-phase reactions of lanthanum dication with small alkanes and the photodissociation of LaC2H4n+ and LaC3H6n+ (n = 1 and 2). J. Am. Chem. Soc. 1992, 114, 9112–9118. [Google Scholar] [CrossRef]
- Petrie, S.; Javahery, G.; Wang, J.; Bohme, D.K. Derivatization of the fullerene dications C602+ and C702+ by ion-molecule reactions in the gas phase. J. Am. Chem. Soc. 1992, 114, 9177–9181. [Google Scholar] [CrossRef]
- Price, S.D.; Manning, M.; Leone, S.R. Bond-forming reactions of gas-phase molecular dications. J. Am. Chem. Soc. 1994, 116, 8673–8680. [Google Scholar] [CrossRef]
- Roithová, J.; Herman, Z.; Schröder, D.; Schwarz, H. Competition of proton and electron transfers in gas-phase reactions of hydrogen-containing dications CHX2+ (X = F, Cl, Br, I) with atoms, nonpolar and polar molecules. Chem. Eur. J. 2006, 12, 2465–2471. [Google Scholar] [CrossRef]
- Roithová, J.; Schröder, D. Bond-forming reactions of molecular dications as a new route to polyaromatic hydrocarbons. J. Am. Chem. Soc. 2006, 128, 4208–4209. [Google Scholar] [CrossRef]
- Roithová, J.; Žabka, J.; Herman, Z.; Thissen, R.; Schröder, D.; Schwarz, H. Reactivity of the CHBr2+ dication toward molecular hydrogen. J. Phys. Chem. A 2006, 110, 6447–6453. [Google Scholar] [CrossRef]
- Roithová, J.; Schröder, D. Bimolecular reactions of molecular dications: Reactivity paradigms and bond-forming processes. Phys. Chem. Chem. Phys. 2007, 9, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Roithová, J.; Schröder, D.; Schwarz, H. The SiF32+ dication: Chemistry counts! Chem. Eur. J. 2009, 15, 9995–9999. [Google Scholar] [CrossRef] [PubMed]
- Blades, A.; Peschke, M.; Verkerk, U.; Kebarle, P. Rates of proton transfer from carboxylic acids to dianions, CO2(CH2)pCO22−, and their significance to observed negative charge states of proteins in the gas phase. J. Phys. Chem. A 2002, 106, 10037–10042. [Google Scholar] [CrossRef]
- Flores, A.E.; Gronert, S. The gas-phase reactions of dianions with alkyl bromides: Direct identification of SN2 and E2 products. J. Am. Chem. Soc. 1999, 121, 2627–2628. [Google Scholar] [CrossRef]
- Gronert, S. Gas phase studies of the competition between substitution and elimination reactions. Acc. Chem. Res. 2003, 36, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Vanadium hydroxyde cluster ions in the gas-phase: Bond-forming reactions of doubly-charged negative ions by SO2-promoted V-O activation. Chem. Eur. J. 2017, 23, 11752–11756. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Sulphur dioxide cooperation in hydrolysis of vanadium oxide and hydroxide cluster dianions. New J. Chem. 2018, 42, 4008–4016. [Google Scholar] [CrossRef]
- de Petris, G.; Cartoni, A.; Troiani, A.; Angelini, G.; Ursini, O. Water activation by SO2+ ions: An effective source of OH radicals. PhysChemChemPhys 2009, 11, 9976–9978. [Google Scholar] [CrossRef]
- de Petris, G.; Troiani, A.; Rosi, M.; Angelini, G.; Ursini, O. Selective Activation of C-Cl and C-F Bonds by SO+ Radical Cations: An Experimental and Computational Study. ChemPlusChem 2013, 78, 1065–1072. [Google Scholar] [CrossRef]
- Troiani, A.; Rosi, M.; Salvitti, C.; de Petris, G. The oxidation of sulfur dioxide by single and double oxygen transfer paths. ChemPhysChem 2014, 15, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Salvitti, C.; de Petris, G. Gas-phase reactivity of carbonate ions with sulfur dioxide: An experimental study of cluster reactions. J. Am. Chem. Soc. Mass Spectrom. 2019, 30, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Effective redox reactions by chromium oxide anions: Sulfur dioxide oxidation in the gas phase. Int. J. Mass Spectrom. 2019, 436, 18–22. [Google Scholar] [CrossRef]
- Salvitti, C.; Rosi, M.; Pepi, F.; Troiani, A.; de Petris, G. Reactivity of transition metal dioxide anions MO2− (M = Co, Ni, Cu, Zn) with sulfur dioxide in the gas phase: An experimental and theoretical study. Chem. Phys. Lett. 2021, 776, 138555. [Google Scholar] [CrossRef]
- Salvitti, C.; Pepi, F.; Troiani, A.; de Petris, G. Intracluster sulphur dioxide oxidation by sodium chlorite anions: A mass spectrometric study. Molecules 2021, 26, 7114. [Google Scholar] [CrossRef] [PubMed]
- Troiani, A.; Rosi, M.; Garzoli, S.; Salvitti, C.; de Petris, G. Iron-Promoted C-C Bond Formation in the Gas Phase. Angew. Chem. Int. Ed. 2015, 54, 14359–14362. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, C.M.; Bryantsev, V.S.; de Jong, W.A.; Diallo, M.S.; Goddard, W.A., III; Groenewold, G.S.; Van Stipdonk, M.J. Addition of H2O and O2 to acetone and dimethylsulfoxide ligated Uranyl(V) dioxocations. J. Phys. Chem. A 2009, 113, 2350–2358. [Google Scholar] [CrossRef]
- Bartmess, J.E.; Georgiadis, R.M. Empirical methods for determination of ionization gauge relative sensitivities for different gases. Vacuum 1983, 33, 149. [Google Scholar] [CrossRef]
- Kuzmic, P. Program DYNAFIT for the analysis of enzyme kinetic data: Application to HIV proteinase. Anal. Biochem. 1996, 237, 260–273. [Google Scholar] [CrossRef]
- Bowers, M.T.; Su, T. Interactions between Ions and Molecules; Plenum Press: New York, NY, USA, 1975. [Google Scholar]
- Habayeb, M.A.; Hileman, O.E., Jr. 51V- FT-nmr investigations of metavanadate ions in aqueous solutions. Can. J. Chem. 1980, 58, 2255–2261. [Google Scholar] [CrossRef]
- Aureliano, M.; Cran, D.C. Decavanadate (V10O286−) and oxovanadates: Oxometalates with many biological activities. J. Inorg. Biochem. 2009, 103, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Walanda, D.K.; Burns, R.C.; Lawrance, G.A.; Von Nagy-Felsobuki, E.I. New isopolyoxovanadate ions identified by electrospray mass spectrometry. Inorg. Chem. Commun. 1999, 2, 487–489. [Google Scholar] [CrossRef]
- Walanda, D.K.; Burns, R.C.; Lawrance, G.A.; Von Nagy-Felsobuki, E.I. Unknown isopolyoxovanadate species detected by electrospray mass spectrometry. Inorg. Chim. Acta 2000, 305, 118–126. [Google Scholar] [CrossRef]
- Al Hasan, N.M.; Johnson, G.E.; Laskin, J. Gas-phase synthesis of singly and multiply charged polyoxovanadate anions employing electrospray ionization and collision induced dissociation. J. Am. Soc. Mass Spectrom. 2013, 24, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Cristy, T.; Graves, S.W.; Hooth, M.J.; Waidyanatha, S. Characterization of aqueous formulations of tetra- and pentavalent forms of vanadium in support of test article selection in toxicology studies. Environ. Sci. Pollut. Res. 2017, 24, 405–416. [Google Scholar] [CrossRef]
- O’Hair, R.A.J.; Freitas, M.A.; Gronert, S.; Schmidt, J.A.R.; Williams, T.D. Concerning the regioselectivity of gas phase reactions of glycine with electrophiles. The cases of the dimethylchlorinium ion and the methoxymethal cation. J. Org. Chem. 1995, 60, 1990–1998. [Google Scholar] [CrossRef]
- Van der Wel, H.; Nibbering, N.M.M. A gas phase study of the regioselective BH4− reduction of some 2-substituted maleic anhydrides. Can. J. Chem. 1998, 66, 2587–2594. [Google Scholar]
- Wernik, M.; Hartmann, P.E.; Sipos, G.; Darvas, F.; Boese, A.D.; Dallinger, D.; Kappe, C.O. On the regioselectivity of the Gould-Jacobs reaction: Gas-phase versus solution-phase thermolysis. Eur. J. Org. Chem. 2020, 2020, 7051–7061. [Google Scholar] [CrossRef]
- Avdeed, V.I.; Tapilin, V.M. Water effect on the electronic structure of active sites of supported Vanadium oxide catalyst VOx/TiO2(001). J. Phys. Chem. C 2010, 114, 3609–3613. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvitti, C.; Pepi, F.; Troiani, A.; de Petris, G. Regioselective Bond-Forming and Hydrolysis Reactions of Doubly Charged Vanadium Oxide Anions in the Gas Phase. Reactions 2022, 3, 254-264. https://doi.org/10.3390/reactions3020019
Salvitti C, Pepi F, Troiani A, de Petris G. Regioselective Bond-Forming and Hydrolysis Reactions of Doubly Charged Vanadium Oxide Anions in the Gas Phase. Reactions. 2022; 3(2):254-264. https://doi.org/10.3390/reactions3020019
Chicago/Turabian StyleSalvitti, Chiara, Federico Pepi, Anna Troiani, and Giulia de Petris. 2022. "Regioselective Bond-Forming and Hydrolysis Reactions of Doubly Charged Vanadium Oxide Anions in the Gas Phase" Reactions 3, no. 2: 254-264. https://doi.org/10.3390/reactions3020019
APA StyleSalvitti, C., Pepi, F., Troiani, A., & de Petris, G. (2022). Regioselective Bond-Forming and Hydrolysis Reactions of Doubly Charged Vanadium Oxide Anions in the Gas Phase. Reactions, 3(2), 254-264. https://doi.org/10.3390/reactions3020019





