Combination of Autohydrolysis and Catalytic Hydrolysis of Biomass for the Production of Hemicellulose Oligosaccharides and Sugars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Characterization of the Feedstock
2.3. Autohydrolysis
2.4. Catalyst Preparation
2.5. Catalytic Hydrolysis Reaction
2.6. HPLC Analysis
2.7. Post-Hydrolysis
2.8. Size Exclusion Chromatography (SEC) Analysis
2.9. Carbon Analysis
2.10. Calculations of Yields and Conversions
3. Results
3.1. Lignocellulosic Starting Materials
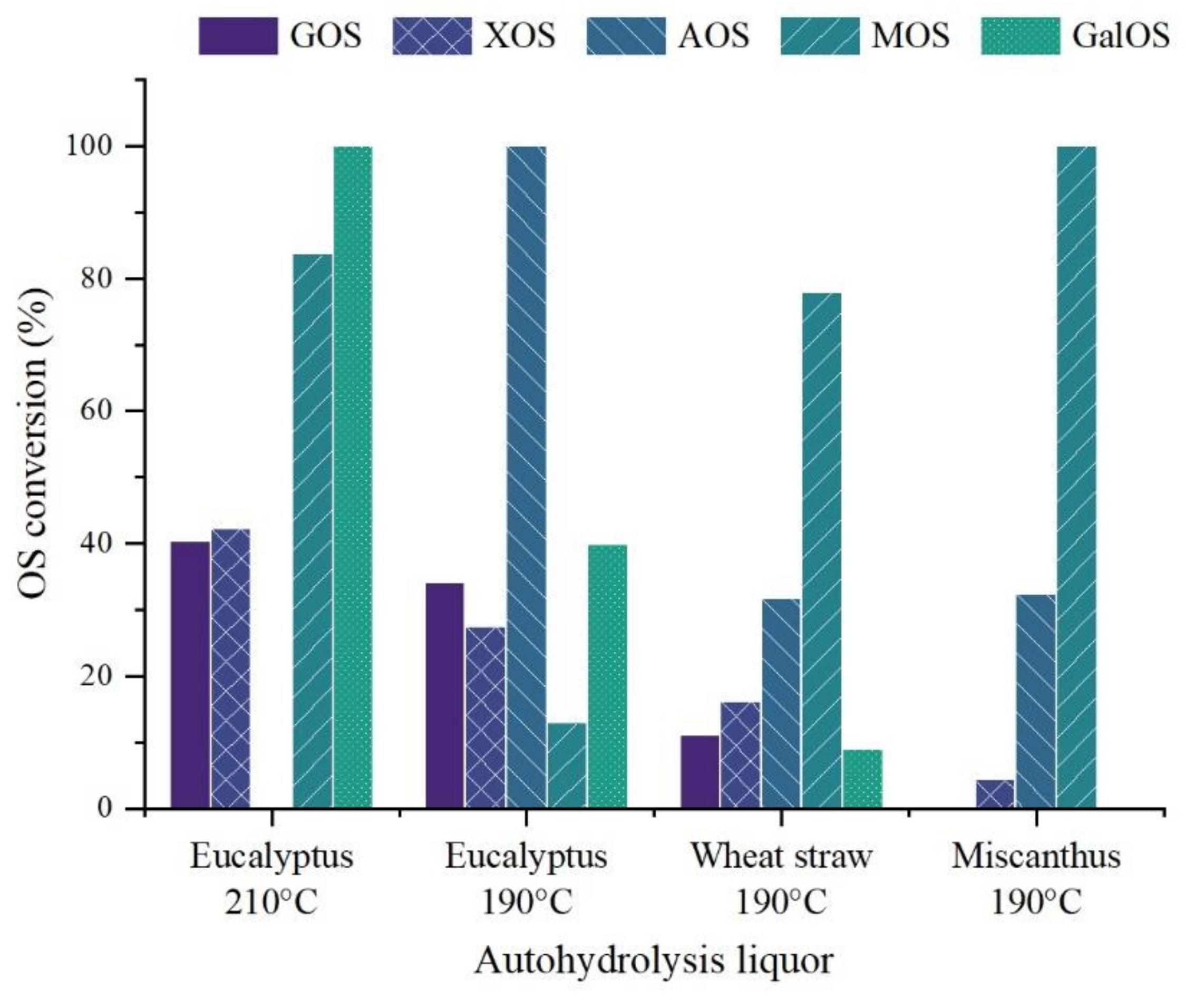
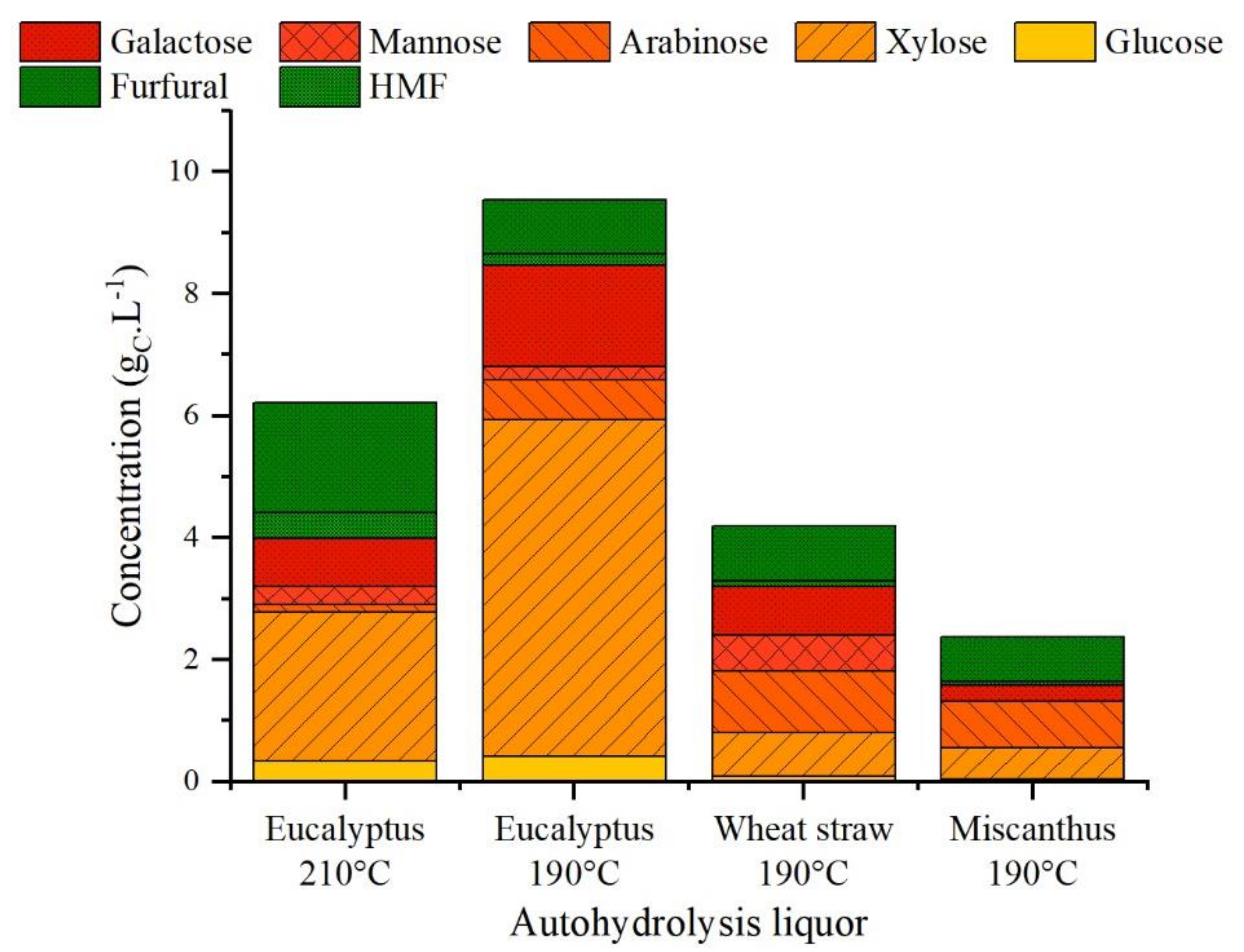
3.2. Autohydrolysis
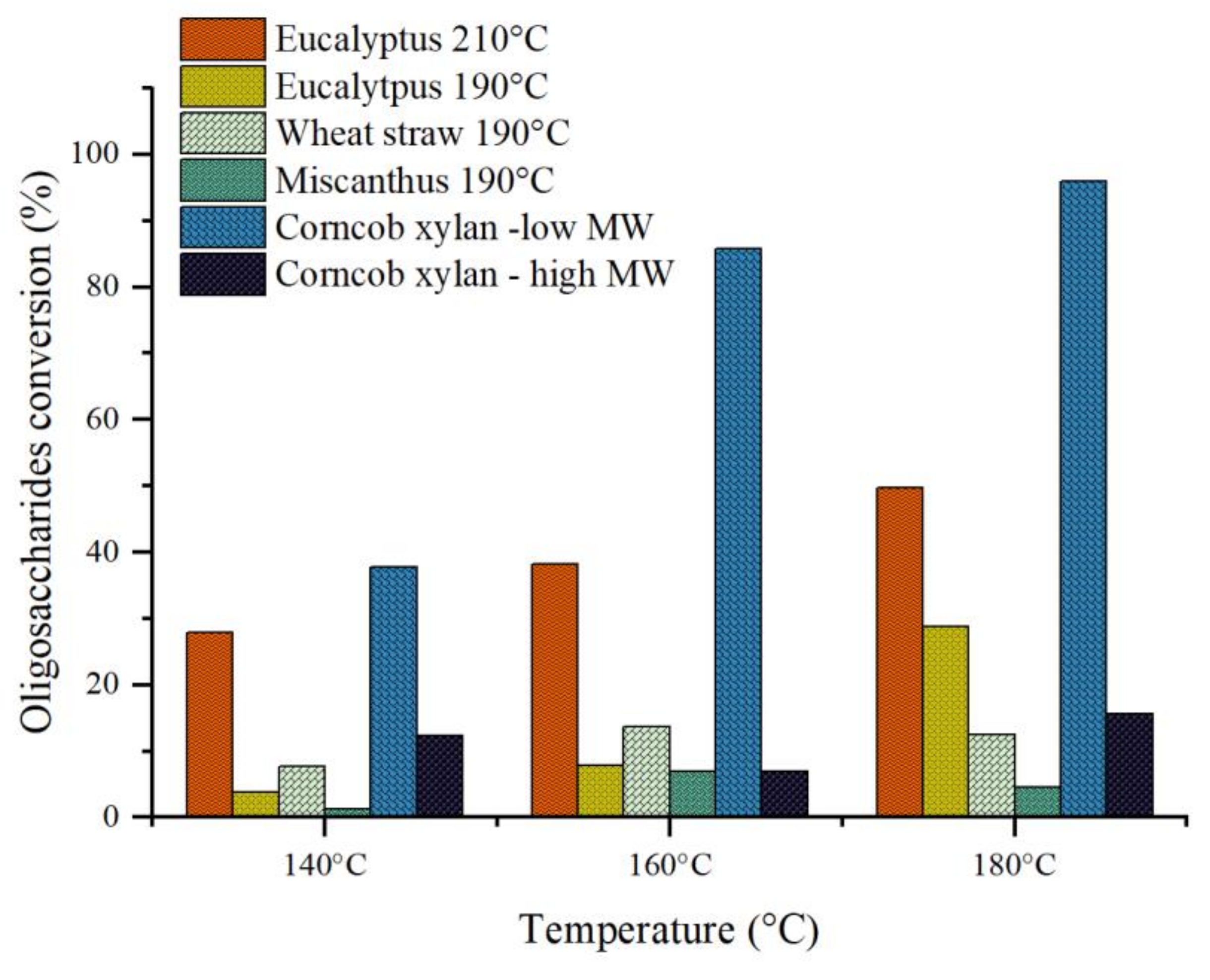
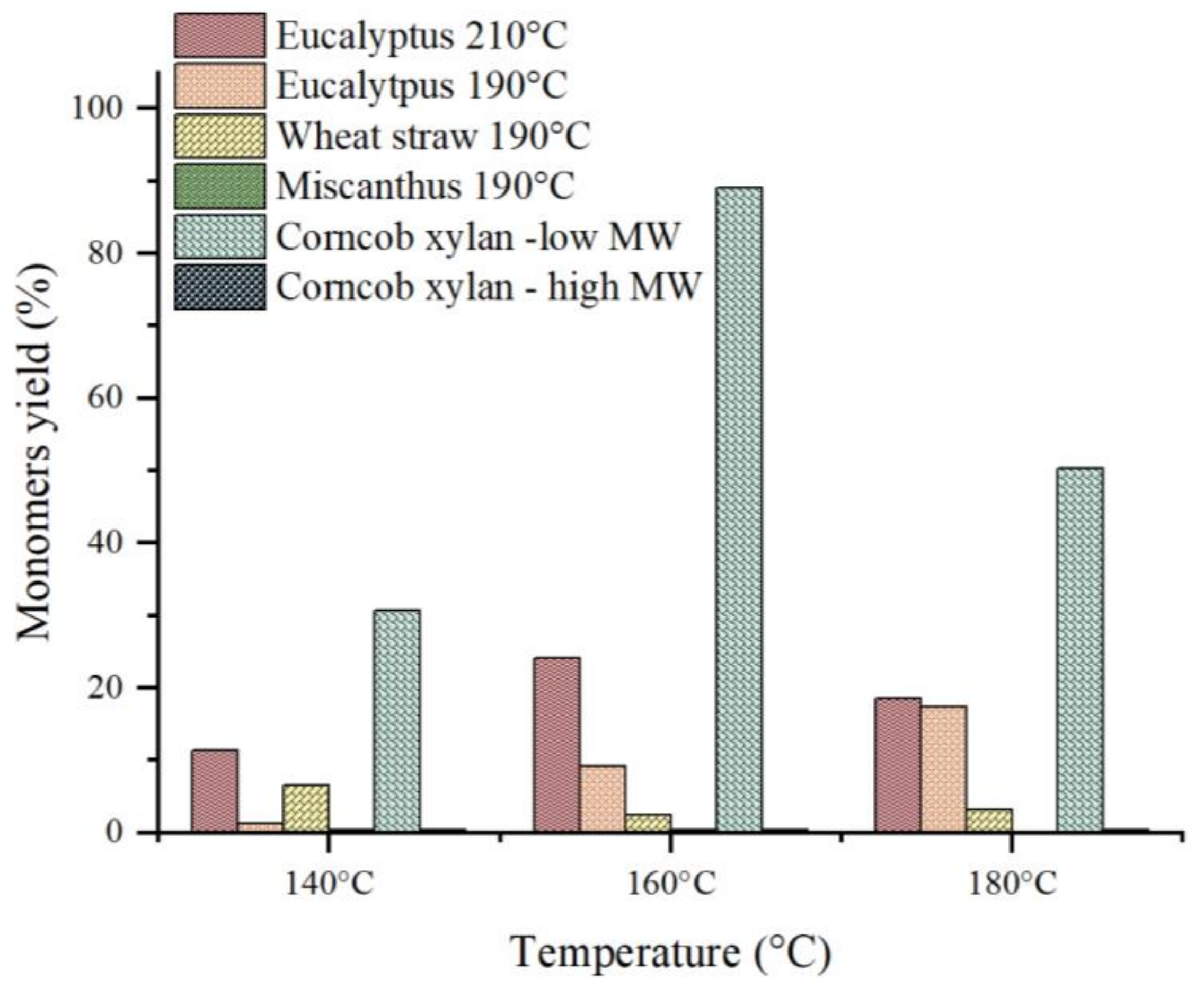
3.3. Catalytic Hydrolysis
4. Discussion
4.1. During Autohydrolysis
4.2. During Catalytic Hydrolysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mauser, W.; Klepper, G.; Zabel, F.; Delzeit, R.; Hank, T.; Putzenlechner, B.; Calzadilla, A. Global biomass production potentials exceed expected future demand without the need for cropland expansion. Nat. Commun. 2015, 6, 8946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäki-Arvela, P.; Salmi, T.; Holmbom, B.; Willför, S.; Murzin, D.Y. Synthesis of Sugars by Hydrolysis of Hemicelluloses—A Review. Chem. Rev. 2011, 111, 5638–5666. [Google Scholar] [CrossRef]
- Yamakawa, C.K.; Qin, F.; Mussatto, S.I. Advances and opportunities in biomass conversion technologies and biorefineries for the development of a bio-based economy. Biomass Bioenergy 2018, 119, 54–60. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z.; Heinze, T. Hemicellulose. In Polysaccharides I; Heinze, T., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 186, pp. 1–67. [Google Scholar]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740. [Google Scholar] [CrossRef]
- Broekaert, W.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J. Prebiotic and Other Health-Related Effects of Cereal-Derived Arabinoxylans, Arabinoxylan-Oligosaccharides, and Xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Delgado Arcaño, Y.; Valmaña García, O.D.; Mandelli, D.; Carvalho, W.A.; Magalhães Pontes, L.A. Xylitol: A review on the progress and challenges of its production by chemical route. Catal. Today 2020, 344, 2–14. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass; NREL Report DOE/GO-102004-1992; 2004. Available online: https://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 30 November 2021).
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural-A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Brosse, N.; Dufour, A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuels Bioprod. Biorefin. 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzyme Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864. [Google Scholar]
- Júnior, D.L.; Ayoub, A.; Venditti, R.A.; Jameel, H.; Colodette, J.L.; Chang, H. min Ethanol precipitation of hetero-polysaccharide material from hardwood by alkaline extraction prior to the kraft cooking process. BioResources 2013, 8, 5319–5332. [Google Scholar] [CrossRef] [Green Version]
- Salas-Veizaga, D.M.; Villagomez, R.; Linares-Pastén, J.A.; Carrasco, C.; Álvarez, M.T.; Adlercreutz, P.; Nordberg Karlsson, E. Extraction of Glucuronoarabinoxylan from Quinoa Stalks (Chenopodium quinoa Willd.) and Evaluation of Xylooligosaccharides Produced by GH10 and GH11 Xylanases. J. Agric. Food Chem. 2017, 65, 8663–8673. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Chang, M.-J.; Chang, W.-J. Comparison of Dilute Acid and Sulfite Pretreatments on Acacia confusa for Biofuel Application and the Influence of Its Extractives. J. Agric. Food Chem. 2014, 62, 10768–10775. [Google Scholar] [CrossRef]
- Lavarack, B.P.; Griffin, G.J.; Rodman, D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy 2002, 23, 367–380. [Google Scholar] [CrossRef]
- Grande, P.M.; Viell, J.; Theyssen, N.; Marquardt, W.; Domínguez de María, P.; Leitner, W. Fractionation of lignocellulosic biomass using the OrganoCat process. Green Chem. 2015, 17, 3533–3539. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Li, Z.; Zuo, Y.; Su, Z.; Hu, C. Effects of γ-Valerolactone/H 2 O Solvent on the Degradation of pubescens for Its Fullest Utilization. J. Agric. Food Chem. 2018, 66, 6094–6103. [Google Scholar] [CrossRef]
- Loow, Y.-L.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Md. Jahim, J.; Mohammad, A.W.; Teoh, W.H. Recent Advances in the Application of Inorganic Salt Pretreatment for Transforming Lignocellulosic Biomass into Reducing Sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, Z.; Jin, Y.; Matsumoto, Y.; Zhai, H. Effects of LiCl/DMSO dissolution and enzymatic hydrolysis on the chemical composition and lignin structure of rice straw. Biomass Bioenergy 2014, 71, 357–362. [Google Scholar] [CrossRef]
- Vanoye, L.; Fanselow, M.; Holbrey, J.D.; Atkins, M.P.; Seddon, K.R. Kinetic model for the hydrolysis of lignocellulosic biomass in the ionic liquid, 1-ethyl-3-methyl-imidazolium chloride. Green Chem. 2009, 11, 390. [Google Scholar] [CrossRef]
- Shamsudin, S.; Md Shah, U.K.; Zainudin, H.; Abd-Aziz, S.; Mustapa Kamal, S.M.; Shirai, Y.; Hassan, M.A. Effect of steam pretreatment on oil palm empty fruit bunch for the production of sugars. Biomass Bioenergy 2012, 36, 280–288. [Google Scholar] [CrossRef]
- Retsina, T.; Pylkkanen, V.; Zebroski, R. Production of Fermentable c5 and c6 Sugars from Lignocellulosic Biomass. U.S. Patent US 2015/0184260 Al, 2 July 2015. [Google Scholar]
- Rissanen, J.V.; Grénman, H.; Xu, C.; Willför, S.; Murzin, D.Y.; Salmi, T. Obtaining Spruce Hemicelluloses of Desired Molar Mass by using Pressurized Hot Water Extraction. ChemSusChem 2014, 7, 2947–2953. [Google Scholar] [CrossRef]
- Sella Kapu, N.; Trajano, H.L. Review of hemicellulose hydrolysis in softwoods and bamboo. Biofuels Bioprod. Biorefin. 2014, 8, 857–870. [Google Scholar] [CrossRef]
- Domínguez, E.; Nóvoa, T.; del Río, P.G.; Garrote, G.; Romaní, A. Sequential two-stage autohydrolysis biorefinery for the production of bioethanol from fast-growing Paulownia biomass. Energy Convers. Manag. 2020, 226, 113517. [Google Scholar] [CrossRef]
- Gullón, P.; Romaní, A.; Vila, C.; Garrote, G.; Parajó, J.C. Potential of hydrothermal treatments in lignocellulose biorefineries. Biofuels Bioprod. Biorefin. 2012, 6, 219–232. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; López, F.; Parajó, J.C. Eucalyptus globulus wood fractionation by autohydrolysis and organosolv delignification. Bioresour. Technol. 2011, 102, 5896–5904. [Google Scholar] [CrossRef] [PubMed]
- Loaiza Rodriguez, J.M.; Colodette, J.L.; García, J.C.; López, F. Autohydrolysis, Pulping, and Bleaching of Eucalyptus urograndis in a Biorefinery Framework. BioResources 2019, 14, 5467–5487. [Google Scholar] [CrossRef]
- Moniz, P.P.; Pereira, H.; Duarte, L.C.; Carvalheiro, F. Hydrothermal production and gel filtration purification of xylo-oligosaccharides from rice straw. Ind. Crops Prod. 2014, 62, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, W.; Smirnova, I. Hydrothermal flow-through treatment of wheat straw: Coupled heat and mass transfer modeling with changing bed properties. J. Supercrit. Fluids 2018, 133, 625–639. [Google Scholar] [CrossRef]
- Silva-Fernandes, T.; Duarte, L.C.; Carvalheiro, F.; Loureiro-Dias, M.C.; Fonseca, C.; Gírio, F. Hydrothermal pretreatment of several lignocellulosic mixtures containing wheat straw and two hardwood residues available in Southern Europe. Bioresour. Technol. 2015, 183, 213–220. [Google Scholar] [CrossRef] [Green Version]
- El Hage, R.; Chrusciel, L.; Desharnais, L.; Brosse, N. Effect of autohydrolysis of Miscanthus x giganteus on lignin structure and organosolv delignification. Bioresour. Technol. 2010, 101, 9321–9329. [Google Scholar] [CrossRef] [PubMed]
- Ligero, P.; van der Kolk, J.C.; de Vega, A.; van Dam, J.E.G. Production of xylo-oligosaccharides from Miscanthus x giganteus by autohydrolysis. BioResources 2011, 6, 4417–4429. [Google Scholar] [CrossRef]
- Garrote, G.; Domınguez, H.; Parajó, J.C. Generation of xylose solutions from Eucalyptus globulus wood by autohydrolysis—posthydrolysis processes: Posthydrolysis kinetics. Bioresour. Technol. 2001, 79, 155–164. [Google Scholar] [CrossRef]
- Duarte, L.C.; Silva-Fernandes, T.; Carvalheiro, F.; Gírio, F.M. Dilute Acid Hydrolysis of Wheat Straw Oligosaccharides. Appl. Biochem. Biotechnol. 2009, 153, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Silva-Fernandes, T.; Duarte, L.C.; Carvalheiro, F.; Marques, S.; Loureiro-Dias, M.C.; Fonseca, C.; Gírio, F. Biorefining strategy for maximal monosaccharide recovery from three different feedstocks: Eucalyptus residues, wheat straw and olive tree pruning. Bioresour. Technol. 2015, 183, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Sakai, Y. Hydrolysis Rate of Pentosan of Hardwood in Dilute Sulfuric Acid. Bull. Agric. Chem. Soc. Jpn. 1956, 20, 1–7. [Google Scholar] [CrossRef]
- Dussan, K.; Girisuta, B.; Lopes, M.; Leahy, J.J.; Hayes, M.H.B. Conversion of Hemicellulose Sugars Catalyzed by Formic Acid: Kinetics of the Dehydration of D-Xylose, L-Arabinose, and D-Glucose. ChemSusChem 2015, 8, 1411–1428. [Google Scholar] [CrossRef]
- Wyman, C.E.; Decker, S.R.; Himmel, M.E.; Brady, J.W.; Skopec, C.E.; Viikari, L. Hydrolysis of Cellulose and Hemicellulose. In Polysaccharides; Dumitriu, S., Ed.; Marcel Dekker, Inc.: Hanover, NH, USA, 2005; pp. 995–1033. [Google Scholar]
- Vilcocq, L.; Castilho, P.C.; Carvalheiro, F.; Duarte, L.C. Hydrolysis of Oligosaccharides Over Solid Acid Catalysts: A Review. ChemSusChem 2014, 7, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Hilpmann, G.; Becher, N.; Pahner, F.-A.; Kusema, B.; Mäki-Arvela, P.; Lange, R.; Murzin, D.Y.; Salmi, T. Acid hydrolysis of xylan. Catal. Today 2016, 259, 376–380. [Google Scholar] [CrossRef]
- Chung, P.-W.; Charmot, A.; Olatunji-Ojo, O.A.; Durkin, K.A.; Katz, A. Hydrolysis Catalysis of Miscanthus Xylan to Xylose Using Weak-Acid Surface Sites. ACS Catal. 2014, 4, 302–310. [Google Scholar] [CrossRef]
- Vilcocq, L.; Spinola, V.; Moniz, P.; Duarte, L.C.; Carvalheiro, F.; Fernandes, C.; Castilho, P. Acid-modified clays as green catalysts for the hydrolysis of hemicellulosic oligosaccharides. Catal. Sci. Technol. 2015, 5, 4072–4080. [Google Scholar] [CrossRef] [Green Version]
- Aellig, C.; Scholz, D.; Dapsens, P.Y.; Mondelli, C.; Pérez-Ramírez, J. When catalyst meets reactor: Continuous biphasic processing of xylan to furfural over GaUSY/Amberlyst-36. Catal. Sci. Technol. 2015, 5, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Murzin, D.Y.; Murzina, E.V.; Tokarev, A.; Shcherban, N.D.; Wärnå, J.; Salmi, T. Arabinogalactan hydrolysis and hydrolytic hydrogenation using functionalized carbon materials. Catal. Today 2015, 257, 169–176. [Google Scholar] [CrossRef]
- Ennaert, T.; Feys, S.; Hendrikx, D.; Jacobs, P.A.; Sels, B.F. Reductive splitting of hemicellulose with stable ruthenium-loaded USY zeolites. Green Chem. 2016, 18, 5295–5304. [Google Scholar] [CrossRef] [Green Version]
- Vilcocq, L.; Rebmann, É.; Cheah, Y.W.; Fongarland, P. Hydrolysis of Cellobiose and Xylan over TiO2 -Based Catalysts. ACS Sustain. Chem. Eng. 2018, 6, 5555–5565. [Google Scholar] [CrossRef]
- Chareonlimkun, A.; Champreda, V.; Shotipruk, A.; Laosiripojana, N. Catalytic conversion of sugarcane bagasse, rice husk and corncob in the presence of TiO2, ZrO2 and mixed-oxide TiO2-ZrO2 under hot compressed water (HCW) condition. Bioresour. Technol. 2010, 101, 4179–4186. [Google Scholar] [CrossRef] [PubMed]
- Putro, J.N.; Kurniawan, A.; Soetaredjo, F.E.; Lin, S.-Y.; Ju, Y.-H.; Ismadji, S. Production of gamma-valerolactone from sugarcane bagasse over TiO2 -supported platinum and acid-activated bentonite as a co-catalyst. RSC Adv. 2015, 5, 41285–41299. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Bastardo, N.; Romero, A.; Alonso, E. Extraction of arabinoxylans from wheat bran using hydrothermal processes assisted by heterogeneous catalysts. Carbohydr. Polym. 2017, 160, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.; Wang, C.; Huang, F.; Wang, F.; Jia, H.; Zhou, H.; Wei, P. Selective hydrolysis of hemicellulose from wheat straw by a nanoscale solid acid catalyst. Carbohydr. Polym. 2015, 131, 384–391. [Google Scholar] [CrossRef]
- Brar, K.K.; Espirito Santo, M.C.; Pellegrini, V.O.A.; DeAzevedo, E.R.; Guimaraes, F.E.C.; Polikarpov, I.; Chadha, B.S. Enhanced hydrolysis of hydrothermally and autohydrolytically treated sugarcane bagasse and understanding the structural changes leading to improved saccharification. Biomass Bioenergy 2020, 139, 105639. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice, 1st ed.; Oxford University Press: Oxford, UK, 1998; ISBN 9780198506980. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Technical Report NREL/TP-510-42623; National Renewable Energy Laboratory: Golden, CO, USA, 2006. [Google Scholar]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Timell, T.E. Wood Hemicelluloses: Part I. In Advances in Carbohydrate Chemistry; Academic Press: New York, NY, USA; London, UK, 1964; Volume 19, pp. 247–302. [Google Scholar]
- Timell, T.E. Recent progress in the chemistry of wood hemicelluloses. Wood Sci. Technol. 1967, 1, 45–70. [Google Scholar] [CrossRef]
- Timell, T.E. Wood Hemicelluloses: Part II. In Advances in Carbohydrate Chemistry; Academic Press: New York, NY, USA; London, UK, 1965; Volume 20, pp. 409–483. [Google Scholar]
- Izydorczyk, M.S.; Biliaderis, C.G. Studies on the structure of wheat-endosperm arabinoxylans. Carbohydr. Polym. 1994, 24, 61–71. [Google Scholar] [CrossRef]
- Vogel, J. Unique aspects of the grass cell wall. Curr. Opin. Plant. Biol. 2008, 11, 301–307. [Google Scholar] [CrossRef]
- Chen, X.; Lawoko, M.; Heiningen, A. van Kinetics and mechanism of autohydrolysis of hardwoods. Bioresour. Technol. 2010, 101, 7812–7819. [Google Scholar] [CrossRef]
- Sebhat, W.; El-Roz, A.; Crepet, A.; Ladavière, C.; Perez, D.D.S.; Mangematin, S.; Almada, C.C.; Vilcocq, L.; Djakovitch, L.; Fongarland, P. Comparative study of solvolysis of technical lignins in flow reactor. Biomass Convers. Biorefin. 2020, 10, 351–366. [Google Scholar] [CrossRef]
- Maruani, V.; Narayanin-Richenapin, S.; Framery, E.; Andrioletti, B. Acidic Hydrothermal Dehydration of D-Glucose into Humins: Identification and Characterization of Intermediates. ACS Sustain. Chem. Eng. 2018, 6, 13487–13493. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C. Hydrothermal Treatments Applied to Agro- and Forest- Industrial Waste to Produce High Added-Value Compounds. Repos. Inst. Conicet Digit. 2017, 12, 2058–2080. [Google Scholar] [CrossRef]
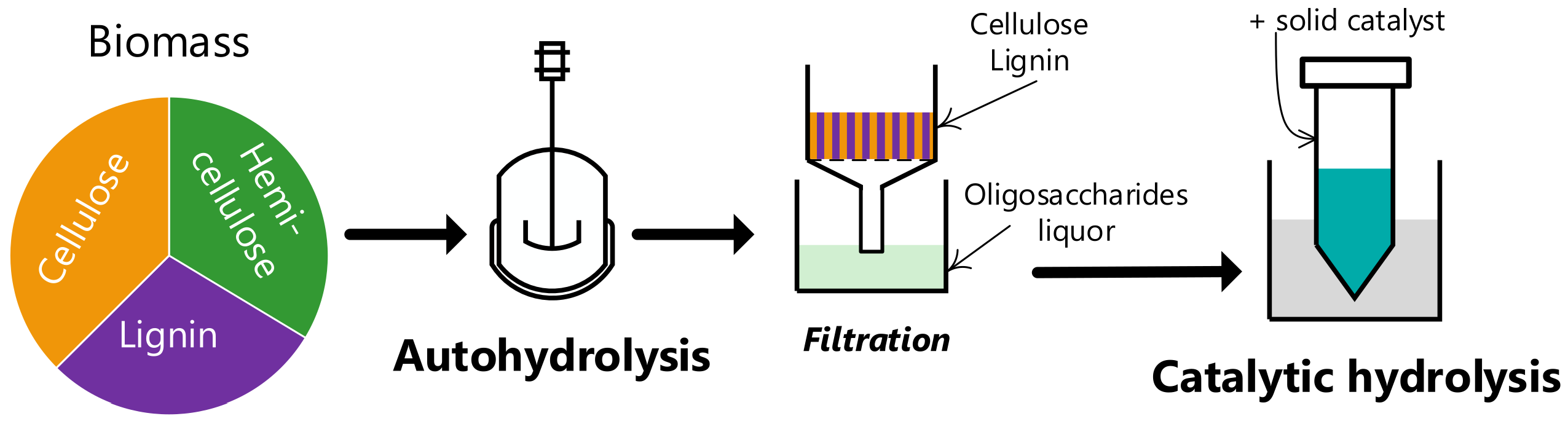
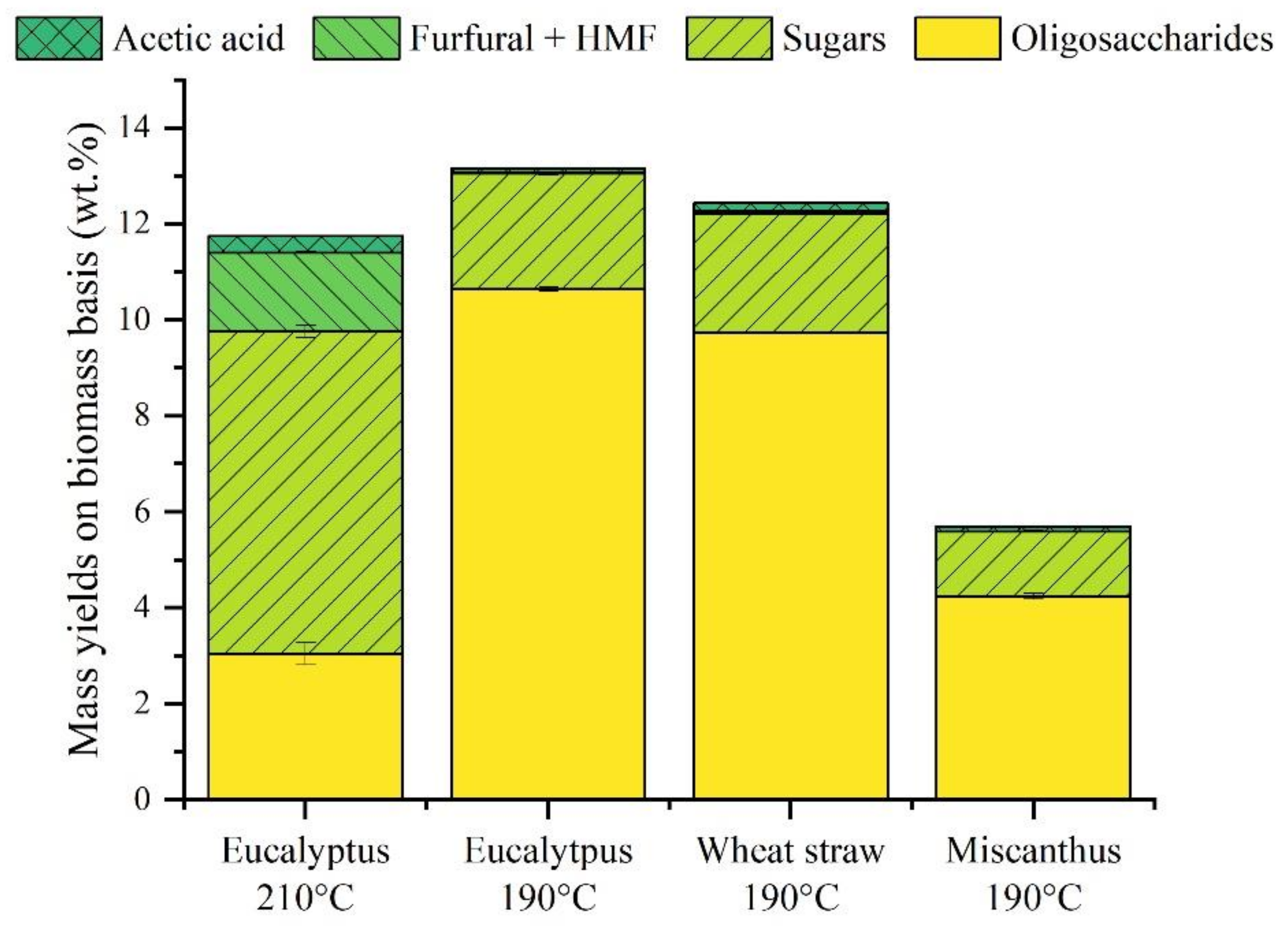
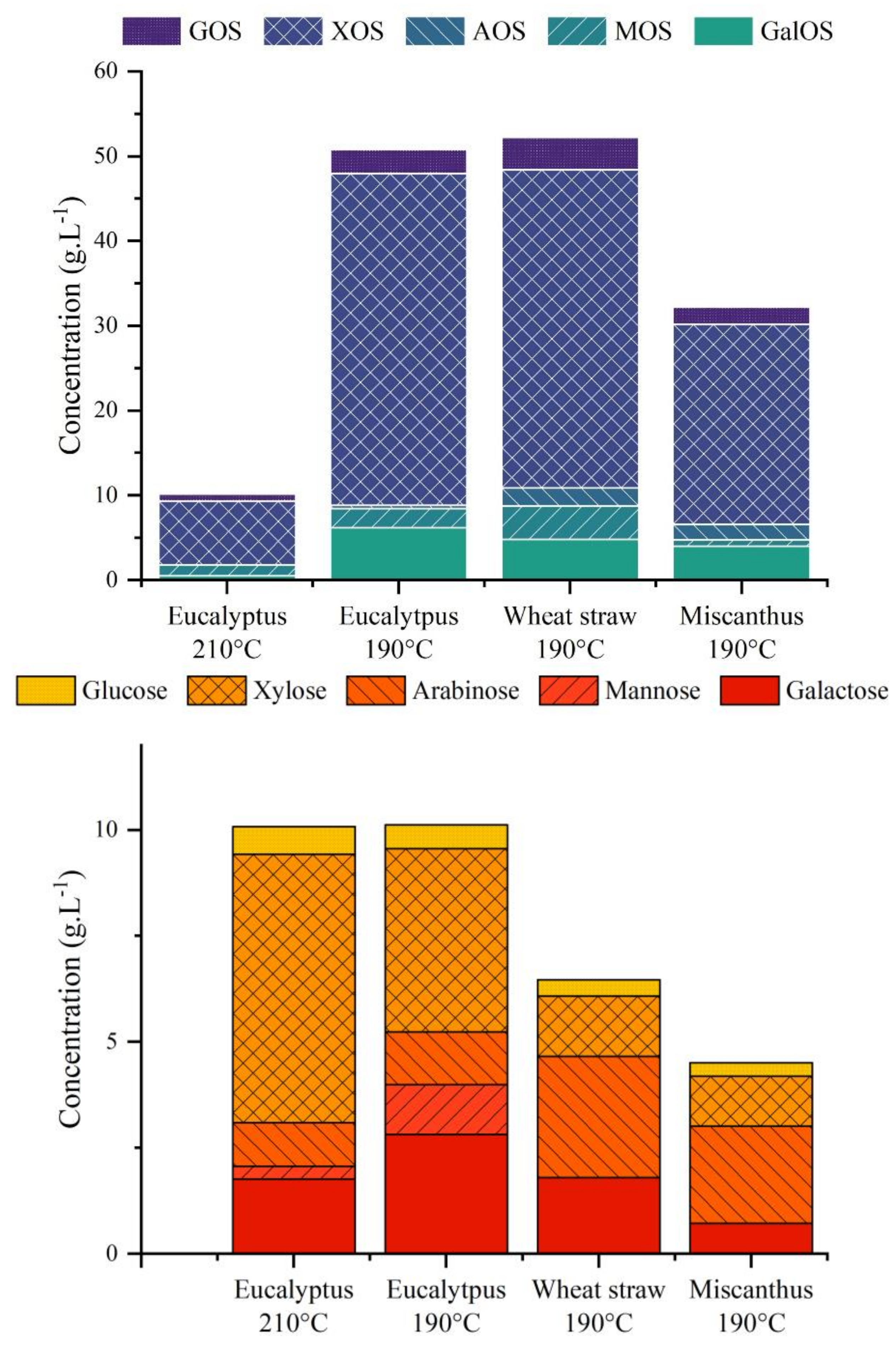
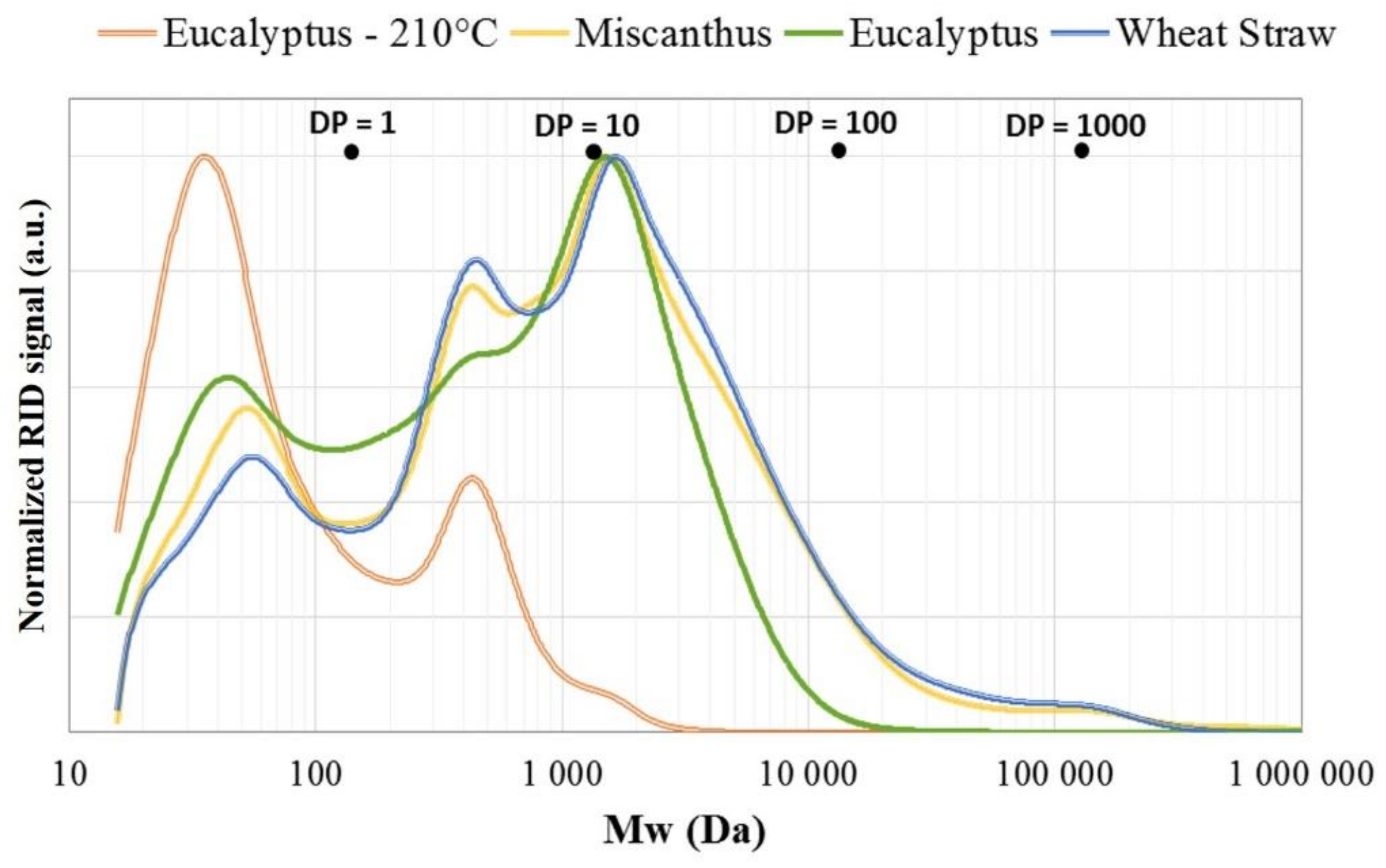
| (wt.%, Dry Basis) | Eucalyptus | Wheat Straw | Miscanthus |
|---|---|---|---|
| Glucan | 36.1 | 33.7 | 41.3 |
| Hemicelluloses | 23.8 | 32.3 | 27.4 |
| Xylan/Mannan/Galactan | 16.9 | 24.2 | n.d. |
| Arabinan | 2.0 | 4.6 | n.d. |
| Acetyl groups | 4.9 | 3.5 | n.d. |
| Klason lignin | 26.7 | 16.8 | 23.1 |
| Ash | 2.4 | 5.0 | 6.4 |
| Protein | 2.8 | 5.4 | n.d. |
| Fat | 1.9 | 1.4 | n.d. |
| Soluble saccharides | 1.7 | 5.3 | n.d. |
| Total | 95.4 | 99.9 | 98.2 |
| Biomass Type | Peak 1 (Monomers) | Peak 2 (Oligomers) | Peak 3 (Polymers) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mw (Da) | PD | Mass% | Mw (Da) | PD | Mass% | Mw (Da) | PD | Mass% | |
| Eucalyptus liquor—210 °C | 52.1 | 1.42 | 69% | 594.6 | 1.34 | 31% | - | - | - |
| Eucalyptus liquor—190 °C | 54.8 | 1.36 | 22% | 339 | 1.19 | 22% | 2 552 | 1.57 | 56% |
| Wheat straw liquor—190 °C | 55.7 | 1.31 | 13% | 342 | 1.14 | 23% | 5 116 | 3.10 | 64% |
| Miscanthus liquor—190 °C | 52.7 | 1.29 | 15% | 326 | 1.15 | 18% | 4 495 | 2.33 | 67% |
| Eucalyptus 210 °C | Eucalyptus 190 °C | Wheat Straw 190 °C | Miscanthus 190 °C | |
|---|---|---|---|---|
| OS (quantified by HPLC—gC.L−1) | 10.2 | 50.8 | 60.1 | 32.3 |
| Sugars (quantified by HPLC—gC.L−1) | 10.1 | 10.1 | 5.9 | 4.5 |
| Others (quantified by HPLC—gC.L−1) | 4.7 | 3.4 | 4.1 | 2.9 |
| Total carbon (TOC analysis—gC.L−1) | 31.6 | 88.2 | 96.4 | 40.2 |
| Unknown compounds (calculation—gC.L−1) | 6.7 | 23.9 | 9.4 | 0.5 |
| Unknown compounds yield (C%—biomass basis) | 2.5 | 5.3 | 1.9 | <0.5 |
| BET specific surface area (m2·g−1) | 97 |
| Average pore diameter (nm) | 13 |
| Crystalline phase | Anatase |
| W loading (wt.%) | 5.6 |
| Acid sites number (µmoleq.NH3.g−1) | 245 |
| Autohydrolysis Yield on Hemicellulose Basis | Catalytic Hydrolysis 180 °C on OS Basis | Global Yield ** on Hemicellulose Basis | ||||||
|---|---|---|---|---|---|---|---|---|
| Biomass | T° (°C) | Yield OS (%) | Yield Sugars (%) | Yield Sugars (%) | Yield Furans (%) | Yield OS (%) | Yield Sugars (%) | Yield Furans (%) |
| Eucalyptus | 210 | 12.8 | 28.2 | −20.59 * | 19.66 | 8.1 | 14.2 | 5.1 |
| Eucalyptus | 190 | 44.5 | 10.0 | 13.41 | 3.29 | 37.0 | 17.7 | 1.5 |
| Wheat straw | 190 | 30.1 | 7.7 | −0.17 * | 3.22 | 28.0 | 4.2 | 0.9 |
| Miscanthus | 190 | 15.2 | 5.0 | −4.99 * | 4.17 | 17.0 | 1.7 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilcocq, L.; Crepet, A.; Jame, P.; Carvalheiro, F.; Duarte, L.C. Combination of Autohydrolysis and Catalytic Hydrolysis of Biomass for the Production of Hemicellulose Oligosaccharides and Sugars. Reactions 2022, 3, 30-46. https://doi.org/10.3390/reactions3010003
Vilcocq L, Crepet A, Jame P, Carvalheiro F, Duarte LC. Combination of Autohydrolysis and Catalytic Hydrolysis of Biomass for the Production of Hemicellulose Oligosaccharides and Sugars. Reactions. 2022; 3(1):30-46. https://doi.org/10.3390/reactions3010003
Chicago/Turabian StyleVilcocq, Léa, Agnès Crepet, Patrick Jame, Florbela Carvalheiro, and Luis C. Duarte. 2022. "Combination of Autohydrolysis and Catalytic Hydrolysis of Biomass for the Production of Hemicellulose Oligosaccharides and Sugars" Reactions 3, no. 1: 30-46. https://doi.org/10.3390/reactions3010003
APA StyleVilcocq, L., Crepet, A., Jame, P., Carvalheiro, F., & Duarte, L. C. (2022). Combination of Autohydrolysis and Catalytic Hydrolysis of Biomass for the Production of Hemicellulose Oligosaccharides and Sugars. Reactions, 3(1), 30-46. https://doi.org/10.3390/reactions3010003







