Mapping Paddy Fields Using Satellite Images and Machine Learning to Identify High Temperature-Induced Sterility in Nankoku, Japan
Abstract
1. Introduction
2. Materials and Methods
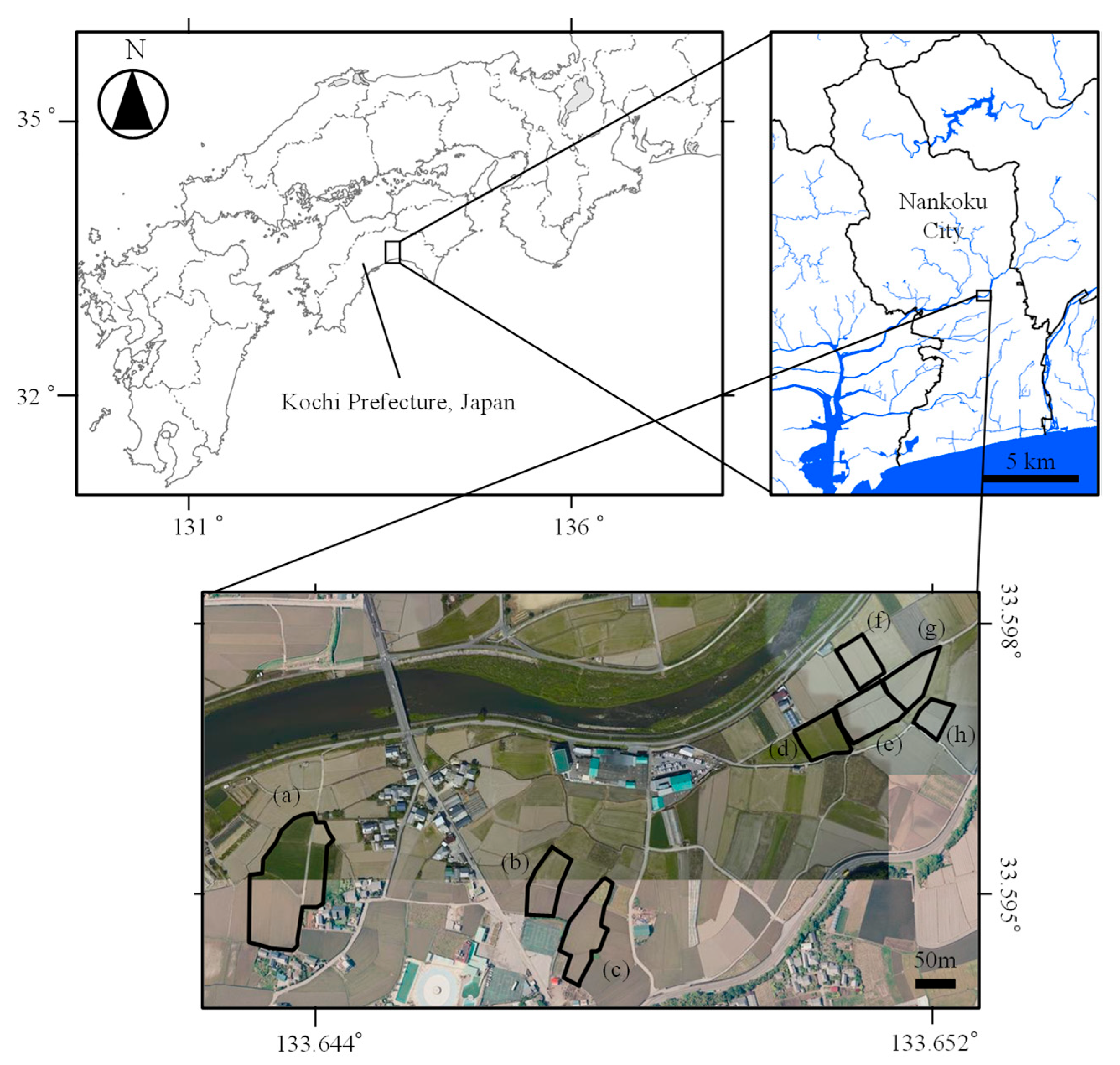
2.1. Study Site
2.1.1. Area for Sterility Rate Mapping
2.1.2. Paddy Fields for Collecting Training Data
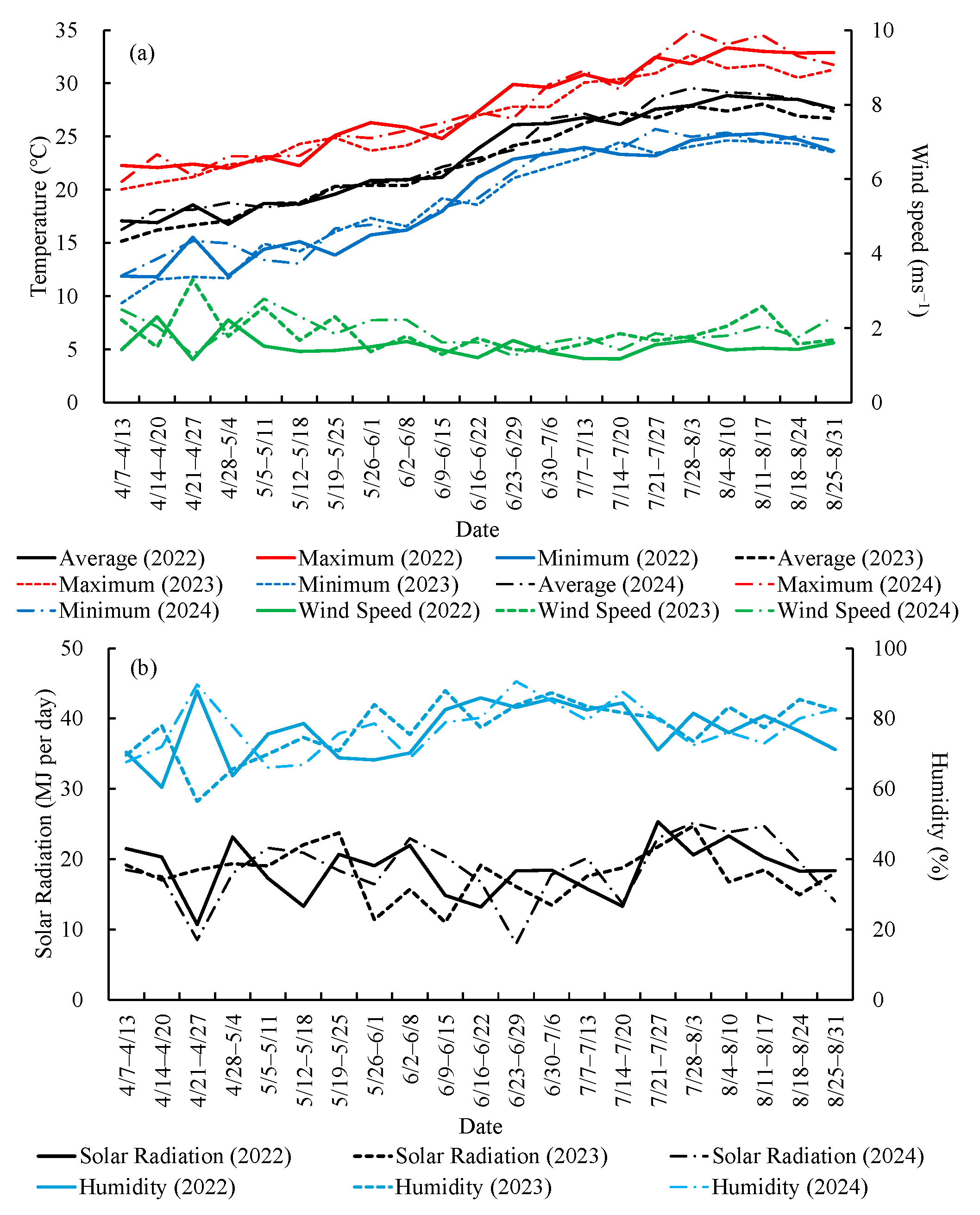
2.1.3. Meteorological Conditions
2.2. Field Survey of Sterility Rate
2.3. Satellite Images
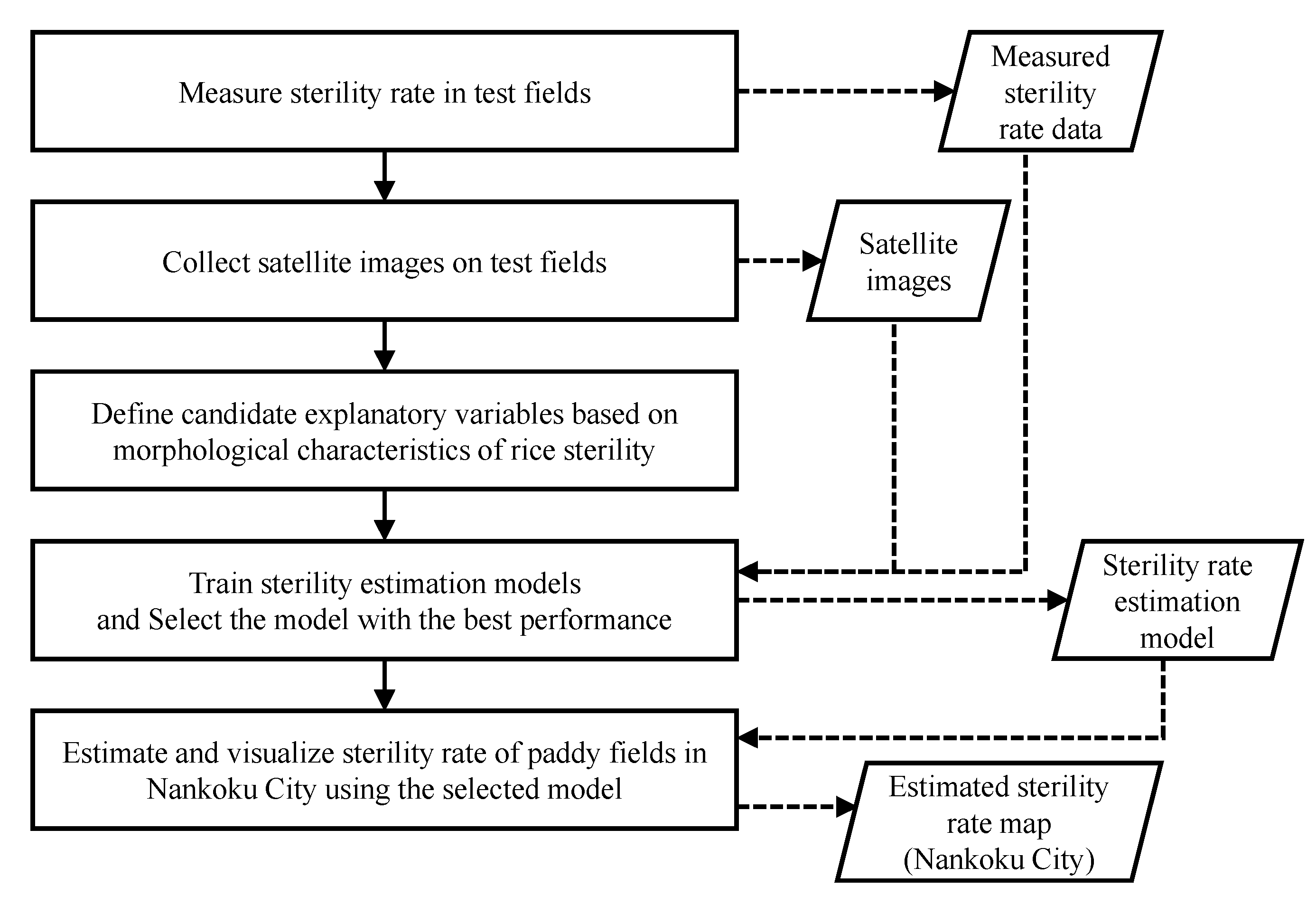
2.4. Building of Sterility Rate Estimation Model
2.4.1. Definition of Explanatory Variables
2.4.2. Creation of Sterility Rate Estimation Model
2.5. Mapping Paddy Fields in Nankoku Based on Estimated Sterility Rate
3. Results
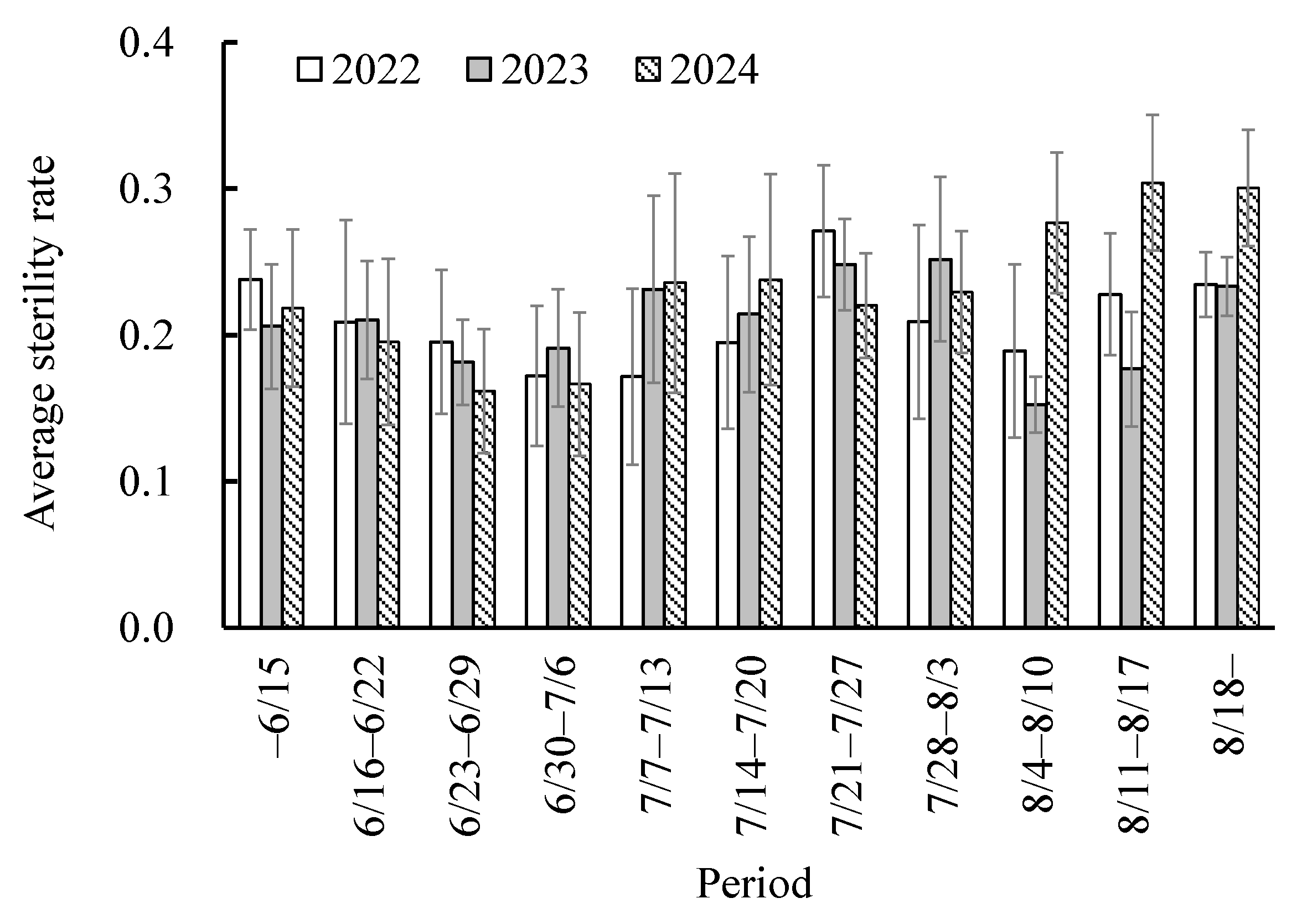
3.1. Sterility Rate in Study Fields
3.2. Sterility Rate Estimation Model
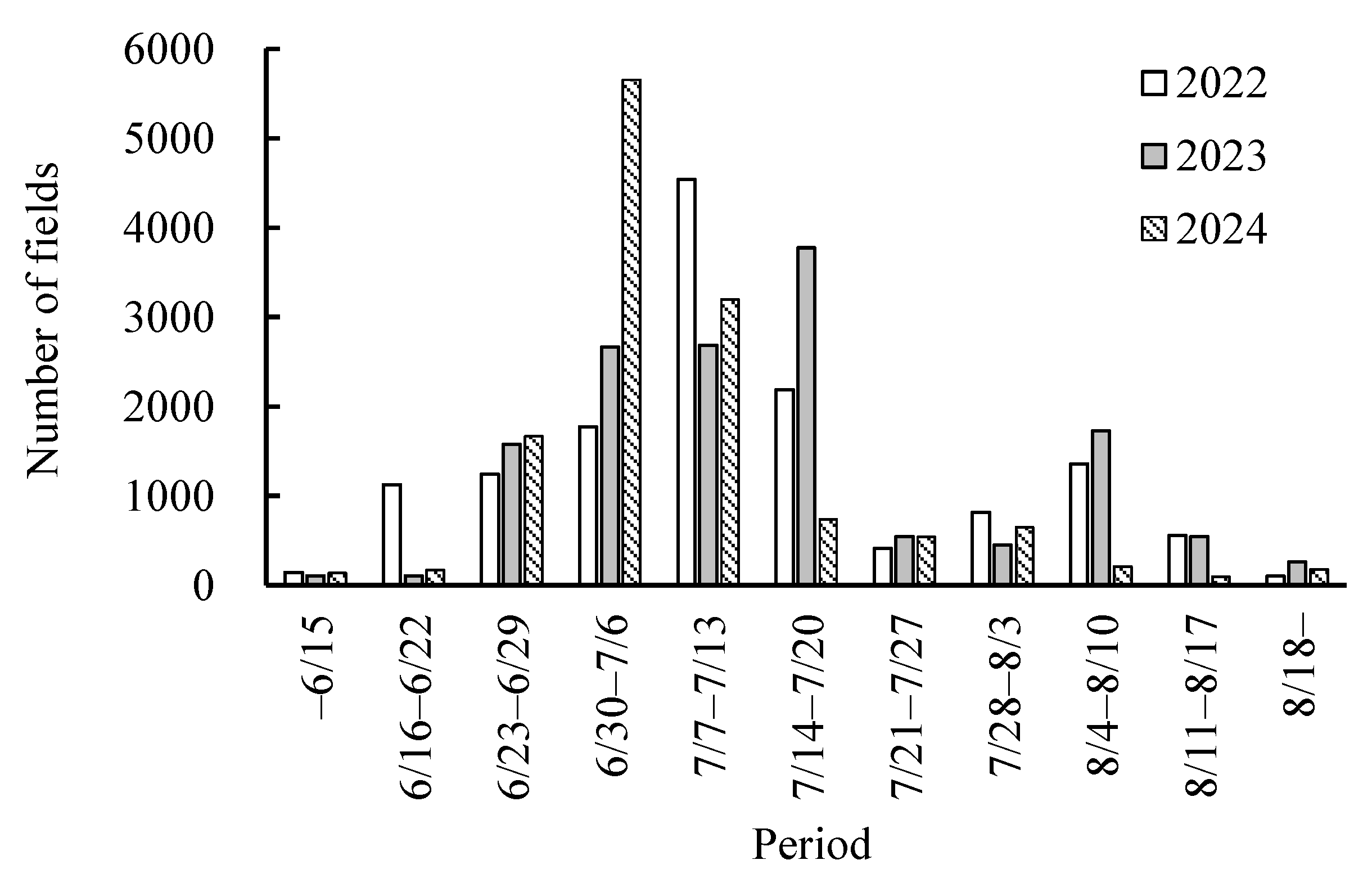
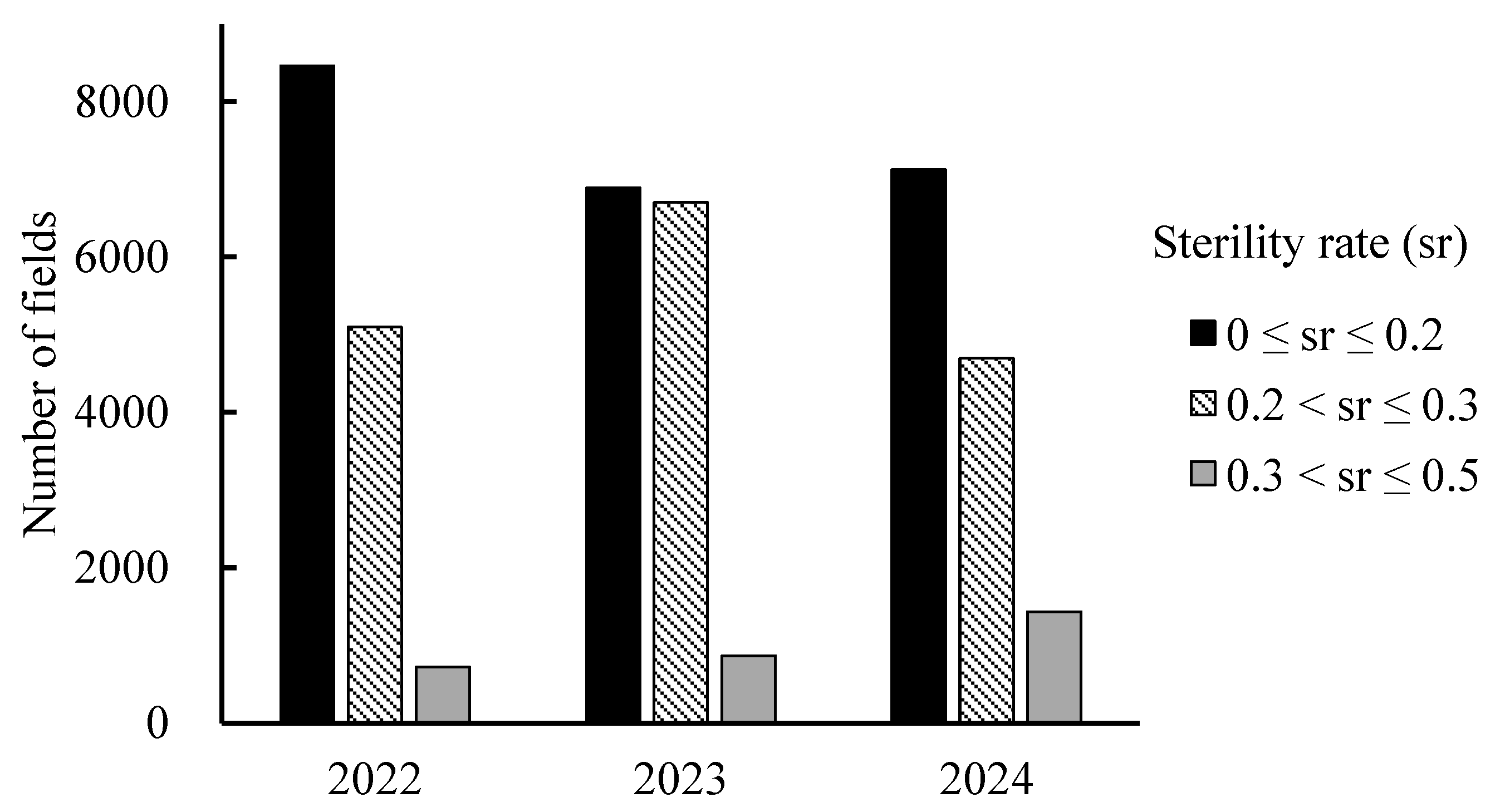
3.3. Estimation of Sterility Occurrence in Nankoku
4. Discussion
4.1. Occurrence of Rice Sterility in the Study Site
4.2. Evaluation of the Sterility Rate Estimation Model
4.3. Estimation of Heading Dates
4.4. Mapping Paddy Fields Using Estimated Sterility
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshimoto, M.; Sakai, H.; Ishigooka, Y.; Kuwagata, T.; Ishimaru, T.; Nakagawa, H.; Maruyama, A.; Ogiwara, H.; Nagata, K. Field survey on rice spikelet sterility in an extremely hot summer of 2018 in Japan. J. Agric. Meteorol. 2021, 77, 262–269. [Google Scholar] [CrossRef]
- Matsui, T. Floret Sterility Induced by High Temperatures at the Flowering Stage in Rice (Oryza sativa L.). Jpn. J. Crop Sci. 2009, 78, 303–311. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ishimaru, T.; Kondo, M.; Kuwagata, T.; Yoshimoto, M.; Fukuoka, M. Spikelet sterility of rice ob-served in the record hot summer of 2007 and the factors associated with its variation. J. Agiric. Meteorol. 2011, 67, 225–232. [Google Scholar] [CrossRef]
- Japan Meteorological Agency. Available online: https://www.data.jma.go.jp/cpdinfo/temp/sum_jpn.html (accessed on 9 April 2025).
- Sato, Y.; Yokoya, S. Effects of male sterility caused by low temperature at the booting stage on out-crossing rates in rice (Oryza sativa L.). Breed. Res. 2008, 10, 127–134. [Google Scholar] [CrossRef][Green Version]
- Morita, S. Eco-Physiological Analysis for High-Temperature Effects on Rice—Grain Ripening. Bull. Natl. Agric. Res. Cent. Kyushu Okinawa Reg. 2009, 52, 1–78. [Google Scholar]
- Matsui, T.; Omasa, K.; Horie, T. High Temperature at Flowering Inhibits Swelling of Pollen Grains, a Driving Force for Thecae Dehiscence in Rice (Oryza sativa L.). Plant Prod. Sci. 2000, 3, 430–434. [Google Scholar] [CrossRef]
- Matsui, T.; Kobayasi, K.; Kagata, H.; Horie, T. Correlation between Viability of Pollination and Length of Basal Dehiscence of the Theca in Rice under a Hot-and-Humid Condition. Plant Prod. Sci. 2005, 8, 109–114. [Google Scholar] [CrossRef]
- Nabeshima, M.; Ebata, M.; Ishikawa, M. Studies on the High Temperature and Dry Wind Injuries in Flowering Rice Plant: Fertilization Injury Induced by High Temperature. Rep. Tokai Br. Crop Sci. Soc. Jpn. 1988, 105, 1–2. [Google Scholar]
- Ishimaru, T.; Okamura, M.; Nagaoka, I.; Yamaguchi, H.; Yoshimoto, M.; Ohdaira, Y. Quantitative assessment on the grain appearance of a new Japanese rice cultivar ‘Niji-no-kirameki’ with a novel heat-avoidance mechanism during ripening. Plant Stress 2022, 4, 100074. [Google Scholar] [CrossRef]
- Weerakoon, W.M.W.; Maruyama, A.; Ohba, K. Impact of Humidity on Temperature-Induced Grain Sterility in Rice (Oryza sativa L). J. Agron. Crop Sci. 2008, 194, 135–140. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Fukuoka, M.; Tsujimoto, Y.; Matsui, T.; Kobayasi, K.; Saito, K.; van Oort, P.A.J.; Inusah, B.I.Y.; Vijayalakshmi, C.; Vijayalakshmi, D.; et al. Monitoring canopy micrometeorology in diverse climates to improve prediction of heat-induced spikelet sterility in rice under climate change. Agric. For. Meteorol. 2022, 316, 108860. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Matsui, T.; Kobayasi, K.; Nakagawa, H.; Fukuoka, M.; Hasegawa, T. Mictometeorological Effects on Heat Induced Spikelet Sterility of Rice Estimated by Energy Balance Model. In Proceedings of the Abstracts of the 224th Meeting of the CSSJ, Kanazawa, Japan, 26–27 September 2007; pp. 162–163. [Google Scholar]
- Sheehy, J.; Elmido, A.; Centeno, G.; Pablico, P. Searching for New Plants for Climate Change. J. Agric. Meteorol. 2005, 60, 463–468. [Google Scholar] [CrossRef]
- Thorp, K.R.; Drajat, D. Deep machine learning with Sentinel satellite data to map paddy rice production stages across West Java, Indonesia. Remote Sens. Environ. 2021, 265, 112679. [Google Scholar] [CrossRef]
- Bridhikitti, A.; Overcamp, T.J. Estimation of Southeast Asian rice paddy areas with different ecosystems from moderate-resolution satellite imagery. Agric. Ecosyst. Environ. 2012, 146, 113–120. [Google Scholar] [CrossRef]
- Hokkaido Central Agricultural Experiment Station. Analysis of damage on paddy rice using satellite remote sensing. Misc. Pub. Hokkaido Prefect Agric. Exp. Stn. 1994, 22, 144–146. [Google Scholar]
- Shimada, H.; Shima, E.; Tanaka, K.; Nagayoshi, T.; Yoshino, K.; Hattori, T.; Kato, W.; Watanabe, K. Monitoring for investigation of field by simple 4 band camera system with stereo photography. J. JSIDRE 2006, 74, 969–972. [Google Scholar]
- Yawata, K.; Yamamoto, T.; Hashimoto, N.; Ishida, R.; Yoshikawa, H. Mixed model estimation of rice yield based on NDVI and GNDVI using a satellite image. In Proceedings of the Remote Sensing for Agriculture, Ecosystems, and Hydrology XXI, Strasbourg, France, 9–12 September 2019; Volume 11149, p. 1114918. [Google Scholar]
- Tanaka, K.; Hama, A.; Kondoh, A. Analysis of high-temperature stress on rice grains by UAV remote sensing. In Proceedings of the General Meeting of the Association of Japanese Geographers 2017s, Tsukuba, Japan, 28–30 March 2017; p. 100277. [Google Scholar]
- Yoshino, S.; Toyofuku, K.; Sone, C.; Ogawa, A. Estimating the Degree of the High-Temperature Damage in Rice Grain Ripening by Proximity Remote Sensing during the Heading Period. Jpn. J. Crop Sci. 2022, 91, 215–222. [Google Scholar] [CrossRef]
- Cropping Type of Paddy in Kochi Prefecture. Available online: https://www.nogyo.tosa.pref.kochi.lg.jp/download/?t=LD&id=6928&fid=59399 (accessed on 8 April 2024).
- Statistical Survey on Crops. Available online: https://www.maff.go.jp/j/tokei/kouhyou/sakumotu/ (accessed on 8 April 2024).
- The Agro-Meteorological Grid Square Data. Available online: https://amu.rd.naro.go.jp/wiki_open/doku.php?id=start (accessed on 8 April 2024).
- Tsuboi, Y.; Hitaka, N. Studies on Wind Damage of Rice Plant (5) Shattering loss caused by strong wind. J. Agric. Meteorol. 1966, 21, 127–130. [Google Scholar] [CrossRef]
- Mitsui, T.; Maruyama, S. Alleviation of Spikelet Sterility of Rice Caused by High Temperatures by Applying Auxin and Jasmonic Acid. Jpn. J. Crop Sci. 2019, 88, 182–186. [Google Scholar] [CrossRef]
- Planet Labs. Planet Labs Constellation Overview: Planetscope. In PlanetScope Product Specifications; Planet Labs: San Francisco, CA, USA, 2023; p. 10. [Google Scholar]
- Planet Surface Reflectance. Available online: https://assets.planet.com/marketing/PDF/Planet_Surface_Reflectance_Technical_White_Paper.pdf (accessed on 11 February 2025).
- GSI Maps. Available online: https://www.gsi.go.jp/ (accessed on 8 April 2024).
- Wang, N.; Guo, Y.; Wei, X.; Zhou, M.; Wang, H.; Bai, Y. UAV-based remote sensing using visible and multispectral indices for the estimation of vegetation cover in an oasis of a desert. Ecol. Indic. 2022, 141, 109155. [Google Scholar] [CrossRef]
- Liu, L.; Xie, Y.; Zhu, B.; Song, K. Rice leaf chlorophyll content estimation with different crop coverages based on Sentinel-2. Ecol. Inform. 2024, 81, 102622. [Google Scholar] [CrossRef]
- Hashimoto, N.; Saito, Y.; Yamamoto, S.; Ishibashi, T.; Ito, R.; Maki, M.; Homma, K. Relationship between Leaf Area Index and Yield Components in Farmers’ Paddy Fields. AgriEngineering 2023, 5, 1754–1765. [Google Scholar] [CrossRef]
- Hirooka, Y.; Homma, K.; Shiraiwa, T.; Kuwada, M. Parameterization of leaf growth in rice (Oryza sativa L.) utilizing a plant canopy analyzer. Field Crops Res. 2016, 186, 117–123. [Google Scholar] [CrossRef]
- Intaravanne, Y.; Sumriddetchkajorn, S. Android-based rice leaf color analyzer for estimating the needed amount of nitrogen fertilizer. Comput. Electron. Agric. 2015, 116, 228–233. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models, An Introduction with R, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Polygons of Land Parcels. Available online: https://www.maff.go.jp/j/tokei/porigon/index.html (accessed on 15 November 2023).
- Nagata, T. The way to use of UAV remote sensing for paddy rice. Hokuno 2021, 88, 12–17. [Google Scholar]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Greenwave Effect) of Natural Vegetation; NASA/GSFC Type III Final Report; NASA: Greenbelt, MD, USA, 1974. [Google Scholar]
- Arai-Sanoh, Y.; Okamura, M.; Mukouyama, T.; Kobayashi, N.; Ogiwara, H.; Yoshida, H.; Kondo, M. Determination of Suitable Harvesting Time of Rice Cultivars with High Yield and High Eating Quality. Jpn. J. Crop Sci. 2020, 89, 102–109. [Google Scholar] [CrossRef]
- Azuma, S.; Takeuchi, A.; Sato, T. Basal-white kernels occurrences after the maturing stage in rice cultivar “Koshihikari” under high temperature condition. In Proceedings of the Abstracts of 234th Meeting of the CSSJ, Sendai, Japan, 9–10 September 2012; p. 292. [Google Scholar]
- Saida, N.; Kameshima, M.; Sakata, M. Evaluation of sterility tolerance to high temperature treatment at the flowering stage of rice using an artificial weather machine. Shikoku J. Crop Sci. 2022, 59, 2–4. [Google Scholar]
- Kikkawa, S.; Shibata, S. Effect of high temperature to first half of ripening period on grain quality in 2019. Tohoku J. Crop Sci. 2020, 63, 19–20. [Google Scholar]
- Nakano, H. Effect of Early-Stage Shading of Direct Seeded Rice on Growth and Yield Components. Jpn. J. Crop Sci. 2000, 69, 182–188. [Google Scholar] [CrossRef]
- Morita, S. Prospect for Developing Measures to Prevent High-Temperature Damage to Rice Grain Ripening. Jpn. J. Crop Sci. 2008, 77, 1–12. [Google Scholar] [CrossRef]
- Kaneta, Y.; Tsumura, M.; Hatakeyama, K.; Kato, K.; Takakai, F.; Sato, T. Characteristics of the high-temperature-ripening Oryza sativa variety Fusaotome under high-temperature conditions. Jpn. J. Soil Sci. Plant Nutr. 2022, 93, 141–147. [Google Scholar]


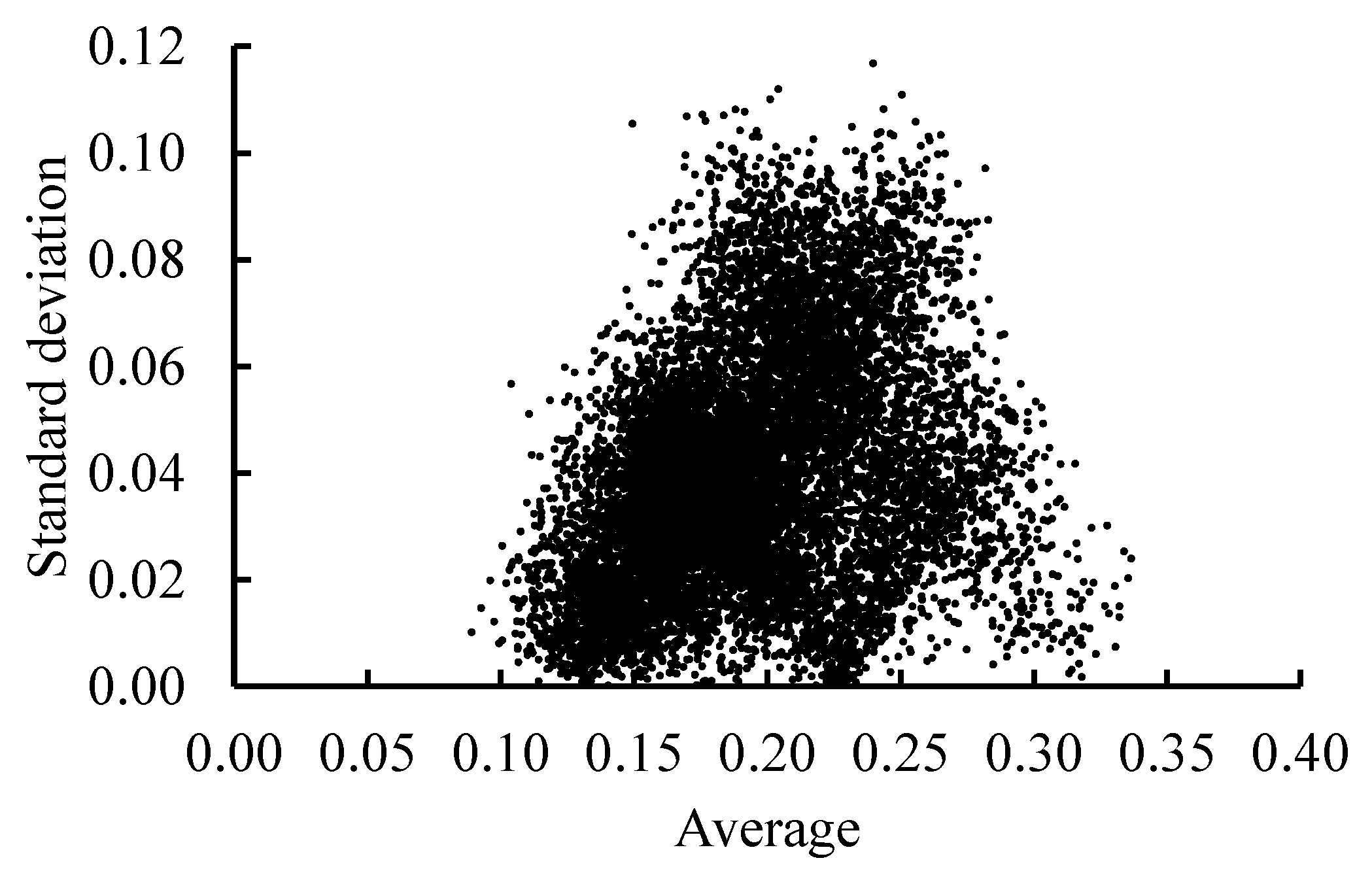
| Year | Field | Cultivar | Test Plot Number | Basal Fertilizer Application (kg per 1000 m2) | Transplanting Date | Heading Date | Harvesting Date |
|---|---|---|---|---|---|---|---|
| 2022 | (a) | Nangokusodachi | 32 | 38 1 | 31 March | 23 June | 21 July |
| (c) | Yosakoibijin | 30 | 32 1 | 4 April | 23 June | 24 July | |
| (b) | Koshihikari (early-season cultivation) | 16 | 33 2 | 10 April | 1 July | 5 August | |
| (d), (e) | Koshihikari (normal-season cultivation) | 30 | 25 3 | 25 May | 29 July | 29 August | |
| 2023 | (d), (f) | Fukuhikari | 20 | 150 4, 30 2 | 24 April | 8, 9 July | 11 August |
| (e), (g), (h) | Koshihikari (normal-season cultivation) | 50 | 150 4, 30 2 | 13, 14 May | 21 July | 21 August |
| Year | Cultivar | Mean Sterility Rate (Standard Deviation) |
|---|---|---|
| 2022 | Nangokusodachi | 0.08 (±0.060) |
| Yosakoibijin | 0.15 (±0.031) | |
| Koshihikari (early-season cultivation) | 0.15 (±0.050) | |
| Koshihikari (normal-season cultivation) | 0.35 (±0.066) | |
| 2023 | Fukuhikari | 0.09 (±0.035) |
| Koshihikari (normal-season cultivation) | 0.31 (±0.079) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, N.; Yamada, H.; Matsuoka, S. Mapping Paddy Fields Using Satellite Images and Machine Learning to Identify High Temperature-Induced Sterility in Nankoku, Japan. AgriEngineering 2025, 7, 122. https://doi.org/10.3390/agriengineering7040122
Hashimoto N, Yamada H, Matsuoka S. Mapping Paddy Fields Using Satellite Images and Machine Learning to Identify High Temperature-Induced Sterility in Nankoku, Japan. AgriEngineering. 2025; 7(4):122. https://doi.org/10.3390/agriengineering7040122
Chicago/Turabian StyleHashimoto, Naoyuki, Haruki Yamada, and Shiho Matsuoka. 2025. "Mapping Paddy Fields Using Satellite Images and Machine Learning to Identify High Temperature-Induced Sterility in Nankoku, Japan" AgriEngineering 7, no. 4: 122. https://doi.org/10.3390/agriengineering7040122
APA StyleHashimoto, N., Yamada, H., & Matsuoka, S. (2025). Mapping Paddy Fields Using Satellite Images and Machine Learning to Identify High Temperature-Induced Sterility in Nankoku, Japan. AgriEngineering, 7(4), 122. https://doi.org/10.3390/agriengineering7040122









