Abstract
A laser-based selective wax ablation method using a 532 nm Nd:YAG laser was developed to improve the foliar uptake efficiency of agrochemicals in citrus leaves. In contrast to conventional applications that suffer major losses, our approach exposes up to 80% of the underlying epidermis (within the irradiated footprint) with no visible tissue damage, thereby substantially enhancing substance penetration. Efficacy was confirmed using two indicators: (1) A fluorescent glucose analog (2-NBDG) exhibited a radial expansion velocity reaching 0.0105 mm/min in treated areas, enabling rapid phloem transport across an 8 cm distance within just three minutes—an 11,280% improvement over untreated controls. (2) Laser-induced breakdown spectroscopy (LIBS) demonstrated a threefold increase in zinc (Zn) uptake (and over fivefold compared to untreated leaves) when using a Zn-based foliar fertilizer. To assess processing efficiency, we quantified the ablation footprint by combining single-pulse laser shots in a 1 cm-diameter region and found that 23.4% of the total area was fully exposed. This selective, non-invasive approach enables precise targeting, potentially reducing fertilizer and pesticide usage while improving crop health. Beyond citrus, it is readily adaptable to other crops, with integration into orchard or greenhouse spraying systems as a promising path for scale-up. Such versatility highlights the technique’s potential to optimize efficacy, cut input costs, and diminish environmental impact in modern precision agriculture.
1. Introduction
Agrochemicals play a crucial role in modern agriculture by enhancing crop health and yield through foliar application. However, several barriers hinder their efficient absorption and utilization [1]. The waxy cuticle on the leaf surface, which serves as a natural barrier against water loss and pathogen entry, also limits the permeability of externally applied soluble compounds, including most agrochemicals [2]. The primary pathway for substance absorption is through stomata, which are predominantly located on the underside of leaves, a less exposed surface [3]. This significantly reduces the functional area available for absorption, leading to inefficient agrochemical uptake. As a result, a substantial portion of applied chemicals is lost to the environment, raising concerns about their ecological impact [4].
Several alternative methods have been developed to improve the efficiency of agrochemical uptake in foliar applications, addressing the limitations imposed by the waxy cuticle and other barriers to absorption. Among these methods, surfactants, direct injection, and nanoformulations have gained significant attention due to their potential to enhance the penetration and utilization of applied substances [5].
Surfactants are widely used to improve the adhesion, spreading, and penetration of agrochemicals on leaf surfaces. By reducing the surface tension of spray droplets, surfactants facilitate uniform coverage and allow greater interaction between the chemical and the cuticle. Some surfactants also alter the cuticle structure, increasing permeability and promoting diffusion into plant tissues. However, despite their effectiveness, excessive use of surfactants can lead to phytotoxicity, causing damage to leaf tissues and reducing plant productivity. Additionally, certain surfactants pose environmental risks, particularly when they enter water systems, where they can negatively affect aquatic organisms [6].
Direct injection methods bypass the leaf surface barriers entirely by delivering agrochemicals directly into the plant’s vascular system. This technique ensures precise and efficient substance uptake, eliminating issues related to cuticular resistance and environmental losses due to runoff or volatilization. Direct injection is particularly useful for systemic agrochemicals, which need to be transported throughout the plant. However, this method is highly invasive and requires specialized equipment and labor-intensive application, making it less practical for large-scale agricultural use. Furthermore, repeated injections can cause mechanical damage to the plant, increasing susceptibility to pathogens [7].
Nanoformulations represent a promising innovation in foliar agrochemical applications. Nanoparticles and nanoencapsulated agrochemicals enhance uptake efficiency by improving solubility, stability, and controlled release of active ingredients. Their small size allows them to penetrate the cuticle more effectively and, in some cases, enter through stomatal openings, facilitating deeper tissue absorption. Additionally, nanoformulations can reduce the required dosage of agrochemicals, minimizing environmental impact. Despite these advantages, concerns remain regarding the long-term ecological effects and potential toxicity of nanomaterials in agricultural ecosystems. The cost of production and regulatory challenges also limit the widespread adoption of nanoformulation-based products [8]. Each of these approaches provides distinct advantages for improving foliar agrochemical uptake, yet they also present specific limitations that must be addressed to ensure sustainable and effective application [5].
To overcome these challenges, laser-based methods have been explored as a means to enhance agrochemical penetration. One approach involves laser micro-perforation, which uses a CO2 laser to create small pores (~250 μm in diameter) by directly ablating the cuticle, epidermis, and part of the palisade parenchyma [9]. Studies have shown that this technique can increase agrochemical penetration by over 2000% compared to untreated leaves. However, this method has limitations: it inevitably removes some live tissue, compromising the integrity of the epidermis, and it requires precise laser focusing, which is challenging under field conditions due to natural variations in leaf position and orientation. Additionally, the 10.6 μm wavelength of the CO2 laser is well known for its strong thermal effects on organic tissues, leading to heat diffusion and potential damage to the surrounding area. This collateral thermal effect can further impact tissue integrity and may limit its applicability in agricultural settings where preserving plant health is critical.
A less invasive alternative was later developed using selective laser ablation, which takes advantage of the different absorption properties of leaf tissues. This method, initially implemented with an Erbium laser at a wavelength of 2.94 μm, enables the partial detachment of the wax cuticle over an extended area using a single laser pulse. The ablation mechanism is based on the strong absorption of this wavelength by water within the epidermis, which, upon rapid overheating, induces the exfoliation of the cuticle. Unlike micro-perforation, this technique preserves the epidermis, allowing the cuticle to regenerate naturally over time [10]. However, thermal effects in the surrounding area must be considered, as the rapid heating process may have localized impacts on tissue integrity.
In this study, we introduce an improved selective wax ablation method based on the optical properties of green leaves across a wide range of plant species. The concept of selective ablation relies on establishing an appropriate energy density window, where the underlying layer (the epidermis) exhibits high reflectance, while the layer to be removed (the wax cuticle) is supposed to have higher absorption. This differential interaction allows for precise material removal while minimizing damage to the remaining tissue.
In many applications, selective ablation is achieved by choosing a laser wavelength where the absorption contrast between layers is significant [11]. However, in this case, the wax cuticle does not exhibit higher absorption than the epidermis in the selected spectral range. As will be discussed later, despite this limitation, it is still possible to adjust the laser parameters, such as pulse duration and fluence, to achieve controlled ablation of the cuticle while preserving the integrity of the epidermis. This approach enables the adaptation of the technique to different plant species and conditions, making it a versatile tool for improving foliar agrochemical uptake.
Figure 1 illustrates the absorption properties of citrus leaves at different wavelengths, highlighting the advantage of using the green spectral region to minimize disruption to the internal structure. Given this spectral behavior, 532 nm is an optimal wavelength for selective wax ablation, and Nd:YAG lasers provide an accessible and cost-effective solution suitable for field applications.
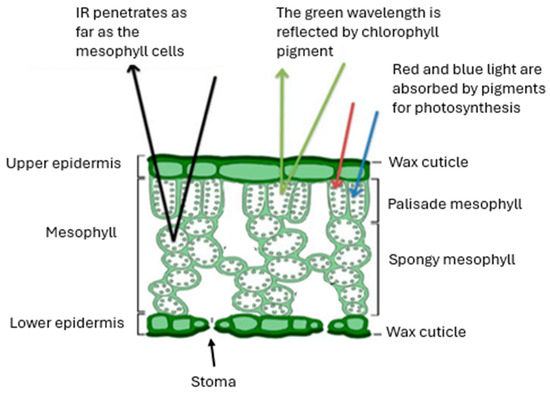
Figure 1.
Schematic representation of light interaction with a leaf’s internal structure.
To validate the effectiveness of this method, we employed two complementary approaches to demonstrate improved penetration of applied substances:
- Fluorescent glucose visualization: A fluorescent glucose analog was applied to the treated areas to track and quantify absorption over time. This enabled a direct comparison between laser-treated and untreated areas, confirming enhanced uptake through wax-free zones [12].
- Laser-induced breakdown spectroscopy (LIBS) analysis: A Zn-based fertilizer was applied to both laser-treated and control leaves. LIBS measurements were then performed to quantitatively assess zinc uptake, providing further evidence of the method’s effectiveness in increasing agrochemical absorption.
As a result of the combination of these analytical techniques, we demonstrate that selective waxy cuticle ablation with a 532 nm Nd:YAG laser effectively enhances foliar penetration, offering a non-invasive, precise, and efficient alternative for optimizing agrochemical application in citrus crops. Beyond citrus, this method holds promise for broader agricultural applications, as the selective removal of the cuticular barrier can facilitate the uptake of essential nutrients, pesticides, and biostimulants across a wide range of crop species.
By enhancing agrochemical efficiency and minimizing losses, this approach promotes sustainable agriculture, reducing environmental impact while improving crop productivity. Additionally, more effective substance penetration can lower agrochemical costs by reducing the required amounts of fertilizers and pesticides.
2. Materials and Method
2.1. Sample Preparation
The study was conducted on ten young navel orange trees, each approximately 1 m in height. Leaf irradiation was performed as shown in Figure 2, and measurements were taken from leaves located in the designated positions but on a different branch.
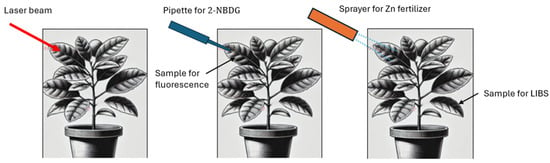
Figure 2.
Schematic representation of the experimental process.
For the fluorescence analysis, a total of ten leaves, one per tree, were extracted and, immediately after being detached from the plant, treated with the laser, and then fluorescent glucose was applied.
For the LIBS analysis, three sample groups were used. The first group consisted of leaves from trees that received neither laser treatment nor glucose application, serving as an untreated control in its natural state. The second group included samples from trees where glucose was applied without prior laser treatment. This group aimed to assess the natural penetration of the substance with the wax cuticle intact, simulating conventional spraying conditions. The third group comprised samples from trees that first underwent laser treatment, followed immediately by the application of glucose to the treated area.
In the two groups where glucose was applied, the treatment was performed on leaves located in the upper canopy. However, for all three groups, the LIBS measurements were conducted on leaves collected from a lower section of the tree, approximately one meter away from the application site. This sampling approach allowed for the evaluation of systemic translocation and absorption efficiency under different conditions.
The sampled leaves were collected one hour after glucose application and analyzed using laser-induced breakdown spectroscopy (LIBS) without requiring any additional preparation, as one of the known advantages of LIBS is its ability to analyze samples without prior treatment. Measurements were taken at five different positions on each leaf, and the average value was considered representative of each sample.
For this analysis, ten trees were examined, with one control leaf and one treated leaf per tree, resulting in a total of 100 spectra being recorded: 50 from the control and 50 from treated samples. The leaves underwent no prior chemical treatment. To eliminate any surface dirt or contamination, including potential fertilizer residues, the leaves were cleaned using a cotton swab moistened with distilled water.
To enhance clarity in the experimental workflow, Figure 3 presents a schematic representation of the methodology used in this study. The process begins with sample selection, where 10 young navel orange trees were chosen. Leaf collection and treatment followed, with 10 leaves designated for fluorescence analysis and 20 leaves (10 control and 10 treated) used for LIBS analysis. The next step involved laser treatment using a 532 nm wavelength laser to selectively remove the wax cuticle. After treatment, fluorescence analysis was performed using NBDG to assess substance penetration, while LIBS analysis measured Zn uptake, generating 100 spectra (50 control, 50 treated). Finally, the data from both analyses were processed and interpreted to compare the uptake efficiency between treated and untreated samples. This structured workflow ensures reproducibility and highlights the sequence of key steps in the study.
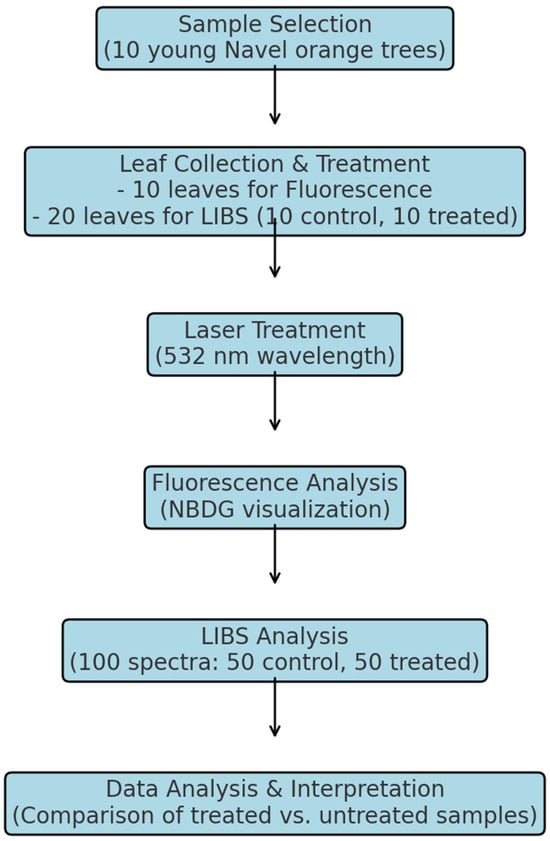
Figure 3.
Flowchart illustrating the experimental workflow, from sample selection and laser treatment to fluorescence and LIBS analysis, followed by data interpretation.
2.2. Nd:Yag Laser for Waxy Cuticle Ablation
To effectively ablate the wax cuticle while minimizing impact on the epidermis, an Nd:YAG laser from Onteko, operating at its second harmonic wavelength of 532 nm, was used. This wavelength was selected due to its low absorption in the green spectral region, where chlorophyll strongly reflects light.
The laser beam was focused on a 3 mm-diameter spot and used to irradiate leaf samples at different energy densities. Each laser treatment consisted of a single pulse. Four different laser energy levels were tested, varying the energy density within the spot area. The fluence levels applied for wax removal across the examined leaves ranged from 1.42 J/cm2 to 9.91 J/cm2. The laser pulse duration was 20 ns, and a single pulse was applied to ablate the targeted area.
2.3. Zn-Based Fertilizer
Zinc (Zn) is an essential micronutrient for plants, playing a critical role in enzyme activation, protein synthesis, and overall plant metabolism. In citrus crops, zinc is essential for leaf expansion, fruit set, and chlorophyll synthesis, directly influencing yield and fruit quality [13]. Zinc deficiency in citrus is commonly associated with symptoms such as small leaves, interveinal chlorosis, and reduced fruit size, leading to lower productivity and poor fruit development.
Zinc sulfate (ZnSO4) is a widely used example of a Zn-based fertilizer, and is frequently applied in agriculture to correct deficiencies. However, various Zn formulations exist, including complexed liquid fertilizers designed for improved absorption and mobility within the plant. One of the main challenges with foliar Zn applications, regardless of the formulation, is that its uptake efficiency is significantly limited by the presence of the wax cuticle barrier.
For this study, we used Opulent Zinc, a 23% liquid Zn fertilizer supplied by Opulent Blends Inc., Sebring, Florida. This formulation was specifically developed to move internally throughout the plant, allowing for rapid uptake and efficient correction of deficiencies. Unlike ZnSO4, Opulent Zinc is complexed with amino acids, does not contain EDTA, and is free of harsh impurities, enhancing its compatibility with plant tissues. The full product description can be found at https://opulentblends.net/products/opulent-zinc (accessed on 2 April 2025).
Additionally, Zn was selected as a traceable marker for penetration analysis. As a metallic element, Zn can be easily detected and quantified using laser-induced breakdown spectroscopy (LIBS). This property makes Zn-based fertilizers, including Opulent Zinc, ideal candidates for assessing the effectiveness of laser treatment in enhancing agrochemical uptake [14].
2.4. Fluorescent Glucose
To visualize the penetration of applied substances, a fluorescent glucose analog, 2-NBDG (2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose, λ Ex/Em (nm) 465/540), was applied to the leaf surface at a concentration of 30 mM Images of the treated leaves were captured using a Pixel 7 smartphone camera, which was acquired from the official Google online store in the United States. The device is from Google, headquartered in Mountain View, CA, USA, and manufactured by Foxconn, Taipei, Taiwan. It was used under illumination from a Model SFA Stereo Microscope Fluorescence Adapter (manufactured by Nightsea, Portland, OR, USA), emitting light in the 440–460 nm wavelength range.
The analog 2-NBDG was selected due to its ability to mimic natural glucose, allowing for the tracking of its absorption and distribution within plant cells. This property makes it an effective tool for assessing foliar penetration efficiency of applied substances [9].
A mechanical pipette (100–1000 µL, model CU201946) was used to apply 1 mL of fluorescent glucose solution to each sample on the laser-treated area.
2.5. Image Capture and Processing
To document the effects of laser treatment on leaf morphology and assess the penetration of applied substances, images were captured using two different imaging systems. For high-magnification imaging, a Supereyes digital microscope was used to examine epidermal morphology after laser treatment, allowing for detailed visualization of structural changes in the cuticle and epidermis. This microscope provided insights into the extent and uniformity of wax ablation at the microscopic level.
For fluorescence imaging, a FinePix S8300 camera (Fujifilm Corp., Tokyo, Japan) equipped with a Nightsea green fluorescence filter was used to capture fluorescence emission from the applied 2-NBDG tracer. This setup ensured the selective detection of fluorescent signals, enabling clear visualization of substance penetration in laser-treated and control leaves. The combination of these imaging techniques provided a comprehensive evaluation of both structural modifications and enhanced uptake efficiency following laser treatment.
To evaluate the extent of the laser-treated area that resulted in exposed epidermis with the cuticle removed, microscope images were processed using a program created in Python 3.11. The cursor tool was used to define the regions of interest. The image processing algorithm was based on color segmentation in the HSV (hue, saturation, value) color space and binary mask processing. The methodology can be mathematically described as follows:
The first step in the processing pipeline was converting the image from BGR (blue, green, red) to HSV (hue, saturation, value) color space. This transformation was chosen because HSV decouples color information (hue and saturation) from illumination effects (value), allowing for more robust segmentation under varying lighting conditions.
The conversion follows these equations:
where each pixel p is transformed according to:
In our image processing pipeline, after converting the image from BGR to HSV color space, we generate a binary mask, M(x,y), that determines whether a pixel belongs to the region of interest. Specifically, a pixel is classified as part of the target region (M(x,y)=1) if its hue, saturation, and value components are all within the adaptive tolerance thresholds relative to reference values. This unified criterion can be expressed as:
where Th, Ts, and Tv are adaptive tolerance values determined through calibration with known reference samples. These thresholds are optimized to account for natural variations in leaf tissue while maintaining segmentation accuracy.
M(x, y) = {1, if | H(x, y) − Href | ≤ Th ∧ | S(x, y) − Sref | ≤ Ts ∧ | V(x, y) − Vref | ≤ Tv
0, otherwise }
0, otherwise }
The quantification of treated regions involves several key measurements:
- Total Area Measurement: Pixel counting within the leaf mask, excluding the background;
- Region-Specific Analysis: Individual area calculations for each segmented region;
- Distribution Analysis: Spatial distribution patterns of treated areas;
- Coverage Calculation: Percentage of the treated area relative to the total leaf area.
The area percentage for each region is calculated as:
where ∑∑M(x,y) represents the sum of pixels in the binary mask and Atotal is the total leaf area. This calculation provides a normalized measure of coverage that can be compared across different samples and experimental conditions.
Finally, the area analysis was conducted by generating binary masks for the selected colors. The area was quantified by counting the number of pixels classified within the epidermis-exposed region. To ensure consistency, the calculated area was normalized relative to the total object area, excluding the background. This approach provided an objective and reproducible assessment of the extent of cuticle removal following laser treatment.
3. Results and Discussion
The schematic representation in Figure 4 illustrates the sequential process of laser-assisted wax cuticle removal and its effect on substance absorption.
Figure 4.
Mechanism of wax cuticle removal and enhanced penetration for sprayed substance.
- (a)
- Initial state prior to laser treatment—The leaf surface is covered by the wax cuticle (gray layer), which acts as a natural barrier limiting the penetration of external substances. Beneath the cuticle, the epidermal and palisade mesophyll cells remain protected.
- (b)
- Laser treatment—The laser selectively interacts with the leaf surface, ablating the wax cuticle without damaging the epidermis. This process exposes the underlying epidermis, creating an access point for improved agrochemical uptake.
- (c)
- Substance application and absorption—Following cuticle removal, agrochemical solutions can be precisely applied to the treated area. The absence of the wax barrier facilitates rapid and efficient penetration of the applied substances into the underlying leaf tissues.
3.1. Laser Treatment and Substance Application Experiment
Figure 2 illustrates the locations where laser treatment was applied, substances were administered, and samples were collected. Laser treatment was consistently performed on leaves from the canopy. A total of six laser pulses per leaf were manually distributed over a 1 cm2 area at the center of the leaf.
For the fluorescence experiment, immediately after laser treatment, a single drop of fluorescent glucose solution was applied using a pipette to the designated samples. The pipette was positioned over the leaf, targeting a 1 cm-diameter area corresponding to the laser-treated zone. In control samples, the pipette was applied in the same manner, with the only difference being the absence of prior laser treatment.
A similar procedure was followed for the zinc fertilizer experiment. In this case, a single spray pulse was applied, directing the spray onto the leaf using the most collimated nozzle setting available. It is important to note that for this experiment, pre-penetration losses were not a concern, as the primary objective was to monitor zinc penetration into the leaf tissue using LIBS analysis.
A precise quantification of the overall spraying efficiency is a complex task, and to the best of our knowledge, no comprehensive reports exist in the literature addressing this aspect. This remains an open area for future investigation.
In the first step (left), the laser beam irradiates the leaf, selectively removing the wax cuticle from the targeted area. In the center, a pipette is used to apply a single drop of fluorescent glucose solution precisely onto the laser-treated area. On the right, the Zn fertilizer spraying experiment is shown.
3.2. Morphological Analysis of Laser-Treated Area
Below, Figure 5 presents images of laser-treated leaf areas, each corresponding to a specific fluence level, as indicated in the accompanying table. These images visually demonstrate the effect of varying laser energy levels on the leaf surface, particularly on the wax cuticle.
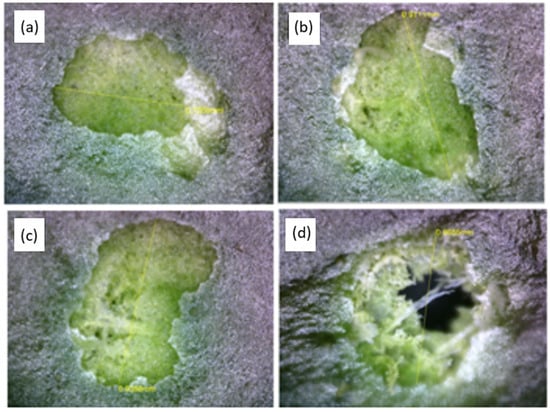
Figure 5.
Laser fluence working window for wax cuticle removal. The images illustrate the laser’s effect on wax cuticle removal at various fluence levels: (a) 1.42 J/cm2, (b) 4.25 J/cm2, (c) 7.08 J/cm2, and (d) 9.91 J/cm2. In subfigures (a,b), the wax cuticle is removed at lower fluence levels, exposing the epidermis without visible damage. At a moderate fluence level in subfigure (c), minimal epidermal ablation is observed, while in subfigure (d) the highest fluence results in pronounced epidermal ablation.
The wax cuticle, which initially appears as a reflective and textured layer, was effectively ablated by the laser, revealing the underlying green epidermis due to its chlorophyll content. The exposed epidermis appears well preserved, with no observable signs of thermal damage, indicating that the laser treatment selectively removed the wax layer while maintaining epidermal integrity.
From a morphological perspective, only the highest fluence level resulted in visible tissue damage, suggesting that there is an optimal working window ranging from 1 J/cm2 to 7 J/cm2, where cuticle removal is achieved without compromising the underlying epidermis.
The calculation of the exposed epidermis area after treatment was performed using microscope images processed by software created in Python. In the example below (Figure 6), the results indicate that 43.44% of the laser spot area had the wax cuticle removed at the lowest fluence level, increasing to 80% at the highest fluence level within the working window (13.3 J/cm2), effectively leaving the epidermis exposed.
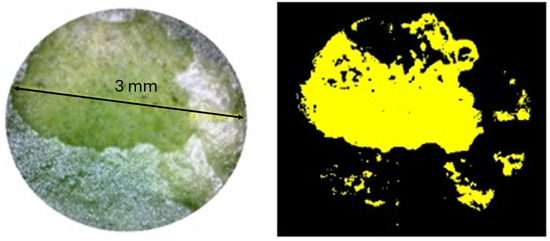
Figure 6.
Example of epidermis exposure percentage calculation. The yellow-highlighted area represents the region where the wax cuticle was removed after laser irradiation at an energy density of 0.7 J/cm2. The exposed area was measured using ImageJ software and expressed as a percentage of the total image area.
To determine the effective area of laser treatment, we considered a total of six laser pulses, each with a spot diameter of 3 mm, applied within a 1 cm-diameter circular region. The area of a single laser spot was calculated as 0.0707 cm2, resulting in a total laser-treated area of 0.424 cm2. This represents 54.0% of the total 1 cm2 region where glucose and zinc fertilizer were later applied for penetration analysis.
Since each laser spot led to 43.4% cuticle exposure, the total exposed cuticle area within the 1 cm treatment region was 0.184 cm2, corresponding to 23.4% of the total area. This quantification is essential for accurately assessing the improvement in substance penetration achieved with laser treatment and for estimating the potential for further optimization if the effective treatment area is increased, which will be the focus of future studies.
The images from Figure 4 demonstrate the high selectivity of the laser process using a 532 nm wavelength and short pulses, as evidenced by the intact epidermis, with no visible thermal or mechanical damage. The absence of morphological alterations, burns, or microfractures in the exposed epidermis confirms that the laser effectively removes the cuticle while preserving the underlying tissue. This indicates that the process operates within an optimal parameter range, ensuring precision and minimizing unintended side effects.
The wax cuticle serves a critical function in plant physiology, acting as a barrier that regulates water retention, protects against pathogen invasion, and mitigates environmental stress. While its selective removal facilitates the penetration of foliar-applied substances, it is necessary to investigate the regeneration dynamics of the cuticle following laser treatment to assess potential long-term effects on plant health. Future research will focus on characterizing the rate and extent of cuticle reformation and evaluating any potential vulnerabilities associated with this process. However, the selective nature of the laser treatment, as evidenced by the preservation of epidermal integrity in post-treatment imaging, suggests that the procedure does not induce significant structural damage. Additionally, the short pulse duration (20 ns) minimizes thermal diffusion, reducing the likelihood of collateral tissue alterations. These factors provide a strong rationale for expecting a rapid and natural restoration of the wax layer, ensuring the sustainability of this approach for enhancing agrochemical uptake.
3.3. Fluorescent Glucose Penetration Analysis
To demonstrate the effectiveness of laser treatment in facilitating the penetration of externally applied substances into the leaf and their movement through the plant vasculature, we applied fluorescent NBDG to laser-treated areas (Figure 6). The images were captured using a fluorescence filter. A control untreated leaf is shown on the right side of Figure 7. In contrast, Figure 7a displays the treated leaf at the moment of glucose application, Figure 7b shows the leaf 10 min after treatment, and Figure 7c presents the fluorescent glucose distribution 2 h post-application.
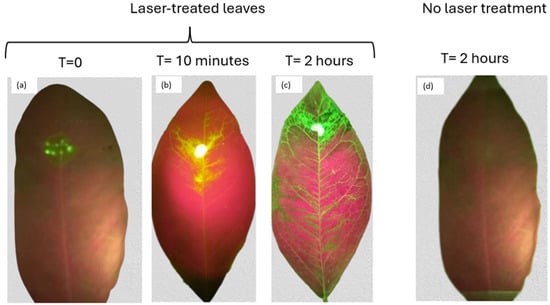
Figure 7.
Evolution of fluorescent glucose propagation inside the leaf: (a) at the moment of glucose application, (b) after 10 min, and (c) after 2 h. On the right (d), a leaf is shown where glucose was applied without prior laser treatment.
In the control leaf (Figure 7d), where no laser treatment was applied, although the same amount of glucose was used as in the laser-treated leaves, no visually detectable propagation of fluorescent glucose is observed. This clearly illustrates the role of the wax cuticle as a barrier against the penetration of the substance. This result highlights the limitations of conventional foliar application methods, where penetration into the plant interior is extremely slow and inefficient. The low diffusion rate causes the applied substance to remain exposed to the environment for an extended period, increasing the evaporation before effective absorption occurs.
In contrast, the enhanced penetration observed in laser-treated leaves demonstrates a clear improvement. Within just two hours, the fluorescent glucose spread throughout the interior of the leaf, covering its entire surface area. This highlights the potential of the laser treatment to significantly increase the efficiency of foliar applications, ensuring rapid and effective uptake while reducing losses due to environmental exposure. For the selective removal of the wax cuticle in the target area, a laser fluence of 1.42 J/cm2 was used.
The importance of the waxy cuticle as a barrier to molecular transport in plant leaves has been well established in the literature. In a seminal study, Schönherr [15] demonstrated that the removal of cuticular waxes significantly increases the permeability of the plant cuticle. Using organic solvents, primarily chloroform, to extract cuticular waxes from isolated cuticular membranes, the author observed that “the permeability of the cuticle to water and organic compounds increases upon wax extraction by factors between 10 and 1000”. This finding underscores the critical role of cuticular waxes in regulating the transport of water and solutes across the plant surface.
To assess the efficiency of substance penetration in leaves following laser-induced cuticle removal, we conducted an experiment using fluorescent glucose as a tracer. The objective was to quantify the spread of the applied substance over time and estimate its radial expansion velocity.
A fluorescent glucose solution was applied as a droplet using a pipette at the center of the leaf, precisely over a 9 mm laser-treated spot. Fluorescence was captured through imaging at two time points: 10 min and 2 h post-application. The recorded images were processed using custom-developed Python software to determine the fluorescence area at each time point, enabling the calculation of the radial expansion velocity.
The image processing algorithm included several preprocessing steps, such as background removal through adaptive thresholding and morphological operations to reduce noise. The fluorescence segmentation involved manual selection of key fluorescence points and the definition of tolerance thresholds. A binary mask was then applied to count the pixels corresponding to fluorescence, and the values were normalized to obtain the percentage of the fluorescent area.
Figure 8 presents an example of image processing used to determine the extent of glucose penetration—in this case, two hours after the substance was applied to the laser-treated area.
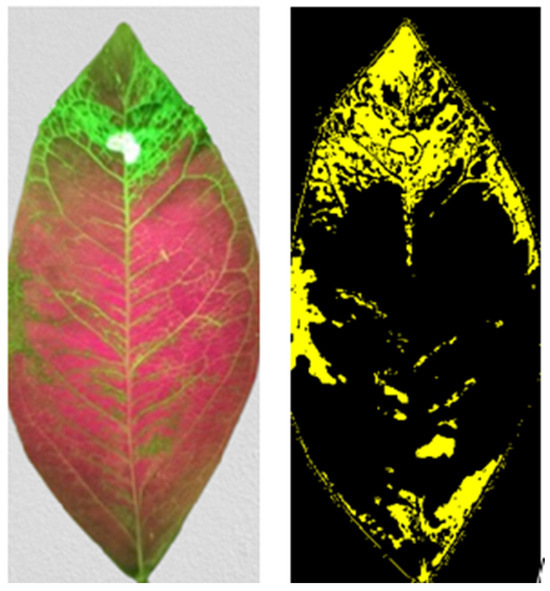
Figure 8.
Example of image processing used to determine the extent of glucose penetration, shown here two hours after application to the laser-treated area. On the left, the original photograph is displayed, while on the right, the processed image highlights the area of epidermis exposed by the laser treatment.
This analysis revealed that at 10 min post-application, the fluorescence-covered area was 7.17%, increasing to 27.6% after 2 h. Assuming an isotropic expansion from the center of the laser-treated region, the radial velocity was estimated using the following relationship:
where r1 and r2 are the effective radii of the fluorescence-covered areas at times t1 (10 min) and t2 (2 h), respectively. The effective radius at each time point was calculated from the fluorescence area using the equation:
where A is the measured fluorescence area. By substituting the measured values, the radial expansion velocity was determined. The calculated radial expansion velocity is approximately 0.0105 mm/min. This represents the rate at which the fluorescent glucose spread outward from the center of the laser-treated area over time.
The results indicate a significant increase in substance diffusion over time, suggesting that laser-induced cuticle removal enhances penetration efficiency. The observed radial velocity provides a quantitative measure of the substance’s spread dynamics, which can be further analyzed to optimize treatment parameters for improved absorption.
Etxeberria et al. [9] demonstrated that laser-induced microperforation using a CO2 laser significantly enhances the uptake and transport of foliar-applied substances in Citrus sinensis leaves. Their method involved creating multiple microscopic perforations in the cuticle to facilitate the penetration of applied compounds. One of their key findings was a 2511% increase in the uptake of fluorescent vancomycin in laser-treated leaves compared to untreated controls, confirming the effectiveness of this approach. Additionally, they measured the velocity of CF–SE transport in the phloem under natural conditions, estimating a rate of 3.94 μm/s ± 2.71 SD. While this measurement corresponds to a different compound, it provides a useful reference point for evaluating the enhancement achieved through laser treatment.
A direct comparison of transport velocities highlights the effectiveness of our approach. In our study, fluorescent glucose NBDG reached a distance of 8 cm from the application point to the petiole within 3 min, corresponding to a linear velocity of 444.4 μm/s. This is substantially higher than the velocity reported by Etxeberria et al. [9] for CF–SE without laser treatment (3.94 μm/s). Assuming that uptake efficiency scales proportionally with transport velocity, our method is estimated to have increased uptake by 11,280% compared to untreated conditions, far exceeding the 2511% improvement achieved by Etxeberria et al. with their CO2 laser microperforation method. These results strongly suggest that our approach provides a dramatic enhancement in the absorption and transport of foliar-applied substances, positioning laser-assisted foliar application as a powerful tool for improving agrochemical delivery in plants.
3.4. Laser Induced Breakdown Spectroscopy Analysis
The analyzed samples consisted of leaves extracted from plants that had been subjected to foliar fertilizer application, following the procedure detailed in the Section 2. The goal of the LIBS analysis was to assess the elemental composition of these leaves and confirm the penetration of the applied fertilizer under different treatment conditions.
To capture the LIBS spectra, each leaf sample was placed on a glass microscope slide and irradiated with laser pulses. Each pulse generated a localized plasma, allowing the capture of a single spectrum per shot. Both the plasma formation and the resulting microcavity were visually verified through the microscope. To ensure accurate measurements, each laser shot was performed in a different area, preventing the formation of larger perforations that could alter the laser fluence. The experimental setup used for LIBS analysis is illustrated in Figure 9, detailing the optical arrangement, laser system, and detection components employed for spectral acquisition.
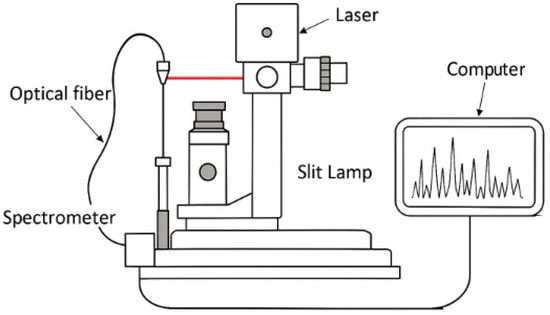
Figure 9.
Schematic representation of the LIBS experimental setup.
The laser-induced breakdown spectroscopy (LIBS) system used in this study was developed by Onteko (https://www.onteko.net/general-clean, accessed on 1 January 2020) and employed a Nd:YAG laser at 1064 nm for plasma generation. The laser operated with a pulse energy of up to 40 mJ; a macropulse of 4 µs composed of a train of 3 micropulses, each 4 ns long; and a wavelength of 1064 nm. Spectral acquisition was performed using an Ocean Optics USB 4000 spectrometer, covering the 300–800 nm range with a spectral resolution of 1.5 nm (FWHM). The spectrometer is manufactured by Ocean Insight, based in Tampa, USA. The spectra were recorded using Ocean View 2.0.8 software, while spectral identification and analysis were carried out using ProLIBSpector 1.2, specialized software developed by Onteko for LIBS-based element detection and quantification.
Figure 10 presents the LIBS spectra obtained for different treatment conditions. The spectral comparison reveals a clear enhancement in Zn detection in the laser-treated sample (blue spectrum). In particular, the characteristic peaks of Zn I (328–330 nm) and Zn II (758.9 nm) are only observed in the laser-treated condition, confirming the successful penetration of the foliar fertilizer into the plant tissue when combined with laser pre-treatment. In contrast, these Zn signals are absent in both the control (green) and the non-treated (red) samples, indicating limited uptake under standard foliar application conditions. The Zn I peaks (328–330 nm) have been reported in previous studies on Zn detection using LIBS due to their intensity, easy detection, and distinctive recognizability [16].
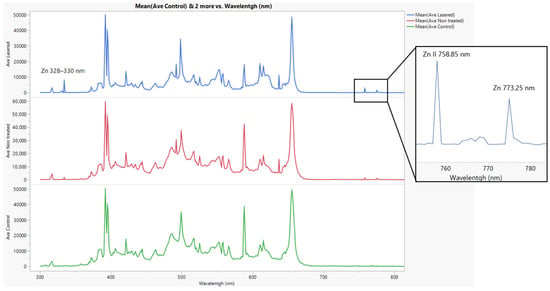
Figure 10.
LIBS spectra of leaf samples under different treatment conditions. The control sample (green) represents the natural elemental composition of the leaf. The non-treated sample (red) corresponds to foliar fertilizer application without laser treatment, while the laser-treated sample (blue) shows the effect of fertilizer application on a laser-treated area, demonstrating enhanced uptake.
It is important to note that the LIBS spectra were captured from a leaf different from the one where the laser treatment was applied, specifically from a position indicated in Figure 3—that is, from leaves collected approximately 1 m away from the leaf where the spray was applied.
On the other hand, due to the multi-pulse plasma emission regime of our laser setup, a high level of ionization was achieved, enabling the detection of ionized zinc (Zn II) at 758.9 nm and 773.5 nm, which were identified as coinciding with the values reported in the NIST database for LIBS analysis (https://physics.nist.gov/PhysRefData/ASD/LIBS/libs-form.html, accessed on 2 April 2025). This capability demonstrates the enhanced sensitivity of our system, allowing the identification of both neutral and ionized species, which is critical for assessing the internalization of agrochemical compounds at different depths within the plant tissue.
These findings suggesting that laser treatment significantly improves the absorption and mobility of foliar-applied nutrients, providing experimental evidence of its effectiveness in enhancing agrochemical delivery.
Figure 11 presents the intensity comparison of the Zn I (328–334 nm) and Zn II (758.9 nm) atomic emission peaks obtained through LIBS analysis under different treatment conditions. The control sample (blue) represents the natural background levels of zinc in the leaf, while the non-lasered sample (red) corresponds to foliar application of Zn fertilizer without laser pre-treatment, mimicking standard application conditions. The laser-treated sample (green) represents the condition where Zn fertilizer was applied to an area that had undergone selective cuticle removal via laser treatment.
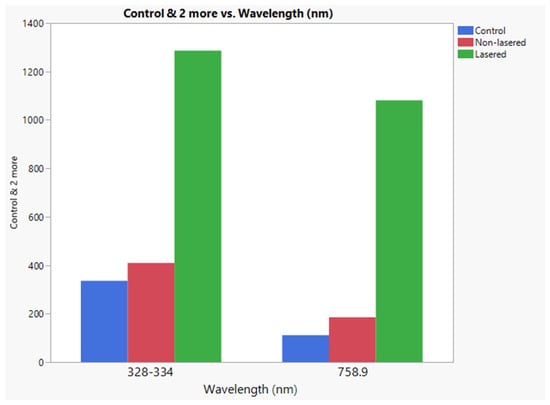
Figure 11.
Comparison of Zn I (328–334 nm) and Zn II (758.9 nm) emission peaks in LIBS analysis under different treatment conditions. The control sample (blue) represents natural background zinc levels, the non-lasered sample (red) corresponds to foliar application without laser pre-treatment, and the laser-treated sample (green) indicates Zn fertilizer applied after selective cuticle removal.
The results demonstrate a significant enhancement in Zn uptake in the laser-treated condition compared to the non-lasered and control samples. Specifically, the LIBS intensity of Zn I (328–334 nm) in the laser-treated sample was approximately three times higher than in the non-lasered sample and more than five times higher than in the control sample. A similar trend was observed for Zn II (758.9 nm), further confirming increased zinc absorption due to laser treatment.
These findings confirm that laser-assisted selective cuticle removal substantially improves zinc penetration into the leaf tissue, as evidenced by the stronger LIBS signal. The enhanced Zn detection in the laser-treated sample confirms that the cuticle layer acts as a major barrier to foliar uptake, and its removal facilitates more efficient absorption of the applied fertilizer.
4. Conclusions
This study demonstrates that laser-assisted selective cuticle removal significantly enhances the penetration and systemic transport of foliar-applied substances in citrus leaves. By employing a 532 nm-wavelength laser, we effectively removed the wax cuticle while preserving the integrity of the epidermis, creating a permeable surface that facilitates agrochemical uptake while minimizing tissue damage.
The enhancement in penetration was confirmed through two complementary analytical techniques:
- Fluorescent Glucose Visualization: The application of fluorescent glucose (NBDG) showed a substantial increase in penetration and mobility within the leaf when applied to laser-treated areas. The transport velocity of NBDG was measured at 444.4 μm/s, more than 100 times higher than previously reported values for passive phloem transport under natural conditions.
- Laser-Induced Breakdown Spectroscopy (LIBS) Analysis: LIBS measurements confirmed a significant increase in Zn absorption in laser-treated leaves compared to untreated controls. The Zn I (328–334 nm) emission intensity in laser-treated leaves was approximately three times higher than in non-lasered samples and over five times higher than in control leaves, demonstrating the improved uptake efficiency facilitated by selective cuticle removal.
These findings confirm that the wax cuticle serves as a major limiting barrier to foliar uptake, and its selective ablation significantly improves the efficiency of agrochemical absorption and translocation. A key advantage of this method is its ability to preserve epidermal integrity while effectively removing the wax cuticle. Unlike previously reported mechanical, laser-based, or chemical approaches, this technique minimizes potential tissue damage. We attribute this superior level of preservation and biocompatibility to two key factors:
- The use of a 532 nm wavelength, which is highly reflected by the epidermis, preventing excessive energy absorption by underlying tissues;
- The short nanosecond pulse duration, which allows for precise ablation of the wax cuticle, while limiting heat diffusion and collateral damage.
Given its potential applications in precision agriculture, this method has been patented by Onteko Inc. (application number 18/450,483), ensuring its protection for further development and commercialization. Future research should focus on evaluating the long-term physiological effects of laser treatment on plant health, optimizing laser parameters for different crop species, and integrating this technology into large-scale precision agriculture applications to maximize its potential impact on agrochemical efficiency and sustainability.
Additionally, further research is required to establish a quantitative relationship between the degree of wax cuticle removal, laser parameters, and optimal concentrations of agrochemicals. The ability to fine-tune these factors could enable more precise application strategies, reducing chemical waste and improving treatment uniformity across different crop species. While this study provides a proof-of-concept demonstration, future investigations should aim to develop predictive models that correlate laser treatment conditions with agrochemical uptake efficiency. Such efforts will be essential for scaling this technology into commercial agricultural practices, ensuring both efficacy and economic viability in real-world applications.
Author Contributions
L.P.-C. led the experimental design, conceived the main idea, and was the primary author of the manuscript. A.P.-F. conducted experiments, performed fluorescent glucose measurements, and processed LIBS data. T.F.-R. contributed to LIBS experimentation and spectral analysis. E.P.-F. developed Python scripts for image processing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are available from the corresponding author upon reasonable request, but restrictions apply to the availability of these data due to confidentiality obligations.
Conflicts of Interest
Author Luis Ponce-Cabrera was employed by the company Onteko Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Brodersen, C.; Narciso, C.; Reed, M.; Etxeberria, E. Phloem production in Huanglongbing-affected citrus trees. HortScience 2014, 49, 59–64. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of huanglongbing or greening disease on orange juice quality, a review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, E.; Gonzalez, P. The use of laser light to enhance penetration of antimicrobials into citrus leaves. Proc. Fla. State Hortic. Soc. 2014, 127, 75–77. [Google Scholar]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar]
- Niu, J.; Liu, C.; Huang, M.; Liu, K.; Yan, D. Effects of foliar fertilization: A review of current status and future perspectives. J. Soil Sci. Plant Nutr. 2021, 21, 104–118. [Google Scholar] [CrossRef]
- Toramana, M.C.; Bayatb, A. Effect of Surfactant Compound Sprays on the Rate of Adsorption on Different Target Surfaces. J. Plant Sci. Crop Protec 2019, 2, 102. [Google Scholar] [CrossRef]
- Ojo, I.; Ampatzidis, Y.; Neto, A.D.O.C.; Batuman, O. Development of an automated needle-based trunk injection system for HLB-affected citrus trees. Biosyst. Eng. 2024, 240, 90–99. [Google Scholar]
- Etxeberria, E.; Gonzalez, P.; Bhattacharya, P.; Sharma, P.; Ke, P.C. Determining the size exclusion for nanoparticles in citrus leaves. HortScience 2016, 51, 732–737. [Google Scholar] [CrossRef]
- Etxeberria, E.; Gonzalez, P.; Fanton Borges, A.; Brodersen, C. The use of laser light to enhance the uptake of foliar-applied substances into citrus (Citrus sinensis) leaves. Appl. Plant Sci. 2016, 4, 1500106. [Google Scholar]
- Cabrera, L.P.; Etxeberria, E.; Gonzalez, P.; Reyes, T.F. Use of non-intrusive laser exfoliation to improve substance uptake into citrus leaves. F1000Research 2023, 12, 303. [Google Scholar] [CrossRef]
- Cabrera, L.P.; Reyes, T.F.; Delgado, A.P.; Ornela, R.E.; Caro, D.P.; De Posada, E.; Arronte, M. Removal of Opuntia thorns by pulsed laser ablation: Bromatological and microbiological analysis. J. Food Eng. 2016, 169, 38–43. [Google Scholar] [CrossRef]
- Zou, C.; Wang, Y.; Shen, Z. 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J. Biochem. Biophys. Methods 2005, 64, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, M.; Ali, I.; Ahmad, S.; Malik, A.M.; Ashraf, H.M.Q.; Parveen, N.; Tareen, M.J.; Saeed, T.; Shah, S.H.; Zulfiqar, B. Efficiency of exogenous zinc sulfate application reduced fruit drop and improved antioxidant activity of ‘Kinnow’mandarin fruit. Braz. J. Biol. 2021, 83, e244593. [Google Scholar]
- Mikkelsted, F.N.; Adén, D.; Nikolajsen, T.; Laursen, K.H. A novel LIBS method for quantitative and high-throughput analysis of macro-and micronutrients in plants. J. Anal. At. Spectrom. 2024, 39, 2008–2020. [Google Scholar]
- Schönherr, J. Water permeability of isolated cuticular membranes: The effect of cuticular waxes on diffusion of water. Planta 1976, 131, 159–164. [Google Scholar] [PubMed]
- Andrade, D.F.; Pereira-Filho, E.R. Direct determination of contaminants and major and minor nutrients in solid fertilizers using laser-induced breakdown spectroscopy (LIBS). J. Agric. Food Chem. 2016, 64, 7890–7898. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).