1. Introduction
Agricultural spraying is a fundamental procedure in plant health management and is the predominant method for applying pesticides and foliar fertilizers. It involves fragmenting a mixture into droplets, which are then applied to specific targets to manage pests, diseases, and weeds [
1]. The process of droplet fragmentation during spraying is complex and influenced by various operating parameters, such as the hydraulic pressure, spray tip orifice diameter, and droplet release velocity [
2]. This fragmentation is a hydrodynamic process that occurs when the pressurized mixture passes through small, calibrated orifices, forming a liquid film that destabilizes and breaks into droplets. These droplets, which vary in size from a few micrometers to several hundred micrometers [
1], play a vital role in ensuring that pesticides are distributed uniformly and efficiently, maximizing the crop yield and minimizing the waste.
The distribution of the droplet spectrum during the spraying process is intrinsically heterogeneous, which directly affects the efficiency of the spraying process. Droplets with a volumetric median diameter (VMD) of <100 µm offer better target coverage but are more likely to be dispersed by wind, thereby increasing the risk of drift. On the other hand, droplets with diameters > 500 µm tend to slide off the leaves, resulting in a lower application efficiency [
3]. Drift refers to the movement and deposition of sprayed droplets on non-target regions. It is one of the primary technical and environmental challenges in agricultural spraying because it not only results in a loss of product to the environment but also includes risks of environmental contamination [
4]. To minimize this issue, several anti-drift technologies have been developed and implemented, such as spray nozzles equipped with air induction technology [
5]. These nozzles are designed to produce larger droplets with air bubbles inside, which are less susceptible to drift. When these droplets land on a surface, they behave similarly to fine droplets in terms of retention and wettability, but with a lower risk of runoff. This makes them a more effective option for controlling drift while maintaining good coverage and minimizing waste [
5].
Another key technology for drift management includes using agricultural adjuvants. These products have emerged as strategic in the application of phytosanitary products as they help optimize the spraying process. These adjuvants, when incorporated into the product formulation or spray mixture, can increase the application efficiency, which may be partly attributed to their effects on reducing the surface tension and altering the viscosity of the mixture and their role as mixture conditioning agents [
6]. Changes in the physicochemical properties of the spray mixture induced by adjuvants directly influence the droplet spectrum. This parameter is characterized quantitatively using a dimensional analysis of the droplets; primarily, the VMD and relative amplitude (RA) are used for this assessment [
7]. Fine droplets are typically formed when the surface tension of the spray solution is reduced by the addition of adjuvants [
8]. Research conducted by Ferreira et al. [
9] reported a 33.5% reduction in droplet size (VMD) after incorporating adjuvants into the spray solution, which could improve the spray coverage but potentially increase drift. However, certain types of adjuvants, such as those that produce oil-enhanced droplets, can increase the droplet size [
10], potentially reducing drift but also decreasing the coverage. This variation in droplet size is critical as it directly impacts the spray performance in the field, balancing the trade-off between maximizing coverage and minimizing drift.
Although a wide variety of adjuvants have been reported to improve the performance of pesticide sprays, quantitative analyses are lacking on the relative effectiveness of different classes of adjuvants as spreaders and humectants [
11]. This study addresses these gaps by exploring the interaction between adjuvants and spray tip architectures, providing a multifactorial analysis of their combined effects on droplet spectra and drift risk. Such an approach not only enhances the understanding of the relationship between adjuvant properties and nozzle performance but also highlights the critical role of physicochemical factors, such as the surface tension and contact angle, in determining the spray application efficiency [
12].
In this study, we aim to test the following hypotheses:
Hypothesis 1: The inclusion of agricultural adjuvants in spray solutions will result in significant changes in the droplet size distribution, with adjuvants lowering the surface tension and leading to different droplet sizes depending on the spray nozzle type, thereby varying the drift potential.
Hypothesis 2: The surface tension and contact angle influence the droplet spectrum and can explain variations in the droplet size based on their measurements.
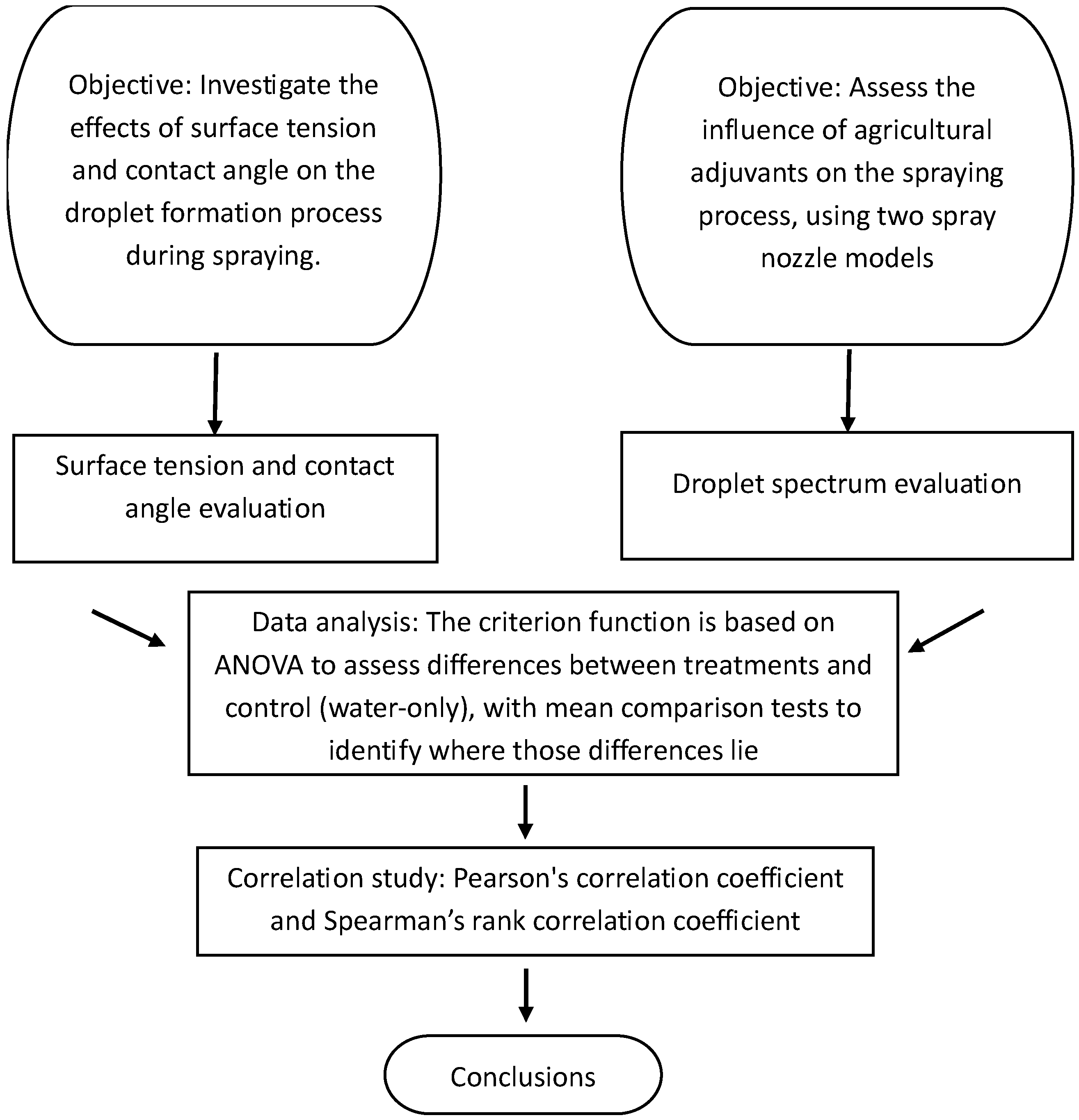
To test these hypotheses, we will compare the spray performance of different adjuvant treatments against a control solution (water-only) by measuring the droplet size distribution using a traditional method (particle image analyzer) and additional measurements of the surface tension and contact angle to explore their influence on the spray dynamics. The statistical significance will be assessed using ANOVA to evaluate differences between the treatments and the control. The results will be interpreted based on p-values to confirm the impact of the adjuvants on the spray performance. Additionally, the relationship between the surface tension, contact angle, and droplet spectrum parameters will be analyzed using a correlation analysis.
2. Materials and Methods
2.1. Experiment SITE
The experiment was performed in August and September 2023 at the Machinery and Mechanization Laboratory (LAMM) of the Federal University of Uberlândia (UFU) (Monte Carmelo campus), Monte Carmelo—MG, Brazil. The weather conditions inside the laboratory were monitored during the tests using an automatic weather station (ITWH1080, Instrutemp, São Paulo, Brazil). The temperature ranged from 27.1 °C to 27.8 °C, and the relative humidity ranged from 60% to 65%. There was no wind inside the room.
2.2. Surface Tension and Contact Angle
The surface tension and contact angle between the droplet and the surface were analyzed using a completely randomized design (CRD), which comprised four treatment solutions: water alone and water mixed with three different adjuvants. The water-only treatment was used as a control to assess the effect of adding the three adjuvants to the spray solution and facilitate the accurate judgment of the treatments’ impact [
12]. Each of the four treatments was replicated six times for each evaluation (surface tension and contact angle). The solutions were prepared following the concentrations recommended by the manufacturers for each adjuvant (
Table 1). The CRD was chosen because the experiment was conducted under controlled laboratory conditions.
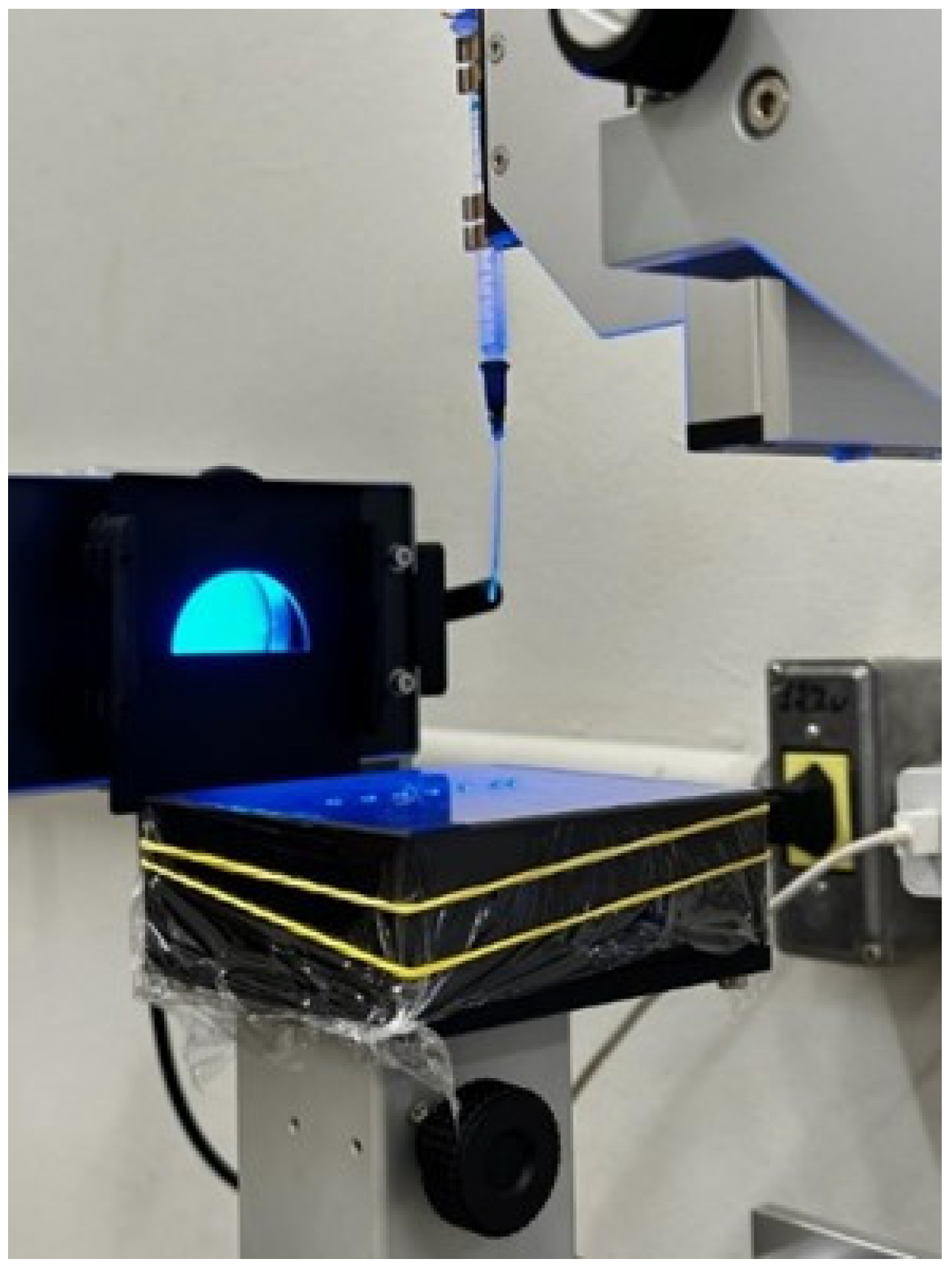
The surface tension and contact angle of the solutions were obtained using a drop shape analyzer—DSA30S (Krüss, Hamburg, Germany) with a resolution of 0.01 mN m
−1 and 0.01°, respectively. This analyzer was equipped with a high-speed, high-resolution CF04 camera (11 × 7 mm CMOS sensor, IR-CUT filter to eliminate disturbances and optical distortions, and manual optical zoom system and 6.5× focus) (
Figure 1). The readings were recorded over a period of 60 s, according to the methodology used by Sijs and Bonn [
10] and Milanowski et al. [
13]. The surface tension and contact angle were measured using the hanging drop method and the sessile drop method, respectively. Both methods used the Young–Laplace equation, adjusted to the image of the droplet through its shape and contour, as described by Marcinkowska et al. [
14].
The drop used to determine the surface tension and contact angle had a volume of 3 µL and was produced by a 1.02 mm needle using a 5 mL syringe. The dynamic surface tension was obtained from the average of the readings taken over 60 s, with one reading recorded every 10 s. The contact angle was determined on a standard surface of Parafilm® (Bemis, Neenah, WI, USA), which is a highly apolar surface.
2.3. Droplet Spectrum
The droplet spectrum analysis was performed using a CRD, under controlled conditions, in a 4 × 2 factorial scheme, with four solutions: water alone and water mixed with three different adjuvants (
Table 1). Two flat-fan spray nozzles were used for the experiments, which included LD 110-02 (without air induction) and ULD 120-02 (with air induction), both from the manufacturer Hypro (New Brighton, MN, USA). Four specimens of each model were randomly selected, with each specimen representing one replicate. A VisiSize Portable P15 particle analyzer (Oxford Lasers, Oxfordshire, UK), adjusted to capture 10,000 particles in suspension, with minimum and maximum diameters of 15 and 1800 µm, respectively, was used (
Figure 2). This is a well-established methodology [
10] that has been widely adopted in laboratories worldwide in this field. It eliminates the issues associated with determining the spread factor when droplets are measured indirectly from their impact on surfaces, and it also prevents interference from the type of surface affecting the measurements.
The spray nozzle was mounted 0.5 m above the optical beam and oscillated across a 180° arc, ensuring the entire spray jet intersected the beam. A pressurized stainless-steel tank, connected to a multi-nozzle body via a hose, supplied the liquid. Compressed air, regulated by a pressure controller, maintained a constant liquid pressure of 3 bar (300 kPa) throughout all of the assessments. The system was programmed to stop acquiring images automatically whenever ten thousand particles had been read in each repetition, thus producing a report containing the characterization of the droplets in each sample.
The software of the equipment assessed the following parameters: the volumetric median diameter (VMD), which reflects the droplet diameter at which 50% of the sprayed liquid volume consists of droplets smaller than this size; the relative amplitude (RA), which is an index that measures the homogeneity of sprayed droplet diameters (Equation (1)) [
7]; and the percentage of volume contained in droplets with diameters < 100 µm (% < 100).
where RA: relative amplitude; Dv0.1: droplet diameter such that 10% of the volume of liquid sprayed comprises droplets smaller than this value; Dv0.5: droplet diameter such that 50% of the volume of liquid sprayed comprises droplets smaller than this value (also called VMD); Dv0.9: droplet diameter such that 90% of the volume of liquid sprayed comprises droplets smaller than this value.
Laboratory methods that correlate with traditional field spray tests are crucial for better understanding and predicting the behavior of spray solutions in the environment. This type of study has been successfully conducted to reduce the potential losses while increasing the application efficacy [
9].
2.4. Statistical Analysis
The statistical design of the experiment included a completely randomized design with a control group for comparison (water-only). The data were analyzed using Analysis of Variance (ANOVA) to evaluate differences between the treatments and the control. When significant differences were detected, mean comparisons were performed using Tukey’s HSD test (p < 0.05). A criterion function was defined to assess the effectiveness of the treatments based on the statistical comparisons.
The assumptions underlying the linear model were assessed through the Shapiro–Wilk test (W) to check the normality of the residuals, the Levene test (L) to evaluate the homogeneity of variances, and the Durbin–Watson test (DW) to test for the independence of residuals.
For the surface tension variable, the assumptions of the linear model were not satisfied, even after applying square root and logarithmic transformations. As a result, non-parametric methods were employed for multiple comparisons, specifically the Kruskal–Wallis test, followed by Dunn’s test for pairwise comparisons. Given the non-normal distribution of the data, median values were used for the analysis in this case.
To explore alternative ways of analyzing the data, the results for the surface tension and contact angle of the solutions were correlated with the droplet spectrum parameters (VMD, RA, and % < 100) obtained for both the nozzle without air induction (LD) and with air induction (ULD). Since the contact angle data met the assumption of normality, Pearson’s correlation coefficient was applied to assess the relationship between the contact angle and droplet spectrum parameters. However, for the surface tension, Spearman’s rank correlation coefficient was used due to the failure to meet normality assumptions for this variable. Pearson’s correlation coefficient is used to quantify the degree to which the relationship between two quantitative variables can be described by a linear function. In contrast, Spearman’s rank correlation coefficient is a non-parametric measure of statistical dependence between two ordinal variables, reflecting how well the relationship can be described by a monotonic function (increasing, decreasing, or constant), which does not necessarily have to be linear. Unlike Pearson’s correlation coefficient (r), Spearman’s correlation coefficient does not require the residuals to be normally distributed, and the presence of outliers does not affect the strength of the association [
15].
All of the statistical analyses were conducted using R software (version 4.2.2) [
16]. In summary, this study can be reproduced following the sequence of the flowchart represented in
Figure 3.
3. Results and Discussion
The contact angles in the aqueous solution, with the addition of the adjuvants, are listed in
Table 2. Compared to the treatment with water alone, all other treatments showed lower contact angles, with polyether–polymethyl exhibiting the lowest mean (25.85°). These results are consistent with those of Sun and Foy [
17], who also observed smaller contact angles for organosilicon adjuvants. Water has a high surface tension, which makes it naturally bead up on surfaces, resulting in a higher contact angle. Adjuvants can enhance the interaction between the solution and the surface by modifying the surface’s energy or by disrupting any hydrophobic properties, enabling better spreading. The vegetable oil exhibited intermediate behavior with respect to the contact angle, which can be attributed to the fact that the reduction in the angle is related to the surfactants present in the formulation rather than on the oil.
A comparative analysis revealed that polyether–polymethyl was significantly better at modifying interfacial properties than the other adjuvants. This organosilicon surfactant promoted more reductions in the contact angle: 86.7%, 142.7%, and 225.6% higher reductions than those obtained using polydimethyl-siloxane, the fatty acid esters-based adjuvant, and the control treatment (water), respectively. This pronounced change in wettability may influence the control effectiveness, although this correlation has not yet been clearly defined in previous studies.
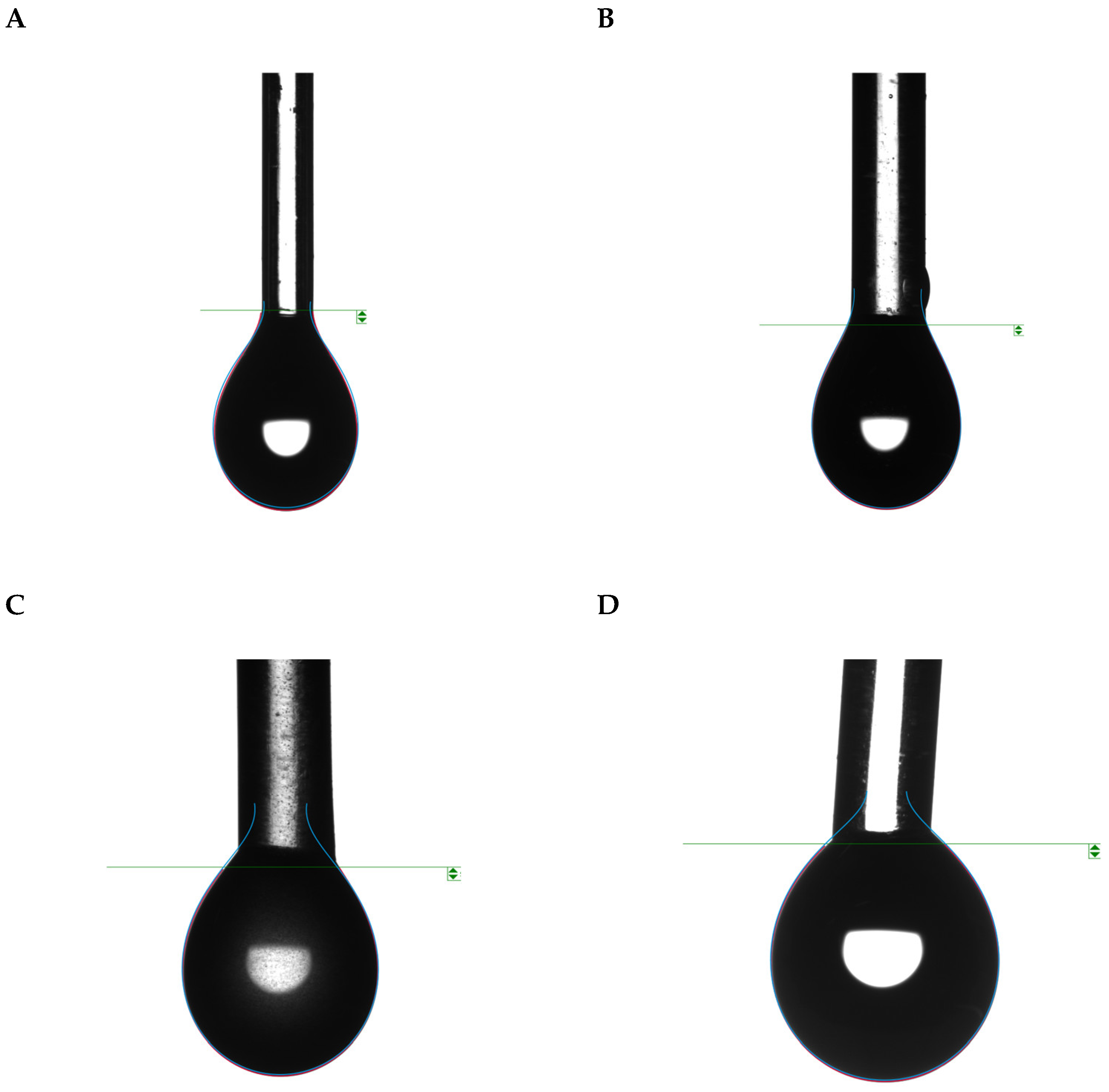
The droplets exhibited a tendency to behave more spherically in the absence of adjuvants (
Figure 4). Smaller contact angles resulted in greater spreading, leading to a consequent increase in the surface contact area. This contribution of adjuvants to reducing the contact angle increased wettability and resulted in a more hydrophilic interaction between the air, surface, and mixture.
According to Kismman [
6], the contact angle depends both on the solution tested and the surface characteristics. In this case, as the water contact angle (84.16°) was less than 90°, the surface used was characterized as hydrophilic. A contact angle lower than 90° indicates that the surface has a tendency to attract water molecules, causing the water droplets to spread more easily across the surface. This behavior is typical of hydrophilic surfaces, where intermolecular forces between the water and surface molecules are stronger than those between the water molecules themselves [
18].
The behavior of the droplet before it detached from the capillary allowed for the measurement of surface tension values, as shown in
Figure 5 (
Table 3).
An analysis of the results showed that the minimum values were consistently observed in solutions containing the silicone-based adjuvants (polyether–polymethyl and polydimethyl-siloxane), corroborating the results previously reported in the scientific literature by Iost and Raetano [
19]. The incorporation of the surfactant polyether–polymethyl led to reductions in the surface tension: 7.0% compared to polydimethyl-siloxane, 66.3% compared to the fatty acid ester-based adjuvant, and 179.8% compared to the control treatment (water), although this difference was significant only when compared to water. The comprehensive analysis of the changes in the interfacial properties (surface tension and contact angle) shows that this organosilicon surfactant exerts a decisive influence on the technological parameters of the application.
According to Montório et al. [
20], silicon surfactants have a more malleable molecular structure due to the carbon–silicon bond, which makes it easier to orient the surfactant’s hydrophobic group more parallel to the droplet’s surface. On the other hand, the hydrophilic group tends to extend further into the drop. This molecular arrangement results in a faster and greater reduction in surface tension compared to non-organosilicon surfactants, which have a more rigid conformation due to carbon–carbon bonds.
It is known that there is a strong correlation between contact angle and surface tension, which is verified in this work. The adjuvants with the lowest surface tension values were the same as those with the lowest contact angle averages. This correlation is based on the physical–chemical principles of liquid–solid interfaces, where high surface tension values result in greater resistance to atomization and, consequently, a greater contact angle, resulting in lower surface wettability [
19].
Regarding the assessment of droplet size (
Table 4), it may be observed that there was a significant interaction between the nozzles and the solutions tested, indicating that there is a dependence between the factors assessed. The VMD ranged from 175.4 to 206.2 µm for the LD 110-02 nozzle and from 334.7 to 363.9 µm for the ULD 120-02 nozzle. In all of the solutions, including water, there was a greater VMD whenever the nozzle with air induction was used. The objective of these nozzles is to incorporate air bubbles into the droplet to increase its size and reduce its propensity to drift. Air induction nozzles are configured with a long fluid chamber and small holes in the chamber upstream from the nozzle orifices. These holes are reported to induce air into the liquid flow due to the Venturi effect and reduce pressure at the nozzle orifice [
21].
The polyether–polymethyl adjuvant exhibited the lowest VMD values regardless of the nozzle tested, being statistically similar to water with the LD 110-02 nozzle and to fatty acid esters with the ULD 120-02 nozzle. The VMD of all of the treatments, including those performed with nozzles equipped with air induction, remained below 500 µm. This suggests the possibility that losses due to surface runoff were reduced with the treatments [
22]. Coarse droplets tend to run off leaves due to their larger volume and weight, which can exceed the adhesion forces between the droplet and the leaf surface. Additionally, the lower surface area-to-volume ratio of coarse droplets reduces their ability to spread and adhere to the leaf surface, especially on plants with waxy or hydrophobic cuticles [
23]. This leads to gravity overcoming the adhesion forces, causing the droplets to slide off [
24].
Regarding the relative amplitude (
Table 5), a significant interaction between the factors tested was observed. The lowest mean values were obtained for the LD 110-02 nozzle with fatty acid esters and polydimethyl. Polydimethyl was the only adjuvant that differed statistically in reducing this parameter when spraying with the ULD120-02 nozzle.
RA is a fundamental parameter in the qualitative evaluation of spraying as it characterizes the degree of homogeneity of the droplet spectrum produced [
22]. Polyether–polymethyl exhibited the highest average RA regardless of the nozzle use. This result was statistically similar to that obtained using water when the nozzle was not equipped for air induction and to that obtained using water and fatty acid esters when the nozzle was equipped for air induction. This indicated that although polyether–polymethyl reduced the contact angle, surface tension, and VMD, there was greater non-uniformity in size among the generated droplets, which may have interfered with the quality of the application.
The analysis of the volumetric percentage of droplets with diameters < 100 µm (considered highly susceptible to drift) showed a significant interaction between adjuvants and spray nozzle designs (
Table 6). The ULD 120-02 air induction nozzle significantly reduced this parameter compared to the conventional LD 110-02 flat-fan nozzle.
The polydimethyl adjuvant resulted in the lowest volumetric percentages of driftable droplets (<100 µm) regardless of the nozzle used. When spraying with the LD 110-02 conventional flat nozzle, the volumetric percentage obtained by this organosilicon surfactant was statistically equivalent to that obtained using the fatty acid esters adjuvant. When the ULD 120-02 air induction nozzle was used, the volumetric percentage of droplets obtained using the polydimethyl adjuvant was statistically similar to those obtained using the fatty acid esters adjuvant as well as using the control treatment (water).
Cunha et al. [
25] analyzed the VMD and the percentage of droplets with diameters <100, 150, and 200 µm, reporting an increase in the droplet size in solutions containing vegetable oil. This increase may be attributed to the fact that vegetable oils raise the viscosity of the mixture, making droplet disintegration more difficult [
26]. The present study corroborated these findings, showing that the vegetable oil-based adjuvant, when used with the LD 110-02 nozzle, caused a significant change in the droplet spectrum, suggesting that it can help reduce drift when used with nozzles lacking air induction technology. However, the same results were not observed with the ULD 120-02 nozzle, likely due to interference from this nozzle model, as a significant interaction was found between the evaluated factors. Further studies are needed to investigate this behavior of the solution as it passes through the air induction nozzle. Despite the higher viscosity of vegetable oil, it is possible that the induced air could have a stabilizing effect, potentially helping to keep the droplets smaller compared to spraying without air or with low-viscosity liquids.
The lower the rate of droplets with diameters <100 µm, the lower the potential for drift during the application of phytosanitary products, as droplets of this size are more susceptible to meteorological effects [
12]. Cunha et al. [
25] suggested that the application of the mixture should contain no more than 15% of the sprayed volume consisting of droplets with diameters <100 µm. However, this percentage depends on factors such as the product type and the characteristics of the surrounding areas. In the present study, the comparative analysis of the data obtained for the different nozzles showed an inversely proportional correlation between the VMD and the percentage of droplets with diameters <100 µm.
Table 7 shows the correlation analyses between the interfacial properties (surface tension and contact angle) of the solutions in relation to the droplet spectrum parameters for the two spray nozzles. Pearson’s correlation coefficient expresses how much an association of quantitative variables may be described by a linear function between continuous quantitative variables. Spearman’s correlation coefficient is a non-parametric correlation measure of association for ordinal data or data that does not follow a normal distribution [
27].
The correlation analysis did not reveal any significant associations between the investigated variables. Consequently, there is insufficient statistical evidence to conclude that these variables are significantly related. This lack of significance may be attributed to several factors, including an inadequate sample size, high data variability, or the absence of a true correlation between the variables. Schampheleire et al. [
28] similarly reported that no clear correlation exists between droplet size and surface tension, with the droplet-generating element exerting a considerable influence on the process.
In certain cases, solutions with lower surface tensions may produce smaller spray droplets. However, the exact relationship between the surface tension and droplet size is dependent on various factors inherent to the spray system, such as the spray pressure, the solution viscosity, and the characteristics of the spraying device. Mandato et al. [
29] noted that the physical–chemical properties of the liquid, including the surface tension, viscosity, and density, contribute to droplet formation. Specifically, an increase in liquid density leads to larger droplet sizes. Given that viscosity and surface tension often vary together when formulating liquids, it remains challenging to establish straightforward correlations between liquid properties and droplet size. Therefore, future research should focus on investigating the individual effects of viscosity and surface tension in greater detail.
The challenge in establishing clear correlations between liquid properties (such as surface tension and viscosity) and droplet size lies in the complex interplay between these factors. While it is known that both viscosity and surface tension influence the behavior of spray droplets, their effects are not always predictable and may vary depending on several variables inherent to the spraying process. For example, while increasing the viscosity typically leads to larger droplets due to the increased resistance to flow, changes in surface tension can either increase or decrease the droplet size depending on the nature of the formulation and the specific spray system used. Therefore, rather than expecting a straightforward linear relationship, it is essential that future studies investigate the individual and combined effects of viscosity and surface tension in more detail, using controlled experimental designs to isolate the effects of each factor. This would help to clarify the role of these physical–chemical properties in determining the droplet size and improve the optimization of spray formulations for different agricultural applications.
4. Conclusions
The results of this study indicate that the polyether–polymethyl adjuvant significantly reduced both the contact angle and surface tension of the tested solutions. However, despite these reductions, the adjuvant did not effectively homogenize the droplet spectrum and reduce the potential risk of drift.
In contrast, the polydimethyl-siloxane adjuvant exhibited similar effects on the surface tension as the polyether–polymethyl adjuvant, but it was more effective in homogenizing the droplet size and reducing the drift potential. These findings suggest that polydimethyl-siloxane may be a more efficient option for controlling drift while also improving droplet uniformity.
The fatty acid esters (oil-based) adjuvant increased the droplet size when applied with a standard flat-fan nozzle. This characteristic positions the fatty acid ester adjuvants as a viable anti-drift solution, particularly in the absence of air induction nozzles. Such properties may prove advantageous in scenarios where drift reduction is a critical factor.
It was further observed that the influence of adjuvants on the droplet spectrum was dependent on the type of spray nozzle used. This underscores the importance of considering the specific characteristics of each application system when evaluating the effects of adjuvants. As a result, it is not possible to generalize the implications of adjuvant use across various spraying systems.
The air induction nozzle was effective in reducing the risk of drift, although it does not always even out the spectrum of the droplets produced.
Moreover, no significant correlation was observed between the surface tension and contact angle with respect to the droplet spectrum parameters. This indicates that the relationship between these variables is not straightforward and may be influenced by other factors not addressed in the present study.
In summary, the study highlights the complexity of the agricultural spraying process, where the effects of adjuvants and spray nozzles vary depending on the conditions and interactions between factors. This reinforces the need for specific analyses for each combination of adjuvant and nozzle in order to optimize the application efficiency and reduce the environmental risks, such as drift. Furthermore, other physicochemical properties should be investigated and correlated with the droplet spectrum to enhance the understanding of the droplet formation process.