Biofilter, Ventilation, and Bedding Effects on Air Quality in Swine Confinement Systems
Abstract
1. Introduction
2. Materials and Methods
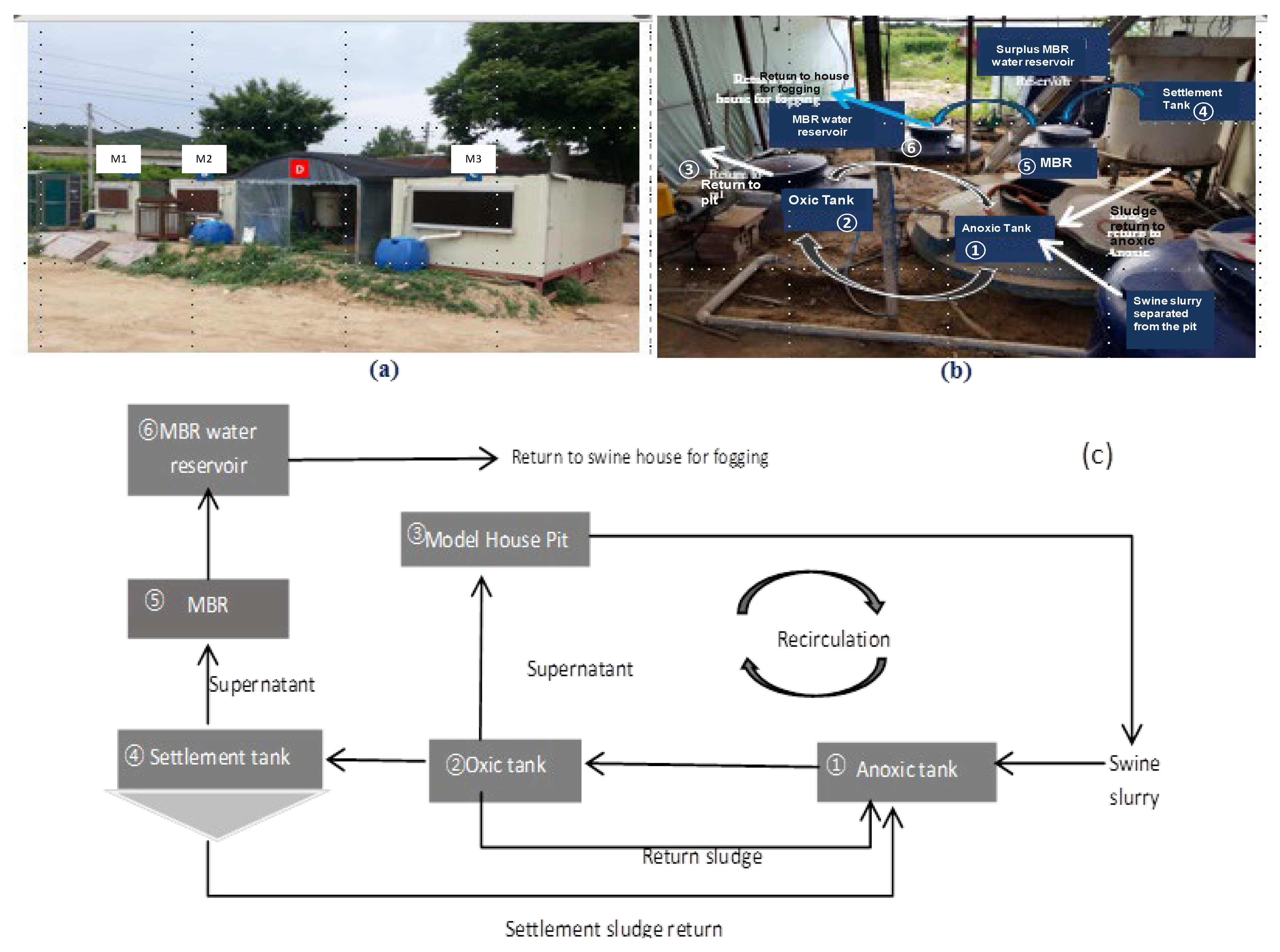
2.1. Experimental Swine Houses Design and Operation
2.2. Analysis of PM and Odorous Compounds
2.3. Airborne Bacteria Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. NH3 and H2S Reduction
3.1.1. Ammonia (NH3) Emissions
3.1.2. Hydrogen Sulfide (H2S) Emissions
3.2. Volatile Organic Compounds (VOCs) and Particulate Matter (PM)
3.2.1. Volatile Organic Compounds (VOCs)
3.2.2. Particulate Matter (PM) Emissions
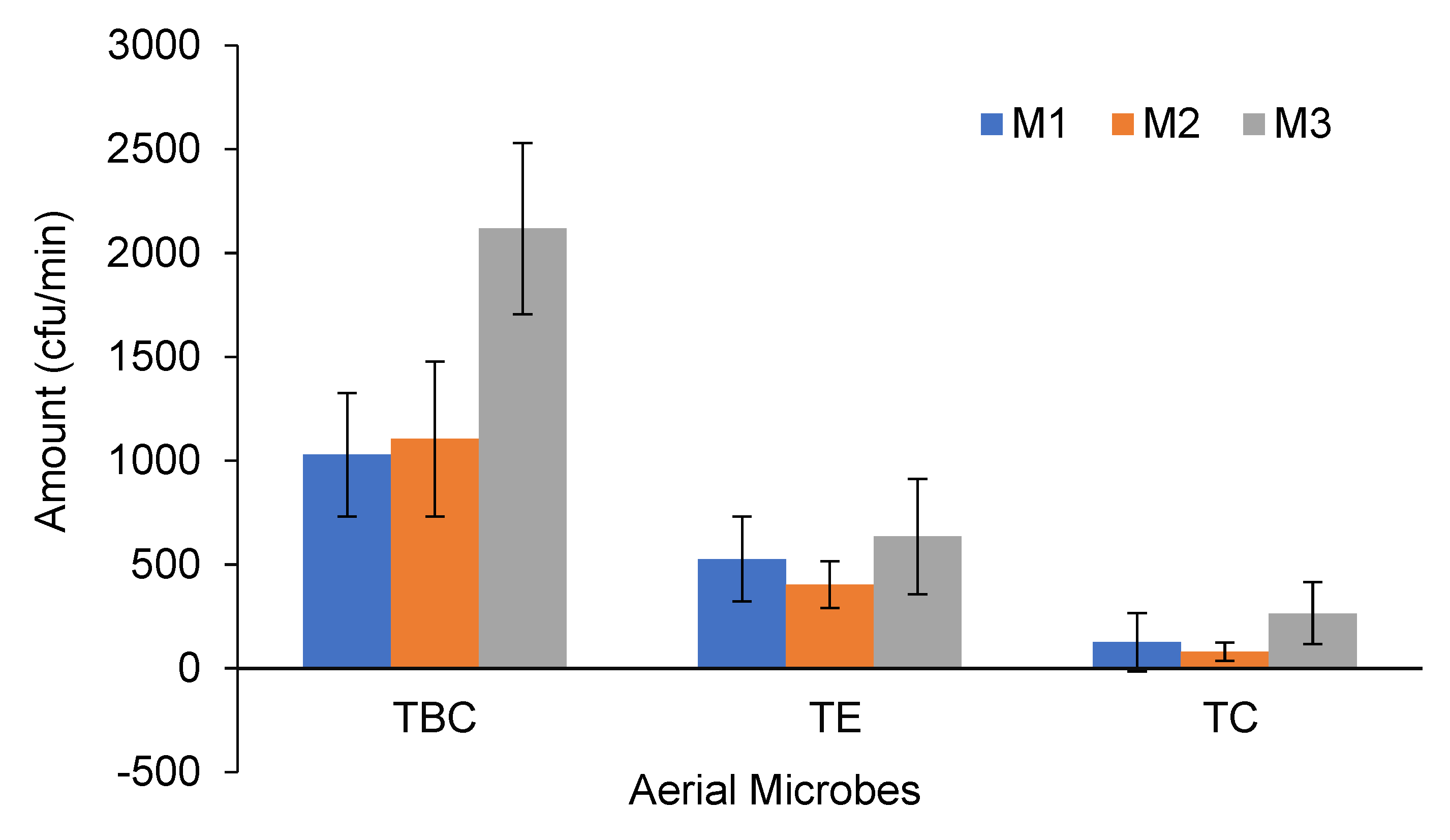
3.3. Aerial Bacterial Concentrations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAFOs | Concentrated Animal Feed Operations |
| CFU | Colony Forming unit |
| FGD | Flue Gas Desulfurization |
| M1 | Model house 1 |
| M2 | Model house 2 |
| M3 | Model house 3 |
| MBR | Membrane Bioreactor |
| ND | Not Detected |
| PM | Particulate Matter |
| ppbv | Parts per billion by volume |
| ppm | Parts per million |
| TBC | Total bacterial Count |
| TCs | Total Coliforms |
| TE | Total Escherichia coli |
| TSPs | Total Suspended Particles |
| VOA | Volatile Organic Acid |
| VOCs | Volatile Organic Compounds |
| VFAs | Volatile Fatty Acids |
References
- Hoff, S.J.; Harmon, J.D. Biofiltration of the Critical Minimum Ventilation Exhaust Air. In Proceedings of the Workshop on Agricultural Air Quality, Washington, DC, USA, 5 June 2006; pp. 760–764. [Google Scholar]
- Nimmermark, S. Influence of odour concentration and individual odour thresholds on the hedonic tone of odour from animal production. Biosyst. Eng. 2011, 108, 211–219. [Google Scholar] [CrossRef]
- ASPCA. A Closer Look at Animals on Factory Farms. Available online: https://www.aspca.org/fight-cruelty/farm-animal-cruelty/pigs-factory-farms (accessed on 7 March 2019).
- Yang, C.; Chen, H.; Zeng, G.; Yu, G.; Luo, S. Biomass accumulation and control strategies in gas biofiltration. Biotechnol. Adv. 2010, 28, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Le Leuch, L.M.; Subrenat, A.; Le Cloirec, P. Hydrogen sulfide adsorption and oxidation onto activated carbon cloths: Applications to odorous gaseous emission treatments. Langmuir 2003, 19, 10869–10877. [Google Scholar] [CrossRef]
- Cai, L.; Koziel, J.A.; Lo, Y.C.; Hoff, S.J. Characterization of volatile organic compounds and odorants associated with swine barn particulate matter using solid-phase microextraction and gas chromatography-mass spectrometry-olfactometry. J. Chromatogr. A 2006, 1102, 60–72. [Google Scholar] [CrossRef]
- Mielcarek, P.; Rzeźnik, W. Odor emission factors from livestock production. Pol. J. Environ. Stud. 2015, 24, 27–35. [Google Scholar] [CrossRef]
- Dumont, E.; Hamon, L.; Lagadec, S.; Landrain, P.; Landrain, B.; Andres, Y. NH3 biofiltration of piggery air. J. Environ. Manag. 2014, 140, 26–32. [Google Scholar] [CrossRef]
- Chung, Y.C.; Ho, K.L.; Tseng, C.P. Two-stage biofilter for effective NH3 removal from waste gases containing high concentrations of H2S. J. Air Waste Manag. Assoc. 2007, 57, 337–347. [Google Scholar] [CrossRef]
- Liu, Z.; Powers, W.; Mukhtar, S. A review of practices and technologies for odor control in swine production facilities. Appl. Eng. Agric. 2014, 30, 477–492. [Google Scholar]
- Ryu, H.W.; Cho, K.S.; Lee, T.H. Reduction of ammonia and volatile organic compounds from food waste-composting facilities using a novel anti-clogging biofilter system. Bioresour. Technol. 2011, 102, 4654–4660. [Google Scholar] [CrossRef]
- Estrada, J.M.; Kraakman, N.B.; Lebrero, R.; Muñoz, R. A sensitivity analysis of process design parameters, commodity prices and robustness on the economics of odour abatement technologies. Biotechnol. Adv. 2012, 30, 1354–1363. [Google Scholar] [CrossRef]
- Martens, W.; Martinec, M.; Zapirain, R.; Stark, M.; Hartung, E.; Palmgren, U. Reduction potential of microbial, odour and ammonia emissions from a pig facility by biofilters. Int. J. Hyg. Environ. Health 2001, 203, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, B.; Curran, T.; Dodd, V.; Colligan, J. Biofiltration of odour and ammonia from a pig unit-a pilot-scale study. Biosyst. Eng. 2002, 82, 441–453. [Google Scholar] [CrossRef]
- Zong, C.; Li, H.; Zhang, G. Ammonia and greenhouse gas emissions from fattening pig house with two types of partial pit ventilation systems. Agric. Ecosyst. Environ. 2015, 208, 94–105. [Google Scholar] [CrossRef]
- Zong, C.; Li, H.; Zhang, G. Airflow characteristics in a pig house with partial pit ventilation system: An experimental chamber study. In Proceedings of the ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; p. 152172138. [Google Scholar]
- Kumari, P.; Lee, J.; Choi, H.L. Characterization of odorant compounds from mechanical aerated pile composting and static aerated pile composting. Asian-Australas. J. Anim. Sci. 2016, 29, 594–598. [Google Scholar] [CrossRef]
- Bottcher, R.W. An environmental nuisance: Odor concentrated and transported by dust. Chem. Senses 2001, 26, 327–331. [Google Scholar] [CrossRef]
- Erisman, J.W.; Schaap, M. The need for ammonia abatement with respect to secondary PM reductions in Europe. Environ. Pollut. 2004, 129, 159–163. [Google Scholar] [CrossRef]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, Y.S.; Roh, Y.M.; Lee, C.M.; Kim, C.N. Quantification of ammonia and hydrogen sulfide emitted from pig buildings in Korea. J. Environ. Manag. 2008, 88, 195–202. [Google Scholar] [CrossRef]
- Sigsgaard, T.; Balmes, J. Environmental effects of intensive livestock farming. Am. J. Respir. Crit. Care Med. 2017, 196, 1092–1093. [Google Scholar] [CrossRef]
- Arogo, J.; Westerman, P.W.; Heber, A.J. A review of ammonia emissions from confined swine feeding operations. Trans. ASAE 2003, 46, 805. [Google Scholar] [CrossRef]
- Huang, G.F.; Wong, J.W.; Wu, Q.T.; Nagar, B.B. Effect of C/N on composting of pig manure with sawdust. Waste Manag. 2004, 24, 805–813. [Google Scholar] [CrossRef]
- Gallmann, E.; Hartung, E.; Jungbluth, T. Long-term study regarding the emission rates of ammonia and greenhouse gases from different housing systems for fattening pigs–final results. In Proceedings of the International Symposium on Gaseous and Odour Emissions from Animal Production Facilities, Horsens, Denmark, 1–4 June 2003; Volume 122. [Google Scholar]
- Cao, Y.; Bai, Z.; Misselbrook, T.; Wang, X.; Ma, L. Ammonia emissions from different pig production scales and their temporal variations in the North China Plain. J. Air Waste Manag. Assoc. 2021, 71, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.Q.; Heber, A.J.; Lim, T.T. Ammonia and hydrogen sulfide in swine production. In Air Quality and Livestock Farming; CRC Press: Boca Raton, FL, USA, 2018; pp. 29–47. [Google Scholar]
- World Health Organization. Guidelines for Air Quality in Livestock Facilities, 4th ed.; WHO Press: Geneva, Switzerland, 2022; Volume 3, pp. 154–196. [Google Scholar]
- Grant, R.H.; Boehm, M.T. Emissions of H2S from hog finisher farm anaerobic manure treatment lagoons: Physical, chemical and biological influence. Atmosphere 2022, 13, 153. [Google Scholar] [CrossRef]
- Hobbs, P.J.; Webb, J.; Mottram, T.T.; Grant, B.; Misselbrook, T.M. Emissions of volatile organic compounds originating from UK livestock agriculture. J. Sci. Food Agric. 2004, 84, 1414–1420. [Google Scholar] [CrossRef]
- Ni, J.Q.; Robarge, W.P.; Xiao, C.; Heber, A.J. Volatile organic compounds at swine facilities: A critical review. Chemosphere 2012, 89, 769–788. [Google Scholar] [CrossRef]
- Chmielowiec-Korzeniowska, A. The concentration of volatile organic compounds [VOCs] in pig farm air. Ann. Agric. Environ. Med. 2009, 16, 249–256. [Google Scholar]
- Bist, R.B.; Chai, L. Advanced Strategies for Mitigating Particulate Matter Generations in Poultry Houses. Appl. Sci. 2022, 12, 11323. [Google Scholar] [CrossRef]
- Hutchings, N.J.; ten Hoeve, M.; Jensen, R.; Bruun, S.; Søtoft, L.F. Modelling the potential of slurry management technologies to reduce the constraints of environmental legislation on pig production. J. Environ. Manag. 2013, 130, 447–456. [Google Scholar] [CrossRef]
- Spiehs, M.J.; Brown-Brandl, T.M.; Parker, D.B.; Miller, D.N. Effect of Bedding Material on Air Quality of Bedded Manure Packs in Livestock Facilities; ASABE Paper No. 12-1337890; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2012. [Google Scholar]
- De Rooij, M.M.; Hoek, G.; Schmitt, H.; Janse, I.; Swart, A.; Maassen, C.B.; Schalk, M.; Heederik, D.J.; Wouters, I.M. Insights into livestock-related microbial concentrations in air at residential level in a livestock dense area. Environ. Sci. Technol. 2019, 53, 7746–7758. [Google Scholar] [CrossRef]
- Lühken, E.; Nicolaisen, T.; Stracke, J.; Schulz, J.; Kemper, N. Microbiological air quality in free-farrowing housing systems for sows. Vet. Anim. Sci. 2019, 8, 100065. [Google Scholar] [CrossRef]
- Bilić, V.; Habrun, B.; Barač, I.; Humski, A. Distribution of airborne bacteria in swine housing facilities and their immediate environment. Arh. Hig. Rada Toksikol. 2000, 51, 199–205. [Google Scholar]
- Zhao, Y.; Aarnink, A.J.; De Jong, M.C.; Groot Koerkamp, P.W. Airborne microorganisms from livestock production systems and their relation to dust. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1071–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Ko, H.J.; Kim, H.T.; Kim, C.N.; Byeon, S.H. Association between pig activity and environmental factors in pig confinement buildings. Aust. J. Exp. Agric. 2008, 48, 680–686. [Google Scholar] [CrossRef]
- Luiken, R.E.; Van Gompel, L.; Bossers, A.; Munk, P.; Joosten, P.; Hansen, R.B.; Knudsen, B.E.; García-Cobos, S.; Dewulf, J.; Aarestrup, F.M.; et al. Farm dust resistomes and bacterial microbiomes in European poultry and pig farms. Environ. Int. 2020, 143, 105971. [Google Scholar] [CrossRef] [PubMed]
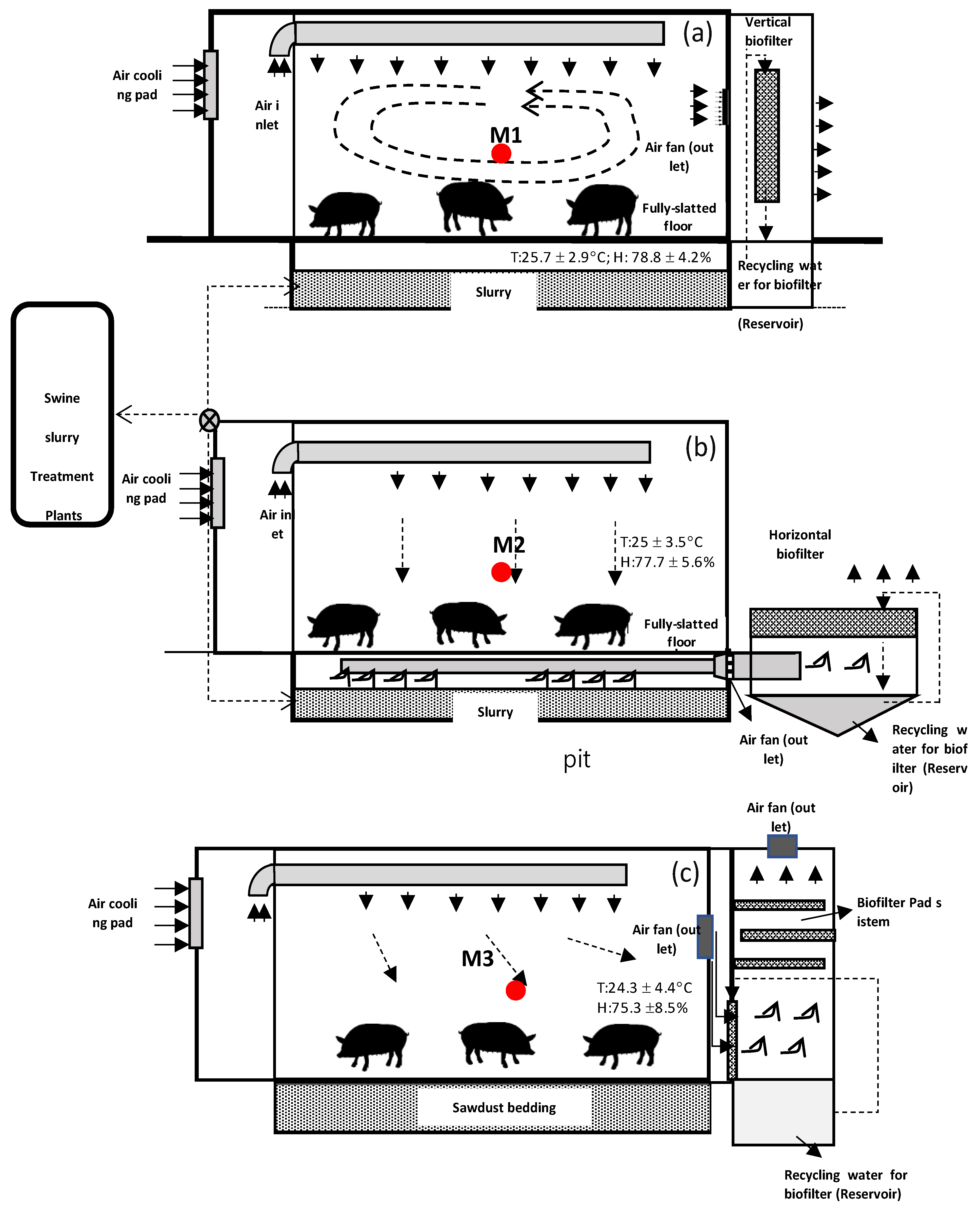



| Characteristics of the Model House | Model M1 | Model M2 | Model M3 | Specification |
|---|---|---|---|---|
| Construction material (roof walls) | 100 mm polyurethane panel 0.8 mm compressed with 0.8 mm metal sheet on both sides | Same as M1 | Same as M1 | Resistance (R) 3.85–4.33 K/W@0.1 m panel |
| Pit | Rectangular | Same as M1 | No pit | 3 m (W) × 4 m (L) × 0.4 m (D) |
| Floor (bedding system) | Fully slatted plastic bed | The same as M1 | Sawdust bed to a depth of 0.4 m | |
| Ventilation mode | Recirculation flow by exhaust fan on the end wall in the middle | Plug flow by exhaust fan on the outlet of a pipe in the pit | Recirculation flow by exhaust fan on the end wall in the middle, connected to biofilter tower | Exhaust fan capacity of 400 m3/h |
| Inlet | Circular inlet duct with perforated holes | The same as M1 | The same as M1 | D 200 mm with perforated holes of Φ10 mm |
| Biofilter composition | Corrugated thin plastic sheet pack | Two cellular pad sheets | Hybrid with Biochar/FGD gypsum layer | M1: 1.000 mm thickness, M2: 400 mm cellulose pad, M3: alternately biochar layer and FGD layer with depth of 100 mm |
| Biofilter orientation | Horizontal | Vertical | Horizontal |
| VOC (ppbv) | Swine House Types | ||
|---|---|---|---|
| Inside M1 | Inside M2 | Inside M3 | |
| Acetic acid | 87.3 ± 2.1 | 73.2 ± 1.8 | 23 ± 0.6 |
| Propionic acid | 29.1 ± 0.7 | 20 ± 0.5 | ND |
| Isobutyric acid | 2.1 ± 0.1 | 1.9 ± 0.1 | 17.4 ± 0.4 |
| Butyric acid | 15.4 ± 0.4 | 10.2 ± 0.2 | ND |
| Isovaleric acid | 5.7 ± 0.1 | 4.2 ± 0.1 | 0.2 |
| Valeric acid | 729.4 ± 17.5 | 489.8 ± 11.8 | 31.6 ± 0.8 |
| P-cresol | ND | 0.5 | ND |
| Indole | 0.9 | 0.4 | 0.6 |
| Skatole | ND | ND | ND |
| Dimethyl sulfide | 13.3 ± 0.3 | 3.2 ± 0.1 | 2.4 ± 0.1 |
| Dimethyl disulfide | 1.1 | 1.9 ± 0.1 | ND |
| Total VFA | 869 ± 20.9 | 599.3 ± 14.4 | 72.3 ± 1.7 |
| Total Phenol | 0.9 | 0.9 | 0.6 |
| Total Sulfuric | 14.4 ± 0.3 | 5.1 ± 0.1 | 2.4 ± 0.1 |
| Total VOA | 884.3 ± 15 | 605.3 ± 10.3 | 75.3 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-L.; Febrisiantosa, A.; Renggaman, A.; Sudiarto, S.I.A.; Yun, C.N.; Suresh, A. Biofilter, Ventilation, and Bedding Effects on Air Quality in Swine Confinement Systems. AgriEngineering 2025, 7, 73. https://doi.org/10.3390/agriengineering7030073
Choi H-L, Febrisiantosa A, Renggaman A, Sudiarto SIA, Yun CN, Suresh A. Biofilter, Ventilation, and Bedding Effects on Air Quality in Swine Confinement Systems. AgriEngineering. 2025; 7(3):73. https://doi.org/10.3390/agriengineering7030073
Chicago/Turabian StyleChoi, Hong-Lim, Andi Febrisiantosa, Anriansyah Renggaman, Sartika Indah Amalia Sudiarto, Chan Nyeong Yun, and Arumuganainar Suresh. 2025. "Biofilter, Ventilation, and Bedding Effects on Air Quality in Swine Confinement Systems" AgriEngineering 7, no. 3: 73. https://doi.org/10.3390/agriengineering7030073
APA StyleChoi, H.-L., Febrisiantosa, A., Renggaman, A., Sudiarto, S. I. A., Yun, C. N., & Suresh, A. (2025). Biofilter, Ventilation, and Bedding Effects on Air Quality in Swine Confinement Systems. AgriEngineering, 7(3), 73. https://doi.org/10.3390/agriengineering7030073







