Dicamba Impacts on Aquatic Bioindicators and Non-Target Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Dicamba Toxicity Tests for Aquatic Bioindicators
2.2. Dicamba Toxicity Tests for Terrestrial Non-Target Plants
2.3. Dicamba Low Doses on Non-Target Plants
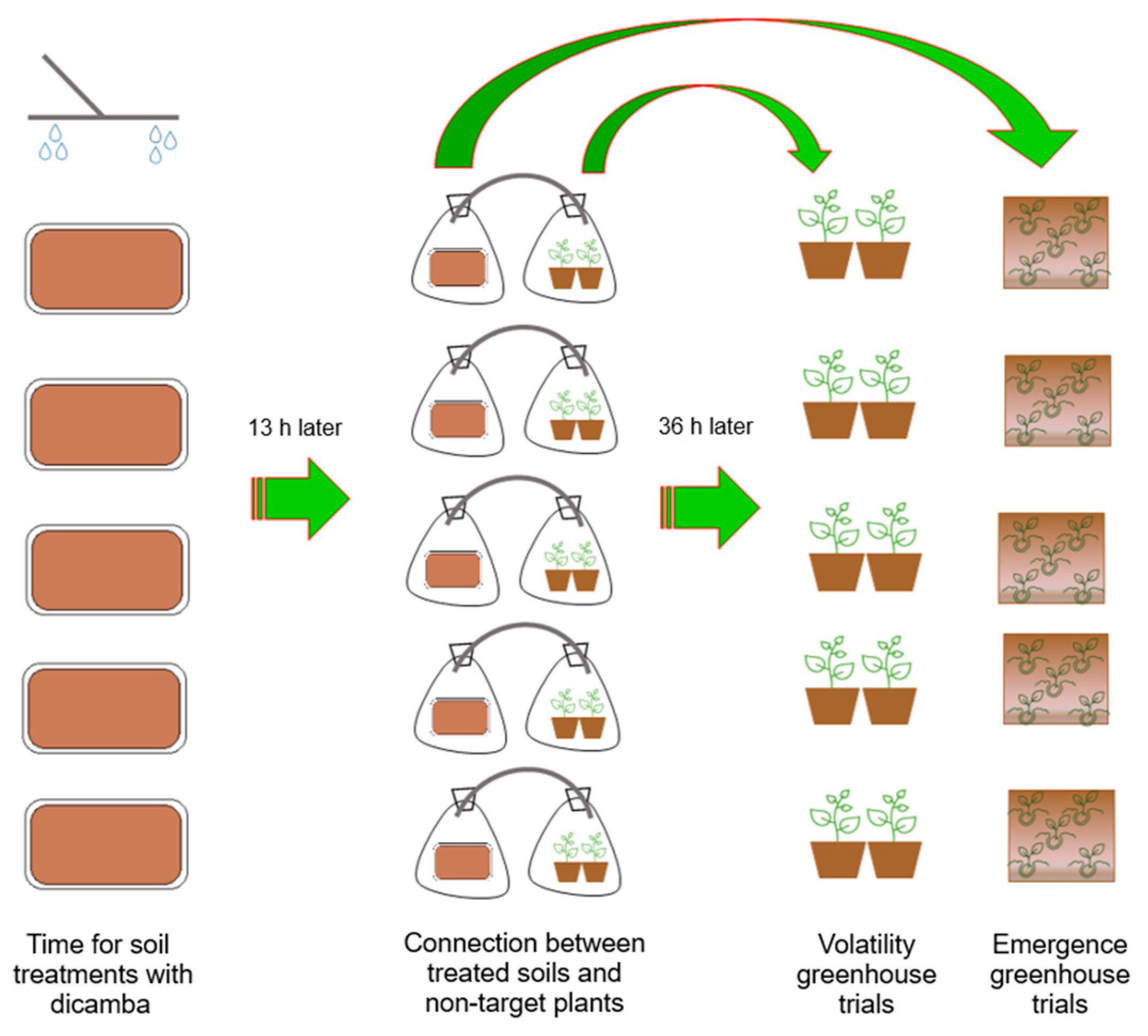
2.4. Dicamba Apparent Volatility on Non-Target Plants
2.5. Statistical Analysis
3. Results
3.1. Dicamba Toxicity Tests for Aquatic Bioindicators
3.2. Toxicity, Low-Dose Applications, and Dicamba Volatility on Cucumber Plants
3.3. Toxicity, Low-Dose Applications, and Dicamba Volatility on Tomato Plants
3.4. Toxicity, Low-Dose Applications, and Dicamba Volatility on Lettuce Plants
3.5. Effects of Dicamba-Treated Soils on Cucumber Seedling Emergence
4. Discussion
4.1. Dicamba Toxicity for Aquatic Bioindicators and Terrestrial Non-Target Plants
4.2. Low-Dose Applications of Dicamba on Non-Target Plants
4.3. Effects of Apparent Volatility of Dicamba and Dicamba-Treated Soils on Non-Target Plants
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LC50 | Lethal concentration 50% |
| EC50 | Effective concentration 50% |
| DMA | Dimethylamine salt |
| DGA | Diglycolamine salt |
| BAPMA | N,N-Bis-(3-aminopropyl)methylamine |
| CTNBio | The national biosafety technical committee |
| BASF | Badische Anilin- und Sodafabrik |
| pka | Electrolytic dissociation constant |
| Kow or P | Partition coefficient |
| Lpg Pow | Partition coefficient |
| Koc | Sorption coefficient on organic matter basis |
| pH | Hydrogenionic potential |
| P | Phosphorus |
| K | Potassium |
| Ca | Calcium |
| mg | Magnesium |
| CTC | Cation exchange capacity |
| V% | Saturation percentage |
| S | Phosphate |
| Zn | Zinc |
| B | Boron |
| Mn | Manganese |
| Cu | Copper |
| Fe | Iron |
| H + Al | Hydrogen + aluminum |
| ºC | Degrees celsius |
| NaCl | Sodium chloride |
| mL | Milliliter |
| mg | Milligrams |
| L | Liter |
| g | Grams |
| ECUA | Ethics Committee for the Use of Animals |
| KCl | Potassium chloride |
| µS | Microsecond |
| cm | Centimeter |
| Kg | Kilo |
| DAA | Days after application |
| CRD | Completely randomized design |
| CO2 | Carbon dioxide |
| km | Kilometer |
| PVC | Addition polymer polyvinyl chloride |
| DAE | Days after exposure |
| DAS | Days after sowing |
| ANOVA | Analysis of variance |
| ha | Hectare |
| LSD | Minimum significant difference |
| CV | Coefficient of variation |
References
- Li, P.; De Marco, A.; Feng, Z.; Anav, A.; Zhou, D.; Paoletti, E. Nationwide ground-level ozone measurements in China suggest serious risks to forests. Environ. Pollut. 2018, 237, 803–813. [Google Scholar] [CrossRef]
- De Oliveira, M.F.; Brighenti, A.M. Herbicide behavior in the environment. In Weed Biology and Management; Oliveira Júnior, R.S., Constantin, J., Inoue, M.H., Eds.; Omnipax: Curitiba, Brazil, 2011; pp. 263–304. [Google Scholar]
- Cruz, C.; Cerveira, W.R.; Pereira, P.C.; Garlich, N.; Vechia, J.F.D.; Carvalho, L.B. Ecotoxicology of herbicides: Applications and Environmental Dynamics. In Matology: Studies on Weeds; Barroso, A.A.M., Murata, A.T., Eds.; Fábrica da Palavra: Jaboticabal, Brazil, 2021; pp. 450–475. [Google Scholar]
- IBAMA—Brazilian Institute of Environment and Renewable Resources. Marketing Reports of Pesticides. 2022. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/relatorios-de-comercializacao-de-agrotoxicos (accessed on 12 March 2024).
- Rodrigues, M.; Raya-Rodriguez, M.T.M. Ecological risk analysis using the Lolium multiflorum bioindicator. J. Braz. Soc. Ecotoxicol. 2012, 7, 9–13. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- De Sotto, R.; Medriano, C.; Cho, Y.; Seok, K.S.; Park, Y.; Kim, S. Meaning of metabolite extraction method for evaluating sulfamethazine toxicity in adult zebrafish using metabolites. Ecotoxicol. Environ. Saf. 2016, 127, 127–134. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, M.; Wang, D.; Yan, J.; Miao, J.; Zhou, Z.; Zhu, W. Enantioselective bioaccumulation following exposure of adult zebrafish (Danio rerio) to epoxiconazole and its effects on metabolomic profile as well as expression genes. Environ. Pollut. 2017, 229, 264–271. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Wang, D.; Teng, M.; Yan, J.; Miao, J.; Zhou, Z. 1h NMR-based metabolites analysis of adult zebrafish (Danio rerio) after exposure to diniconazole as well as its bioaccumulation behavior. Chemosphere 2017, 168, 1571–1577. [Google Scholar] [CrossRef]
- Ferard, J.-F.; Blaise, C. Encyclopedia of Aquatic Ecotoxicology; Springer: Dordrecht, Netherlands, 2013; 1221p. [Google Scholar]
- Leonel, K.P.; Morandi, J.M.; Brunetti, I.A.; Aparicio, L.D.B.; da Cruz, C. Acute toxicity of insecticides and spreaders used in vector control for the bioindicator caramujo (Pomacea canaliculata). Ciência Cultura 2016, 12, e062016. [Google Scholar] [CrossRef]
- Boteon, B.F.; Silva, R.O.; Schedenffeldt, B.F.; Spricigo, H.; Hirata, A.C.S.; Monquero, P.A. Crop sensitivity to dicamba and 2.4-d applied to commercial and subdose levels. Agrociencia 2024, 58, 3015. [Google Scholar] [CrossRef]
- Gressel, J.; Gassmann, A.J.; Owen, M.D. How well will stacked transgenic pest/herbicide resistance delay pests from evolving resistance? Pest Manag. Sci. 2017, 73, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Mithila, J.; Hall, J.C.; Johnson, W.G.; Kelley, K.B.; Riechers, D.E. Evolution of resistance to auxinic herbicides: Historical perspectives, mechanisms of resistance, and implications for broadleaf management in agronomic crops. Weed Sci. 2011, 59, 445–457. [Google Scholar] [CrossRef]
- Bunch, T.R.; Gervais, J.A.; Buhl, K.; Stone, D. Dicamba Technical Fact Sheet; National Pesticide Information Center: Corvallis, OR, USA, 2012. [Google Scholar]
- IBAMA—Brazilian Institute of Environment and Renewable Resources. Environmental Profile Dicamba 1918-00-9. 2019. Available online: https://www.gov.br/ibama/pt-br/assuntos/quimicos-e-biologicos/agrotoxicos/arquivos/perfis-ambientais/2019/Perfil%20Ambiental%20-%20Dicamba%20-%2002_10_2019.pdf (accessed on 3 September 2025).
- Zhang, J.; Huang, Y.; Reddy, K.N.; Wang, B. Assessing crop damage from dicamba on non-dicamba-tolerant soybean by hyperspectral imaging through machine learning. Pest Manag. Sci. 2019, 75, 3260–3272. [Google Scholar] [CrossRef]
- Mueller, T.C.; Wright, D.R.; Remund, K.M. Effect of formulation and application time of day on detecting dicamba in the air under field conditions. Weed Sci. 2013, 61, 586–593. [Google Scholar] [CrossRef]
- Vieira, B.C.; Butts, T.R.; Rodrigues, A.O.; Schleier, J.J.; Fritz, B.K.; Kruger, G.R. Particle drift potential of glyphosate plus 2, 4-D choline pre-mixing formulation in a low-speed wind tunnel. Weed Technol. 2020, 34, 520–527. [Google Scholar] [CrossRef]
- Inman, M.D.; Vann, M.C.; Fisher, L.R.; Gannon, T.W.; Jordan, D.L.; Jennings, K.M. Evaluation of dicamba retention in spray tanks and its impact on flue-cured tobacco. Weed Technol. 2021, 35, 35–42. [Google Scholar] [CrossRef]
- Bish, M.D.; Bradley, K.W. Survey of Missouri pesticide applicator practices, knowledge, and perceptions. Weed Technol. 2017, 31, 165–177. [Google Scholar] [CrossRef]
- Hager, A. Observations of Midwest weaved extensists. In Proceedings of the 72nd Annual Meeting of the North Central Weed Science Society, St. Louis, MO, USA, 4–7 December 2017; North Central Weed Science Society: St. Louis, MO, USA, 2017; p. 98. [Google Scholar]
- Johnson, V.A.; Fisher, L.R.; Jordan, D.L.; Edmisten, K.E.; Stewart, A.M.; York, A.C. Cotton, peanut, and soybean respond to sublethal rates of dicamba, glufosinate, and 2, 4-D. Weed Technol. 2012, 26, 195–206. [Google Scholar] [CrossRef]
- Egan, J.F.; Mortensen, D.A. Quantifying vapor drift of dicamba herbicides applied to soybean. Environ. Toxicol. Chem. 2012, 31, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Penner, D.; Michael, J. Bioassay for evaluating herbicide volatility from soil and plants. In 33rd Symposium on Pesticide Formulation and Delivery Systems: “Sustainability: Contributions from formulation Technology”; ASTM International: West Conshohocken, PA, USA, 2014; pp. 36–43. [Google Scholar] [CrossRef]
- Mueller, T.C.; Steckel, L.E. Spray mixture pH as affected by dicamba, glyphosate, and spray additives. Weed Technol. 2019, 33, 547–554. [Google Scholar] [CrossRef]
- Colquhoun, J.B.; Heider, D.J.; Rittmeyer, R.A. Relationship between visual injury from synthetic auxin and glyphosate herbicides and snap bean and potato yield. Weed Technol. 2014, 28, 671–678. [Google Scholar] [CrossRef]
- Egan, J.F.; Barlow, K.M.; Mortensen, D.A. A Meta-Analysis on the Effects of 2,4-D and Dicamba Drift on Soybean and Cotton. Weed Sci. 2014, 6, 193–206. [Google Scholar] [CrossRef]
- Mohseni-Moghadam, M.; Doohan, D. Response from bell Pepper and broccoli to simulated drift rates of 2, 4-D and dicamba. Weed Technol. 2015, 29, 226–232. [Google Scholar] [CrossRef]
- Mohseni-Moghadam, M.; Wolfe, S.; Dami, I.; Doohan, D. Response of wine grape crops to simulated drift rates of 2, 4-D, dicamba, and glyphosate, and 2, 4-D or dicamba plus glyphosate. Weed Technol. 2016, 30, 807–814. [Google Scholar] [CrossRef]
- Culpepper, A.S.; Sosnoskie, L.M.; Shugart, J.; Leiffit, N.; Curry, M.; Gray, T. Effects of low-dose applications of 2, 4-D and dicamba on watermelon. Weed Technol. 2018, 32, 267–272. [Google Scholar] [CrossRef]
- Hand, L.C.; Eason, K.M.; Randell, T.M.; Gray, T.L.; Culpepper, A.S. 2, 4-D and dicamba removal from the surface of plastic mulch using overhead irrigation: Analytical analysis and cucurbit bioassay crop response. Weed Technol. 2021, 35, 662–668. [Google Scholar] [CrossRef]
- Hand, L.C.; Vance, J.C.; Randell, T.M.; Shugart, J.; Gray, T.; Luo, X.; Culpepper, A.S. Effects of low-dose applications of 2, 4-D and dicamba on cucumber and cantaloupe. Weed Technol. 2021, 35, 357–362. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Costa, R.N.; Giovanelli, B.F.; Velini, E.D. Evaluating methods and factors that affect dicamba volatility. Adv. Weed Sci. 2022, 40, e020220014. [Google Scholar] [CrossRef]
- Westberg, D.E.; Adams, A. Application stewardship of Engenia herbicide in dicamba tolerant crops, Abstract 187. In Proceedings of the Southern Weed Science Society 70th Annual Meeting, Birmingham, AL, USA, 23–26 January 2017; p. 155. [Google Scholar]
- Oseland, E.; Bish, M.; Steckel, L.; Bradley, K. Identification of environmental factors that influence the likelihood of off-target movement of dicamba. Pest Manag. Sci. 2020, 76, 3282–3291. [Google Scholar] [CrossRef] [PubMed]
- OECD—Organization for Economic Co-Operation and Development. Guidelines for the Testing of Chemicals, Lemna sp. Growth Inhibition Test; OECD—Organization for Economic Co-Operation and Development: Paris, France, 2002; 22p. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants Without Soils; California Agricultural Experimental Station: Berkeley, CA, USA, 1950. [Google Scholar]
- NBR 15088:2022; Aquatic Ecotoxicology—Acute Toxicity-Test Method with Fish (Cyprinidae). ABNT—Brazilian Association of Technical Standards: Sao Paulo, Brazil, 2022.
- OECD—Organization for Economic Co-Operation and Development. Guideline for the testing of chemicals. In Proposal for a New Guideline 227—Terrestrial Plant Test: Vegetative Vigour Test; OECD—Organization for Economic Co-Operation and Development: Paris, France, 2003; 16p. [Google Scholar]
- Pádua, A.C.C.; Pereira, J.A.; Araujo, F.R.; Silva, A.B.; Casagrande, H.; Diamantino, E.M.; Garlich, N.; Cruz, C. Efficacy of the herbicide mixture 2,4-D + aminopyralid with the addition of adjuvants in controlling weeds and sensitivity of signal grass (Urochloa decumbens). Ciência Cult. 2023, 19, e231901. [Google Scholar] [CrossRef]
- Ferreira, P.H.U.; Thiesen, L.V.; Pelegrini, G.; Ramos, M.F.T.; Pinto, M.M.D.; Ferreira, M.C. Physicochemical properties, droplet size and volatility of dicamba with herbicides and adjuvants on tank-mix. Sci. Rep. 2020, 10, 18833. [Google Scholar] [CrossRef] [PubMed]
- USEPA—United States Environmental Protection Agency. Technical Overview of Ecological Risk Assessment—Analysis Phase: Ecological Effects Characterization; USEPA—United States Environmental Protection Agency: Washington, DC, USA, 2023. [Google Scholar]
- Barbosa, J.C.; Maldonado Junior, W. AgroEstat: System for Statistical Analysis of Agronomic Assays; FCAV/UNESP: Jaboticabal, Brazil, 2015; 396p. [Google Scholar]
- Tunić, T.; Knežević, V.; Kerkez, Đ.; Tubić, A.; Šunjka, D.; Lazić, S.; Brkić, D.; Teodorović, I. Some arguments in favor of a Myriophyllum aquaticum growth inhibition test in a water-segment system as an additional test in risk assessment of herbicides. Environ. Toxicol. Chem. 2015, 34, 2104–2115. [Google Scholar] [CrossRef]
- Sanford, M.; Washuck, N.; Carr, K.; Prosser, R.S. Pulsed exposure of the macrophyte Lemna minor to herbicides and the mayfly Neocloeon triangulifer to diamide insects. Chemosphere 2021, 273, 128582. [Google Scholar] [CrossRef]
- Park, J.; Brown, M.T.; Depuydt, S.; Kim, J.K.; Won, D.S.; Han, T. Comparing the acute sensitivity of growth and photosynthetic endpoints in three Lemna species exposed to four herbicides. Environ. Pollut. 2017, 220, 818–827. [Google Scholar] [CrossRef]
- De Arcaute, C.R.; Soloneski, S.; Larramendy, M.L. Opposite effects of mixtures of commercial formulations of glyphosate with auxinic herbicides on the ten spotted live-bearer fish Cnesterodon decemmaculatus (Pisces, Poeciliidae). Environ. Pollut. 2018, 240, 858–866. [Google Scholar] [CrossRef]
- Felisbino, K.; Kirsten, N.; da Silva Milhorini, S.; Marçal, I.S.; Bernert, K.; Schiessl, R.; Nominato-Oliveira, L.; Guiloski, I.C. Teratogenic effects of the dicamba herbicide in Zebrafish (Danio rerio) embryos. Environ. Pollut. 2023, 334, 122187. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Silva, A.; Shiogiri, N.; Garlich, N.; PitellI, R. Efficacy of the herbicide imazapyr in the control of floating macrophytes and ecotoxicology for non-target organisms. Planta Daninha 2015, 33, 103–108. [Google Scholar] [CrossRef]
- Pinto, A.; Azenha, M.; Fidalgo, F.; Teixeira, J. 2, 4-dichlorophenoxyacetic acid detoxication occurs primarily in tomato leaves by the glutathione S-transferase phi members 4 and 5. Sci. Hortic. 2023, 321, 112214. [Google Scholar] [CrossRef]
- Della Vechia, J.F.; da Silva Peres, L.R.; Castro, P.P.; da Cruz, C. Determination of indicator plants for residues of bentazone, atrazine, and clomazone in the soil. Ciência Cult. 2021, 17, e211707. [Google Scholar] [CrossRef]
- Silva, T.R.; Brunetti, I.A.; Perez, L.H.O.; Pereira, P.C.; Santos, K.P.; Cruz, C. Effect of low doses of sulfentrazone in aqueous plants and ecotoxicology for bioindicators. Ciência Cult. 2020, 16, e201606. [Google Scholar] [CrossRef]
- Dittmar, P.J.; Ferrell, J.A.; Fernandez, J.V.; Smith, H. Effect of glyphosate and dicamba drift timing and rates in bell Pepper and yellow squash. Weed Technol. 2016, 30, 217–223. [Google Scholar] [CrossRef]
- Costa, E.M.; Jakelaitis, A.; Zuchi, J.; Pereira, L.S.; Ventura, M.V.A.; Oliveira, G.S.D.; Souza, G.D.; Silva, J.N. Simulated drift of dicamba and 2,4-D on soybeans: Effects of dose and time application. Biosci. J. 2020, 36, 857–864. [Google Scholar] [CrossRef]
- Silva, R.O.D.; Silva, D.N.D.; Aguiar, C.M.D.; Novello, D.P.; Silva, A.A.D.; Basso, J. Drift of 2, 4-D and dicamba applied to soybean at vegetative and reproductive growth stage. Ciência Rural 2018, 48, e20180179. [Google Scholar] [CrossRef]
- Rubert, J.; Somavilla, I.; Leichtweiss, E.; Avila Neto, R.; Thomasi, R.; Tarouco, C.; Berghetti, A.; Nicoloso, F.; Ulguim, A. The influence of 2,4-D and dicamba on the physiology of olive seedlings. Pesqui. Agropecuária Bras. 2024, 59, e03233. [Google Scholar] [CrossRef]
- Sims, K.C.; Jennings, K.M.; Monks, D.W.; Jordan, D.L.; Hoffmann, M.; Mitchem, W.E. Effect of simulated 2,4-D and dicamba drift on strawberry (Fragaria × ananassa) plant and fruit development. Weed Technol. 2025, 39, e38. [Google Scholar] [CrossRef]
- Werle, R.; Oliveira, M.C.; Jhala, A.J.; Proctor, C.A.; Rees, J.; Klein, R. Survey of Nebraska farmer’s adoption of dicamba-resistant soybean technology and dicamba off-target movement. Weed Technol. 2018, 32, 754–761. [Google Scholar] [CrossRef]
- Rubert, J.; Tarouco, C.P.; Wesz, A.M.; Bortolin, E.S.; Reis, C.B.V.; Dornelles, S.H.B.; Ulguim, A.R. Simulated drift of 2,4-D and dicamba in pecan (Carya illinoinensis K. Koch) and olive trees (Olea europaea L.). Ciência Florest. 2024, 34, e69073. [Google Scholar] [CrossRef]
- Carlsen, S.C.K.; Spliid, N.H.; Syensmark, B. Drift of 10 herbicides after tractor spray application. 2. Primary drift (droplet drift). Chemosphere 2006, 64, 778–786. [Google Scholar] [CrossRef]
- Godinho Júnior, J.D.; Caixeta, L.; Pereira, L.O.A.; Ruas, R.A.A.; Faria, V.R.; Carvalho Filho, A. Herbicide drift 2,4-D in hydraulic jet tapes type leque. Rev. Bras. Ciências Agrárias 2017, 12, 550–554. [Google Scholar] [CrossRef]
- Pretto, M.; Polito, R.A.; Dysarz, R.; Cinelli, R.; Heck, T.; Nunes, A.L. Performance of the isolated or mixed application of auxin mimicking herbicides in the control of Conyza spp. Braz. J. Dev. 2020, 6, 53083–53095. [Google Scholar] [CrossRef]
- Santos, D.P.D.; Braga, R.R.; Guimarães, F.A.R.; Passos, A.B.R.D.; Silva, D.V.; Santos, J.B.D.; Nery, M.C. Determination of bioindicator species of residues of auxinic herbicides. Rev. Ceres 2013, 60, 354–362. [Google Scholar] [CrossRef]
- Grossmann, K. Auxin herbicides: Current status of mechanism and mode of action. Pest Manag. Sci. 2010, 66, 113–120. [Google Scholar] [CrossRef]
- Figueiredo, M.R.A.; Küpper, A.; Malone, J.M.; Petrovic, T.; Figueiredo, A.B.T.B.; Campagnola, G.; Peersen, O.B.; Prasad, K.V.S.K.; Patterson, E.L.; Reddy, A.S.N.; et al. An in-frame deletion mutation in the degron tail of auxin coreceptor IAA2 confers resistance to the herbicide 2,4-D in Sisymbrium orientale. Proc. Natl. Acad. Sci. USA 2022, 119, e2105819119. [Google Scholar] [CrossRef]
- Garcia, N.S.; Dayan, F.E.; Camargo, E.R.; Ceolin, B.C.; Deuner, S.; Avila, L.A. Auxin-mimic herbicides dilema: Their benefits and limitations. Pest Manag. Sci. 2025, 81, 4973–4992. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Calderon-Villalobos, L.I.A.; Sharon, M.; Zheng, C.; Robinson, C.V.; Estelle, M.; Zheng, N. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 2007, 446, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Dinesh, D.C.; Villalobos, L.; Abel, S. Structural biology of nuclear auxin action. Trends Plant Sci. 2016, 21, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, C.; Ong, E.K.; Dalling, M.J.; Stevenson, T.W. Regulation of genes associated with auxin, ethylene and ABA pathways by 2,4-dichlorophenoxyacetic acid in Arabidopsis. Funct. Integr. Genom. 2006, 6, 60–70. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Osipitan, O.A.; Scott, J.E. Sensitivity of grape and tomato to micro-rates of dicamba-based herbicides. J. Hortic. Sci. Biotech. 2018, 5, 1000229. [Google Scholar] [CrossRef]
- Saygideger, S.D.; Okkay, O. Effect of 2, 4-dichlorophenoxyacetic acid on growth, protein and chlorophyll-a content of Chlorella vulgaris and Spirulina platensis cells. J. Environ. Biol. 2008, 29, 175–178. [Google Scholar]
- Ouse, D.; Gifford, J.M.; Schleier, J.; Simpson, D.D.; Tank, H.H.; Jennings, C.J.; Annangudi, S.P.; Valverde-Garcia, P.; Masters, R.A. A New Approach to Quantify Herbicide Volatility. Weed Technol. 2018, 32, 691–697. [Google Scholar] [CrossRef]
- Nishimura, J.; Gazzo, K.; Budd, R. Environmental Fate and Toxicology of Dicamba; California Department of Pesticide Registration: Sacramento, CA, USA, 2015. [Google Scholar]
- Zaccaro-Gruener, M.L.; Norsworthy, J.K.; Brabham, C.B.; Barber, L.T.; Butts, T.R.; Roberts, T.L.; Mauromoustakos, A. Evaluation of dicamba volatilization when mixed with glyphosate using imazethapyr as a tracer. J. Environ. Manag. 2022, 317, 115303. [Google Scholar] [CrossRef]
- Comfort, S.D.; Inskeep, W.P.; Macur, R.E. Degradation and transport of dicamba in a clay soil. J. Environ. Qual. 1992, 21, 653–658. [Google Scholar] [CrossRef]
- Silva, D.R.O.; Cuchi, M.L.; Silva, A.A.A.; Novello, B.D.; Basso, C.J. Simulated rainfall following the preplant application of 2,4-D and dicamba in soybean. Pesqui. Agropecuária Trop. 2020, 50, e62780. [Google Scholar] [CrossRef]
- De Souza Cruz, A.B.; Rocha, P.R.R.; Albuquerque, J.A.; Alves, J.M.A.; de Souza Cruz, D.L.; Finoto, E.L.; dos Santos, G.X.L. Selectivity of herbicides applied in pre and post-emergence in cowpea crop in the Amazon Savana. Native 2018, 6, 625–630. [Google Scholar]
- Johnson, N.M.; Baucom, R.S. Dicamba drift alters plant–herbivore interactions at the agro-ecological interface. Ecosphere 2022, 13, e4274. [Google Scholar] [CrossRef]

| Non-Target Plants | Cucumber (Cucumis sativus) | Tomato (Solanum lycopersicum) | Lettuce (Lactuca sativa) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Injury Symptoms | Toxicity | Low Doses | Volatility | Toxicity | Low Doses | Volatility | Toxicity | Low Doses | Volatility |
| Leaf edge chlorosis | 1 (light) | 1 (light) | 1 (light) | 1 (light) | 1 (light) | 0 (no effect) | 0 (no effect) | 0 (no effect) | 1 (light) |
| Loss of pigmentation | 2 (light) | 0 (no effect) | 0 (no effect) | 2 (light) | 0 (no effect) | 0 (no effect) | 2 (light) | 2 (light) | 0 (no effect) |
| Leaf/stem wilting | 3 (light) | 0 (no effect) | 0 (no effect) | 3 (light) | 0 (no effect) | 0 (no effect) | 3 (light) | 3 (light) | 0 (no effect) |
| Irregular growth of branches and irregular leaf development | 4 (light) | 4 (light) | 0 (no effect) | 4 (light) | 4 (light) | 4 (light) | 4 (light) | 4 (light) | 0 (no effect) |
| Partial or total necrosis | 0 (no effect) | 5 (moderate) | 0 (no effect) | 5 (moderate) | 5 (moderate) | 0 (no effect) | 0 (no effect) | 0 (no effect) | 0 (no effect) |
| Leaf wilting | 0 (no effect) | 6 (moderate) | 0 (no effect) | 6 (moderate) | 6 (moderate) | 0 (no effect) | 0 (no effect) | 0 (no effect) | 0 (no effect) |
| Apical bud necrosis | 0 (no effect) | 0 (no effect) | 0 (no effect) | 0 (no effect) | 9 (severe) | 0 (no effect) | 0 (no effect) | 9 (severe) | 0 (no effect) |
| Death | 10 (severe) | 10 (severe) | 0 (no effect) | 10 (severe) | 10 (severe) | 0 (no effect) | 10 (severe) | 10 (severe) | 0 (no effect) |
| Low Doses (g ha−1) | Injury Symptoms (%) (1 to 21 Days After Application) | Biometric Variables (21 Days After Application) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ------------------------------------------------------------------------------- | ----------------------------------------------------------------------------------- | ||||||||
| 1 | 3 | 7 | 14 | 21 | Shoot Length (cm) | Root Length (cm) | Fresh Mass (g) | Dry Mass (g) | |
| Cucumber (Cucumis sativus) | |||||||||
| 0 | - | - | - | - | - | 99.9 a | 24.4 a | 60.28 a | 9.08 a |
| 15 | 50.9d | 51.9d | 54.9d | 56.0d | 57.0 a | 28.3 b | 27.0 a | 39.97 b | 5.72 b |
| 30 | 51.9c | 52.9c | 56.1c | 57.3ab | 62.2 a | 20.5 bc | 21.6 a | 24.69 bc | 2.96 bc |
| 60 | 52.9 b | 54.9 b | 56.9 b | 58.8 ab | 72.0 a | 13.1 c | 17.0 a | 15.23 c | 1.99 c |
| 120 | 53.9 a | 55.9 a | 58.4 a | 67.4 a | 75.4 a | 12.7 c | 18.1 a | 13.25 c | 1.69 c |
| LSD (5%) | 0.38 | 0.38 | 0.60 | 10.38 | 19.02 | 12.07 | 14.12 | 15.45 | 2.83 |
| CV | 0.60 | 0.58 | 0.88 | 14.39 | 23.69 | 27.22 | 51.39 | 39.63 | 50.88 |
| F | 166.67 ** | 333.33 ** | 85.29 ** | 3.56 * | 2.91 * | 150.90 ** | 1.43 NS | 26.04 ** | 19.58 ** |
| Tomato (Solanum lycopersicum) | |||||||||
| 0 | - | - | - | - | - | 58.8 a | 17.9 a | 19.72 a | 6.09 a |
| 15 | 50.9d | 51.9d | 52.9 c | 54.0 c | 88.0 ab | 25.2 b | 18.3 a | 14.86 ab | 3.07 b |
| 30 | 52.10 c | 53.10 c | 54.10 b | 65.4 b | 66.4 c | 20.3 b | 16.35 a | 10.61 abc | 2.35 bc |
| 60 | 53.10 b | 54.10 b | 54.10 b | 81.0 a | 83.80 b | 19.9 b | 14.2 ab | 7.33 bc | 1.51 bc |
| 120 | 54.10 a | 54.90 a | 56.4 a | 81.0 a | 96.0 a | 8.3 b | 4.80 b | 2.66 c | 0.52 c |
| LSD (5%) | 0.3809 | 0.3809 | 0.8301 | 8.098 | 10.06 | 17.58 | 9.91 | 9.43 | 2.29 |
| CV | 0.6017 | 0.5911 | 1.26 | 9.54 | 10.02 | 52.21 | 54.49 | 67.25 | 66.67 |
| F | 187.67 ** | 168.00 ** | 45.39 ** | 38.69 ** | 22.39 ** | 19.08 ** | 5.07 ** | 7.90 ** | 13.74 ** |
| Lettuce (Lactuca sativa) | |||||||||
| 0 | - | - | - | - | - | 29.4 a | 21.3 a | 32.91 a | 6.31 a |
| 15 | 0.0 c | 40.5 b | 28.10 c | 30.00 c | 35.00 d | 21.5 b | 18.45 a | 31.78 a | 3.94 b |
| 30 | 0.0 c | 41.1 a | 39.10 a | 49.00 bc | 53.00 c | 14.0 c | 19.3 a | 27.15 a | 2.91 b |
| 60 | 20.5 b | 34.64 d | 37.10 b | 69.40 ab | 73.00 b | 7.5 d | 7.9 b | 10.28 b | 0.95 c |
| 120 | 21.1 a | 35.24 c | 39.2 a | 77.50 a | 100.0 a | 0.0 e | 0.0 c | 0.0 c | 0.0 c |
| LSD (5%) | 0.2129 | 0.2770 | 0.5039 | 21.34 | 13.99 | 5.40 | 5.89 | 7.85 | 1.37 |
| CV | 1.6977 | 0.6071 | 1.1661 | 31.38 | 17.81 | 29.36 | 34.64 | 30.25 | 38.28 |
| F | 46,275.40 ** | 2202.27 ** | 1588.71 ** | 14.50 ** | 57.42 ** | 73.37 ** | 38.63 ** | 55.70 ** | 53.35 ** |
| Doses (g ha−1) | Injury Symptoms (%) (14–21 Days After Exposure) | Biometric Variables (21 Days After Exposure) | ||||
|---|---|---|---|---|---|---|
| ---------------------------------- | --------------------------------------------------------------------------------- | |||||
| 14 | 21 | Shoot Length (cm) | Root Length (cm) | Fresh Mass (g) | Dry Mass (g) | |
| Cucumber (Cucumis sativus) | ||||||
| 0 | - | - | 75.8 a | 19.0 ab | 21.87 ab | 3.89 a |
| 45 | 1.9 e | 6.1 e | 69.6 a | 13.3 b | 20.35 b | 3.75 a |
| 90 | 2.9 d | 7.1 d | 87.1 a | 16.1 ab | 25.24 ab | 4.31 a |
| 180 | 4.1 c | 8.1 c | 72.0 a | 16.0 ab | 24.10 ab | 4.38 a |
| 360 | 5.0 b | 9.1 b | 72.4 a | 20.0 a | 29.37 a | 4.62 a |
| 720.0 | 6.1 a | 10.1 a | 67.60 a | 16.7 ab | 28.35 a | 4.14 a |
| LSD (5%) | 0.35 | 0.40 | 26.89 | 5.71 | 7.81 | 1.30 |
| CV | 7.07 | 3.90 | 27.48 | 25.65 | 23.76 | 23.59 |
| F | 345.0 ** | 250.0 ** | 1.17 NS | 3.05 * | 3.57 ** | 1.06 NS |
| Tomato (Solanum lycopersicum) | ||||||
| 0 | - | - | 71.8 a | 24.8 a | 46.62 a | 12.09 ab |
| 45 | 2.0 e | 2.9 e | 71.0 a | 19.4 b | 44.96 a | 13.62 a |
| 90 | 30.1 d | 70.11 d | 73.9 a | 19.4 b | 38.36 a | 10.35 ab |
| 180 | 30.2 c | 72.10 c | 70.8 a | 19.5 b | 35.32 a | 8.76 b |
| 360 | 30.49 c | 74.9 b | 77.7 a | 19.4 b | 40.83 a | 10.25 ab |
| 720 | 30.89 a | 79.9 a | 66.2 a | 18.7 b | 38.71 a | 9.19 b |
| LSD (5%) | 0.035 | 0.35 | 12.55 | 4.94 | 12.61 | 4.16 |
| CV | 0.11 | 0.47 | 13.21 | 18.52 | 23.40 | 29.38 |
| F | 2,021,095.00 ** | 128,648.71 ** | 1.60 NS | 3.69 ** | 2.01 NS | 3.40 ** |
| Lettuce (Lactuca sativa) | ||||||
| 0 | - | - | 26.1 a | 13.8 a | 60.40 a | 7.21 ab |
| 45 | 0.0 | 6.1 d | 25.5 a | 13.1 a | 55.51 ab | 7.02 ab |
| 90 | 0.0 | 7.1 c | 25.2 a | 14.2 a | 44.33 b | 5.74 b |
| 180 | 0.0 | 8.1 b | 27.1 a | 15.8 a | 58.12 ab | 8.32 a |
| 360 | 0.0 | 9.0 b | 22.8 a | 15.6 a | 44.77 b | 6.14 b |
| 720 | 0.0 | 10.0 a | 25.6 a | 13.6 a | 54.52 ab | 6.78 ab |
| LSD (5%) | - | 0.36 | 4.62 | 4.60 | 14.22 | 2.01 |
| CV | - | 3.50 | 13.77 | 24.26 | 20.32 | 22.13 |
| F | - | 306.50 ** | 1.67 NS | 1.01 NS | 4.01 ** | 3.53 ** |
| Doses (g ha−1) | Soil 1 | Soil 2 | Soil 3 |
|---|---|---|---|
| 0 | 100 Aa | 100 Aa | 100 Aa |
| 45 | 67 Aab | 71 Aab | 100 Aa |
| 90 | 46 * Bbc | 95 * Aab | 36 * Bbc |
| 180 | 29 * Bbc | 81 * Aab | 50 *ABab |
| 360 | 38 * Bbc | 95 * Aab | 45 * Bab |
| 720 | 25 * ABc | 52 * Ab | 0 Cc |
| Average | 41 B | 79 A | 46 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, P.C.; Brunetti, I.A.; da Silva, A.B.; de Oliveira, A.C.; da Cruz, C.; Duke, S.O.; Carvalho, L.B.d. Dicamba Impacts on Aquatic Bioindicators and Non-Target Plants. AgriEngineering 2025, 7, 336. https://doi.org/10.3390/agriengineering7100336
Pereira PC, Brunetti IA, da Silva AB, de Oliveira AC, da Cruz C, Duke SO, Carvalho LBd. Dicamba Impacts on Aquatic Bioindicators and Non-Target Plants. AgriEngineering. 2025; 7(10):336. https://doi.org/10.3390/agriengineering7100336
Chicago/Turabian StylePereira, Pâmela Castro, Isabella Alves Brunetti, Ana Beatriz da Silva, Ana Carolina de Oliveira, Claudinei da Cruz, Stephen Oscar Duke, and Leonardo Bianco de Carvalho. 2025. "Dicamba Impacts on Aquatic Bioindicators and Non-Target Plants" AgriEngineering 7, no. 10: 336. https://doi.org/10.3390/agriengineering7100336
APA StylePereira, P. C., Brunetti, I. A., da Silva, A. B., de Oliveira, A. C., da Cruz, C., Duke, S. O., & Carvalho, L. B. d. (2025). Dicamba Impacts on Aquatic Bioindicators and Non-Target Plants. AgriEngineering, 7(10), 336. https://doi.org/10.3390/agriengineering7100336






