From Waste to Resource: Phosphorus Adsorption on Posidonia oceanica Ash and Its Application as a Soil Fertilizer
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Characterization of Adsorbent
2.2. Batch and Semi-Batch Adsorption Experiments
2.3. Leaching-Desorption Studies
2.4. Adsorption Kinetics
2.5. Adsorption Isotherms
3. Results and Discussion
3.1. Characterization of Adsorbent
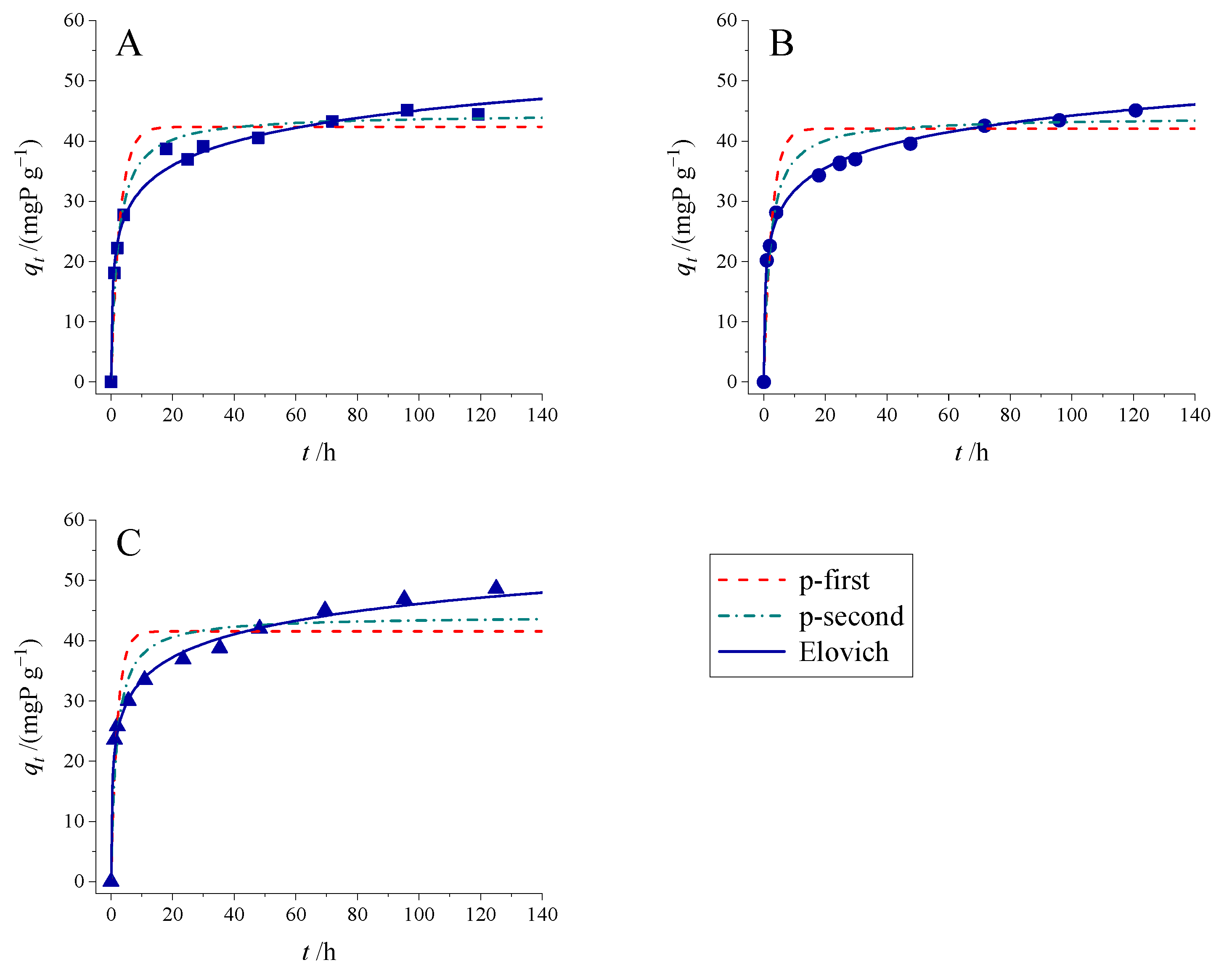
3.2. Adsorption Kinetic Studies
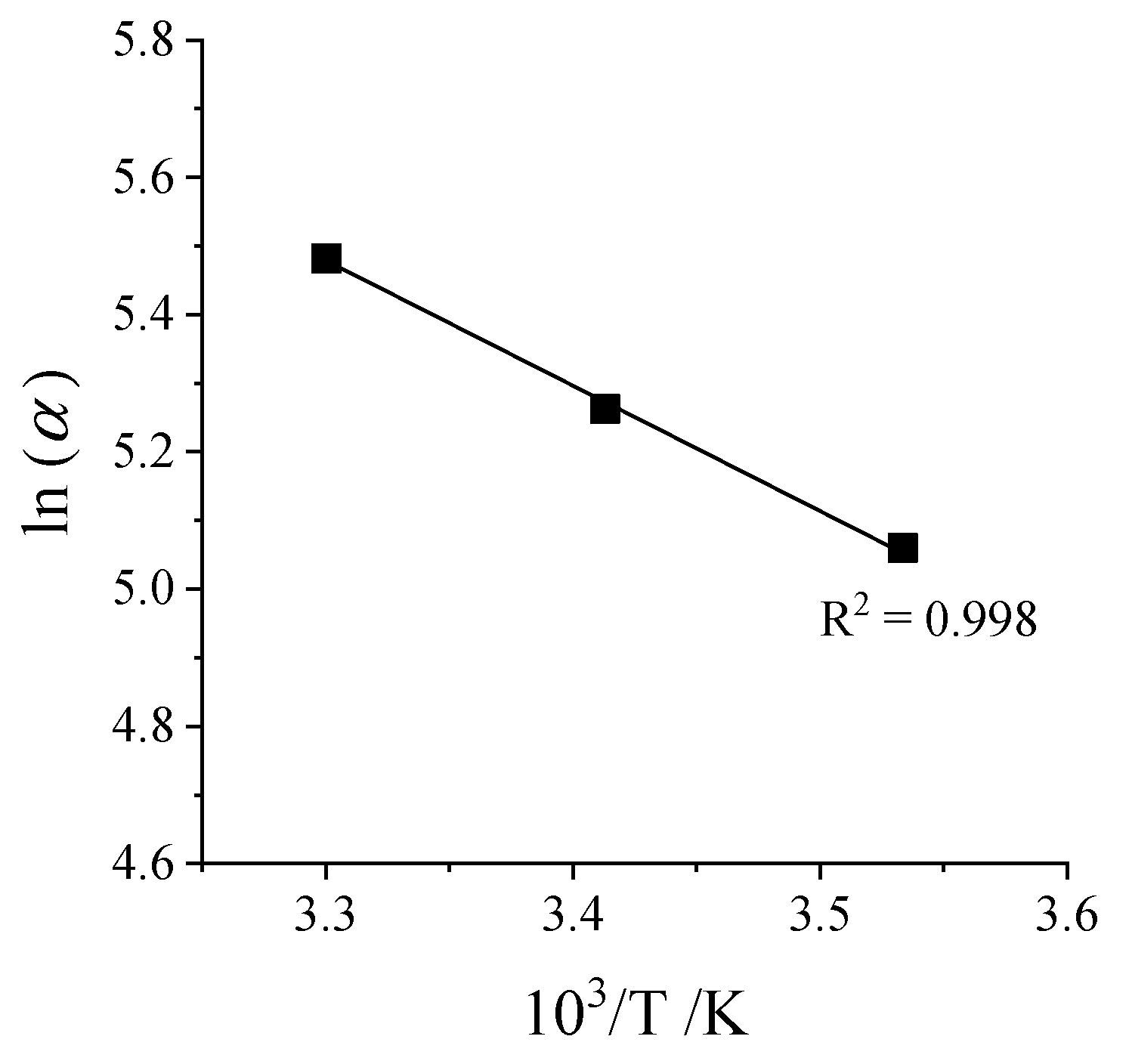
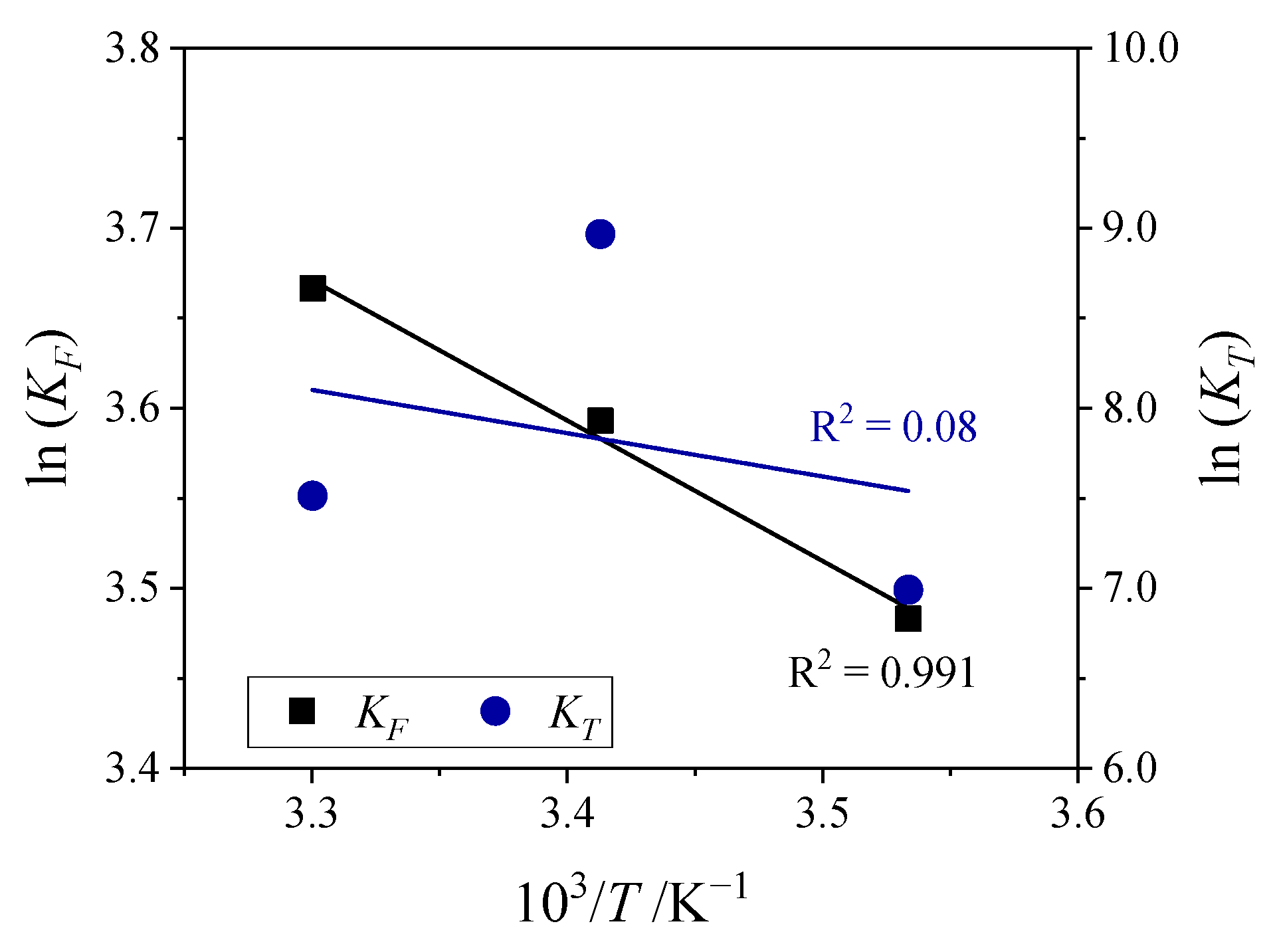
Activation Energy—Arrhenius Plot
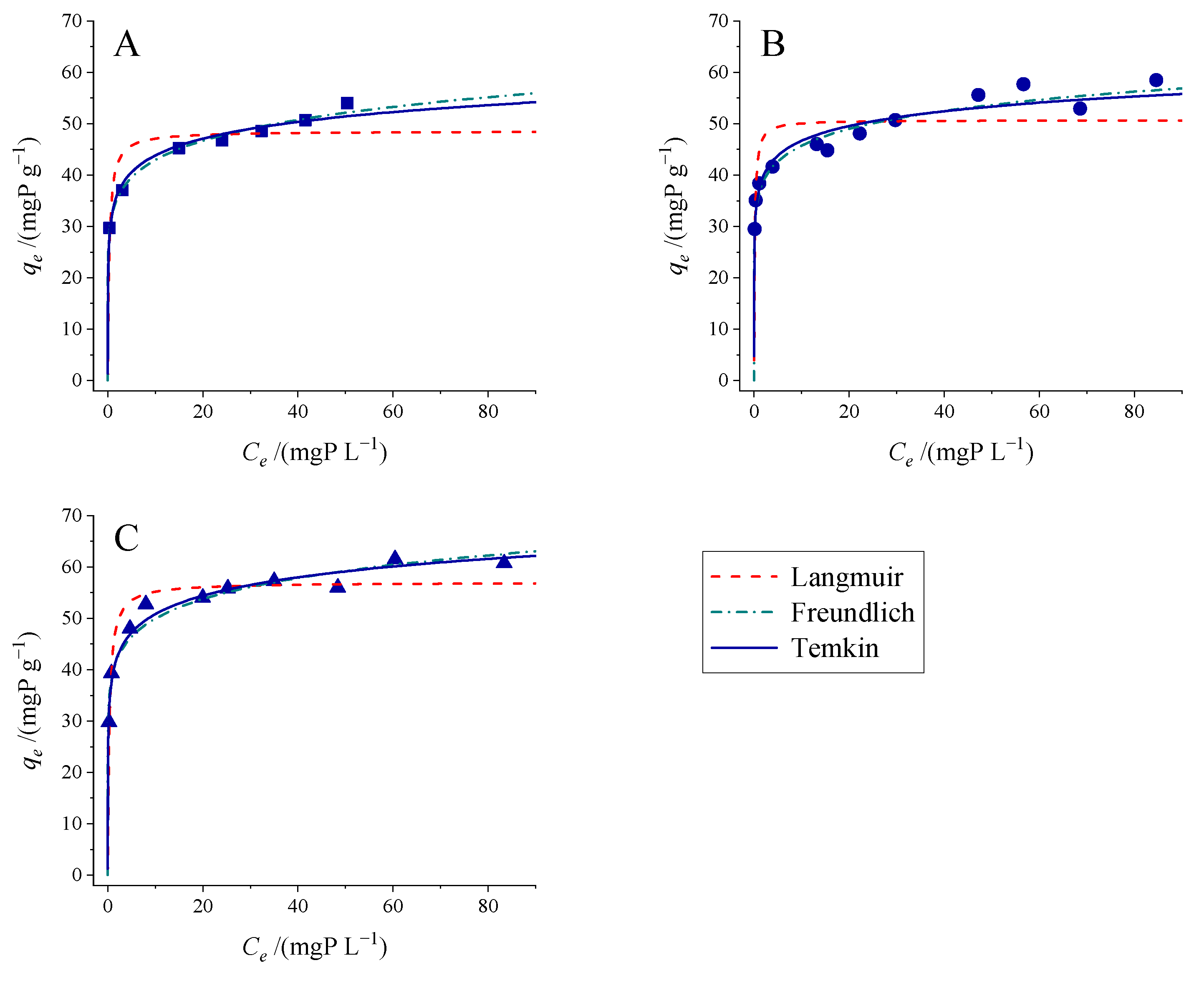
3.3. Adsorption Isotherm Studies
3.3.1. Effect of Temperature
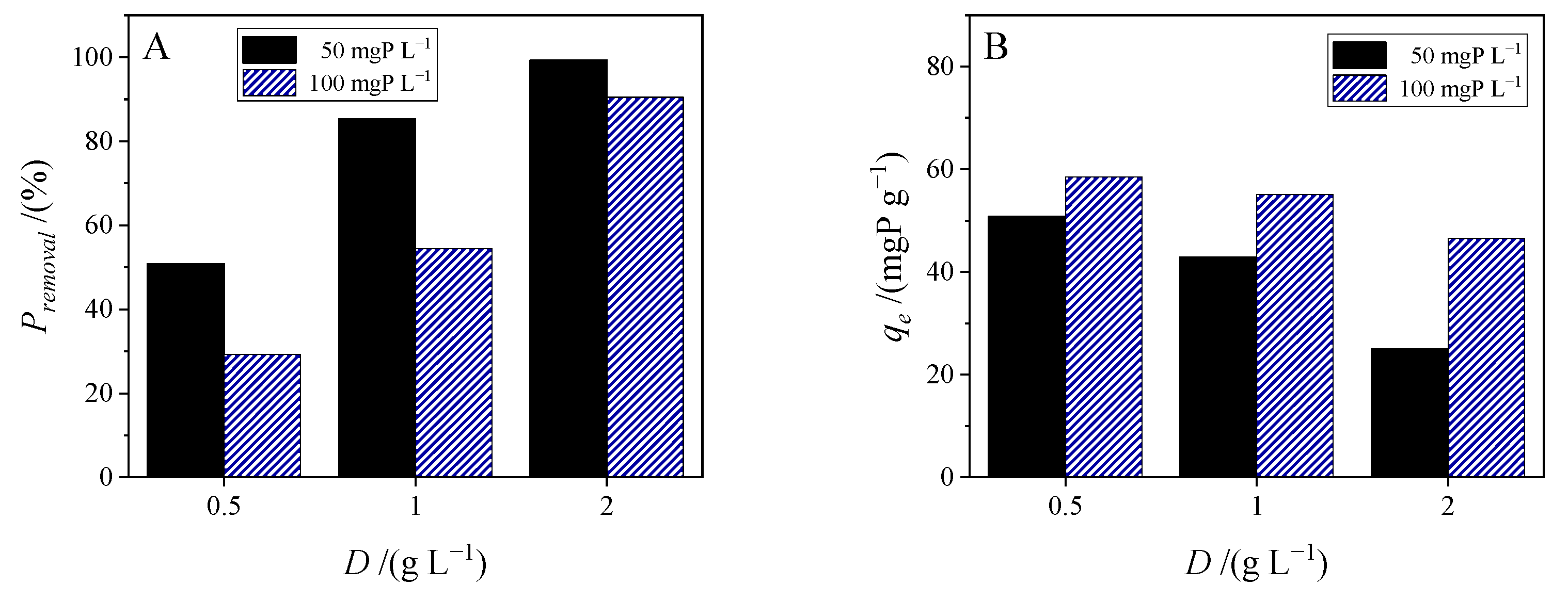
3.3.2. Effect of Adsorbent Dose
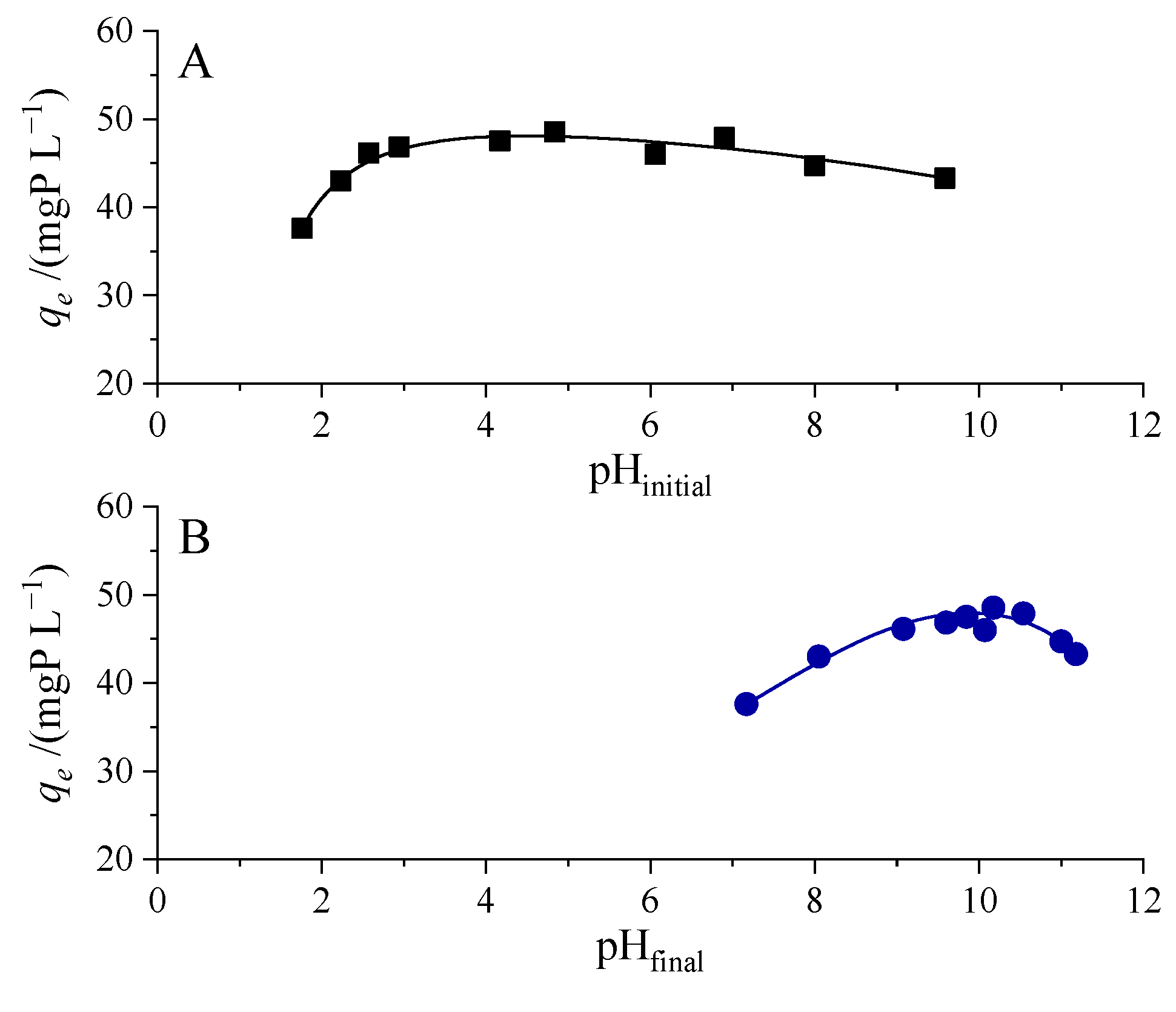
3.3.3. Effect of pH on the Removal Process
3.3.4. Phosphate Adsorption Performance
3.4. Leaching-Desorption Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OECD | Organization for Economic Cooperation and Development |
| PO | Posidonia oceanica fiber |
| POA | Posidonia oceanica ash |
| POA-P | Phosphorus-saturated Posidonia oceanica ash |
| ICP | Inductively Coupled Plasma |
| OES | Optical Emission Spectrometry |
| SEM | Scanning Electron Microscopy |
| PZC | Point of Zero Charge |
| PXRD | Powder X-ray diffraction |
| TGA | Thermogravimetric analyses |
| DTA | Differential thermal analyses |
References
- Vigiak, O.; Udías, A.; Grizzetti, B.; Zanni, M.; Aloe, A.; Weiss, F.; Hristov, J.; Bisselink, B.; de Roo, A.; Pistocchi, A. Recent Regional Changes in Nutrient Fluxes of European Surface Waters. Sci. Total Environ. 2023, 858, 160063. [Google Scholar] [CrossRef]
- Fadiran, A.O.; Dlamini, S.C.; Mavuso, A. A Comparative Study of the Phosphate Levels in Some Surface and Ground Water Bodies of Swaziland. Bull. Chem. Soc. Ethiop. 2008, 22, 197–206. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. Harmful Algal Blooms: Causes, Impacts and Detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, G.M.; Johnson, R.A. Eutrophication of Freshwater and Coastal Ecosystems. Encycl. Sustain. Technol. Second Ed. 2024, 2, 710–721. [Google Scholar] [CrossRef]
- Esfandi, F.; Mahvi, A.H.; Mosaferi, M.; Armanfar, F.; Hejazi, M.A.; Maleki, S. Assessment of Temporal and Spatial Eutrophication Index in a Water Dam Reservoir. Glob. J. Environ. Sci. Manag. 2018, 4, 153–166. [Google Scholar] [CrossRef]
- Moon, J.Y.; Lee, K.; Tanhua, T.; Kress, N.; Kim, I.N. Temporal Nutrient Dynamics in the Mediterranean Sea in Response to Anthropogenic Inputs. Geophys. Res. Lett. 2016, 43, 5243–5251. [Google Scholar] [CrossRef]
- Zhu, F.; Cakmak, E.K.; Cetecioglu, Z. Phosphorus Recovery for Circular Economy: Application Potential of Feasible Resources and Engineering Processes in Europe. Chem. Eng. J. 2023, 454, 140153. [Google Scholar] [CrossRef]
- Cheng, M.; Shi, C.; Hao, L.; Wang, X.; Guo, X.; Liu, R.; Hao, X. Sustainable Development of Phosphorus Recovery: From a Product Perspective. Sustain. Prod. Consum. 2023, 41, 275–290. [Google Scholar] [CrossRef]
- Chirilă Băbău, A.M.; Micle, V.; Damian, G.E.; Sur, I.M. Lead and Copper Removal from Sterile Dumps by Phytoremediation with Robinia Pseudoacacia. Sci. Rep. 2024, 14, 9842. [Google Scholar] [CrossRef]
- Pergent-Martini, C.; Pergent, G.; Monnier, B.; Boudouresque, C.F.; Mori, C.; Valette-Sansevin, A. Contribution of Posidonia Oceanica Meadows in the Context of Climate Change Mitigation in the Mediterranean Sea. Mar. Environ. Res. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Gómez-Pujol, L.; Orfila, A.; Álvarez-Ellacuría, A.; Terrados, J.; Tintoré, J. Posidonia Oceanica Beach-Cast Litter in Mediterranean Beaches: A Coastal Videomonitoring Study. J. Coast. Res. 2013, 65, 1768–1773. [Google Scholar] [CrossRef]
- Mnafki, R.; Morales, A.; Sillero, L.; Khiari, R.; Moussaoui, Y.; Labidi, J. Integral Valorization of Posidonia Oceanica Balls: An Abundant and Potential Biomass. Polymers 2024, 16, 164. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Giosafatto, C.V.L.; Mirpoor, S.F.; Cammarota, M.; Hejazi, S.; Mariniello, L.; Schiraldi, C.; Porta, R. Sustainable Exploitation of Posidonia Oceanica Sea Balls (Egagropili): A Review. Int. J. Mol. Sci. 2023, 24, 7301. [Google Scholar] [CrossRef] [PubMed]
- Hashim, K.S.; Ewadh, H.M.; Muhsin, A.A.; Zubaidi, S.L.; Kot, P.; Muradov, M.; Aljefery, M.; Al-Khaddar, R. Phosphate Removal from Water Using Bottom Ash: Adsorption Performance, Coexisting Anions and Modelling Studies. Water Sci. Technol. 2021, 83, 77–89. [Google Scholar] [CrossRef]
- Drapanauskaite, D.; Buneviciene, K.; Silva, M.; Slepetiene, A.; Baltrusaitis, J. Phosphate Removal from Simulated Wastewater Using Industrial Calcium-Containing Solid Waste. J. Environ. Chem. Eng. 2021, 9, 106575. [Google Scholar] [CrossRef]
- Carricondo Anton, J.M.; González Romero, J.A.; Mengual Cuquerella, J.; Turegano Pastor, J.V.; Oliver Villanueva, J.V. Alternative Use of Rice Straw Ash as Natural Fertilizer to Reduce Phosphorus Pollution in Protected Wetland Ecosystems. Int. J. Recycl. Org. Waste Agric. 2020, 9, 61–74. [Google Scholar] [CrossRef]
- Carricondo, J.M.; Oliver-Villanueva, J.V.; Turégano, J.V.; González, J.A.; Mengual, J. Use of Phragmites Australis for Controlling Phosphorus Contamination in Anthropogenic Wetland Ecosystems. Environ. Technol. 2021, 42, 3055–3064. [Google Scholar] [CrossRef]
- Bala, R.; Dhankhar, R.; Chhikara, S.K. Sustainable Phosphate Removal with Acid-Modified Fly Ash: Kinetic, Isothermal, and Thermodynamic Insights. Nat. Environ. Pollut. Technol. 2025, 24, B4242. [Google Scholar] [CrossRef]
- Abbas, M.N. Phosphorus Removal from Wastewater Using Rice Husk and Subsequent Utilization of the Waste Residue. Desalin. Water Treat. 2015, 55, 970–977. [Google Scholar] [CrossRef]
- Karageorgiou, K.; Paschalis, M.; Anastassakis, G.N. Removal of Phosphate Species from Solution by Adsorption onto Calcite Used as Natural Adsorbent. J. Hazard. Mater. 2007, 139, 447–452. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, C.; Cheng, G.; Von Lau, E. Towards Carbon Neutrality: A Comprehensive Study on the Utilization and Resource Recovery of Coal-Based Solid Wastes. Int. J. Coal Sci. Technol. 2025, 12, 34. [Google Scholar] [CrossRef]
- Kim, J.A.; Vijayaraghavan, K.; Reddy, D.H.K.; Yun, Y.S. A Phosphorus-Enriched Biochar Fertilizer from Bio-Fermentation Waste: A Potential Alternative Source for Phosphorus Fertilizers. J. Clean. Prod. 2018, 196, 163–171. [Google Scholar] [CrossRef]
- Ganem, H.E.; Cohen, K.; Gildor, O.; Sha, M.; Wang, Q.; Radian, A. From Effluent to Fertilizer: Phosphorus Recovery and Reuse Using Granulated Iron-Oxyhydroxide-Montmorillonite Composites. Sci. Total Environ. 2025, 981, 179613. [Google Scholar] [CrossRef]
- ISO 18122:2022; Solid Biofuels—Determination of Ash Content. International Organization for Standardization: Geneva, Switzerland, 2022.
- González, J.A.; Mengual, J.; Palomares, A.E. Phosphorus Control and Recovery in Anthropogenic Wetlands Using Their Green Waste—Validation of an Adsorbent Mixture Model. Sustainability 2025, 17, 6153. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L.; Association, A.P.H.; Association, A.W.W.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- UNE-EN 15958 2012; Aenor Fertilizers-Extraction of Water Soluble Phosphorus. European Committee for Standardization: Brussels, Belgium, 2012.
- Lagergren, S. Zur Theorie Der Sogenannten Adsorption Geloster Stoffe. Kungliga Svenska Ventenskapsakademiens. Handlinger 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Aharoni, C.; Tompkins, F.C. Kinetics of Adsorption and Desorption and the Elovich Equation. Adv. Catal. 1970, 21, 1–49. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. In Advanced Sorption Process Applications; Edebali, S., Ed.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: Oxford, UK, 2004; ISBN 9780198038344. [Google Scholar]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Zeitschrift für Phys. Chemie 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption Equilibrium and the Kinetics of Processes on Nonhomogeneous Surfaces and in the Interaction between Adsorbed Molecules. Zh. Fiz. Chim. 1941, 15, 296–332. [Google Scholar]
- Bolster, C.H.; Hornberger, G.M. On the Use of Linearized Langmuir Equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.J.; Chao, H.P. Effect of Pyrolysis Temperatures and Times on the Adsorption of Cadmium onto Orange Peel Derived Biochar. Waste Manag. Res. 2016, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.J.; Hosseini-Bandegharaei, A.; Chao, H.P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Plis, A.; Lasek, J.; Skawińska, A.; Kopczyński, M. Thermo-Chemical Properties of Biomass from Posidonia Oceanica. Chem. Pap. 2014, 68, 879–889. [Google Scholar] [CrossRef]
- Plis, A.; Lasek, J.A.; Zuwała, J.; Yu, C.C.; Iluk, A. Combustion Performance Evaluation of Posidonia Oceanica Using TGA and Bubbling Fluidized-Bed Combustor (Batch Reactor). J. Sustain. Min. 2016, 15, 181–190. [Google Scholar] [CrossRef]
- Khiari, R.; Mhenni, M.F.; Belgacem, M.N.; Mauret, E. Chemical Composition and Pulping of Date Palm Rachis and Posidonia Oceanica—A Comparison with Other Wood and Non-Wood Fibre Sources. Bioresour. Technol. 2010, 101, 775–780. [Google Scholar] [CrossRef]
- Mencarelli, A.; Greco, R.; Cavalli, R.; Zilio, C.; Pighi, A.; Grigolato, S. Production and Quality Assessment of Pellets Made from Spruce Sawdust and Posidonia oceanica (L.) Delile. In Proceedings of the Biosystems Engineering Promoting Resilience to Climate Change-AIIA 2024-Mid-Term Conference, Padova, Italy, 17–19 June 2024; Sartori, L., Tarolli, P., Guerrini, L., Zuecco, G., Pezzuolo, A., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 457–464. [Google Scholar]
- Jiang, H.; Lu, P.; Xue, Z.; Zhao, D.; Bu, C.; Ge, H.; Song, T. Prediction and Evaluation on Fuel Properties and Pyrolysis Characteristics of Combustible Industrial Solid Wastes. J. Energy Inst. 2022, 105, 232–241. [Google Scholar] [CrossRef]
- Voca, N.; Grubor, M.; Peter, A.; Kricka, T. Evaluation of Posidonia Oceanica Waste as a Biomass Source for Energy Generation. Bioenergy Res. 2019, 12, 1104–1112. [Google Scholar] [CrossRef]
- Boumanchar, I.; Charafeddine, K.; Chhiti, Y.; M’hamdi Alaoui, F.E.; Sahibed-dine, A.; Bentiss, F.; Jama, C.; Bensitel, M. Biomass Higher Heating Value Prediction from Ultimate Analysis Using Multiple Regression and Genetic Programming. Biomass Convers. Biorefinery 2019, 9, 499–509. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Adeniyi, A.G. A Mini-Review of the Morphological Properties of Biosorbents Derived from Plant Leaves. SN Appl. Sci. 2020, 2, 509. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Qian, G. Phosphate Adsorption on Metal Oxides and Metal Hydroxides: A Comparative Review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Carricondo Anton, J.M.; Oliver-Villanueva, J.V.; Turégano Pastor, J.V.; Raigón Jiménez, M.D.; González Romero, J.A.; Mengual Cuquerella, J. Reduction of Phosphorous from Wastewater Through Adsorption Processes Reusing Wood and Straw Ash Produced in Bioenergy Facilities. Water Air Soil Pollut. 2020, 231, 128. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A Review of the Kinetics Adsorption Models and Their Application to the Adsorption of Lead by an Activated Carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Liu, J. Adsorptive Removal of Phosphate from Aqueous Solutions Using Iron Oxide Tailings. Water Res. 2004, 38, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of Elovich Equation Used for the Analysis of Adsorption Kinetics in Dye-Chitosan Systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Goh, K.H.; Lim, T.T. Geochemistry of Inorganic Arsenic and Selenium in a Tropical Soil: Effect of Reaction Time, PH, and Competitive Anions on Arsenic and Selenium Adsorption. Chemosphere 2004, 55, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cui, W.; Yang, Y.; Li, S.; Song, F.; Wang, M.; Shao, X.; Xu, X. Surfactant-Functionalized Fly Ash from Waste into Wealth: Collaboratively Scavenge of Heavy Metals and Antibiotics from Multi-Pollutant Wastewater. Sep. Purif. Technol. 2025, 375, 133862. [Google Scholar] [CrossRef]
- Sugiura, N. Further Analysts of the Data by Akaike’s Information Criterion and the Finite Corrections. Commun. Stat. Theory Methods 2007, 7, 13–26. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Mor, S.; Chhoden, K.; Ravindra, K. Application of Agro-Waste Rice Husk Ash for the Removal of Phosphate from the Wastewater. J. Clean. Prod. 2016, 129, 673–680. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Zhu, Z.; Li, L.; Yao, X.; Rudolph, V.; Haghseresht, F. Phosphate Removal from Wastewater Using Red Mud. J. Hazard. Mater. 2008, 158, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; He, Z.; Mahmood, Q.; Liu, D.; Yang, X.; Islam, E. Phosphate Removal from Solution Using Steel Slag through Magnetic Separation. J. Hazard. Mater. 2008, 152, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Hassine, R.B.; Jellali, S. Posidonia oceanica (L.) Fibers as a Potential Low-Cost Adsorbent for the Removal and Recovery of Orthophosphate. J. Hazard. Mater. 2011, 191, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wahab, M.A.; Hassine, R.B.; Jellali, S. Removal of Phosphorus from Aqueous Solution by Posidonia oceanica Fibers Using Continuous Stirring Tank Reactor. J. Hazard. Mater. 2011, 189, 577–585. [Google Scholar] [CrossRef]
- Riahi, K.; Chaabane, S.; Thayer, B. Ben A Kinetic Modeling Study of Phosphate Adsorption onto Phoenix dactylifera L. Date Palm Fibers in Batch Mode. J. Saudi Chem. Soc. 2017, 21, S143–S152. [Google Scholar] [CrossRef]
- Jung, K.W.; Hwang, M.J.; Ahn, K.H.; Ok, Y.S. Kinetic Study on Phosphate Removal from Aqueous Solution by Biochar Derived from Peanut Shell as Renewable Adsorptive Media. Int. J. Environ. Sci. Technol. 2015, 12, 3363–3372. [Google Scholar] [CrossRef]
- Park, J.H.; Ok, Y.S.; Kim, S.H.; Cho, J.S.; Heo, J.S.; Delaune, R.D.; Seo, D.C. Evaluation of Phosphorus Adsorption Capacity of Sesame Straw Biochar on Aqueous Solution: Influence of Activation Methods and Pyrolysis Temperatures. Environ. Geochem. Health 2015, 37, 969–983. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Z.; Deng, L. Adsorption Behaviors and Removal Efficiencies of Inorganic, Polymeric and Organic Phosphates from Aqueous Solution on Biochar Derived from Sewage Sludge of Chemically Enhanced Primary Treatment Process. Water 2018, 10, 869. [Google Scholar] [CrossRef]
- Tao, X.; Huang, T.; Lv, B. Synthesis of Fe/Mg-Biochar Nanocomposites for Phosphate Removal. Materials 2020, 13, 816. [Google Scholar] [CrossRef]
- González, J.A.; Mengual, J. Enhanced Phosphate Adsorption Using Chemically Modified Walnut Shell Biochar: A Comparative Study of Activation Methods, Isotherm Uncertainty Analysis and Modelling. J. Water Process Eng. 2025, 77, 108468. [Google Scholar] [CrossRef]
- Bal Krishna, K.C.; Niaz, M.R.; Sarker, D.C.; Jansen, T. Phosphorous Removal from Aqueous Solution Can Be Enhanced through the Calcination of Lime Sludge. J. Environ. Manag. 2017, 200, 359–365. [Google Scholar] [CrossRef]
- Mendez, J.C.; Hiemstra, T. Ternary Complex Formation of Phosphate with Ca and Mg Ions Binding to Ferrihydrite: Experiments and Mechanisms. ACS Earth Sp. Chem. 2020, 4, 545–557. [Google Scholar] [CrossRef]
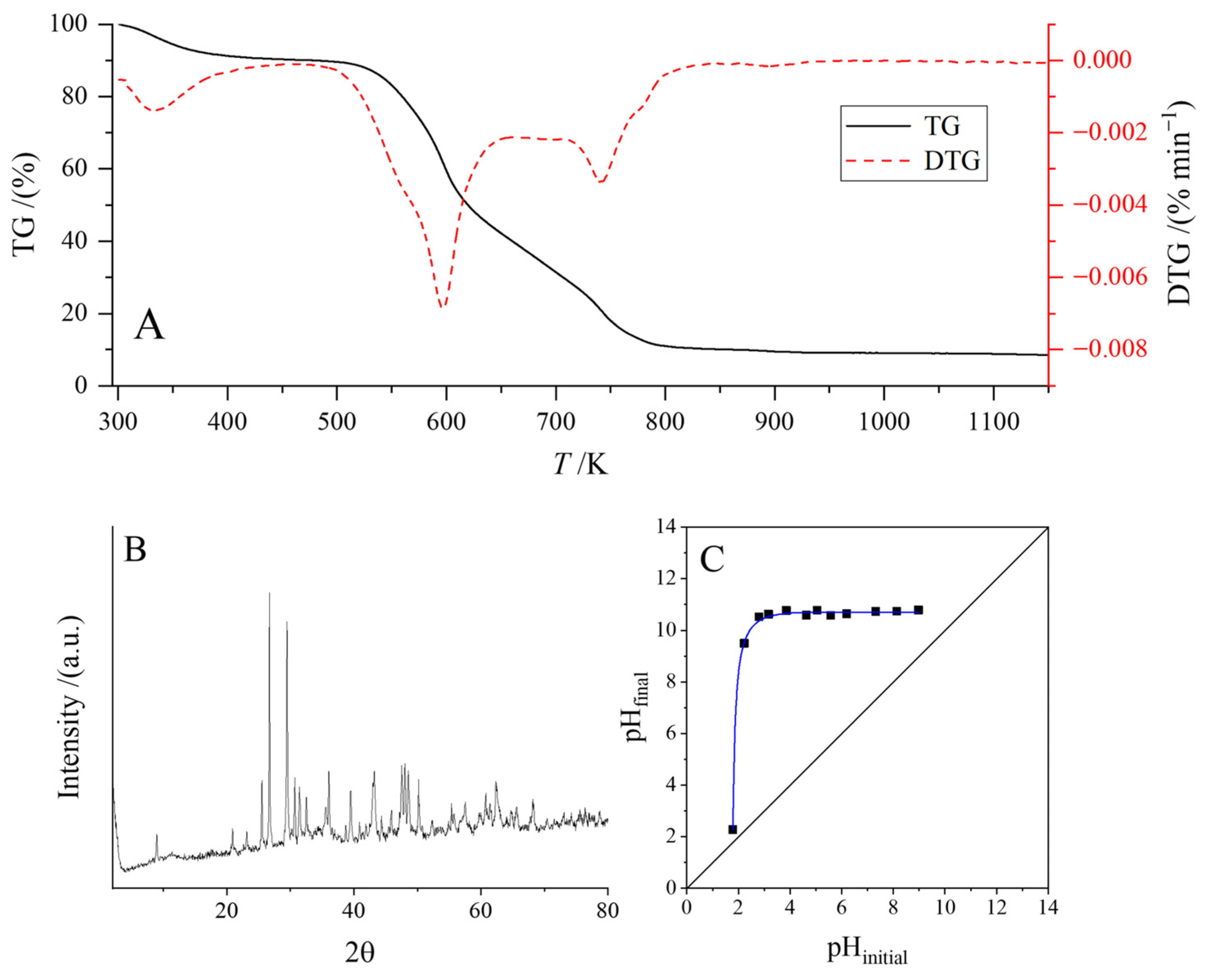
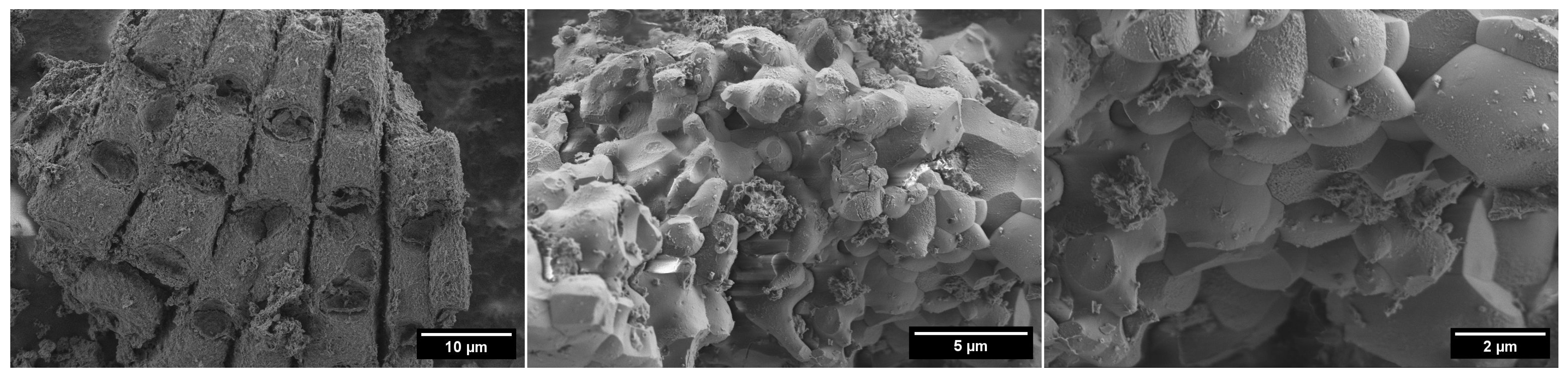
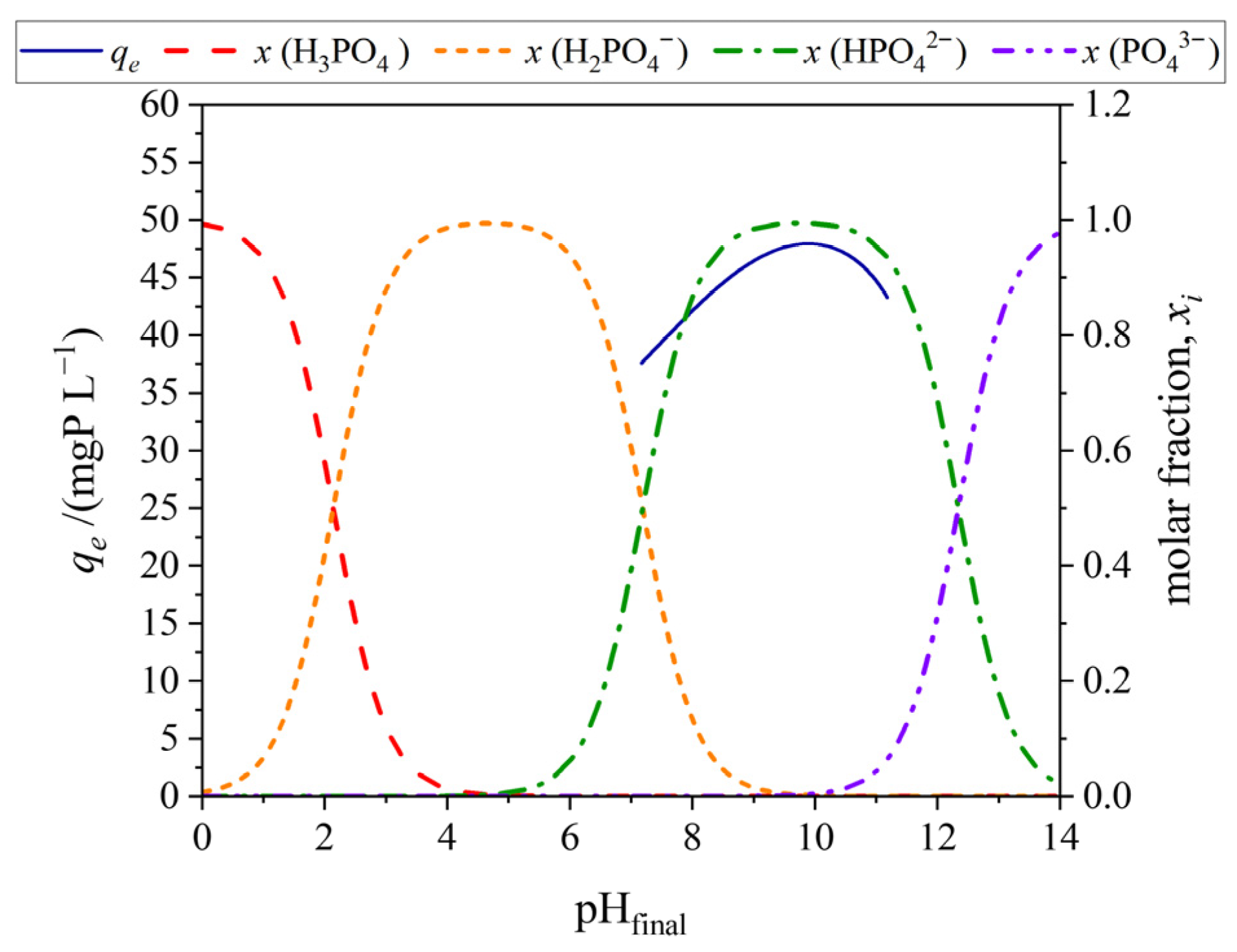
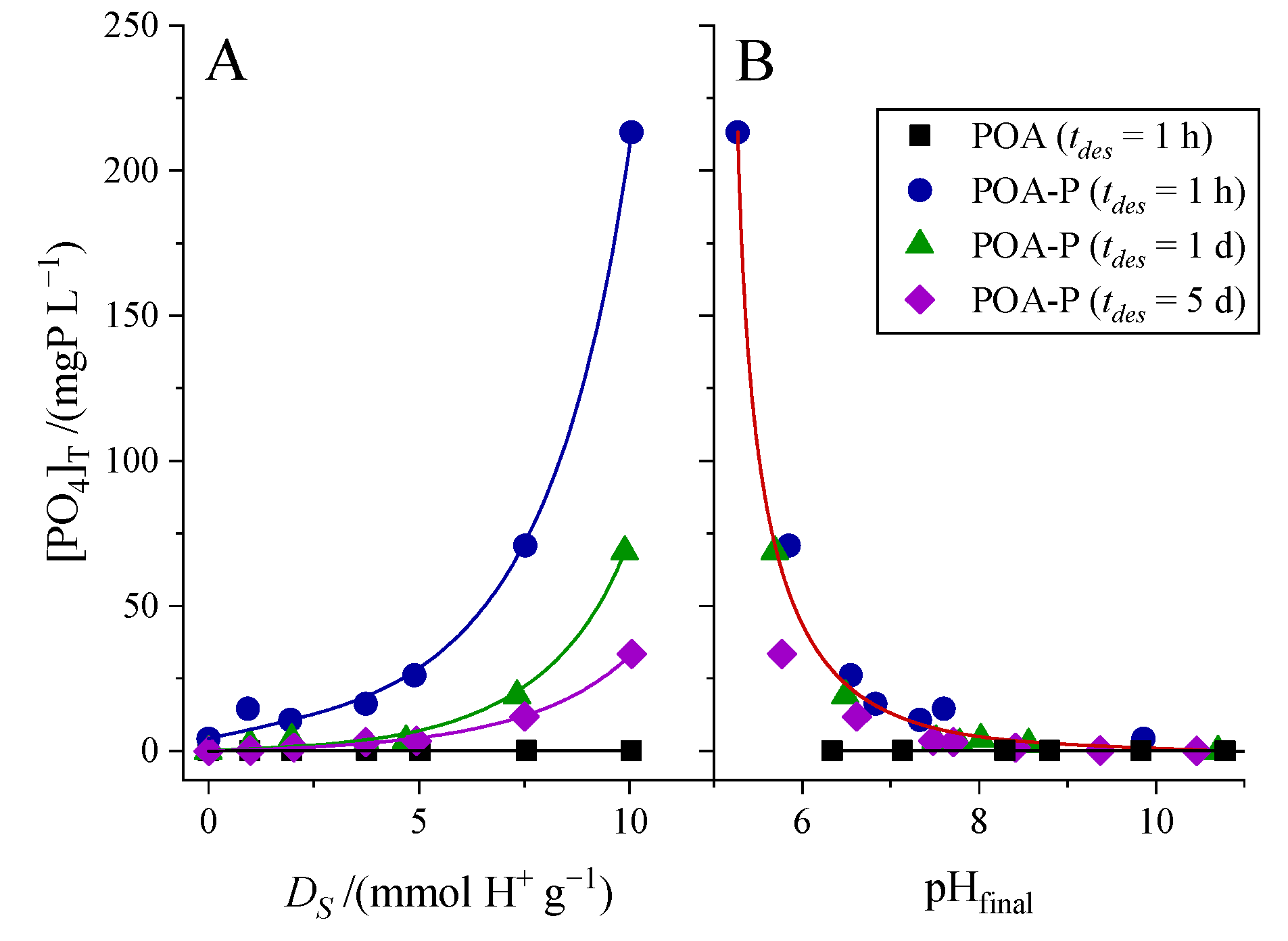
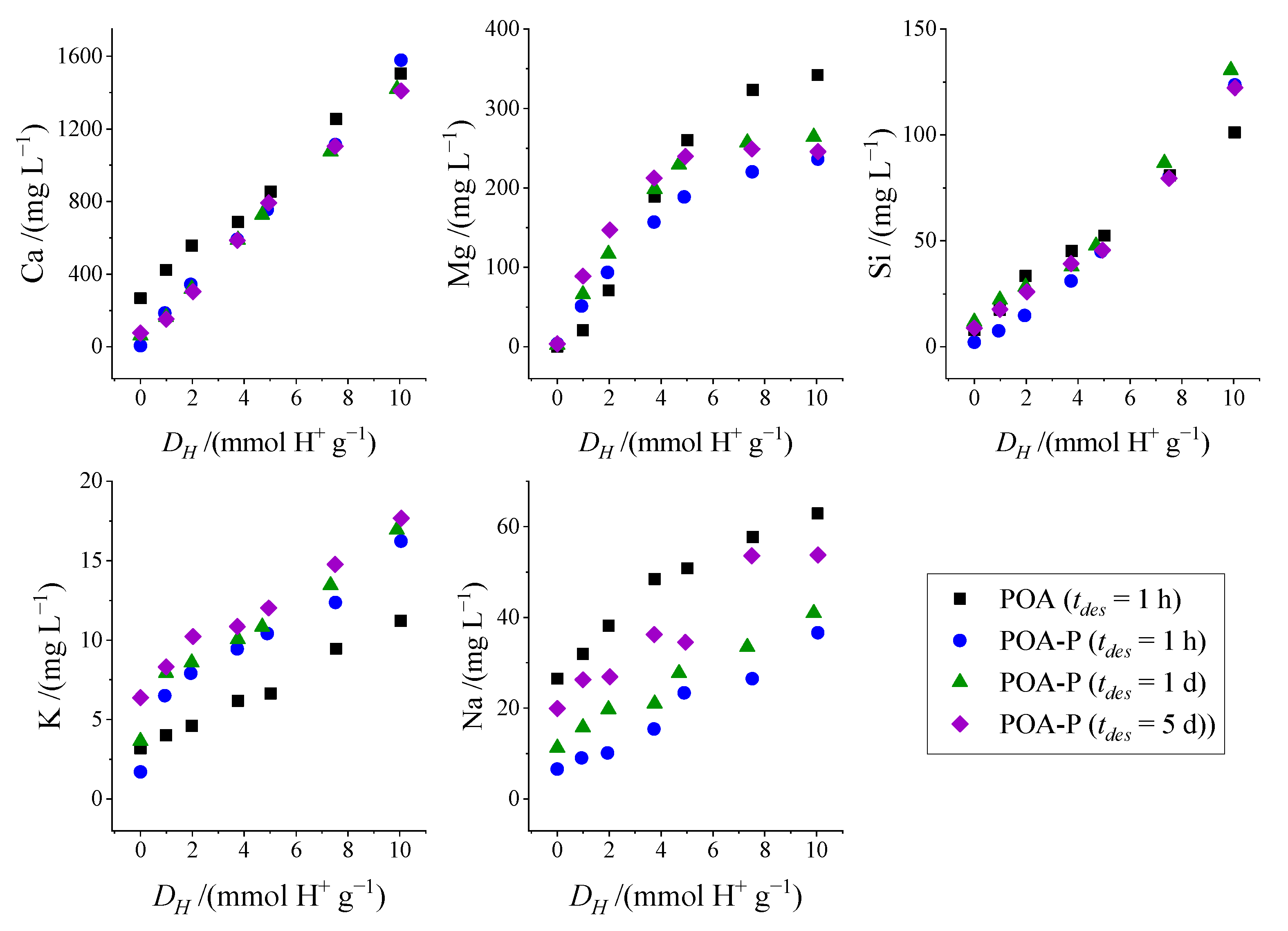
| Samples | PO | POA |
|---|---|---|
| pHPZC | n.d. | 10.7 |
| BET surface area/(m2 g−1) | n.d. | 13 |
| External surface area/(%) | n.d. | 86 |
| Elementary analysis/(wt. %) | ||
| Carbon | 39.22 | 3.42 |
| Nitrogen | 0.72 | 0.00 |
| Hydrogen | 5.05 | 0.48 |
| Sulfur | 0.70 | 1.85 |
| Oxygen | 37.04 | n.d. |
| Composition/(wt. %) | ||
| Si | n.d. | 11.0 |
| Al | n.d. | 1.6 |
| Fe | n.d. | 4.0 |
| Mg | n.d. | 5.6 |
| Ca | n.d. | 11.4 |
| Na | n.d. | 1.2 |
| K | n.d. | 0.8 |
| P | n.d. | 0.2 |
| Models | T/K | 283 | 293 | 303 |
|---|---|---|---|---|
| Pseudo-first-order | ||||
| qe/(mgP g−1) | 42.4 | 42.1 | 41.5 | |
| k1/(h−1) | 0.348 | 0.388 | 0.487 | |
| R2 | 0.84 | 0.72 | 0.53 | |
| RMSE | 4.3 | 5.1 | 5.6 | |
| Pseudo-second-order | ||||
| qe/(mgP g−1) | 44.5 | 44.0 | 44.1 | |
| k2/(g mgP−1 h−1) | 0.011 | 0.012 | 0.013 | |
| R2 | 0.925 | 0.85 | 0.74 | |
| RMSE | 2.8 | 3.7 | 4.2 | |
| Elovich | ||||
| α/(mgP g−1 h−1) | 157.6 | 193.0 | 240.3 | |
| β/(g mgP−1) | 0.176 | 0.185 | 0.181 | |
| R2 | 0.972 | 0.993 | 0.972 | |
| RMSE | 1.7 | 0.78 | 1.4 |
| Models | T/K | 283 | 293 | 303 |
|---|---|---|---|---|
| Langmuir | ||||
| qmax/(mg g−1) | 48.5 | 50.7 | 57.0 | |
| KL/(mg−1 L) | 3.265 | 7.382 | 3.063 | |
| R2 | 0.76 | 0.60 | 0.906 | |
| RMSE | 4.69 | 6.02 | 3.25 | |
| ΔAICc | 49.8 | |||
| Freundlich | ||||
| 1/n | 0.120 | 0.100 | 0.106 | |
| KF/(mg1−(1/n) L1/n g−1) | 32.6 | 36.3 | 39.1 | |
| R2 | 0.987 | 0.940 | 0.938 | |
| RMSE | 1.06 | 2.33 | 2.64 | |
| ΔAICc | 0.0 | |||
| Temkin | ||||
| B/(mg g−1) | 4.71 | 4.14 | 5.18 | |
| KT/(mg−1 L) | 1088 | 7847 | 1834 | |
| R2 | 0.972 | 0.915 | 0.963 | |
| RMSE | 1.53 | 2.76 | 2.03 | |
| ΔAICc | 2.4 |
| Precursor Material | Treatment | Dose/(g L−1) | q/(mgP g−1) | Ref. |
|---|---|---|---|---|
| Posidonia oceanica fiber | Raw | 10 | 3.0 | [60] |
| Posidonia oceanica fiber | Raw | 2 | 5.0 | [61] |
| Date palm | Raw | 6 | 5.85 | [62] |
| Peanut shell | Biochar | 1 | 7.6 | [63] |
| Sesame straw | Biochar | 2 | 9.4 | [64] |
| Sewage sludge | Biochar | 1 | 15.2 | [65] |
| Walnut shell | Biochar | 2 | 6.4 | [66] |
| Walnut shell | Biochar | 1 | 4.7 | [67] |
| Rice straw | Ash | 10 | 4.5 | [25] |
| Rice husk | Ash | 2 | 1.6 | [57] |
| Reed | Ash | 1.5 | 22.5 | [25] |
| Lime sludge | Ash | 1 | 20.8 | [68] |
| Paulownia | Ash | 5 | 12.0 | [48] |
| Wheat straw | Ash | 5 | 8.4 | [48] |
| Barley straw | Ash | 5 | 12.0 | [48] |
| Posidonia oceanica fiber | Ash | 0.5–2 | 33.5–58.7 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, J.A.; Mengual, J.; Palomares, A.E. From Waste to Resource: Phosphorus Adsorption on Posidonia oceanica Ash and Its Application as a Soil Fertilizer. AgriEngineering 2025, 7, 333. https://doi.org/10.3390/agriengineering7100333
González JA, Mengual J, Palomares AE. From Waste to Resource: Phosphorus Adsorption on Posidonia oceanica Ash and Its Application as a Soil Fertilizer. AgriEngineering. 2025; 7(10):333. https://doi.org/10.3390/agriengineering7100333
Chicago/Turabian StyleGonzález, Juan A., Jesús Mengual, and Antonio Eduardo Palomares. 2025. "From Waste to Resource: Phosphorus Adsorption on Posidonia oceanica Ash and Its Application as a Soil Fertilizer" AgriEngineering 7, no. 10: 333. https://doi.org/10.3390/agriengineering7100333
APA StyleGonzález, J. A., Mengual, J., & Palomares, A. E. (2025). From Waste to Resource: Phosphorus Adsorption on Posidonia oceanica Ash and Its Application as a Soil Fertilizer. AgriEngineering, 7(10), 333. https://doi.org/10.3390/agriengineering7100333






