Concept of a Cyber–Physical System for Control of a Self-Cleaning Aquaponic Unit
Abstract
1. Introduction
2. Basic Types of Aquaponic Systems
3. Materials and Methods
3.1. Components of the Aquaponic System
3.2. Laser Processing of the Polypropylene
3.3. Biofilm Experiments
3.3.1. Microorganism and Culture Conditions
3.3.2. SEM Investigations
3.3.3. Evaluation of Bacterial Viability
4. Maintaining Balance in Aquaponic Systems
4.1. Temperature (t)
4.2. Power of Hydrogen (pH)
4.3. Water Hardness
4.4. Dissolved Oxygen
4.5. Carbon Dioxide CO2
4.6. UV Radiation
4.7. Total Nitrogen
4.8. Electrical Conductivity (EC) and Total Dissolved Solids (TDSs)
4.9. Mechanical Filtration
4.10. Bacterial Development and Biofiltration
4.11. Surface Treatment of the Fish Tank
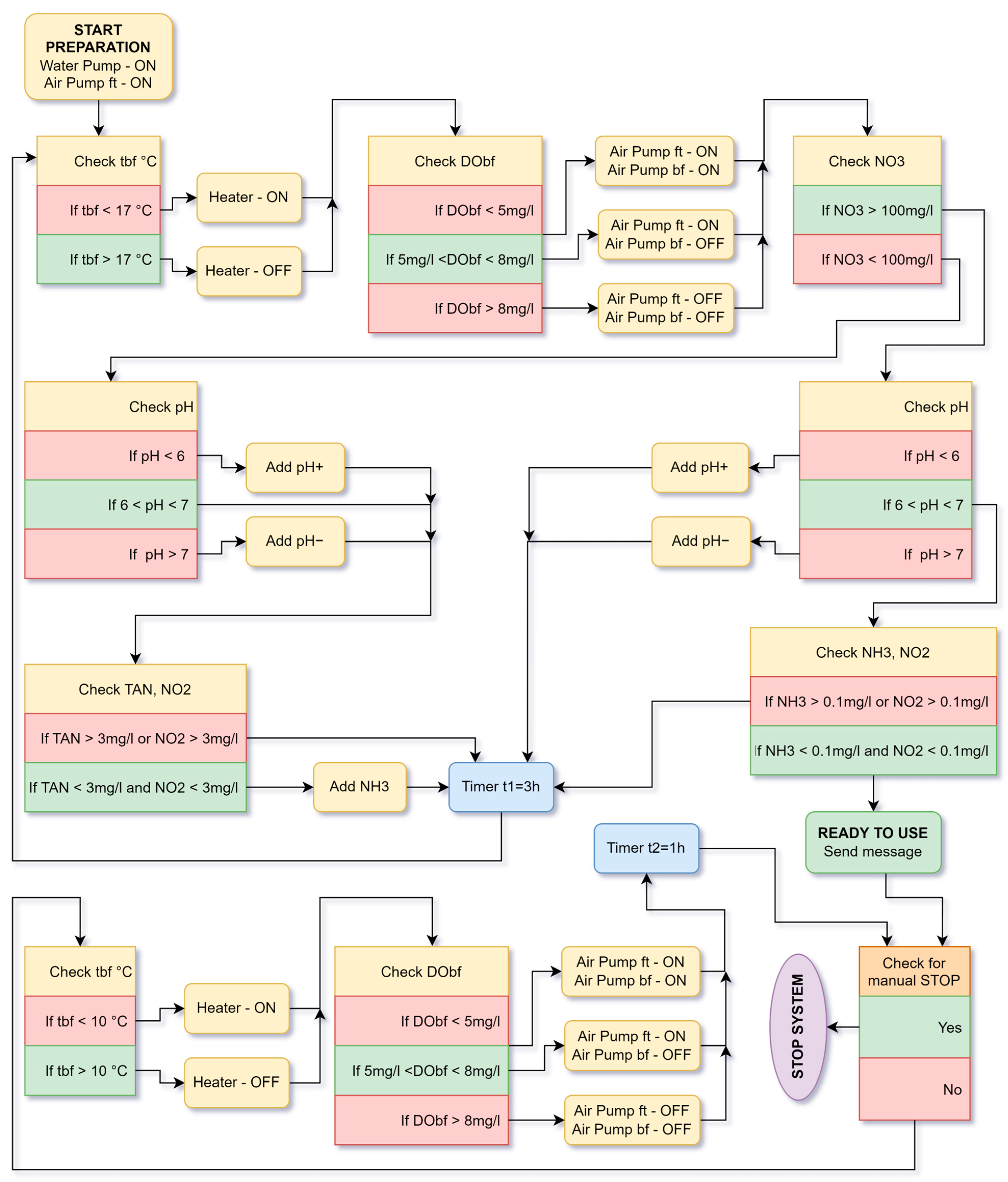
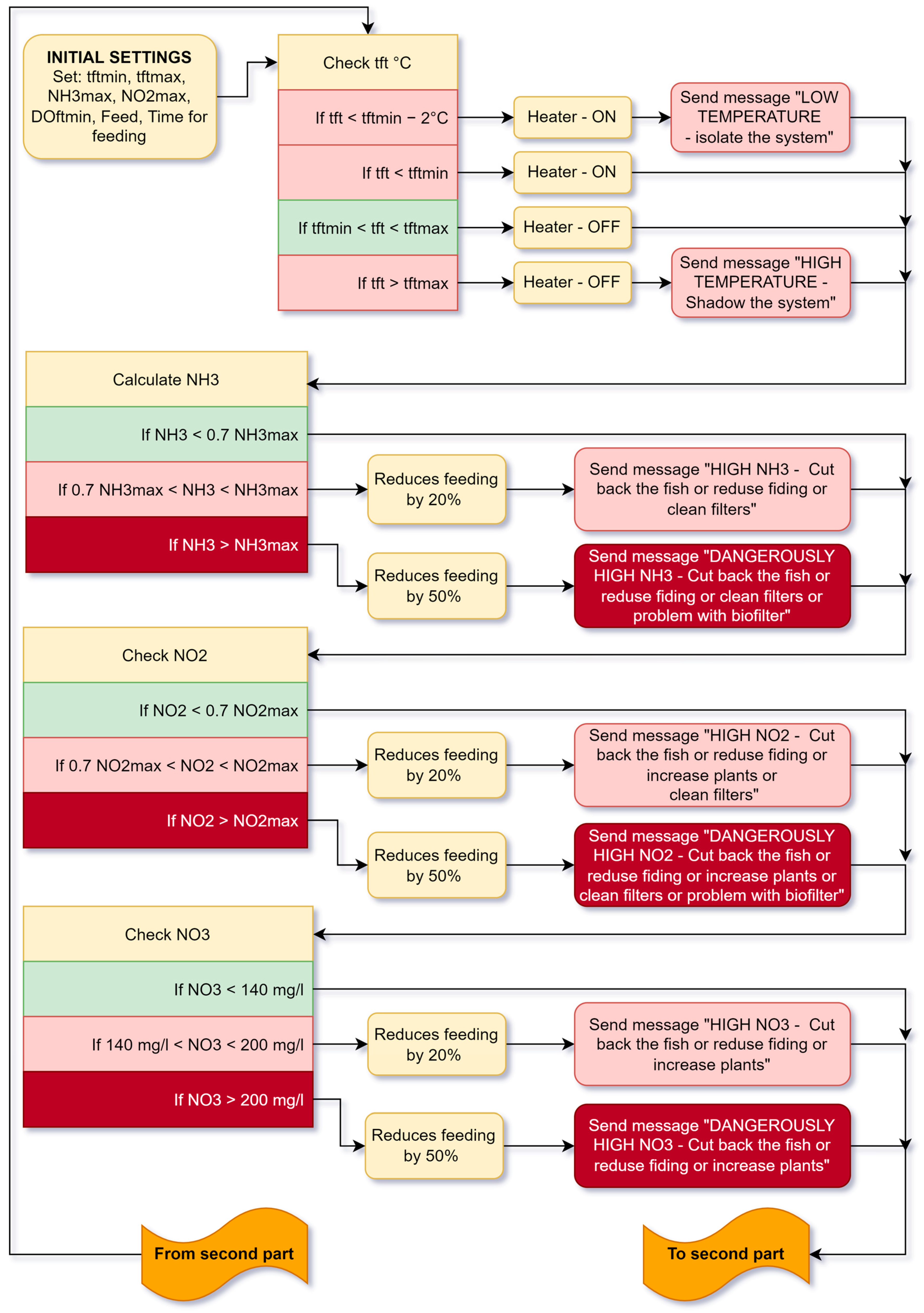
5. Algorithms of Cyber Physical System Operation
5.1. Bacterial Growth Startup Cycle in Aquaponic System
5.2. Working Algorithm of the Aquaponic System
5.3. Feeding Cycle
5.4. Water Addition Cycle
6. Test and Result
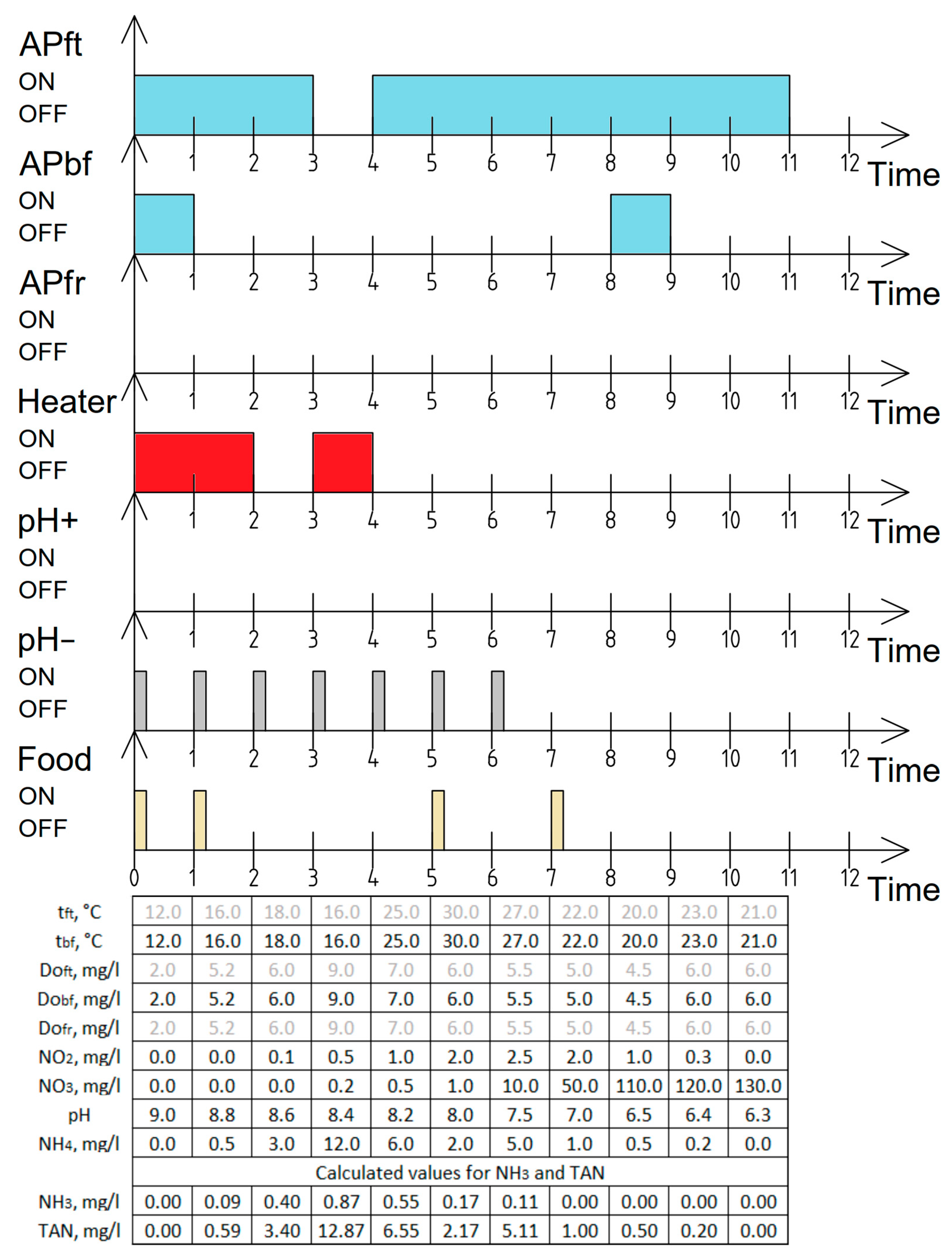
6.1. Test of Cyber–Physical System Work
6.2. Surface Treatment
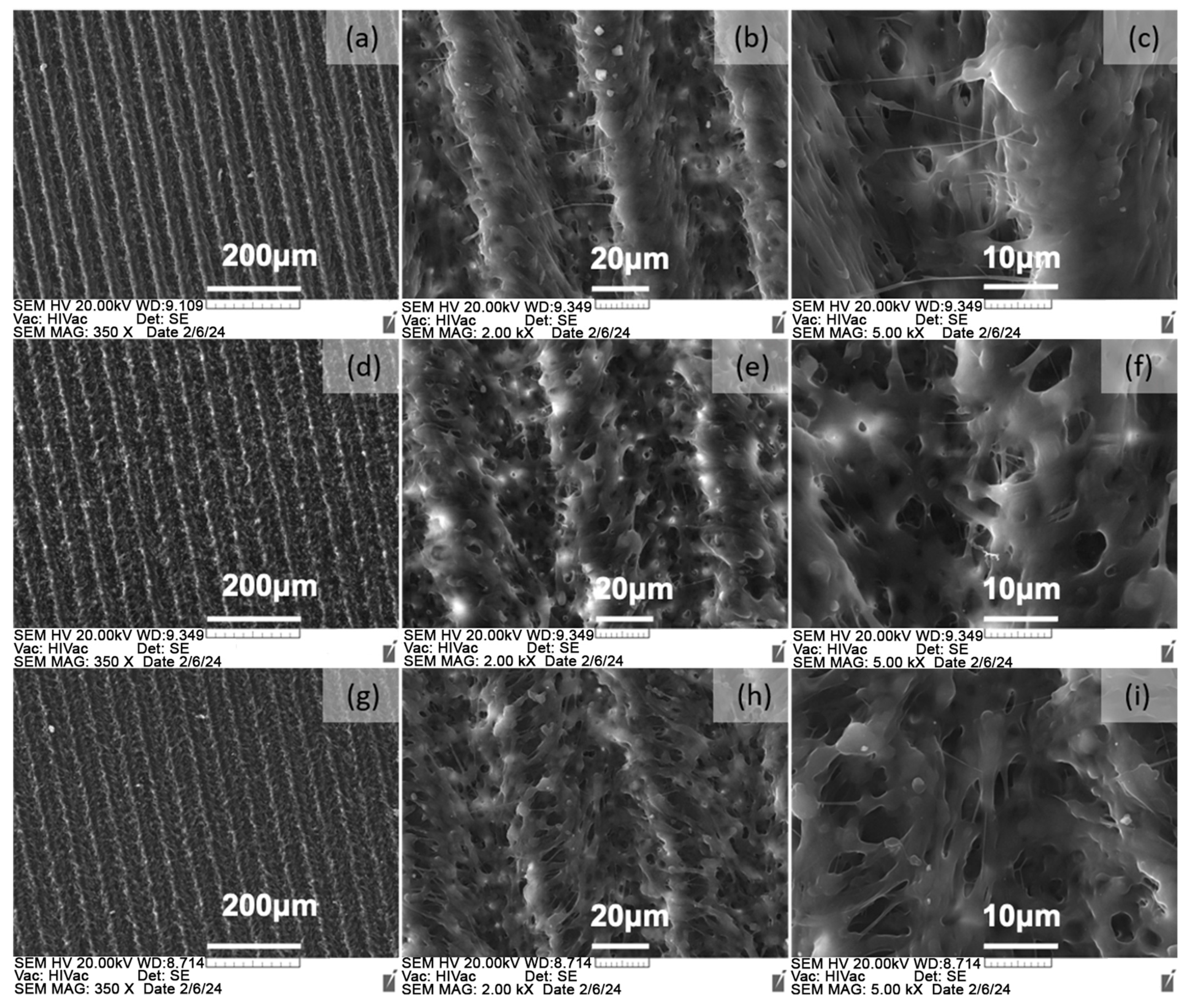
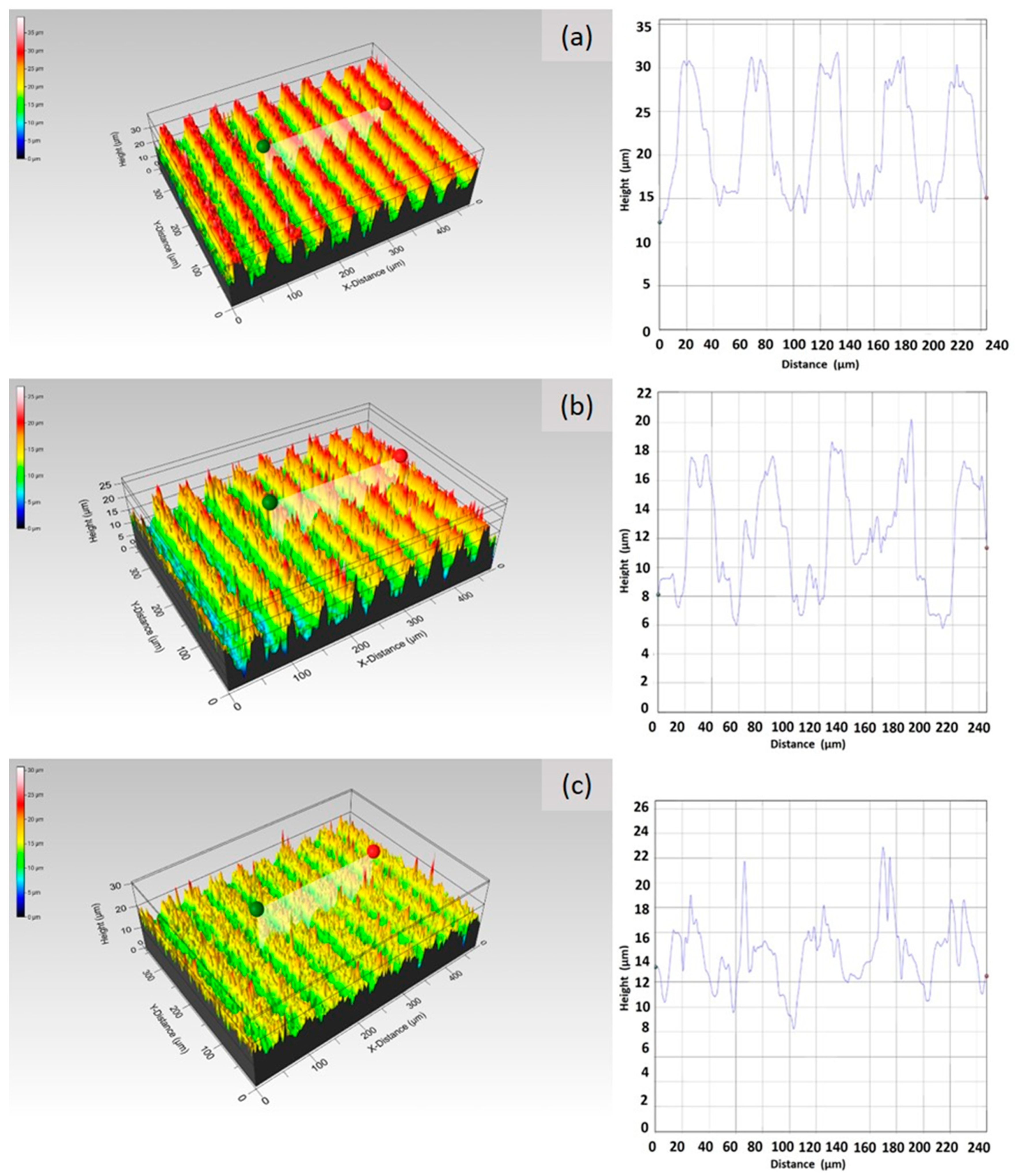
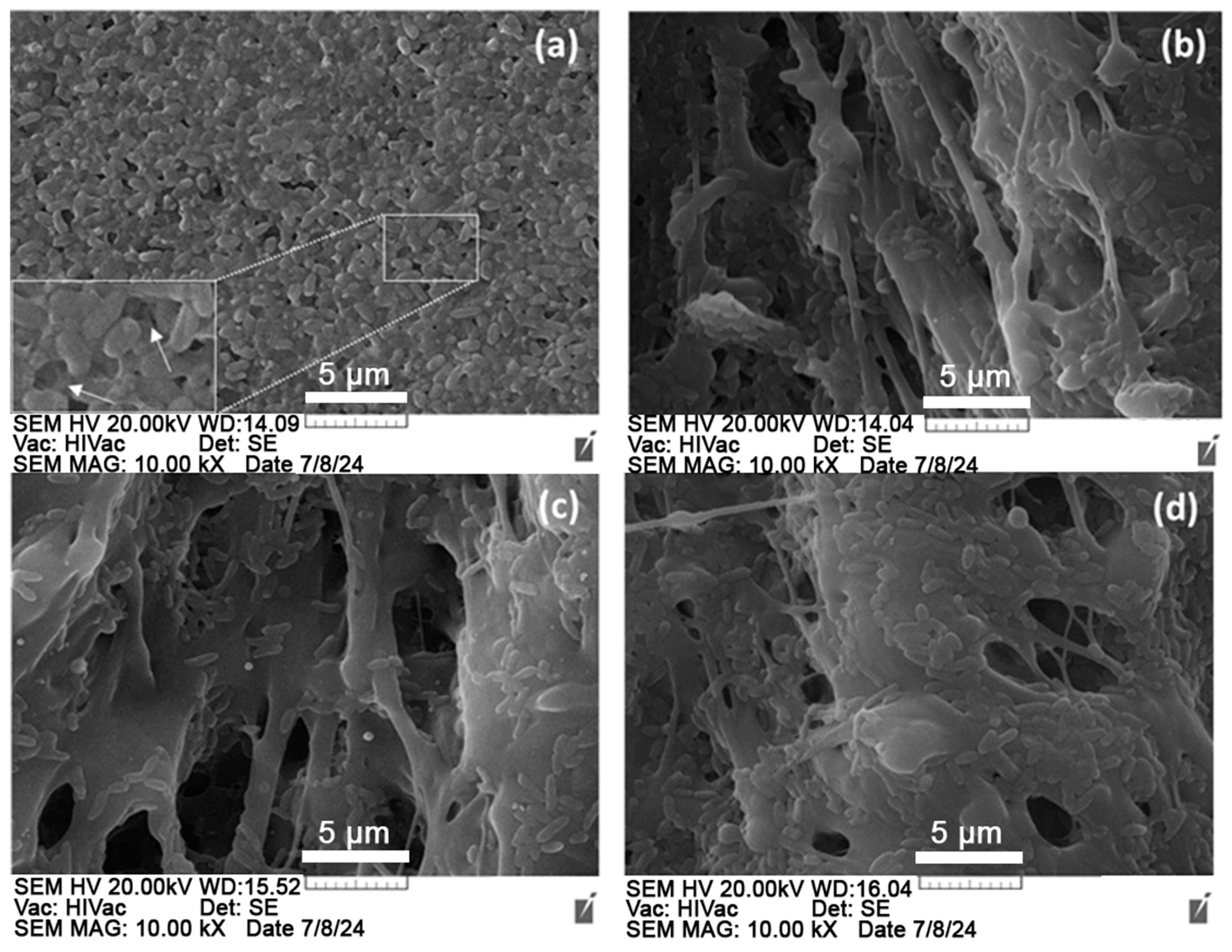
6.2.1. Surface Morphology Analysis
6.2.2. Surface Topography Analysis
6.2.3. Effects of the Surface Topography on the Biofilm Formation
6.2.4. Effects on Bacterial Viability
7. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Total Production of Fishery Products (Million Tonnes of Live Weight, EU, 2000–2021). Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Aquaculture_statistics (accessed on 1 February 2024).
- Isaacson, M.; Allyn, N.; Loverich, G. Development of a net pen system for aquaculture farming. Can. J. Civ. Eng. 2011, 20, 189–200. [Google Scholar] [CrossRef]
- van Rijn, J. Waste treatment in recirculating aquaculture systems. Aquac. Eng. 2013, 53, 49–56. [Google Scholar] [CrossRef]
- Wahyuningsih, S.; Effendi, H.; Wardiatno, Y. Nitrogen removal of aquaculture wastewater in aquaponic recirculation system. Aquac. Aquar. Conserv. Legis. 2015, 8, 491–499. [Google Scholar]
- Li, X.; Li, J.; Wang, Y.; Fu, L.; Fu, Y.; Li, B.; Jiao, B. Aquaculture industry in China: Current state, challenges, and outlook. Rev. Fish. Sci. 2011, 19, 187–200. [Google Scholar] [CrossRef]
- Somerville, C.; Cohen, M.; Pantanella, E.; Stankus, A.; Lovatelli, A. Small-Scale Aquaponic Food Production: Integrated Fish and Plant Farming; FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2014. [Google Scholar]
- Van Stappen, G.; Salie, K.; Stander, H. Inoculating Fish Sludge from Aquaponics with Microbes to Enhance Mineralisation of Phosphorus; Ghent University: Flanders, Belgium, 2020. [Google Scholar]
- Resources, N. Food Production and Water Conservation in a Recirculating Aquaponic System in Saudi Arabia at Different Ratios of Fish Feed to Plants. J. World Aquac. Soc. 2008, 39, 510–520. [Google Scholar]
- Graber, A.; Junge, R. Aquaponic Systems: Nutrient recycling from fish wastewater by vegetable production. Desalination 2009, 246, 147–156. [Google Scholar] [CrossRef]
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of sustainable and commercial aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Cai, W.; De La Fuente, L.; Arias, C.R. Biofilm Formation by the Fish Pathogen Flavobacterium columnare: Development and Parameters Affecting Surface Attachment. Appl. Environ. Microbiol. 2013, 79, 5633–5642. [Google Scholar] [CrossRef]
- Schwibbert, K.; Richter, A.M.; Krüger, J.; Bonse, J. Laser TexturedSurfaces: A Way to Control Biofilm Formation? Laser Photon-Rev. 2024, 18, 2300753. [Google Scholar] [CrossRef]
- Ge, X.; Ren, C.; Ding, Y.; Chen, G.; Lu, X.; Wang, K.; Ren, F.; Yang, M.; Wang, Z.; Li, J.; et al. Micro/nano-structured TiO2 surface with dual-functional antibacterial effects for biomedical applications. Bioact. Mater. 2019, 4, 346–357. [Google Scholar] [CrossRef]
- Li, X.; Du, S.; Ma, C.; Shi, T.; Qi, W.; Yang, H. Nano-SiO2 based anti-corrosion superhydrophobic coating on Al alloy with mechanical stability, anti-pollution and self-cleaning properties. Ceram. Int. 2024, 50, 9469–9478. [Google Scholar] [CrossRef]
- Roalkvam, I.; Drønen, K.; Dahle, H.; Wergeland, H. Microbial Communities in a Flow-Through Fish Farm for Lumpfish (Cyclopterus lumpus L.) During Healthy Rearing Conditions. Front. Microbiol. 2019, 10, 1594. [Google Scholar] [CrossRef]
- Bondad-Reantaso, M.G.; MacKinnon, B.; Karunasagar, I.; Fridman, S.; Alday-Sanz, V.; Brun, E.; Le Groumellec, M.; Li, A.; Surachetpong, W.; Karunasagar, I.; et al. Review of alternatives to antibiotic use in aquaculture. Rev. Aquac. 2023, 15, 1421–1451. [Google Scholar] [CrossRef]
- Amri, A.; Sari, S.; Yusoff, A.; Roslan, H.; Ibrahim, N.; Muji, S.Z.M. Development of Indoor Aquaponic System with Monitoring Mechanism for Tilapia and Pak Choy. Evol. Electr. Electron. Eng. 2022, 3, 879–887. [Google Scholar]
- Masłon, A.; Tomaszek, J.A. A Study on the Use of the BioBall® as a Biofilm Carrier in a Sequencing Batch Reactor. Bioresour. Technol. 2015, 196, 577–585. [Google Scholar] [CrossRef]
- Sheikh, B. Hydroponics: Key to sustain agriculture in water stressed and urban environment. Pak. J. Agric. Agril. Eng. Vet. Sci. 2006, 22, 53–57. [Google Scholar]
- Sallenave, R. Important Water Quality Parameters in Aquaponics Systems; Circular 680, College of Agricultural, Consumer and Environmental Sciences, New Mexico State University: Las Cruces, NM, USA, 2016. [Google Scholar]
- Thorarinsdottir, R. Aquaponics Guidelines; Haskolaprent: Reykjavik, Iceland, 2015; ISBN 978-9935-9283-1-3. [Google Scholar] [CrossRef]
- Palm, H.W.; Bissa, K.; Knaus, U. Significant factors affecting the economic sustainability of closed aquaponic systems. Part II: Fish and plant growth. Aquac. Aquar. Conserv. Legis. Int. J. Bioflux Soc. 2014, 7, 3. [Google Scholar]
- Wongkiew, S.; Hu, Z.; Chandran, K.; Lee, J.W.; Khanal, S.K. Nitrogen transformations in aquaponic systems: A review. Aquac. Eng. 2017, 76, 9–19. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M.; Wheaton, F.W.; Summerfelt, S.T.; Vinci, B.J. Recirculating Aquaculture Systems, 2nd ed.; Cayuga Aqua Ventures: New York, NY, USA, 2002. [Google Scholar]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E.M. Reconciling water parameters impacting nitrification in aquaponics: The pH levels. Proc. Fla. State Hortic. Soc. 2004, 117, 79–83. [Google Scholar]
- Wedemeyer, G.A. Physiology of Fish in Intensive Culture Systems; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Luo, S.; Wu, B.; Xiong, X.; Wang, J. Short-term toxicity of ammonia, nitrite, and nitrate to early life stages of the rare minnow (Gobiocypris rarus). Environ. Toxicol. Chem. 2016, 35, 1422–1427. [Google Scholar] [CrossRef]
- Jensen, F.B. Nitrite disrupts multiple physiological functions in aquatic animals. Comp. Biochem. Physiol. A 2003, 135, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.M.; Eddy, F.B. Chloride uptake in freshwater teleosts and its relationship to nitrite uptake and toxicity. J. Comp. Physiol. B 1986, 156, 867–872. [Google Scholar] [CrossRef]
- Kroupova, H.; Machova, J.; Svobodova, Z. Nitrite influence on fish: A review. Vet. Med. 2005, 11, 461–471. [Google Scholar] [CrossRef]
- Espinal, C.A.; Matulić, D. Recirculating Aquaculture Technologies. In Aquaponics Food Production Systems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 35–76. [Google Scholar]
- Zhou, L.; Boyd, C.E. An assessment of total ammonia nitrogen concentration in Alabama (USA) ictalurid catfish ponds and the possible risk of ammonia toxicity. In Aquaculture and Aquatic Sciences; School of Fisheries, Auburn University: Auburn, AL, USA, 2015. [Google Scholar]
- Zhou, L.; Boyd, C.E. Ammonia Nitrogen Management in Aquaculture Ponds. In Aquaculture and Aquatic Sciences; School of Fisheries, Auburn University: Auburn, AL, USA, 2015. [Google Scholar]
- Calculation of Unionized Ammonia in Fresh Water Storet Parameter Code 00619, FLORIDA DEPARTMENT OF ENVIRONMENTAL PROTECTION CHEMISTRY LABORATORY METHODS MANUAL, TALLAHASSEE. Revision 2. Ph.D. Thesis, Florida DEP Laboratory, Tallahassee, FL, USA, 2001.
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous ammonia equilibrium calculations: Effect of pH and temperature. J. Fish. Res. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Francis-Floyd, R.; Watson, C.; Petty, D.; Pouder, D.B. Ammonia in Aquatic Systems; University of Florida: Gainesville, FL, USA, 2022. [Google Scholar] [CrossRef]
- Purwono, A.R.; Muhammad, H.; Mochamad, A.B. Ammonia-Nitrogen (NH3-N) and Ammonium-Nitrogen (NH4+-N) Equilibrium on The Process of Removing Nitrogen by Using Tubular Plastic Media. J. Mater. Environ. Sci. 2017, 8, 4915–4922. [Google Scholar]
- Yogev, U.; Sowers, K.R.; Mozes, N.; Gross, A. Nitrogen and carbon balance in a novel near-zero water exchange saline recirculating aquaculture system. Aquaculture 2017, 467, 118–126. [Google Scholar] [CrossRef]
- Boyd, C.E. Overview of aquaculture feeds: Global impacts of ingredient use. In Feed and Feeding Practices in Aquaculture, 1st ed.; Davis, D.A., Ed.; Woodhead Publishing: Cambridge/London, UK, 2015; pp. 3–25. [Google Scholar]
- Neori, A.; Krom, M.D.; van Rijn, J. Biogeochemical processes in intensive zero-effluent marine fish culture with recirculating aerobic and anaerobic biofilters. J. Exp. Mar. Biol. Ecol. 2007, 349, 235–247. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, M.P.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Masser, M.P.; James Rakocy, J.; Thomas, M.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: An Overview of Critical Considerations; SRAC Publication No. 452; SRAC Publication: Stoneville, MS, USA, 1999. [Google Scholar]
- Thangam, Y. Histopathological studies on nitrite toxicity to freshwater fish, Cirrhinus mrigala. IOSR JESTFT 2014, 8, 2319–2402. [Google Scholar] [CrossRef]
- Buzby, K.M.; Lin, L.S. Scaling aquaponics systems: Balancing plant uptake with fish output. Aquac. Eng. 2014, 63, 39–44. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil-, and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Kasozi, N.; Abraham, B.; Kaiser, H.; Wilhelmi, B. The complex microbiome in aquaponics: Significance of the bacterial ecosystem. Ann. Microbiol. 2021, 71, 1. [Google Scholar] [CrossRef]
- Keesman, K.J.; Körner, O.; Wagner, K.; Urban, J.; Karimanzira, D. Thomas Rauschenbach, Simon Goddek, Aquaponics Systems Modelling, In Aquaponics Food Production Systems; Springer: Berlin/Heidelberg, Germany, 2019; pp. 267–299. [Google Scholar]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Mori, J.; Smith, R. Transmission of waterborne fish and plant pathogens in aquaponics and their control with physical disinfection and filtration: A systematized review. Aquaculture 2019, 504, 380–395. [Google Scholar] [CrossRef]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar] [CrossRef]
- Gnilitskyi, I.; Rymar, S.; Iungin, O.; Vyshnevskyy, O.; Parisse, P.; Potters, G.; Zayats, A.V.; Moshynets, O. Femtosecond laser modified metal surfaces alter biofilm architecture and reduce bacterial biofilm formation. Nanoscale Adv. 2023, 5, 6659–6669. [Google Scholar] [CrossRef]
- Lutey, A.H.A.; Gemini, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef]
- Helbig, R.; Günther, D.; Friedrichs, J.; Rößler, F.; Lasagni, A.; Werner, C. The impact of structure dimensions on initial bacterial adhesion. Biomater. Sci. 2016, 4, 1074–1078. [Google Scholar] [CrossRef]
- Chen, H.; Wei, Z.; Sun, G.; Su, H.; Liu, J.; Hu, B.; Zhou, X.; Lou, L. Formation of biofilms from new pipelines at both ends of the drinking water distribution system and comparison of disinfection by-products formation potential. Environ. Res. 2020, 182, 109150. [Google Scholar] [CrossRef]
- Sharan, M.; Vijay, D.; Dhaka, P.; Bedi, J.S.; Gill, J.P.S. Biofilms as a microbial hazard in the food industry: A scoping review. J. Appl. Microbiol. 2022, 133, 2210–2234. [Google Scholar] [CrossRef] [PubMed]
- Damyanova, T.; Dimitrova, P.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An overview of biofilm-associated infections and the role of phytochemicals and nanomaterials in their control and prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef] [PubMed]
- Stoitsova, S.R.; Paunova-Krasteva, T.S.; Borisova, D.B. Modulation of biofilm growth by Sub-inhibitory amounts of antibacterial substances. In Microbial Biofilms; Dhanasekaran, D., Thajuddin, N., Eds.; InTech: London, UK, 2016. [Google Scholar]
- World Health Organization. Prioritization of Pathogens to Guide Discovery, Research and Development of New Antibiotics for Drug-Resistant Bacterial Infections, Including Tuberculosis; World Health Organization: Geneva, Switzerland, 2017.
- Flemming, H.-C.; van Hullebusch, E.D.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. The biofilm matrix: Multitasking in a shared space. Nat. Rev. Microbiol. 2023, 21, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Kouider, N.; Hamadi, F.; Mallouki, B.; Bengoram, J.; Mabrouki, M.; Zekraoui, M.; Ellouali, M.; Latrache, H. Effect of stainless steel surface roughness on Staphylococcus aureus adhesion. Int. J. Pure Appl. Sci. 2010, 4, 1–7. [Google Scholar]
- Daskalova, A.; Sezanova, K.; Angelova, L.; Paunova-Krasteva, T.; Gergulova, R.; Kovacheva, D.; Rabadjieva, D. Ultra-Short Laser-Assisted Micro-Structure Formations on Mg/Zn Double-Doped Calcium Phosphate Ceramics for Enhanced Antimicrobial Activity. Materials 2023, 16, 6626. [Google Scholar] [CrossRef]
- Yanes, A.R.; Martinez, P.; Ahmad, R. Towards automated aquaponics: A review on monitoring, IoT, and smart systems. J. Clean. Prod. 2020, 263, 121571. [Google Scholar] [CrossRef]







| Description | Description |
|---|---|
| Ammonia Monitoring | The CPS shall monitor the critical water parameter ammonia (NH3) within the fish tank. |
| Nitrite Monitoring | The CPS shall monitor the critical water parameter nitrite (NO2−) to ensure safe levels in the aquaponic system. |
| Nitrate Monitoring | The CPS shall monitor the critical water parameter nitrate (NO3−) to ensure safe levels in the aquaponic system. |
| pH Level Monitoring | The CPS shall monitor the pH level in the water and provide alerts when pH deviates from the set range. |
| Temperature Monitoring | The CPS shall monitor the water temperature in both the fish tank and biofilter, ensuring it stays within species-specific ranges. |
| Dissolved Oxygen Monitoring | The CPS shall monitor the dissolved oxygen (DO) levels in the fish tank, biofilter, and floating rafts to maintain optimal conditions for fish and bacteria. |
| Water Level Monitoring | The CPS shall monitor the water levels in the fish tank and biofilter and control the solenoid valve for maintaining water levels. |
| Automated Fish Feeding | The CPS shall control the fish feeding mechanism through an auger, adjusting the amount and frequency of feed based on the system’s conditions. |
| Air Pump Control | The CPS shall control the air pumps in the fish tank, biofilter, and floating rafts, maintaining oxygen levels within the set parameters. |
| pH Regulation | The CPS shall regulate the pH levels by controlling augers that dispense pH-altering substances when necessary. |
| Continuous Operation | The CPS shall operate continuously with minimal manual intervention, ensuring stable and autonomous system functionality. |
| Alarm and Messaging System | The CPS shall provide alarms and a messaging system to notify operators in case of parameter deviations, such as temperature, oxygen levels, or pH fluctuations. |
| Data Logging | The CPS shall log real-time data from all monitored parameters for analysis and troubleshooting. |
| System Scalability | The CPS shall allow scalability, enabling the addition of new sensors and control mechanisms as the aquaponic unit expands. |
| User Interface | The CPS shall have an intuitive user interface for operators to monitor system status and manually adjust parameters when necessary. |
| Water Quality Management | The CPS shall maintain water quality by automatically adjusting parameters such as oxygen, ammonia, and pH, to ensure healthy conditions for both fish and plants. |
| Nutrient Management | The CPS shall monitor and manage nutrient levels, ensuring that plants receive adequate nutrients for optimal growth. |
| Adaptive Feeding Management | To prevent overfeeding, the CPS shall adjust fish feeding rates based on water quality parameters such as ammonia and nitrites. |
| Sensors | Locations |
|---|---|
| Water temperature, t °C | Fish tank, biofilter |
| Dissolved oxygen, DO | Fish tank, biofilter, floating rafts |
| Nitrite, NO2− | Fish tank |
| Nitrate, NO3− | Fish tank |
| Power of hydrogen, pH | Fish tank |
| Ammonium NH4+ | Fish tank |
| Ammonia NH3 (Calculated) | - |
| Total ammonia nitrogen TAN (Calculated) | - |
| Water level sensor | Fish tank, biofilter |
| Control systems | Locations |
| Water pump | Biofilter |
| Air pump | Biofilter, Biofilter, Floating rafts |
| Heater | Fish tank |
| Auger for adding pH− | Biofilter |
| Auger for adding pH+ | Biofilter |
| Auger for adding fish food | Fish tank |
| Electric solenoid water valve | Biofilter |
| Model | Measurement | Measuring Range | Resolution for |
|---|---|---|---|
| TriOxmatic 700 IQ | DO | 0–60 mg/L | 0.1 mg/L |
| Temperature | −5–60 °C | 0.1 °C | |
| AmmoLyt Plus 700 IQ | Ammonium, NH4+ | 0.1–129 mg/L | 0.1 mg/L |
| NiCaVis 701 IQ NI | Nitrites NO2− | 0.1–120 mg/L | 0.1 mg/L |
| Nitrates NO3− | 0.1–300 mg/L | 0.1 mg/L | |
| SensoLyt® 700 IQ | pH | 0.00–14.00 | 0.01 |
| SL-M5 | Water level | - | - |
| Water Parameter | Measurement Location | Tolerance Range | Maintained by the System |
|---|---|---|---|
| Temperature, t °C | Fish tank (working cycle) | Depending on fish type above 10 °C | Not lower than the set |
| Biofilter (startup cycle) | 17 | Not lower than 17 | |
| Power of hydrogen, pH | Fish tank | 6–7 | 6–7 |
| Dissolved oxygen, DO, mg/L | Fish tank | 4–6 *, 6–8 ** | 5–6 *, 6–8 ** |
| Biofilter | 4–8 | 5–8 | |
| Floating rafts | Above 3 | 4–8 | |
| Ammonia, NH3, mg/L | Fish tank *** | <3 *, <1 ** | Not higher than the set according to the specific fish species **** |
| Nitrate, NO3−, mg/L | Fish tank | <1 | Not higher than the set according to the specific fish species **** |
| Nitrite, NO2−, mg/L | Fish tank | <300 | <200 **** |
| Abbreviations | Description |
|---|---|
| tft, °C | Water temperature in fish tank |
| tbf, °C | Water temperature in biofilter |
| tftmin, °C | Set minimum water temperature in fish tank |
| tftmax, °C | Set maximum water temperature in fish tank |
| tbfmin, °C | Set minimum water temperature in biofilter |
| DOft, mg/L | Concentration of dissolved oxygen in fish tank |
| DObf, mg/L | Concentration of dissolved oxygen in biofilter |
| DOfr, mg/L | Concentration of dissolved oxygen in floating raft |
| DOftmin, mg/L | Set minimum concentration of dissolved oxygen in fish tank |
| pH | Power of hydrogen in fish tank |
| NO2, mg/L | Nitrite in fish tank |
| NO2max, mg/L | Set maximum nitrate in fish tank |
| NO3, mg/L | Nitrate in fish tank |
| NH3, mg/L | Ammonia in fish tank |
| NH3max, mg/L | Set maximum ammonia in fish tank |
| NH4, mg/L | Ammonium in fish tank |
| TAN, mg/L | Total ammonia nitrogen in fish tank |
| Feed, sec | Auger feeding time |
| Air pump ft | Air pump in fish tank |
| Air pump bf | Air pump in biofilter |
| Air pump fr | Air pump in floating raft |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrov, K.; Chivarov, N.; Chivarov, S.; Paunova-Krasteva, T.; Filipov, E.; Daskalova, A. Concept of a Cyber–Physical System for Control of a Self-Cleaning Aquaponic Unit. AgriEngineering 2024, 6, 3843-3874. https://doi.org/10.3390/agriengineering6040219
Dimitrov K, Chivarov N, Chivarov S, Paunova-Krasteva T, Filipov E, Daskalova A. Concept of a Cyber–Physical System for Control of a Self-Cleaning Aquaponic Unit. AgriEngineering. 2024; 6(4):3843-3874. https://doi.org/10.3390/agriengineering6040219
Chicago/Turabian StyleDimitrov, Kristiyan, Nayden Chivarov, Stefan Chivarov, Tsvetelina Paunova-Krasteva, Emil Filipov, and Albena Daskalova. 2024. "Concept of a Cyber–Physical System for Control of a Self-Cleaning Aquaponic Unit" AgriEngineering 6, no. 4: 3843-3874. https://doi.org/10.3390/agriengineering6040219
APA StyleDimitrov, K., Chivarov, N., Chivarov, S., Paunova-Krasteva, T., Filipov, E., & Daskalova, A. (2024). Concept of a Cyber–Physical System for Control of a Self-Cleaning Aquaponic Unit. AgriEngineering, 6(4), 3843-3874. https://doi.org/10.3390/agriengineering6040219







