Abstract
Low doses of glyphosate from application drift can be phytotoxic or stimulate growth of glyphosate-susceptible crops. The application of Si can prevent herbicide-caused plant stress. The effects of Si application (3 mM Si) on low doses (0, 36, 72, and 180 g a.e. ha−1) of glyphosate were determined on Sorghum bicolor in a greenhouse study. Growth parameters, mineral content, metabolite content, and glyphosate and aminomethylphosphonic acid (AMPA) content were measured. Increasing glyphosate content, but no AMPA, was found with increasing glyphosate application rates. Shoot dry weight was increased by 72 g ha−1 of glyphosate when pretreated with Si, and plant height increased in Si-treated plants treated with 72 g ha−1 of glyphosate. Si alone had no effects on growth. Shikimate content was increased by the highest glyphosate rate. Phenylalanine content was generally increased by all glyphosate treatments with or without Si, except for 72 g ha−1 glyphosate without Si. Tyrosine content was increased by 36 and 180 g ha−1 glyphosate without Si. Caffeate content was decreased by Si in the control, and ferulate content was increased with 180 g ha−1 glyphosate in Si-treated plants. Ca levels were reduced by Si at 180 g ha−1 glyphosate. Mn levels were lower than those of the control without Si for all other treatments with Si. The increases in shikimate with the highest glyphosate dose indicated that the herbicide reached its herbicide target and should be causing stress, but the only growth effect was the stimulation of some growth parameters at 72 g ha−1 of glyphosate with Si pretreatment. Similarly, there were increases in some metabolites at some glyphosate concentrations with or without Si. Our results indicate that the rates that we used cause little stress and that Si pretreatment could potentiate glyphosate hormesis for some parameters.
1. Introduction
Sorghum (Sorghum bicolor (L.) Moench.) is a major crop in many arid and semi-arid regions of the world [1]. This crop is grown for both grain and animal fodder with functional properties in healthy diets [2]. Sorghum is tolerant to many biotic and abiotic stresses [3], and is less sensitive to several climate change vulnerabilities than other crops [4]. Although sorghum is a major crop in several countries, there are no herbicide-resistant varieties of this crop [5]. The projection of world sorghum production, in the 2023/2024 harvest, is 60.01 million tons, with the Brazilian production of 3.6 million tons [6]. However, sorghum cultivation faces challenges arising from substantial weed competition limiting increases in sorghum yield [7,8,9]. Herbicides have been the main method of weed management in sorghum [8]. However, some herbicides can negatively affect crops of agricultural interest [10,11], especially non-selective herbicides.
Use of non-selective, post-emergence herbicides, such as glyphosate, requires care to avoid drift, defined, according to ANDEF [12], as a process of transporting the applied product away from the intended target. Crops that are susceptible to these herbicides can be injured [13] by both endodrift (coming from directed-jet applications in the crop itself) and exodrift (coming from applications in adjacent areas). In the case of sorghum, there is no recommendation to apply glyphosate post-emergence of the crop [14]; therefore, the risk of exodrift becomes a concern when the crop is sown close to areas where glyphosate will be applied. Exodrift-caused injury can occur from preemergence use of glyphosate on nearby fields that are planted later or from post-emergence use on glyphosate-resistant crops such as soybean, cotton, or maize. Considering the widespread use of glyphosate, glyphosate exodrift is a common problem.
Studies on the effects of herbicide drift on cultivated plants have been carried out through dose–response experiments, applying low doses of the products to simulate drift [15,16,17,18]. The subdoses used in these studies vary, with high simulated drift doses of up to 50% of the recommended dose for good weed control [19]. In the case of glyphosate, the minimum recommended dose is 720 g of acid equivalent ha−1 [14]. Glyphosate is only applied to emerged weeds, although it can be absorbed by both the leaves and roots [20]. However, it is a foliar-applied herbicide with little or no soil activity because it strongly binds to several soil components, greatly reducing its bioavailability to plants [21]. It can be degraded into aminomethylphosphonic acid (AMPA) by plants or microorganisms [21,22,23,24], a metabolite that is weakly phytotoxic [23,25,26]. It can also be degraded by microbes by cleaving the C-P bond, producing sarcosine and inorganic phosphate, but it is unclear if this occurs significantly in plants [21].
The phytotoxicity of glyphosate is caused solely by the inhibition of the enzyme 5-enol pyruvyl shikimate 3-phosphate synthase (EPSPS), an enzyme of the shikimate pathway [21,27]. The inhibition of EPSPS can lead to the accumulation of shikimic acid, used as the most sensitive marker for susceptible plant exposure to glyphosate [28]. Aromatic amino acids (phenylalanine, tryptophan, and tyrosine) are produced by the shikimate pathway [29]. In addition to their role in protein synthesis, derivatives of these amino acids have functions in many aspects of plant growth and development [21,30,31]. For example, plastoquinone, a requirement for carotenoid synthesis and photosystem II activity, is derived from tyrosine [21]. Phenolic compounds are also synthesized via the shikimate pathway [32,33].
Use of silicon (Si) is a promising strategy to mitigate various types of stress in crops, including stress caused by herbicides [34,35,36,37]. Si is not an essential nutrient for plants, but it is classified as a beneficial or useful element due to its roles in metabolic, physiological, and structural processes, which enhance plant survival under adverse growth conditions [38,39,40]. How Si attenuates herbicide toxicity is not yet well understood [35,36].
In this study, we evaluated physiological, nutritional, and metabolic responses of sorghum plants (S. bicolor) exposed to simulated drift of glyphosate, testing the possible attenuating effect of prior treatment with Si.
2. Materials and Methods
2.1. Plant Material and Growing Conditions
This study was conducted from November to January 2023, in a greenhouse located at 21°14′47.4″ S and 48°18′06.2″ WGr, in Jaboticabal, SP, Brazil. The gable roof greenhouse was 15 m long, 5 m wide, and 3 m high, and entirely covered with plastic material with no vents. It was illuminated with natural lighting, with an average photoperiod of 13 h and 18 min, with no temperature and humidity controls.
Polyethylene pots with a capacity of 6 dm3 were used, filled with 5 dm3 of a substrate derived from an Acrisol with the following physicochemical characteristics: 59% clay, 18% silt, and 23% sand, 5.6 pH (CaCl2); 17 g dm−3 organic matter; 34 mg dm−3 P; 7 mg dm−3 S; 23 mmolc dm−3 Ca; 12 mmolc dm−3 Mg; 3.6 mmolc dm−3 K; 0 mmolc dm−3 Al; 20 mmolc dm−3 H + Al; a 38.3 mmolc dm−3 sum of bases; a 58.1 cation exchange capacity; 66% base saturation; and 6.1 mg dm−3 Si.
At the time of sowing, 3 g of the 8-28-16 formulation was applied to supply nitrogen, phosphorus, and potassium as planting fertilization.
The cultivar FS57PWV of the sorghum (Sorghum bicolor (L.) Moench) crop was sown at a density of five seeds per pot. Ten days after emergence (DAE), thinning was carried out, maintaining the population of three plants per pot. Irrigation was carried out daily to maintain the water status of each pot at 70% of the soil’s water retention capacity, using deionized water.
2.2. Treatments and Experimental Design
The experimental treatments were arranged into two factors: prior treatment with Si (factor 1) and subsequent exposure to glyphosate (factor 2). Factor 1 presented two levels, consisting of previous Si-based treatment (with or without treatment), while factor 2 presented four levels, consisting of exposure to subdoses of glyphosate (without exposure or with exposure to three subdoses of the herbicide). Therefore, a 2 × 4 factorial scheme was used, with treatments in a completely randomized design, and five replications.
The Si treatment was carried out through foliar applications of a potassium silicate solution (K2SiO3, 128 g L−1 Si and 126.5 g L−1 K2O) at a concentration of 3 mmol L−1 of Si, adapted from the methodology used by Barbosa [37]. The Si solution was prepared at the time of application. The solution was stabilized with sorbitol (90% potassium silicate and 10% sorbitol) and the pH was adjusted to 6.8 ± 0.1 with 1 N hydrochloric acid (HCl). In plants not treated with Si, potassium was balanced by applying a potassium chloride (KCl) solution for ionic balance.
Si applications were carried out with a manual sprayer, using a volume of approximately 50 mL of solution per vessel, based on Barbosa [37]. Treatment began at 15 DAE, being complemented with four more weekly applications of Si prior to exposure to glyphosate. Prior to Si applications, the surface of the pots was covered with aluminum foil to prevent Si deposition on the soil, and the floor of the greenhouse was wet to obtain a relative humidity greater than 70%. Applications took place in the late afternoon, with an average temperature of 27 °C. The herbicide glyphosate (commercial formulation: Roundup WG® (Monsanto, São José dos Campos, SP, Brazil), N-(phosphonomethyl) glycine ammonium salt, 720 g kg−1 acid equivalent–g ha−1) was applied at 40 DAE (corresponding to 19 days after the end of the silicon treatment—DAST), using four doses (36, 72, and 180 g ha−1, in addition to 0 g ha−1), simulating a drift of 5%, 10%, and 25% of the minimum recommended dose (720 g ha−1), based on Cerveira Júnior et al. [41].
The application of glyphosate was carried out with a CO2-pressurized backpack sprayer (Herbicat, Catanduva, SP, Brazil), equipped with Teejet XR110.02 tips (Teejet, Cotia, SP, Brazil), and a pressure of 2.0 bar and calibrated for a flow rate of 200 L ha−1. The herbicide sprays were prepared and immediately applied directly to the plants, at a height of 50 cm from the canopy. The plants were removed from the greenhouse, and the application took place early in the morning, in the open air with a temperature of 20 °C, relative humidity of 74%, and few clouds and minimal wind (<3 km h−1). One hour after application, the plants were placed again inside the greenhouse.
2.3. Measurements
At 68 DAE (corresponding to 47 DAST), the plant height (cm); stem diameter (mm); number of leaves (units); shoot dry mass (g plant−1); content of Si (mg kg−1); macronutrients—N, P, K, Ca, Mg, and S (g kg−1); micronutrients—B, Cu, Fe, Mn, and Zn (mg kg−1); glyphosate; AMPA (ng g−1 of dry mass); shikimic acid; phenylalanine; tyrosine; and tryptophan (μg g−1 of dry mass) were determined in leaf tissues of sorghum plants.
Plant height was determined using a ruler graduated in millimeters, from the base of the stem to the insertion of the last leaf. The diameter of the stem was determined using a digital caliper, three centimeters from the ground. Direct counting of non-senescent green leaves was performed to determine the number of leaves.
After completing the evaluations of the previous variables, the shoot of the plants was collected, separated into the stem and leaves, and washed in the following sequence: distilled water, a neutral detergent solution (0.2%), and an HCl solution (0.1%) and thrown twice with deionized water to remove the remains of particles and other contaminants [42]. After washing, the shoot was stored in paper bags and dried in an oven with forced air circulation at 60 °C until constant mass. The dried material was weighed on a precision scale (0.001 g) to determine the dry mass.
The dry leaf material was ground in a Wiley-type micro-mill (Marconi, TE-840, Piracicaba, SP, Brazil), with a 20-micrometer mesh sieve. Part of the ground material (~1 g) was sent to a certified private laboratory (Athenas Consultoria Agrícola e Laboratório, Jaboticabal, SP, Brazil) for the extraction and quantification of mineral elements, by using the following processes: sulfur digestion and titration (N); nitro-perchloric digestion and spectrophotometry (P and S); nitro-perchloric digestion and atomic absorption spectrometry (K, Ca, Mg, Fe, Cu, Mn, and Zn); and incineration and spectrophotometry (B and Si). Based on the mineral element concentrations, the accumulation of Si (mg plant−1) and each macro- (mg plant−1) and micronutrient (μg plant−1) was determined.
Another part of the ground material (~0.5 g) was sent to the research laboratory at the School of Agriculture at UNESP, Campus of Botucatu, SP, Brazil, for the extraction and quantification of glyphosate, AMPA, shikimic acid, phenylalanine, tyrosine, tryptophan, and benzoic, caffeic, chlorogenic, coumaric, ferulic, and salicylic acids, using the method of Bortolheiro et al. [43], through high-performance liquid chromatography and mass spectrometry, using a chromatograph system (HPLC, Shimadzu, Kyoto, Japan) coupled to a triple quadrupole mass spectrometer (LS-MS/MS AB Sciex API 4500, Applied Biosystems, Foster City, CA, USA).
2.4. Statistical Analysis
Data were previously tested for normality of residuals (Shapiro–Wilk test) and for homogeneity of variances (Bartlett test). When both tests were not significant, that is, with a normal distribution of residues and homogeneity of variances, a parametric analysis of the data was carried out, using the Tukey HSD test for multiple comparisons between treatments. When the normal distribution of residues and/or homogeneity of variances was not observed, a non-parametric analysis of the data was carried out, using the Kruskal–Wallis test for multiple comparisons between treatments. The statistical software Statistica® (Statsoft Inc., version 8.0, Tulsa, OK, USA) was used for all analyses, with a 5% probability of error.
3. Results
Plants treated with Si and exposed to 72 g ha−1 of glyphosate were taller (49.3 cm) than plants treated with Si without exposure to the herbicide (39.3 cm), and plants not treated with Si and exposed to 180 g ha−1 of glyphosate (40.5 cm) (Figure 1A). The stem diameter varied from 8.8 to 10.1 mm (Figure 1B), while the number of leaves varied from six to eight units (Figure 1C), with no significant difference between the treatments tested. Plants treated with Si and exposed to 72 g ha−1 of glyphosate showed a greater accumulation of shoot dry mass (12.9 g plant−1) than plants not treated with Si and without exposure to glyphosate (10.1 g plant−1) or with exposure to 72 and 180 g ha−1 of the herbicide (10.1 and 9.8 g plant−1, respectively) (Figure 1D).
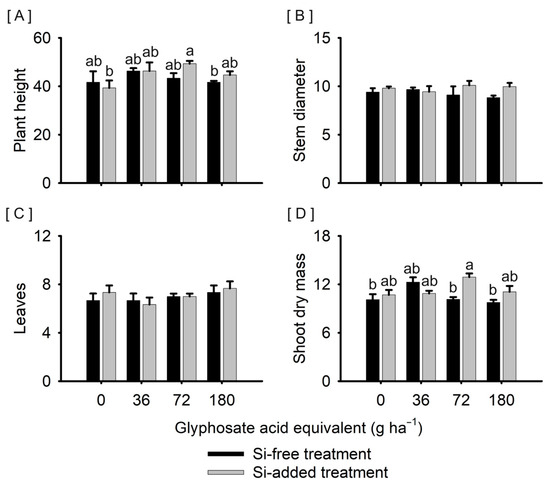
Figure 1.
The plant height ((A), cm), stem diameter ((B), mm), number of leaves ((C), units), and shoot dry mass ((D), g plant−1) of Sorghum bicolor submitted or not to foliar treatment with silicon and with further exposure or not to glyphosate sublethal doses (0, 36, 72, and 180 g ha−1), at 68 days after emergence. Vertical lines represent the standard deviation of the mean, and different lowercase letters represent significant differences by the Tukey HSD test (p < 0.05). Graphs with no letters indicate no significant differences by the analysis of variance.
Glyphosate was detected in plants exposed to the herbicide, whether treated or not with Si (Figure 2A). Glyphosate levels ranged from 11.0 to 53.3 ng g−1, being higher in plants exposed to 72 and 180 g ha−1 (approximate average of 41.6 and 52.6 ng g−1, respectively) than in plants exposed to 36 g ha−1 (approximate average of 13.2 ng g−1) of the herbicide, regardless of Si treatment. AMPA was not detected in tissues of plants from any of the treatments (Figure 2B). Plants exposed to 180 g ha−1 of glyphosate had higher levels of shikimic acid than plants not exposed to the herbicide, regardless of Si treatment, with an approximate average of 22.1 and 12.4 μg g−1, respectively (Figure 2C).
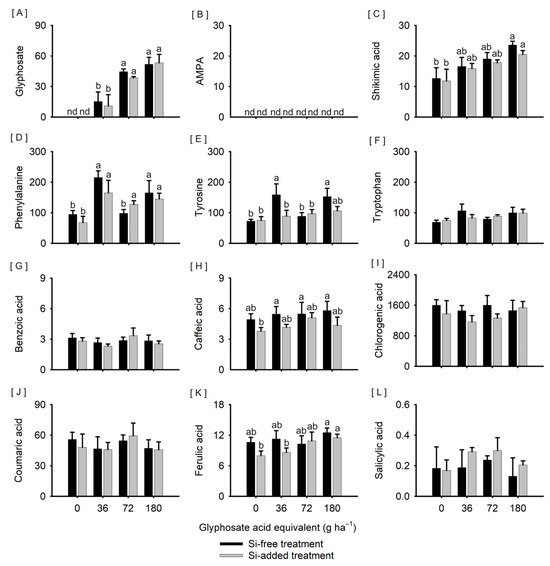
Figure 2.
Content of glyphosate; aminomethylphosphonic acid—AMPA ((A,B), ng g−1 dry mass); shikimic acid; the aromatic amino acids phenylalanine, tyrosine, and tryptophan ((C–F), μg g−1 dry mass); and the phenolic compounds benzoic, caffeic, chlorogenic, coumaric, ferulic, and salicylic acids ((G–L), μg g−1 dry mass) in leaf tissues of Sorghum bicolor submitted or not to foliar treatment with silicon and with further exposure or not to glyphosate sublethal doses (0, 36, 72, and 180 g ha−1), at 68 days after emergence. Vertical lines represent the standard deviation of the mean, and different lowercase letters represent significant differences by the Tukey HSD test (p < 0.05), except for shikimic acid by the Kruskal–Wallis test (p < 0.05). nd means non-detected. Graphs with no letters indicate no significant differences by the analysis of variance.
Plants exposed to 36 and 180 g ha−1 of glyphosate, regardless of Si treatment, and plants treated with Si and exposed to 72 g ha−1 of the herbicide showed higher phenylalanine levels (215.1 and 165.0 μg g−1; 165.2 and 145.2 μg g−1; and 127.2 μg g−1, respectively) than plants exposed to 72 g ha−1 of glyphosate without prior Si treatment (97.9 μg g−1) and plants not exposed to the herbicide, treated or not with Si (94.4 and 67.8 μg g−1, respectively) (Figure 2D). Furthermore, plants not treated with Si and exposed to 36 and 180 g ha−1 of glyphosate had higher tyrosine levels (158.7 and 152.8 μg g−1, respectively) than plants treated with Si and exposed to 36 g ha−1 of glyphosate (88.8 μg g−1) and, regardless of Si treatment, when not exposed (72.3 and 74.9 μg g−1, respectively) or exposed to 72 g ha−1 of glyphosate (88.4 and 97.1 μg g−1, respectively) (Figure 2E). The tryptophan content varied from 69.0 to 106.6 μg g−1, with no significant difference between the treatments tested (Figure 2F).
Plants not treated with Si and exposed to 36, 72, and 180 g ha−1 of glyphosate had higher caffeic acid levels (5.5, 5.5, and 5.8 μg g−1, respectively) than plants treated with Si and not exposed to glyphosate (3.8 μg g−1) (Figure 2H). Plants exposed to 180 g ha−1 of glyphosate, regardless of Si treatment (12.5 and 11.5 μg g−1, respectively, without Si and with Si), had higher levels of ferulic acid than plants not treated with Si exposed or not to 36 g ha−1 of glyphosate (8.6 and 8.0 μg g−1, respectively) (Figure 2L). The contents of benzoic, chlorogenic, coumaric, and salicylic acids varied from 2.7 to 3.4 μg g−1 (Figure 2G), 1161.5 to 1600.2 μg g−1 (Figure 2I), 45.7 to 59.4 μg g−1 (Figure 2J), and 0.13 to 0.29 μg g−1 (Figure 2L), respectively, with no significant difference between the treatments tested.
Plants treated with Si and exposed to 72 g ha−1 of glyphosate had higher Si content (555.2 mg kg−1) than plants not treated with Si, with (404.1 mg kg−1) or without (391.3 mg kg−1) exposure to the same dose of the herbicide (Figure 3A). The N, P, K, and S contents varied, respectively, from 6.0 to 8.2 g kg−1 (Figure 3B), 1.9 to 2.3 g kg−1 (Figure 3C), 19.8 to 24.2 g kg−1 (Figure 3D), and 0.6 to 0.8 g kg−1 (Figure 3G), with no significant difference between the treatments tested. On the other hand, plants exposed to 72 and 180 g ha−1 of glyphosate previously treated with Si had lower Ca content (2.1 and 2.0 g kg−1, respectively) than plants not exposed to the herbicide without prior treatment with Si (3.2 g kg−1) (Figure 3E); and plants exposed to 180 g ha−1 of glyphosate previously treated with Si had lower Mg content (1.3 g kg−1) than plants exposed to the same dose without prior treatment with Si (1.8 g kg−1) (Figure 3F). The Cu, Fe, and Zn contents varied, respectively, from 2.5 to 3.0 mg kg−1 (Figure 3I), 67.7 to 83.0 mg kg−1 (Figure 3J), and 14.1 to 17.5 mg kg−1 (Figure 3L), with no difference between the treatments tested. On the other hand, plants treated with Si had higher B content (9.3 mg kg−1) than plants not treated with Si (6.8 mg kg−1) when exposed to 72 g ha−1 of glyphosate (Figure 3H). Plants treated with Si, exposed or not to glyphosate, had lower Mn content (35.8 and 33.3 mg kg−1; 35.3 and 36.5 mg kg−1, respectively) than plants not treated with Si and without exposure to the herbicide (51.9 mg kg−1) (Figure 3K).
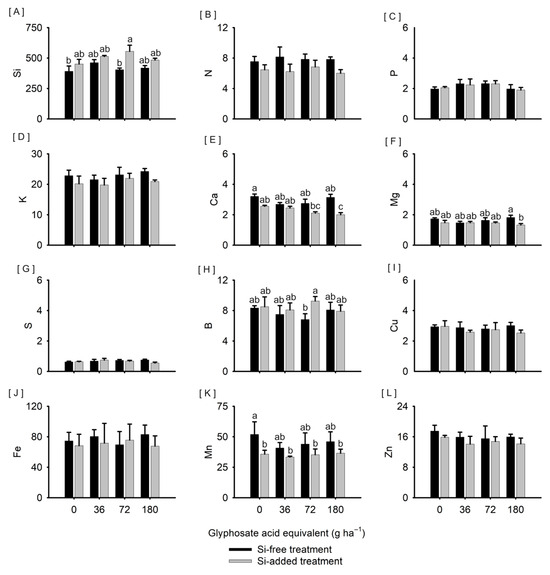
Figure 3.
Content of Si ((A), mg kg−1); macronutrients N, P, K, Ca, Mg, and S ((B–G), g kg−1); and micronutrients B, Cu, Fe, Mn, and Zn ((H–L), mg kg−1) in leaf tissues of Sorghum bicolor submitted or not to foliar treatment with silicon and with further exposure or not to glyphosate sublethal doses (0, 36, 72, and 180 g ha−1), at 68 days after emergence. Vertical lines represent the standard deviation of the mean, and different lowercase letters represent significant differences by the Kruskal–Wallis test (p < 0.05). Graphs with no letters indicate no significant differences by the analysis of variance.
Plants treated with Si and exposed to 72 g ha−1 of glyphosate had the highest Si accumulation (7.2 mg plant−1); except with glyphosate applied at 36 g ha−1, plants treated with Si had a higher accumulation of Si (Figure 4A). Plants not treated with Si and exposed to 36 g ha−1 of glyphosate had higher N accumulation (100.3 mg plant−1) than Si-treated plants exposed to the same herbicide dose (67.7 mg plant−1) similarly to that observed with 180 g ha−1 of glyphosate (Figure 4B). The accumulation of P (29.8 mg plant−1, Figure 4C), K (975.8 mg plant−1, Figure 4D), S (8.8 mg plant−1, Figure 4G), and B (119.5 μg plant−1, Figure 4H) was higher in plants treated with Si and exposed to 72 g ha−1 of glyphosate than plants not treated with Si at the same dose (23.6 mg plant−1, 705.3 mg plant−1, 7.3 mg plant−1, and 69.2 μg plant−1, respectively). Plants not treated with Si had a higher accumulation of K (36 g ha−1 of glyphosate, Figure 4D), Ca (0, 36, and 180 g ha−1 of glyphosate, Figure 4E), Mg (180 g ha−1 of glyphosate, Figure 4F), and Mn (0 and 36 g ha−1 of glyphosate, Figure 4K). On the other hand, the accumulation of Cu, Fe, and Zn varied, respectively, from 28.1 to 35.4 μg plant−1 (Figure 4I), 705.3 to 987.1 μg plant−1 (Figure 4J), and 153.1 to 195.3 μg plant−1 (Figure 3L), with no significant difference between the treatments tested.
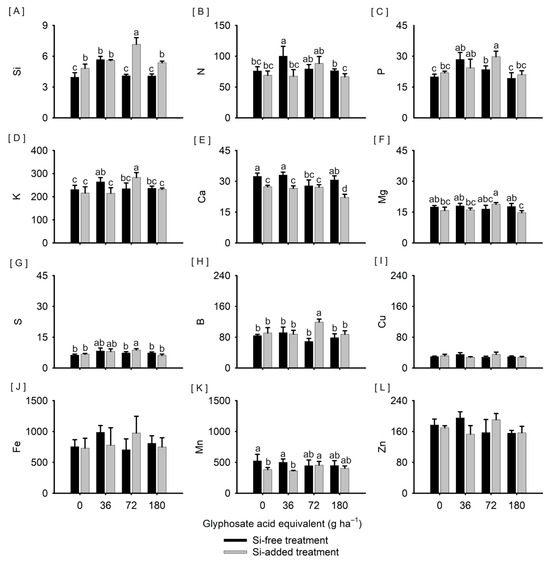
Figure 4.
The accumulation of Si ((A), mg plant−1); macronutrients N, P, K, Ca, Mg, and S ((B–G), mg plant−1); and micronutrients B, Cu, Fe, Mn, and Zn ((H–L), μg plant−1) in leaf tissues of Sorghum bicolor submitted or not to foliar treatment with silicon and with further exposure or not to glyphosate sublethal doses (0, 36, 72, and 180 g ha−1), at 68 days after emergence. Vertical lines represent the standard deviation of the mean, and different lowercase letters represent significant differences by the Kruskal–Wallis test (p < 0.05). Graphs with no letters indicate no significant differences by the analysis of variance.
4. Discussion
Physiological, nutritional, and metabolic responses of S. bicolor might be altered when sorghum plants are exposed to simulated drift of glyphosate. Furthermore, the role of a prior foliar treatment with Si in attenuating the potential toxic effects of glyphosate sublethal doses remains unclear from our results.
4.1. Si in Sorghum
Sorghum is a Si accumulating genus [44,45,46]. Si has been shown to be more abundant in the cuticle and certain epidermal cells of S. halepense [44]. Plants not treated with glyphosate and treated via foliar application with Si accumulated 23% more Si (4.8 mg plant−1) than plants not treated with silicon (3.9 mg plant−1) (Figure 4A), even growing in a substrate with 6.1 mg dm−3 of Si (soil analysis). Monosilicic acid (H4SiO4) is taken up through roots and distributed to the shoot [47,48]. Si can also be taken up by leaves [49,50]. High concentrations of Si induce defenses against plant stress [51,52]. For example, the formation of a double subcuticular layer in Si-treated sorghum plants [53,54] could reduce the rate of herbicide penetration into leaf tissues. This apparently did not happen, as the amount of glyphosate taken up was unaffected by Si treatment (Figure 2A). However, in the case of abiotic stress caused by glyphosate, sorghum response might be dependent on the herbicide dose, as observed on shoot dry mass (Figure 1D).
4.2. Glyphosate and Its Effects on Sorghum
Sorghum plants absorbed the applied glyphosate in a dose-dependent manner (Figure 2A) and probably did not degrade it because AMPA was not detected (Figure 2B). AMPA is the primary product of glyphosate degradation in plants [21,55,56]. However, grass species, such as maize, degrade little or none of the glyphosate that is taken up [21]. There are no previous reports of an assay for AMPA in glyphosate-treated S. bicolor; others have found low levels of AMPA accumulation in glyphosate-treated S. halepense [57].
Glyphosate blocked the shikimate pathway in treated plants in our study, with a consequent accumulation of shikimic acid (Figure 2C). Shikimic acid accumulation in leaf tissues is the most commonly used biomarker for glyphosate activity in plant tissues [21,43,58]. Marked shikimic acid accumulation results from the inhibition of EPSPS [21,24], the molecular target of glyphosate [59].
In our study, this pathway was weakly inhibited since sorghum plants did not die due to glyphosate exposure. There was a stimulatory effect (called hormesis) with a low glyphosate dose (e.g., using 72 g ha−1 of glyphosate on plant height treated with Si, Figure 1A). Hormesis with glyphosate was previously described in barley [60], coffee [61], common bean [43], eucalyptus [41], soybean [62], sugarcane [63], safflower [64], etc. Glyphosate-caused hormesis is reviewed extensively by Brito et al. [65]. This stimulatory effect of glyphosate at low doses varies, depending on the crop, genotype, growth stage, planting time, climatic conditions, etc. [41,43,61,66,67,68]. But the biochemical changes causing the plant to increase plant growth remain unknown.
The inhibition of EPSPS halts the production of aromatic amino acids [32,33,69]. Unexpectedly, absorbed glyphosate did not reduce and, in some cases, even increased the contents of aromatic amino acids (Figure 2D–F). It may occur due to a partial blocking of the shikimate pathway, as discussed above. Similar increases have been observed in rapeseed [70], sugarcane [71], peas [72], and common bean [43]. In addition, the increases in the free amino acid pool concentrations may be related to the rate of protein degradation and the release of these amino acids into the free amino acid pools [71]. Also, free amino acid pool sizes are affected by the use rates of specific amino acids in synthetic pathways, such as phenolic compound synthesis in the case of aromatic amino acids. Furthermore, the effect of glyphosate on amino acid pool sizes will vary with the plant organ evaluated and the time after exposure to glyphosate [69].
Aromatic amino acids are precursors of phenolic compounds through the shikimate pathway [32,43]. Salicylic, ferulic, benzoic, caffeic, coumaric, and chlorogenic acids are biosynthesized from phenylalanine, while coumaric and caffeic acids are also produced from tyrosine [33]. As discussed above, some plants exposed to glyphosate increased phenylalanine (Figure 2D) and tyrosine (Figure 2E) levels. A few plants exposed to glyphosate also increased caffeic (Figure 2H) and ferulic (Figure 2K) acids, with no changes in the contents of benzoic (Figure 2G), chlorogenic (Figure 2I), coumaric (Figure 2J), and salicylic (Figure 2L) acids. Concentrations of some phenolic compounds were found to be altered, while others were not changed after glyphosate application in Codonopsis lanceolata [73] and beans [43]. At growth-inhibiting doses of glyphosate, levels of secondary compounds derived from aromatic amino acids are almost always found to be reduced [21].
The slight increases in some phenolic compounds in this study might be related to the increases in aromatic amino acids found. Aromatic amino acids have important roles in plants, such as protein building blocks and synthesis of phenolic pigments, lignin, antioxidants, hormones, enzyme cofactors, and redox compounds [32,33]. The increased levels of these compounds could be related to increased plant growth [43], as we observed for sorghum plant height (Figure 1A). However, the plant response is dependent on the growing conditions, as observed for beans [43].
Controversial results on the effects of glyphosate on plant nutrition have been reported in common bean, coffee, maize, soybean, sugarcane, sunflower, etc. [63,74,75,76,77,78,79,80]. We observed reduction only in the content and accumulation of Ca when sorghum plants were exposed to glyphosate (Figure 3E and Figure 4E). In comparison to herbicide-free plants, glyphosate applied at 180 g ha−1 reduced the Ca content (23.1%) in sorghum plants treated with Si. In addition, in comparison to herbicide-free plants, glyphosate applied at 72 g ha−1 reduced the Ca accumulation (14.5%) in sorghum plants not treated with Si. Glyphosate chelates divalent metal cations [75,78,81], so this effect is not surprising [80].
Reduction in the Ca content was previously reported in plants treated with low doses of glyphosate [75,78,82,83,84]. Glyphosate can indirectly reduce root mineral absorption by disrupting the many processes requiring products of the shikimate pathway [85,86]. This herbicide can also interfere with the translocation of nutrients [74,87]. Glyphosate is transported by the phloem to metabolic sinks. In the phloem, it could directly interact with mineral nutrients and thereby influence plant nutrient balance [88]. Others have claimed effects of glyphosate on plant mineral nutrition, especially for divalent metal cations that glyphosate chelates (e.g., Mg, Cu, Fe, Mn, and Zn) (e.g., 74, 75, 78), but we did not observe them in this study, and many other studies have shown that such effects are due to indirect effects of glyphosate on plant functions due to the inhibition of the shikimate pathway, as reviewed by Duke [21].
The effect of glyphosate on plant nutrition was attributed to adverse effects of the herbicide on the physiology of plants [21,82]. At low doses of glyphosate, these changes might also be responsible for stimulatory effects, as we observed on the accumulation of Si, N, P, K, S, and B (Figure 4). Despite no stimulatory effect being observed on nutrient content (Figure 3), glyphosate applied at 36 g ha−1 increased the accumulation of Si (42.5%), N (31.5%), and P (42.5%) in sorghum plants not treated with Si, in comparison to the herbicide-free plants (Figure 4). Moreover, glyphosate applied at 72 g ha−1 increased the accumulation of Si (50.0%), P (36.1%), K (31.3%), S (29.7%), and B (31.4%) in sorghum plants treated with Si, while the accumulation of P also increased (17.9%) in sorghum plants not treated with Si, in comparison to the herbicide-free plants (Figure 4).
Increased nutrient accumulation with no change in nutrient content per unit of mass indicates that the plant has extracted more nutrients from the soil to maintain their concentration in plant tissues. Therefore, it confirms the first-time occurrence of hormesis with low doses of glyphosate in sorghum plant nutrition. Once glyphosate can interfere with the absorption and translocation of nutrients, as discussed above, we believe that the changes in the plant physiological state, derived from the exposure to this herbicide [25,82,85], are responsible for the stimulatory effect on the accumulation of nutrients. We believe that the activity of genes involved in nutrient uptake could also be involved in this glyphosate stimulatory effect. For example, once phosphate group transporters aid glyphosate absorption across the plasma membrane [89,90,91], low doses of this herbicide possibly indirectly induce the activity of genes involved in P absorption and, as a consequence, should increase the P content in the plant [63,92]. The findings that transgenic maize with a glyphosate-resistant EPSPS is 100-fold resistant to glyphosate [93] and numerous studies that find that glyphosate does not affect mineral content of glyphosate-resistant crops treated with much higher glyphosate applications than used in the present study, e.g., Reddy et al. [94], are strong support for the view that any effect of glyphosate on the mineral nutrition of plants is an indirect effect of its activity on EPSPS. Certainly, any effect of glyphosate on the nutrient composition of plants is complex [78], and the mechanisms that explain the occurrence of the glyphosate-dependent stimulatory effects remain unclear.
4.3. Combined Effects of Si and Glyphosate on Sorghum
Finally, an interesting, combined effect of the Si treatment and the exposure to glyphosate was observed in our study. Comparing the plants treated with Si and exposed to 72 g ha−1 of glyphosate to the herbicide-free plants not treated with silicon, we observed that the stimulatory effect of glyphosate sometimes was dependent on the Si treatment, e.g., the shoot dry mass (Figure 1D); the contents of phenylalanine (Figure 2D), Si (Figure 3A), and B (except for hormesis, Figure 3H); and the accumulation of Si (Figure 4A), P (Figure 4C), K (Figure 4D), S (Figure 4G), and B (Figure 4H). The positive effect of Si treatment on growth and health status of plants under biotic [95] and abiotic stresses is commonly accepted [96], including for herbicide-induced stresses [34,35,36].
Saudy and Mubarak [34] observed that the treatment of wheat plants with fenoxaprop-p-ethyl plus Si enhanced plant height and chlorophyll content as compared to fenoxaprop-p-ethyl alone and then concluded that use of Si has potential for protecting wheat from damage from fenoxaprop-p-ethyl. Soares et al. [36] indicated the potential of Si to reduce glyphosate-induced oxidative stress in tomato plants. Such effects have been associated with increases in antioxidants and abiotic stress tolerance [97,98,99], such as the improvement in the redox homeostasis to reduce glyphosate-caused toxicity to tomato plants [36]. However, Tripthi et al. [35] suggested that the Si-governed mitigation of butachlor toxicity in rice plants is related to changes in photosynthetic energy flux and plant anatomy, the lowering of oxidative stress, and the up-regulation of the Si channel and transporter genes, the ascorbate–glutathione cycle, and nutrient uptake. Despite these effects, the Si treatment sometimes might inhibit the stimulatory effect of the herbicide, as observed in plants exposed to 36 g ha−1 of glyphosate, e.g., the content of tyrosine (Figure 2E) and the accumulation of N (Figure 4B) and K (Figure 4D).
5. Conclusions
In summary, we have found that Si treatment before glyphosate treatment can sometimes enable glyphosate to stimulate certain plant growth and physiological parameters, depending on the glyphosate dose and the measured parameter. This phenomenon might occur under field conditions in which there is sufficient glyphosate drift to Si-treated sorghum crops. However, considering the many environmental and crop developmental parameters that influence hormesis, the prediction of such an effect in the field would be problematic.
Author Contributions
Conceptualization, L.B.d.C.; methodology, L.A.Y.S., L.B.d.C., C.A.C. and D.O.V.; formal analysis, L.B.d.C.; investigation, L.A.Y.S.; resources, L.B.d.C. and C.A.C.; data curation, L.B.d.C.; writing—original draft preparation, L.B.d.C., L.A.Y.S., S.O.D. and D.O.V.; writing—review and editing, L.B.d.C. and S.O.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
The authors would like to thank the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES) for the scholarship granted to the first author and the United States Department of Agriculture (USDA) for partial funds associated with the Cooperative Agreement 58-6060-1-001 Grant to the University of Mississippi. A special thanks to Renato de Mello Prado for technical support on silicon treatments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Khoddami, A.; Messina, V.; Komala, V.; Faranhaky, A.; Blanchard, C.L.; Roberts, T.H. Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 2023, 63, 1170–1186. [Google Scholar] [CrossRef] [PubMed]
- Proietti, I.; Frazzoli, C.; Mantovani, A. Exploiting nutritional value of staple foods in the world’s semi-arid areas: Risks, benefits, challenges and opportunities of sorghum. Healthcare 2015, 3, 172–193. [Google Scholar] [CrossRef]
- Kresovich, S.; Barbazuk, B.; Bedell, J.A.; Borrell, A.; Buell, C.R.; Burke, J. Toward sequencing the sorghum genome. A US National Science Foundation-sponsored Workshop Report. J. Plant Physiol. 2005, 138, 1898–1902. [Google Scholar]
- Chadalalavada, K.; Kumari, B.D.R.; Kumar, S. Sorghum mitigates climate variability and change on crop yield and quality. Planta 2021, 253, 113. [Google Scholar] [CrossRef] [PubMed]
- Pandian, B.A.; Sexton-Bowser, S.; Prasad, P.V.V.; Jugulam, M. Current status and prospects of herbicide-resistant grain sorghum (Sorghum bicolor). Pest. Manag. Sci. 2022, 78, 409–415. [Google Scholar] [CrossRef]
- USDA. World Agricultural Production; USDA—United States Department of Agriculture: Washington, DC, USA, 2024. Available online: https://apps.fas.usda.gov/psdonline/circulars/production.pdf (accessed on 13 July 2024).
- Bajwa, A.A.; Nawaz, A.; Farooq, M.; Chauhan, B.S.; Adkins, S. Herbicide program to control Parthenium hysterophorus in grain sorghum in an arid environment. Crops 2023, 3, 292–301. [Google Scholar] [CrossRef]
- Freitas, H.C.; Corrêa, F.R.; Silva, N.F.; Silva, W.S.; Cavalcante, D.; Ribeiro, F.R.E. Efeitos fitotécnicos do manejo de herbicidas aplicados em pré e pós emergência na cultura do sorgo. Braz. J. Sci. 2023, 24, 64–75. [Google Scholar] [CrossRef]
- Tkalich, Y.; Tsyliuryk, O.; Havryushenko, O.; Mytsyk, O.; Kozechko, V.; Rudakov, Y. Weed chemical control in grain sorghum at the steppe zone of Ukraine. Ecol. Quest. 2023, 34, 109–115. [Google Scholar] [CrossRef]
- Werle, R.; Tenhumberg, B.; Linquist, J.L. Modeling shattercane dynamics in herbicide-tolerant grain sorghum cropping systems. Ecol. Model. 2017, 343, 131–141. [Google Scholar] [CrossRef]
- Metlinga, G.V.; Vasilchenko, S.A. Efficacy of Ballerina herbicide on grain sorghum. Grain Econ. Rus. 2021, 73, 68–72. [Google Scholar] [CrossRef]
- ANDEF—Associação Nacional de Defesa Vegetal. Manual de Tecnologia de Aplicação de Produtos Fitossanitários, 1st ed.; Linea Creativa: Campinas, SP, Brazil, 2010; pp. 1–50. [Google Scholar]
- Cunha, J.P.A.R.; Teixeira, M.M.; Coury, J.R.; Ferreira, L.R. Evaluation of strategies to reduce pesticide spray drift. Planta Daninha 2003, 21, 325–332. [Google Scholar] [CrossRef]
- MAPA. Agrofit—Sistema de Agrotóxicos Fitossanitários; MAPA—Ministério da Agricultura, Pecuária e Abastecimento: Brasilia, DF, Brazil, 2003. Available online: https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 13 July 2024).
- Reis, L.A.C.; Carvalho, F.P.; França, A.C.; Francino, D.M.T.; Pinto, N.A.V.D.; Freitas, A.F. Leaf morphoanatomy and biochemical variation on coffee cultivars under drift simulation of glyphosate. Planta Daninha 2018, 36, e018143560. [Google Scholar] [CrossRef]
- Andrade, T.C.G.T.; Bacha, A.L.; Camargo, B.M.; Carvalho, L.B. Influence of phosphorus fertilization on the response of pinus genotypes to glyphosate subdoses. New For. 2022, 53, 143–160. [Google Scholar] [CrossRef]
- Nunes, R.T.; Albrecht, A.J.P.; Albrecht, L.P.; Lorenzety, J.B.; Danilussi, M.T.Y.; Silva, R.M.; Silva, A.F.M.; Barroso, A.A.M. Soybean injury caused by the application of subdoses of 2,4-D or dicamba, in simulated drift. J. Environ. Sci. Health Part B 2023, 58, 327–333. [Google Scholar] [CrossRef]
- Zampiroli, R.; Cunha, J.; Alvarenga, C.B. Simulated drift of dicamba and glyphosate on coffee crop. Plants 2023, 12, 3525. [Google Scholar] [CrossRef]
- Cederlund, H. Effects of spray drift of glyphosate on nontarget terrestrial plants: A critical review. Environ. Toxicol. Chem. 2017, 36, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Lin, M.; Mao, J.; Xing, B.; Li, Y.; Hou, R. Capability of phytoremediation of glyphosate in environment by Vulpia myuros. Ecotoxicol. Environ. Saf. 2023, 265, 115511. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate: Uses other than in glyphosate-resistant crops, mode of action, degradation in plants, and effects on non-target plants and agricultural microbes. Rev. Environ. Contam. Toxicol. 2021, 255, 1–65. [Google Scholar]
- Van Eerd, L.L.; Hoagland, R.E.; Zablotowicz, R.M.; Hall, J.C. Pesticide metabolism in plants and microorganisms. Weed Sci. 2003, 51, 472–495. [Google Scholar] [CrossRef]
- Reddy, K.N.; Rimando, A.M.; Duke, S.O. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J. Agric. Food Chem. 2004, 52, 5139–5143. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate degradation in glyphosate-resistant and –susceptible crops and weeds. J. Agric. Food Chem. 2011, 59, 5835–5841. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; Smedbol, A.; Chalifour, L.; Hénault-Ethier, M.; Labrecque, L.L.; Lucotte, M.; Juneau, P. Alteration of plant physiology by glyphosate and its by-product aminomethylphosphonic acid: An overview. J. Exp. Bot. 2014, 65, 4691–4703. [Google Scholar] [CrossRef]
- Guilherme, S.; Santos, M.; Gaivão, I.; Pacheco, M. DNA and chromosomal damage induced in fish (Anguilla anguilla L.) by aminomethylphosphonic acid (AMPA)—The major environmental breakdown product of glyphosate. Environ. Sci. Pollut. Res. 2014, 21, 8730–8739. [Google Scholar] [CrossRef] [PubMed]
- Yanniccari, M.; Tambussi, E.; Istilart, C.; Castro, A.M. Glyphosate effects on gas exchange and chlorophyll fluorescence responses of two Lolium perenne L. biotypes with differential herbicide sensitivity. Plant Physiol. Biochem. 2012, 57, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Zulet-González, A.; Barco-Antoñanzas, M.; Gil-Monreal, M.; Royuela, M.; Zabalza, A. Increased glyphosate-induced gene expression in the shikimate pathway is abolished in the presence of aromatic amino acids and mimicked by shikimate. Front. Plant Sci. 2020, 29, 459. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Zeier, J. New insights into the regulation of plant immunity by amino acid metabolic pathways. Plant Cell Environ. 2013, 36, 2085–2103. [Google Scholar] [CrossRef]
- Häusler, R.E.; Ludewig, F.; Krueger, S. Amino acids: A life between metabolism and signaling. Plant Sci. 2014, 229, 225–237. [Google Scholar] [CrossRef]
- Herrmann, K.M. The Shikimate pathway: Early steps in the biosynthesis of aromatic compounds. Plant Cell. 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Marchiosi, R.; Santos, W.D.; Constantin, R.P.; Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; Oliveira, D.M.; Foletto-Felipe, M.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Saudy, H.S.; Mubarak, M. Mitigating the detrimental impacts of nitrogen deficit and fenoxaprop-p-ethyl herbicide on wheat using silicon. Commun. Soil. Sci. Plant Anal. 2015, 46, 897–907. [Google Scholar] [CrossRef]
- Tripthi, D.K.; Varma, R.K.; Singh, S.; Sachan, M.; Guerriero, G.; Kushwaha, B.K.; Bhardwaj, S.; Ramawat, N.; Sharma, S.; Singh, V.P.; et al. Silicon tackles butachlor toxicity in rice seedlings by regulating anatomical characteristics, ascorbate-glutathione cycle, proline metabolism and levels of nutrients. Sci. Rep. 2020, 10, 14078. [Google Scholar] [CrossRef] [PubMed]
- Soares, C.; Nadais, P.; Sousa, B.; Pinto, E.; Ferreira, I.M.P.L.V.O.; Pereira, R.; Fidalgo, F. Silicon Improves the redox homeostasis to alleviate glyphosate toxicity in tomato plants—Are nanomaterials relevant? Antioxidants 2021, 10, 1320. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, I. Silício como atenuador de estresse causado por glifosato em mudas de laranjeira Valência. Master Thesis, São Paulo State University (UNESP), Jaboticabal, SP, Brazil, 2017. [Google Scholar]
- Prado, R.M. Benefits of Silicon in the Nutrition of Plants, 1st ed.; Springer: Cham, Switzerland, 2023; pp. 1–378. [Google Scholar]
- Olivera-Viciedo, D.; Aguilar, D.S.; Prado, R.M.; Calzada, K.P.; Hurtado, A.C.; Piccolo, M.C.; Soares, M.B.; Toledo, R.L.; Alves, G.R.; Ferreira, D.; et al. Silicon-mediated adjustments in C:N:P ratios for improved beetroot yield under ammonium-induced stress. Agronomy 2024, 14, 1104–1114. [Google Scholar] [CrossRef]
- Olivera-Viciedo, D.; Oliveira, K.S.; de Mello Prado, R.; Habermann, E.; Martínez, C.A.; de Moura Zanine, A. Silicon uptake and utilization on Panicum maximum grass modifies C:N:P stoichiometry under warming and soil water deficit. Soil. Tillage Res. 2024, 235, 105884. [Google Scholar] [CrossRef]
- Cerveira Júnior, W.R.; Costa, Y.K.S.; Carboni, C.A.; Duke, S.O.; Aguiar, P.A.C.; Carvalho, L.B. Growth, morphological, metabolic and photosynthetic responses of clones of eucalyptus to glyphosate. For. Ecol. Manag. 2020, 470–471, 118218. [Google Scholar] [CrossRef]
- Prado, R.M. Introduction to Plant Nutrition. In Mineral Nutrition of Tropical Plants, 1st ed.; Prado, R.M., Ed.; Springer: Cham, Switzerland, 2021; pp. 1–38. [Google Scholar]
- Bortolheiro, F.P.A.P.; Brunelli-Nascentes, M.C.; Carbonari, C.A.; Velini, E.D.; Silva, M.A. Low doses of glyphosate do not damage the secondary metabolism of common bean. J. Environ. Sci. Health Part B 2023, 58, 465–476. [Google Scholar] [CrossRef]
- McWhorter, G.G.; Paul, R.N. The involvement of cork-silica cell pairs in the production of wax filaments in Johnsongrass (Sorghum halepense) leaves. Weed Sci. 1989, 37, 458–470. [Google Scholar] [CrossRef]
- Deshmukh, R.; Sonah, H.; Belanger, R.R. New evidence defining the evolutionary path of aquaporins regulating silicon uptake in land plants. J. Exp. Bot. 2020, 71, 6775–6788. [Google Scholar] [CrossRef]
- Hurtado, A.C.; Chiconato, D.A.; Prado, R.M.; Souza Júnior, G.S.; Gratão, P.L.; Felisberto, G.; Viciedo, D.O.; Santos, D.M.M. Different methods of silicon application attenuate salt stress in sorghum and sunflower by modifying the antioxidative defense mechanism. Ecotoxicol. Environ. Saf. 2020, 203, 110964. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, A.; Tandon, A.; Sharma, V. Insights into the molecular mechanisms of uptake, phytohormone interactions and stress alleviation by silicon: A beneficial but non-essential nutrient for plants. Plant Growth Regul. 2023, 101, 1–13. [Google Scholar] [CrossRef]
- Wani, A.H.; Mir, S.H.; Kumar, S.; Malik, M.A.; Tyub, S.; Rashid, I. Silicon en route-from loam to leaf. Plant Growth Regul. 2023, 99, 465–476. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional role of silicon to activate resilient plant growth and to mitigate abiotic stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef] [PubMed]
- Naidu, S.; Pandey, J.; Mishra, L.C.; Chakraborty, A.; Roy, A.; Singh, I.K.; Singh, A. Silicon nanoparticles: Synthesis, uptake and their role in mitigation of biotic stress. Ecotoxicol. Environ. Saf. 2023, 255, 114783. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Yamaji, N.; Mitani-Ueno, N. Transport of silicon from roots to panicles in plants. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 377–385. [Google Scholar]
- Roohizadeh, G.; Majd, A.; Arbabian, S. The effect of sodium silicate and silica nanoparticles on seed germination and growth in the Vicia faba L. Trop. Plant Res. 2015, 2, 85–89. [Google Scholar]
- Lux, A.; Luxová, M.; Hattori, T.; Inanaga, S.; Sugimoto, Y. Silicification in sorghum (Sorghum bicolor) cultivars with different drought tolerance. Physiol. Plant. 2002, 115, 87–92. [Google Scholar] [CrossRef]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public. Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Tresnakova, N.; Stara, A.; Velisek, J. Effects of glyphosate and its metabolite AMPA on aquatic organisms. Appl. Sci. 2021, 11, 9004. [Google Scholar] [CrossRef]
- Vasquez-Garcia, J.G.; Palma-Bautista, C.; Rojano-Delgadao, A.M.; de Prado, R.; Menendez, J. The first case of glyphosate-resistance in johnsongrass (Sorghum halepense (L.) in Europe. Plants 2020, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Silva, L.; Araújo, T.O.; Nunes-Nesi, A.; Ribeiro, C.; Costa, A.C.; Silva, L.C. Evaluation of morphological and metabolic responses to glyphosate exposure in two neotropical plant species. Ecol. Indic. 2020, 113, 106246. [Google Scholar] [CrossRef]
- Amrhein, N.; Deus, B.; Gehrke, P.; Steinrucken, H.C. The site of the inhibition of the shikimate pathway by glyphosate. Plant Physiol. 1980, 66, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Cedergreen, N.; Felby, C.; Porter, J.R.; Streibig, J.C. Chemical stress can increase crop yield. Field Crops Res. 2009, 114, 54–57. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Alves, P.L.C.A.; Duke, S. Hormesis with glyphosate depends on coffee growth stage. An. Acad. Bras. Cienc. 2013, 85, 813–822. [Google Scholar] [CrossRef]
- Velini, E.D.; Alves, E.; Godoy, M.C.; Meschede, D.K.; Souza, R.T.; Duke, S.O. Glyphosate applied at low doses can stimulate plant growth. Pest. Manag. Sci. 2008, 64, 489–496. [Google Scholar] [CrossRef]
- Pincelli-Souza, R.P.; Bortolheiro, F.P.A.P.; Carbonari, C.A.; Velini, E.D.; Silva, M.A. Hormetic effect of glyphosate persists during the entire growth period and increases sugarcane yield. Pest. Manag. Sci. 2020, 76, 2388–2394. [Google Scholar] [CrossRef]
- Santos, J.C.C.; Silva, D.M.R.; Amorim, D.J.; Sab, M.P.V.; Almeida Silva, M. Glyphosate hormesis mitigates the effect of water deficit in safflower (Carthamus tinctorius L.). Pest. Manag. Sci. 2021, 77, 2029–2044. [Google Scholar] [CrossRef]
- Brito, P.F.S.; Tropaldi, L.; Carbonari, C.A.; Velini, E.D. Hormetic effects of glyphosate on plants. Pest. Manag. Sci. 2018, 74, 1064–1070. [Google Scholar] [CrossRef]
- Belz, R.G.; Cedergreen, N. Parthenin hormesis in plants depends on growth conditions. Environ. Exp. Bot. 2010, 69, 293–301. [Google Scholar] [CrossRef]
- Carvalho, L.B.; Duke, S.O.; Alves, P.L.C.A. Physiological responses of Eucalyptus x urograndis to glyphosate are dependent on the genotype. Sci. For. 2018, 46, 177–187. [Google Scholar] [CrossRef]
- Bortolheiro, F.P.A.P.; Brunelli-Nascentes, M.C.; Nascentes, R.F.; Silva, M.A. Glyphosate at low doses changes the physiology and increases the productivity of common bean as affected by sowing seasons. J. Environ. Sci. Health Part B 2022, 57, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Orcaray, L.; Igal, M.; Marino, D.; Zabalza, A.; Royuela, M. The possible role of quinate in the mode of action of glyphosate and acetolactate synthase inhibitors. Pest. Manag. Sci. 2010, 66, 262–269. [Google Scholar] [CrossRef]
- Petersen, I.L.; Hansen, H.C.B.; Ravn, H.W.; Sorensen, J.C.; Sorensen, H. Metabolic effects in rapeseed (Brassica napus L.) seedlings after root exposure to glyphosate. Pestic. Biochem. Phys. 2007, 89, 220–229. [Google Scholar] [CrossRef]
- Carbonari, C.A.; Gomes, G.L.G.C.; Velini, E.D.; Machado, R.F.; Simões, P.S.; Macedo, G.C. Glyphosate effects on sugarcane metabolism and growth. Am. J. Plant Sci. 2014, 5, 3585–3593. [Google Scholar] [CrossRef]
- Zabalza, A.; Orcaray, L.; Fernandez-Escalada, M.; Zulet-González, A.; Royuela, M. The Pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic. Biochem. Physiol. 2017, 141, 96–102. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Son, N.Y.; Kim, S.H.; Yu, C.Y.; Chung, I.M. Evaluating water deficit and glyphosate treatment on the accumulation of phenolic compounds and photosynthesis rate in transgenic Codonopsis lanceolata (Siebold & Zucc.) Trautv. over-expressing c-Tocopherol methyltransferase (c-Tmt) gene. Biotech 2017, 7, 167. [Google Scholar]
- Eker, S.; Ozturk, L.; Yazici, A.; Erenoglu, B.; Romheld, V.; Cakmak, I. Foliar-applied glyphosate substantially reduced uptake and transport of iron and manganese in sunflower (Helianthus annus L.) plants. J. Agric. Food Chem. 2006, 54, 10019–10025. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Yazici, A.; Tutus, Y.; Ozturk, L. Glyphosate reduced seed and leaf concentrations of calcium, manganese, magnesium, and iron in non-glyphosate resistant soybean. Eur. J. Agron. 2009, 31, 114–119. [Google Scholar] [CrossRef]
- Costa, F.R.; Rech, R.; Duke, S.O.; Carvalho, L.B. Lack of effects of glyphosate and glufosinate on growth, mineral content, and yield of glyphosate- and glufosinate-resistant maize. GM Crops Food 2018, 9, 189–198. [Google Scholar] [CrossRef]
- Duke, S.O.; Rimando, A.M.; Reddy, K.N.; Cizdziel, J.V.; Bellalui, N.; Shaw, D.R.; Williams, M.M.; Maul, J.E. Lack of transgene and glyphosate effects on yield and mineral and amino acid content of glyphosate-resistant soybean. Pest. Manag. Sci. 2018, 74, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Bortolheiro, F.P.A.P.; Silva, M.A. Low doses of glyphosate can affect the nutrient composition of common beans depending on the sowing season. Sci. Total Environ. 2021, 794, 148733. [Google Scholar] [CrossRef] [PubMed]
- Costa, Y.K.S.; Ribeiro, N.M.; Moura, G.C.P.; Oliveira, A.R.; Bianco, S.; Alcántara, R.; Carvalho, L.B. Effect of glyphosate and P on the growth and nutrition of Coffea arabica cultivars and on weed control. Sci. Rep. 2021, 11, 8095. [Google Scholar] [CrossRef] [PubMed]
- Bidóia, V.S.; Santos Neto, J.C.; Maciel, C.D.G.; Tropaldi, L.; Carbonari, C.A.; Duke, S.O.; Carvalho, L.B. Lack of significant effects of glyphosate on glyphosate-resistant maize in different field locations. Agronomy 2023, 13, 1071. [Google Scholar] [CrossRef]
- Duke, S.O. Glyphosate. In Herbicides: Chemistry, Degradation, and Mode of Action, 1st ed.; Kearney, P.C., Kaufman, D.D., Eds.; Dekker: New York, NY, USA, 1988; Volume 3, pp. 1–70. [Google Scholar]
- Duke, S.O.; Wauchope, R.D.; Hoagland, R.E.; Wills, G.D. Influence of glyphosate on uptake and translocation of calcium ion in soybean seedlings. Weed Res. 1983, 23, 133–139. [Google Scholar] [CrossRef]
- Schoenherr, J.; Schreiber, L. Interactions of calcium ions with weakly acidic active ingredients slow cuticular penetration: A case study with glyphosate. J. Agric. Food Chem. 2004, 52, 6546–6551. [Google Scholar] [CrossRef]
- Mueller, T.C.; Main, C.L.; Thompson, A.; Steckel, L.E. Comparison of glyphosate salts (isopropylamine, diammonium, and potassium) and calcium and magnesium concentrations on the control of various weeds. Weed Technol. 2006, 20, 164–171. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499–510. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J.; Koskinen, W.C.; Moorman, T.B.; Chaney, R.L.; Hammerschmidt, R. Glyphosate effects on mineral nutrition, crop rhizosphere microbiota, and plant disease in glyphosate-resistant crops. J. Agric. Food Chem. 2012, 60, 10375–10397. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, A.; Eker, S.; Gokmen, O.; Romheld, V.; Cakmak, I. Glyphosate inhibition of ferric reductase activity in iron deficient sunflower roots. New Phytol. 2008, 177, 899–906. [Google Scholar] [CrossRef]
- Harris, W.D.; Sammons, R.D.; Grabiak, R.C.; Mehrsheikh, A.; Bleeke, M.S. Computer simulation of the interactions of glyphosate with metal ions in phloem. J. Agric. Food Chem. 2012, 60, 6077–6087. [Google Scholar] [CrossRef] [PubMed]
- Pipke, R.; Schulz, A.; Amrhein, N. Uptake of glyphosate by an Arthrobacter sp. Appl. Environ. Microbiol. 1987, 53, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Hetherington, P.R.; Marshall, G.; Kirkwood, R.C.; Warner, J.M. Absorption and efflux of glyphosate by cell suspensions. J. Exp. Bot. 1998, 29, 527–533. [Google Scholar] [CrossRef]
- Rabello, W.S.; Monnerat, P.H.; Campanharo, M.; Espindula, M.C.; Ribeiro, G. Growth and phosporus absorption by common bean ‘Xodó’ genotype under effect of glyphosate reduced rates. Rev. Bras. Herbic. 2012, 11, 204–212. [Google Scholar]
- Perim, L.; Prando, M.B.; Rosolem, C.A. Cinética de absorção de fósforo em soja transgênica após aplicação de glyphosate. Rev. Bras. Herbic. 2011, 10, 143–150. [Google Scholar] [CrossRef]
- Hetherington, P.R.; Reynolds, T.L.; Marshall, G.; Kirkwood, R.C. The absorption, translocation and distribution of the herbicide glyphosate in maize expressing the CP-4 transgene. J. Exp. Bot. 1999, 50, 1567–1576. [Google Scholar] [CrossRef]
- Reddy, K.N.; Cizdziel, J.V.; Williams, M.M.; Maul, J.E.; Rimando, A.M.; Duke, S.O. Glyphosate resistance technology has minimal effect on maize mineral nutrition and yield. J. Agric. Food Chem. 2018, 66, 10139–10146. [Google Scholar] [CrossRef]
- Ma, J.F. Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil. Sci. Plant Nutr. 2004, 50, 11–18. [Google Scholar] [CrossRef]
- Ranganathan, S.; Suvarchala, V.; Rajesh, Y.B.R.D.; Prasad, M.S.; Padmakumari, A.P.; Voleti, S.R. Effects of silicon sources on its deposition, chlorophyll content, and disease and pest resistance in rice. Biol. Plant. 2006, 50, 713–716. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q.I.N.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef]
- Gong, H.J.; Chen, K.M.; Chen, G.C.; Wang, S.M.; Zhang, C.L. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Liang, Y.; Si, J.; Romheld, V. Silicon uptake and transport is an active process in Cucumis sativus. New Phytol. 2005, 167, 797–804. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).