Biomass of Eichhornia crassipes as an Alternative Substrate for the Formation of Lettuce Seedlings
Abstract
1. Introduction
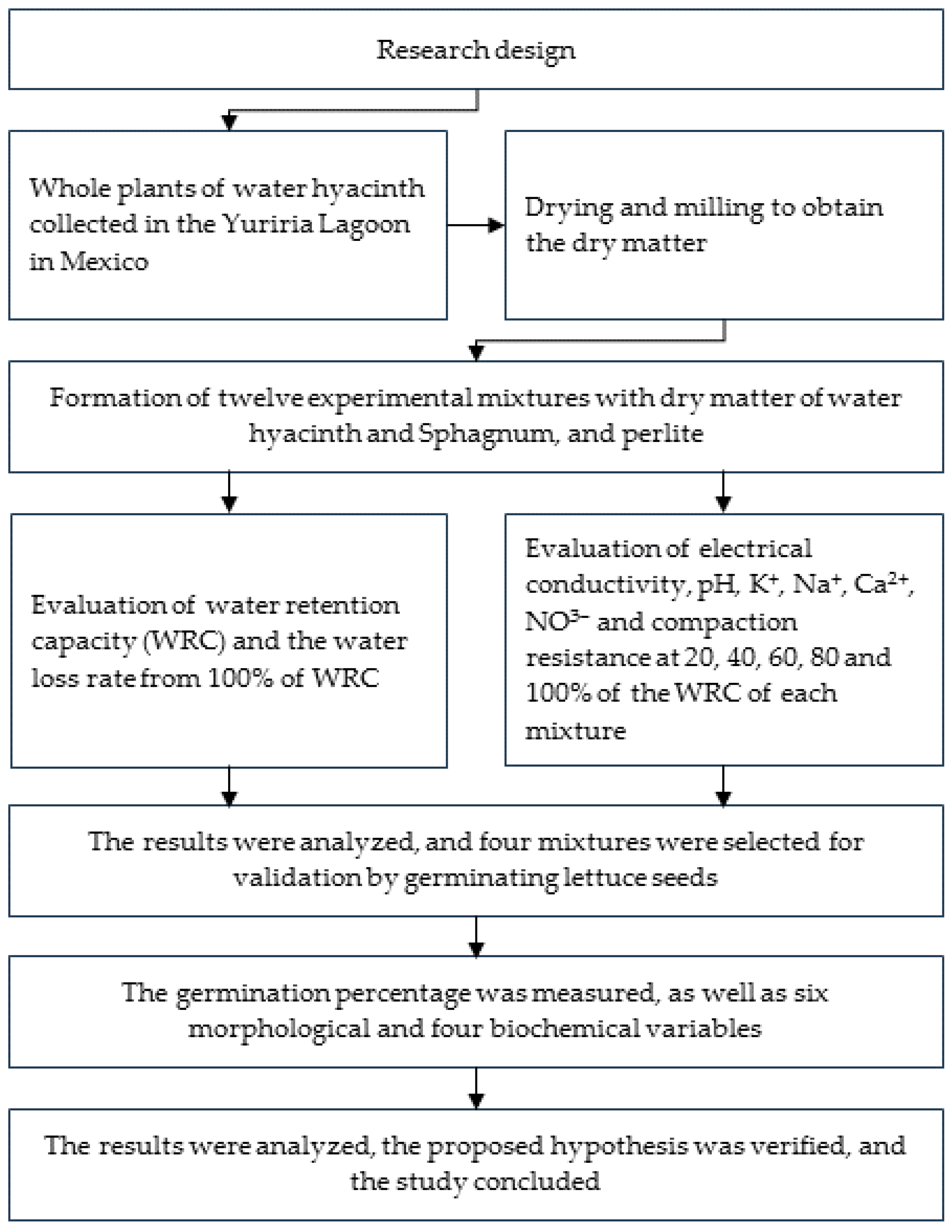
2. Materials and Methods
2.1. Plant Material and Experimental Mixtures
2.2. Variables Evaluated in the Mixtures
2.3. Lettuce Seedling Formation and Variables Evaluated
2.4. Data Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macwan, J.; Pandya, D.; Pandya, H.; Mankad, A. Review on soilless method of cultivation: Hydroponics. Int. J. Recent Sci. Res. 2020, 11, 37122–37127. [Google Scholar]
- Schuch, M.W.; Tomaz, Z.F.P.; Casarin, J.V.; Moreira, R.M.; Silva, J.B.D. Advances in vegetative propagation of Olive tree. Rev. Bras. Frutic. 2019, 41, e003. [Google Scholar] [CrossRef]
- Sharma, N.; Acharya, S.; Kumar, K.; Singh, N.; Chaurasia, O.P. Hydroponics as an advanced technique for vegetable production: An overview. J. Soil Water Conserv. 2018, 17, 364–371. [Google Scholar] [CrossRef]
- Eid, A.R.; Negm, A. Improving agricultural crop yield and water productivity via sustainable and engineering techniques. In Conventional Water Resources and Agriculture in Egypt; The Handbook of Environmental Chemistry; Negm, A.M., Ed.; Springer: Cham, Switzerland, 2018; Volume 74. [Google Scholar]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic solutions for soilless production systems: Issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Szeląg-Sikora, A.; Sikora, J.; Niemiec, M.; Gródek-Szostak, Z.; Kapusta-Duch, J.; Kuboń, M.; Komorowska, M.; Karcz, J. Impact of integrated and conventional plant production on selected soil parameters in carrot production. Sustainability 2019, 11, 5612. [Google Scholar] [CrossRef]
- Chiomento, J.L.T.; Frizon, P.; Costa, R.C.; Trentin, N.S.; Nardi, F.S.; Calvete, E.O. Water retention of substrates potentiates the quality of lettuce seedlings. Adv. Hortic. Sci. 2019, 33, 197–204. [Google Scholar]
- Bello, A.S.; Ahmed, T.A.; Ben-Hamadou, R. Hydroponics: Innovative option for growing crops in extreme environments—The case of the Arabian Peninsula (A Review). J. Agric. Res. 2019, 4, 000235. [Google Scholar]
- Sela Saldinger, S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae 2023, 9, 51. [Google Scholar] [CrossRef]
- Okpara, D.A.; Kharlamova, M.; Grachev, V. Proliferation of household waste irregular dumpsites in Niger Delta region (Nigeria): Unsustainable public health monitoring and future restitution. Sustain. Environ. Res. 2021, 31, 4. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Orden, L.; Postemsky, P.; Iocoli, G.; Mockel, G.; Marinangeli, P. Agrowaste compost as a component of substrates for ornamental plants. Hortic. Argent. 2022, 41, 7. [Google Scholar]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A Review on hydroponics and the technologies associated for medium-and small-scale operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Castoldi, G.; Freiberger, M.B.; Pivetta, L.A.; Pivetta, L.G.; Echer, M.D.M. Alternative substrates in the production of lettuce seedlings and their productivity in the field. Rev. Ciência Agronômica 2014, 45, 299–304. [Google Scholar] [CrossRef]
- Zuffo, A.M.; González Aguilera, J.; Lima, R.E.; Zaratin Alves, C. Substrates for the production of lettuce seedlings. Eur. J. Hortic. Sci. 2020, 85, 372–379. [Google Scholar] [CrossRef]
- Ayanda, O.I.; Ajayi, T.; Asuwaju, F.P. Eichhornia crassipes (Mart.) Solms: Uses, challenges, threats, and prospects. Sci. World J. 2020, 2020, 3452172. [Google Scholar] [CrossRef] [PubMed]
- Dechassa, N. Origin, distribution, impact and management of water hyacinth (Eichhornia crassipes (Martius) Solm): A review. J. Environ. Earth Sci. 2020, 10, 13–18. [Google Scholar]
- Adelodun, A.A. Appraising the control and benefits of water hyacinth (Eichhornia crassipes [Mart.] Solms). Focus 2022, 89, 89. [Google Scholar]
- Gaurav, G.K.; Mehmood, T.; Cheng, L.; Klemeš, J.J.; Shrivastava, D.K. Water hyacinth as a biomass: A review. J. Clean. Prod. 2020, 277, 122214. [Google Scholar] [CrossRef]
- Datta, A.; Maharaj, S.; Prabhu, G.N.; Bhowmik, D.; Marino, A.; Akbari, V.; Rupavatharam, S.; Sujeetha, J.A.R.P.; Anantrao, G.G.; Poduvattil, V.K.; et al. Monitoring the spread of water hyacinth (Pontederia crassipes): Challenges and future developments. Front. Ecol. Evol. 2021, 9, 631338. [Google Scholar] [CrossRef]
- El Mekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and agricultural wastes as substrates for bioelectrochemical system (BES): The synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Sahmat, S.S.; Rafii, M.Y.; Oladosu, Y.; Jusoh, M.; Hakiman, M.; Mohidin, H. A systematic review of the potential of a dynamic hydrogel as a substrate for sustainable agriculture. Horticulturae 2022, 8, 1026. [Google Scholar] [CrossRef]
- SIAP—Servicio de Información Agroalimentaria y Pesquera, Secretaria, Secretaría de Agricultura y Desarrollo Rural, Gobierno de México. 2024. Available online: https://www.gob.mx/siap (accessed on 6 July 2024).
- Bates, L.S.; Waldren RP y Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dudek, G.; Strzelewicz, A.; Krasowska, M.; Rybak, A.; Turczyn, R. A spectrophotometric method for plant pigments determination and herbs classification. Chem. Pap. 2014, 68, 579–583. [Google Scholar] [CrossRef]
- Higuti, J.; Martens, K. Invasive South American floating plants are a successful substrate for native Central African pleuston. Biol. Invasions 2016, 18, 1191–1201. [Google Scholar] [CrossRef]
- Feng, W.; Xiao, K.; Zhou, W.; Zhu, D.; Zhou, Y.; Yuan, Y.; Xiao, N.; Wan, X.; Hua, Y.; Zhao, J. Analysis of utilization technologies for Eichhornia crassipes biomass harvested after restoration of wastewater. Bioresour. Technol. 2017, 223, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Taskila, S.; Särkelä, R.; Tanskanen, J. Valuable applications for peat moss. Biomass Convers. Biorefinery 2016, 6, 115–126. [Google Scholar] [CrossRef]
- Chimona, C.; Rhizopoulou, S. Water economy via oriented root elongation of mediterranean plants: Physiological parameters. World J. Res. Rev. 2018, 6, 262671. [Google Scholar] [CrossRef]
- Gebresilasie, K.G.; Berhe, G.G.; Tesfay, A.H.; Gebre, S.E. Assessment of some physicochemical parameters and heavy metals in hand-dug well water samples of Kafta Humera Woreda, Tigray, Ethiopia. Int. J. Anal. Chem. 2021, 2021, 8867507. [Google Scholar] [CrossRef]
- Fan, R.; Luo, J.; Yan, S.; Wang, T.; Liu, L.; Gao, Y.; Zhang, Z. Use of Water Hyacinth (Eichhornia crassipes) Compost as a Peat Substitute in Soilless Growth Media. Compos. Sci. Util. 2015, 23, 237–247. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Neina, D. The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. 2019, 2019, 5794869. [Google Scholar] [CrossRef]
- Gillespie, D.P.; Kubota, C.; Miller, S.A. Effects of low pH of hydroponic nutrient solution on plant growth, nutrient uptake, and root rot disease incidence of basil (Ocimum basilicum L.). HortScience 2020, 55, 1251–1258. [Google Scholar] [CrossRef]
- Abawari, R.A.; Tuji, F.A.; Yadete, D.M. Phosphate solubilizing biofertilizers and their role in bioavailable P nutrient: An overview. Int. J. Appl. Agric. Sci. 2020, 6, 162. [Google Scholar]
- Tyagi, A.; Sharma, S.; Srivastava, H.; Singh, A.; Kaila, T.; Ali, S.; Gaikwad, A.B.; Singh, N.K.; Gaikwad, K. Transcriptome profiling of two contrasting pigeon pea (Cajanus cajan) genotypes in response to waterlogging stress. Front. Genet. 2023, 13, 1048476. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Sun, Q.; Xia, M.; Wen, Z.; Yao, Z. The resource utilization of water hyacinth (Eichhornia crassipes [Mart.] Solms) and its challenges. Resources 2018, 7, 46. [Google Scholar] [CrossRef]
- Kaleem Abbasi, M.; Mahmood Tahir, M.; Sabir, N.; Khurshid, M. Impact of the addition of different plant residues on nitrogen mineralization–immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth 2015, 6, 197–205. [Google Scholar] [CrossRef]
- Markoska, V.; Spalevic, V.; Gulaboski, R. A research on the influence of porosity on perlite substrate and its interaction on porosity of two types of soil and peat substrate. Poljopr. Sumar. 2018, 64, 15–29. [Google Scholar] [CrossRef]
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The ability of conservation agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Anjos, M.L.D.; Bória-Fernandez, J.A.; Peixoto-Henares, M.N. Use of Eichhornia crassipes dry matter as organic substrate for germination and initial growth of corn. Ecosistemas Recur. Agropecu. 2018, 5, 97–102. [Google Scholar] [CrossRef]
- Yan, S.H.; Song, W.; Guo, J.Y. Advances in management and utilization of invasive water hyacinth (Eichhornia crassipes) in aquatic ecosystems—A review. Crit. Rev. Biotechnol. 2017, 37, 218–228. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Oliwa, J.; Kornas, A.; Skoczowski, A. A low ratio of red/far-red in the light spectrum accelerates senescence in nest leaves of Platycerium bifurcatum. Acta Biol. Cracoviensia Ser. Bot. 2017, 59, 17–30. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and opportunities of light-emitting diode (Led) as key to modulate antioxidant compounds in plants. A review. Antioxidants 2020, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Ibny, F.Y.; Jaiswal, S.K.; Mohammed, M.; Dakora, F.D. Symbiotic effectiveness and ecologically adaptive traits of native rhizobial symbionts of Bambara groundnut (Vigna subterranea L. Verdc.) in Africa and their relationship with phylogeny. Sci. Rep. 2019, 9, 12666. [Google Scholar] [CrossRef]
- Ban, Q.; Wang, X.; Pan, C.; Wang, Y.; Kong, L.; Jiang, H.; Xu, Y.; Wang, W.; Pan, Y.; Li, Y.; et al. Comparative analysis of the response and gene regulation in cold resistant and susceptible tea plants. PLoS ONE 2017, 12, e0188514. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Ni, R.; Yang, S.; Pu, Y.; Qian, M.; Yang, Y.; Yang, Y. Functional characterization of the Stipa purpurea P5CS gene under drought stress conditions. Int. J. Mol. Sci. 2021, 22, 9599. [Google Scholar] [CrossRef]
- Hussain, İ.; Akhtar, S.; Ashraf, M.A.; Rasheed, R.; Siddiqi, E.H.; Ibrahim, M. Response of maize seedlings to cadmium application after different time intervals. ISRN Agron. 2013, 2013, 169610. [Google Scholar] [CrossRef]

| WRC | Mixture | EC ** | pH * | K+ ** | Na+ ** | Ca2+ ** | NO3− ** | CR ** |
|---|---|---|---|---|---|---|---|---|
| 20% | SP100 | 8.6 yz | 6.3 b | 0.6 f | 2.2 m | 1.0 c | 14.2 a:c | 225.0 f:k |
| SP85PE15 | 9.4 x:z | 6.1 b | 0.4 f | 4.2 lm | 1.2 c | 11.8 a:f | 339.0 c:k | |
| SP70PE30 | 3.6 z | 6.3 b | 0.4 f | 7.0 lm | 1.8 c | 7.0 d:f | 479.0 c:j | |
| SP55PE45 | 2.8 z | 6.2 b | 0.1 f | 4.0 lm | 1.0 c | 7.6 c:f | 610.6 bc | |
| SP50WH50 | 18.8 u:y | 5.7 bc | 8.6 f | 3.0 m | 58.0 a | 8.6 b:f | 207.8 f:k | |
| SP42.5WH42.5PE15 | 31.4 r:u | 6.1 b | 5.2 f | 7.0 lm | 1.0 c | 15.6 ab | 144.2 k | |
| SP35WH35PE30 | 26 s:w | 6.0 b | 5.2 f | 7.8 k:m | 1.2 c | 15.8 a | 214.4 f:k | |
| SP27.5WH27.5PE45 | 23.6 s:x | 6.9 b | 18.0 f | 59.0 cd | 4.2 c | 13.6 a:e | 250.2 d:k | |
| WH100 | 23.6 s:x | 7.4 a | 45.4 f | 115.2 a | 5.8 c | 13.6 a:e | 191.4 h:k | |
| WH85PE15 | 37.8 q:s | 6.2 b | 51.2 f | 86.4 b | 3.4 c | 13.8 a:d | 192.8 h:k | |
| WH70PE30 | 27.6 s:v | 7.0 ab | 26.4 f | 12.4 j:m | 1.0 c | 13.4 a:e | 166.0 j:k | |
| WH55PE45 | 43.8 o:r | 7.2 a | 24.0 f | 51.8 c:e | 1.8 c | 13.4 a:e | 201.2 f:k | |
| 40% | SP100 | 20.4 t:y | 6.4 b | 0.1 f | 1.0 m | 1.2 c | 8.4 c:f | 192.6 h:k |
| SP85PE15 | 19.6 u:y | 6.7 b | 0.2 f | 1.8 m | 1.0 c | 10.6 a:f | 267.2 d:k | |
| SP70PE30 | 12.6 w:z | 7.0 ab | 0.2 f | 2.6 m | 1.8 c | 6.8 d:f | 390.8 c:k | |
| SP55PE45 | 13.6 v:z | 6.0 b | 0.1 f | 3.4 m | 1.2 c | 7.0 d:f | 381.4 c:k | |
| SP50WH50 | 44.2 o:r | 5.7 bc | 10.0 f | 1.8 m | 49.4 a | 6.8 d:f | 200.4 g:k | |
| SP42.5WH42.5PE15 | 49.4 n:q | 5.8 bc | 3.8 f | 8.0 k:m | 1.0 c | 10.6 a:f | 259.8 d:k | |
| SP35WH35PE30 | 52.4 n:q | 6.0 b | 4.0 f | 6.2 l:m | 1.2 c | 11.6 a:f | 248.2 d:k | |
| SP27.5WH27.5PE45 | 38.2 p:s | 6.8 b | 15.8 f | 41.2 d:g | 5.0 c | 8.6 b:f | 172.2 i:k | |
| WH100 | 45.0 o:r | 7.3 a | 21.2 f | 48.2 de | 4.0 c | 10.6 a:f | 170.4 i:k | |
| WH85PE15 | 56.8 j:o | 6.2 b | 23.2 f | 52.6 c:e | 2.4 c | 9.2 a:f | 155.6 k | |
| WH70PE30 | 57.4 i:o | 7.2 a | 25.0 f | 51.4 c:e | 2.0 c | 12.0 a:f | 177.6 h:k | |
| WH55PE45 | 54.8 k:o | 7.3 a | 16.6 f | 34.0 e:j | 2.0 c | 10.2 a:f | 155.0 k | |
| 60% | SP100 | 49.8 n:q | 6.6 b | 0.1 f | 1.8 m | 1.2 c | 8.4 c:f | 215.0 f:k |
| SP85PE15 | 33.4 r:u | 7.5 a | 0.1 f | 1.0 m | 0.8 c | 8.0 c:f | 387.4 c:k | |
| SP70PE30 | 30.6 r:u | 6.9 b | 0.2 f | 6.6 lm | 1.4 c | 7.6 c:f | 352.6 c:k | |
| SP55PE45 | 34.6 r:t | 6.3 b | 0.1 f | 6.0 lm | 1.0 c | 7.8 c:f | 520.0 c:g | |
| SP50WH50 | 62.6 f:n | 5.9 bc | 7.2 f | 1.2 m | 26.8 b | 6.2 f | 243.2 d:k | |
| SP42.5WH42.5PE15 | 63.2 f:n | 5.5 c | 2.8 f | 6.0 lm | 1.2 c | 8.6 b:f | 224.8 f:k | |
| SP35WH35PE30 | 55.6 j:o | 5.8 bc | 2.2 f | 7.4 lm | 1.0 c | 10.0 a:f | 253.4 d:k | |
| SP27.5WH27.5PE45 | 58.0 h:o | 6.8 b | 14.8 f | 32.0 e:k | 4.8 c | 8.8 a:f | 207.4 f:k | |
| WH100 | 57.4 i:o | 7.5 a | 25.6 f | 73.4 bc | 4.6 c | 11.4 a:f | 188.0 h:k | |
| WH85PE15 | 70.0 b:j | 5.7 bc | 26.4 f | 42.4 d:f | 2.8 c | 11.0 a:f | 173.0 i:k | |
| WH70PE30 | 67.6 d:l | 7.5 a | 17.2 f | 37.20 d:i | 1.8 c | 10.2 a:f | 488.6 c:i | |
| WH55PE45 | 67.2 e:m | 7.2 a | 10.8 f | 20.40 f:m | 1.4 c | 8.0 c:f | 207.8 f:k | |
| 80% | SP100 | 68.8 c:l | 6.3 b | 0.1 f | 1.2 m | 1.0 c | 10.0 a:f | 396.0 c:k |
| SP85PE15 | 52.8 m:p | 6.2 b | 0.2 f | 1.8 m | 0.8 c | 8.2 c:f | 385.8 c:k | |
| SP70PE30 | 54.2 l:o | 6.3 b | 0.1 f | 3.4 m | 1.2 c | 8.8 a:f | 547.4 b:e | |
| SP55PE45 | 60.0 g:n | 5.8 bc | 1.0 f | 4.4 lm | 1.4 c | 8.2 c:f | 562.8 b:d | |
| SP50WH50 | 70.2 b:j | 5.6 bc | 6.6 f | 1.4 m | 32.6 b | 7.4 c:f | 283.0 d:k | |
| SP42.5WH42.5PE15 | 73.4 a:g | 5.5 c | 2.4 f | 5.8 lm | 1.0 c | 8.0 c:f | 253.8 d:k | |
| SP35WH35PE30 | 61 f:n | 5.7 bc | 1.8 f | 5.8 lm | 1.0 c | 9.4 a:f | 496.4 c:h | |
| SP27.5WH27.5PE45 | 73.4 a:g | 6.9 b | 9.2 f | 17.60 g:m | 4.0 c | 8.0 c:f | 229.6 e:k | |
| WH100 | 71.8 a:i | 7.3 a | 19.4 f | 38.8 d:h | 4.8 c | 9.6 a:f | 232.6 e:k | |
| WH85PE15 | 78.8 a:e | 5.9 bc | 11.0 f | 17.0 g:m | 1.4 c | 7.4 c:f | 211.0 f:k | |
| WH70PE30 | 70.2 b:j | 7.3 a | 13.2 f | 28.2 e:l | 1.6 c | 10.0 a:f | 499.2 c:h | |
| WH55PE45 | 78.6 a:e | 7.3 a | 8.2 f | 16.0 h:m | 1.2 c | 6.6 ef | 229.0 e:k | |
| 100% | SP100 | 84.2 ab | 7.1 ab | 374.4 c:e | 3.8 lm | 1.4 c | 11.0 a:f | 374.4 c:k |
| SP85PE15 | 75.2 a:f | 6.0 b | 847.4 ab | 3.6 m | 1.2 c | 8.6 b:f | 847.4 ab | |
| SP70PE30 | 69.0 c:k | 7.3 a | 617.4 bc | 4.0 lm | 1.6 c | 7.8 c:f | 617.4 bc | |
| SP55PE45 | 63.8 f:n | 6.5 b | 985.8 a | 5.0 lm | 1.0 c | 8.0 c:f | 985.8 a | |
| SP50WH50 | 72.2 a:h | 5.7 bc | 394.2 c:e | 1.6 m | 29.2 b | 7.8 c:f | 394.2 c:k | |
| SP42.5WH42.5PE15 | 79.6 a:e | 5.5 c | 464.4 c:e | 5.2 lm | 1.0 c | 8.8 a:f | 464.4 c:k | |
| SP35WH35PE30 | 68 d:l | 5.9 bc | 522.2 c:d | 6.2 lm | 1.0 c | 9.2 a:f | 522.2 c:f | |
| SP27.5WH27.5PE45 | 86.0 a | 6.8 b | 399.0 c:e | 14.0 i:m | 4.0 c | 7.8 c:f | 399.0c:k | |
| WH100 | 82.2 a:d | 7.2 a | 328.6 de | 32.6 e:j | 3.6 c | 10.4 a:f | 328.6 c:k | |
| WH85PE15 | 83.2 a:c | 6.0 b | 325.6 de | 17.20 g:m | 1.6 c | 8.2 c:f | 325.6 c:k | |
| WH70PE30 | 72 a:i | 7.4 a | 373.8 c:e | 23.6 f:m | 1.2 c | 8.4 c:f | 373.8 c:k | |
| WH55PE45 | 83.4 a:c | 7.0 ab | 249.4 e:f | 22.2 f:m | 1.6 c | 8.0 c:f | 249.4 d:k | |
| SLAB | p < 0.01 | p > 0.05 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 |
| Variable | WH100 | WH70PE30 | SP35WH35PE30 | SP70PE30 | SP100 |
|---|---|---|---|---|---|
| GE ** | 90.2 ab | 85.2 b | 84.8 b | 89.4 ab | 92.4 a |
| RL ** | 2.0 c | 2.6 b | 2.4 b | 2.6 b | 3.0 a |
| PL ** | 1.5 c | 1.6 c | 2.0 b | 2.2 ab | 2.5 a |
| CA ** | 56.0 d | 50.1 e | 65.5 c | 70.5 b | 75.2 a |
| L ** | 72.9 b | 71.5 c | 72.7 b | 75.0 a | 75.5 a |
| a | −12.5 a | −12.4 a | −12.8 a | −13.1 a | −13.8 a |
| b * | 16.0 bc | 15.6 c | 17.0 b | 19.1 a | 19.9 a |
| PRL ** | 13.3 b | 16.0 a | 2.3 d | 4.7 c | 1.3 d |
| Chlt ** | 31.5 d | 39.7 c | 44.3 b | 33.7 d | 47.6 a |
| Chla ** | 22.7 c | 25.9 b | 29.0 a | 25.3 b | 29.8 a |
| Chlb ** | 8.9 c | 13.5 b | 15.6 b | 8.7 c | 18.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laguna-Estrada, M.I.; Ruiz-Nieto, J.E.; Lopez-Nuñez, A.R.; Ramírez-Pimentel, J.G.; Raya-Pérez, J.C.; Aguirre-Mancilla, C.L. Biomass of Eichhornia crassipes as an Alternative Substrate for the Formation of Lettuce Seedlings. AgriEngineering 2024, 6, 2612-2622. https://doi.org/10.3390/agriengineering6030152
Laguna-Estrada MI, Ruiz-Nieto JE, Lopez-Nuñez AR, Ramírez-Pimentel JG, Raya-Pérez JC, Aguirre-Mancilla CL. Biomass of Eichhornia crassipes as an Alternative Substrate for the Formation of Lettuce Seedlings. AgriEngineering. 2024; 6(3):2612-2622. https://doi.org/10.3390/agriengineering6030152
Chicago/Turabian StyleLaguna-Estrada, María Isabel, Jorge Eric Ruiz-Nieto, Adolfo R. Lopez-Nuñez, Juan G. Ramírez-Pimentel, Juan Carlos Raya-Pérez, and Cesar L. Aguirre-Mancilla. 2024. "Biomass of Eichhornia crassipes as an Alternative Substrate for the Formation of Lettuce Seedlings" AgriEngineering 6, no. 3: 2612-2622. https://doi.org/10.3390/agriengineering6030152
APA StyleLaguna-Estrada, M. I., Ruiz-Nieto, J. E., Lopez-Nuñez, A. R., Ramírez-Pimentel, J. G., Raya-Pérez, J. C., & Aguirre-Mancilla, C. L. (2024). Biomass of Eichhornia crassipes as an Alternative Substrate for the Formation of Lettuce Seedlings. AgriEngineering, 6(3), 2612-2622. https://doi.org/10.3390/agriengineering6030152






