Influence of Post-Harvest Processing and Drying Techniques on Physicochemical Properties of Thai Arabica Coffee
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. True Density Determination
2.3. Color Determination
2.4. Moisture Content (MC)
2.5. Water Activity (aw)
2.6. Caffeine, Trigonelline, Chlorogenic Acid, and Caffeic Acid
2.7. Measurement of Sugar Content (Sucrose, Glucose, Fructose)
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Drying Techniques on Weight Loss
3.2. Physicochemical Properties of Green and Roasted Coffee Beans
3.2.1. True Density (TD)
3.2.2. Color
3.2.3. Moisture Content (MC)
3.2.4. Water Activity (aw)
3.3. Biochemical Properties of Green and Roasted Coffee Beans
3.3.1. Caffeine
3.3.2. Trigonelline
3.3.3. Chlorogenic Acid (CGA)
3.3.4. Caffeic Acid
3.4. Sugar Content of Green and Roasted Coffee Beans
3.5. Principal Component Analysis (PCA) of Green and Roasted Coffee Beans
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azavedo, M. The from farm to cup specialty coffee trend in Thailand and Vietnam. A major assumption about shortening supply chains disproven. Tech. Soc. Sci. J. 2021, 23, 540–556. [Google Scholar]
- Ruiz, X.F.Q.; Nigmann, T.; Schreiber, C.; Neilson, J. Collective Action Milieus and Governance Structures of Protected Geographical Indications for Coffee in Colombia, Thailand and Indonesia. Int. J. Commons 2020, 14, 329–343. [Google Scholar] [CrossRef]
- Noppakoonwong, U.; Khomarwut, C.; Hanthewee, M.; Jarintorn, S.; Hassarungsee, S.; Meesook, S.; Daoruang, C.; Naka, P.; Lertwatanakiat, S.; Satayawut, K.; et al. Research and development of Arabica coffee in Thailand. In Proceedings of the 25th International Conference on Coffee Science (ASIC), Armenia, Colombia, 8–13 September 2014; pp. 8–13. [Google Scholar]
- Humphries, U.W.; Waqas, M.; Hlaing, P.T.; Wangwongchai, A.; Dechpichai, P. Determination of crop water requirements and potential evapotranspiration for sustainable coffee farming in response to future climate change scenarios. Smart Agric. Technol. 2024, 8, 100435. [Google Scholar] [CrossRef]
- Ghosh, P.; Venkatachalapathy, N. Processing and drying of coffee—A review. Int. J. Eng. Res. Technol. 2014, 3, 784–794. [Google Scholar]
- Shofinita, D.; Lestari, D.; Aliwarga, L.; Sumampouw, G.A.; Ambarwati, S.A.; Gunawan, K.C.; Achmadi, A.B. Drying methods of coffee extracts and their effects on physicochemical properties: A review. Food Bioprocess Technol. 2024, 17, 47–72. [Google Scholar] [CrossRef]
- Aryal, B. Study of Physicochemical Properties of Coffee Bean from Different Processing Methods. Ph.D. Dissertation, Department of Food Technology, Central Campus of Technology, Institute of Science and Technology, Tribhuvan University, Kirtipur, Nepal, 2023. [Google Scholar]
- Bustos-Vanegas, J.D.; Correa, P.C.; Martins, M.A.; Baptestini, F.M.; Campos, R.C.; de Oliveira, G.H.H.; Nunes, E.H.M. Developing predictive models for determining physical properties of coffee beans during the roasting process. Ind. Crops Prod. 2018, 112, 839–845. [Google Scholar] [CrossRef]
- Tsai, C.F.; Jioe, I.P.J. The analysis of chlorogenic acid and caffeine content and its correlation with coffee bean color under different roasting degree and sources of coffee (Coffea arabica typica). Processes 2021, 9, 2040. [Google Scholar] [CrossRef]
- Levate Macedo, L.; da Silva Araujo, C.; Costa Vimercati, W.; Gherardi Hein, P.R.; Pimenta, C.J.; Henriques Saraiva, S. Evaluation of chemical properties of intact green coffee beans using near-infrared spectroscopy. J. Sci. Food Agric. 2021, 101, 3500–3507. [Google Scholar] [CrossRef] [PubMed]
- Adnan, A.; Horsten, D.V.; Pawelzik, E. Rapid prediction of moisture content in intact green coffee beans using near infrared spectroscopy. Foods 2017, 6, 38. [Google Scholar] [CrossRef]
- Pittia, P.; Nicoli, M.C.; Sacchetti, G. Effect of moisture and water activity on textural properties of raw and roasted coffee beans. J. Texture Stud. 2007, 38, 116–134. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Frankowski, R.; Zgoła-Grześkowiak, A.; Płatkiewicz, J. Comprehensive Analysis of Metabolites in Brews Prepared from Naturally and Technologically Treated Coffee Beans. Antioxidants 2022, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Kim, T.; Silva, J.L.; Hu, W.Y.; Chen, B.Y. Effects of roasting degrees on phenolic compounds and antioxidant activity in coffee beans from different geographic origins. LWT 2022, 168, 113965. [Google Scholar] [CrossRef]
- Heo, J.; Adhikari, K.; Choi, K.S.; Lee, J. Analysis of caffeine, chlorogenic acid, trigonelline, and volatile compounds in cold brew coffee using high-performance liquid chromatography and solid-phase microextraction—Gas chromatography-mass spectrometry. Foods 2020, 9, 1746. [Google Scholar] [CrossRef]
- Girma, B.; Gure, A.; Wedajo, F. Influence of Altitude on Caffeine, 5-caffeoylquinic acid, and nicotinic acid contents of arabica coffee varieties. J. Chem. 2020, 2020, 3904761. [Google Scholar] [CrossRef]
- Mengistu, M.W.; Workie, M.A.; Mohammed, A.S. Biochemical compounds of Arabica coffee (Coffea arabica L.) varieties grown in northwestern highlands of Ethiopia. Cogent Food Agric. 2020, 6, 1741319. [Google Scholar] [CrossRef]
- de Oliveira Junqueira, A.C.; de Melo Pereira, G.V.; Medina, J.D.C.; Alvear, M.C.; Rosero, R.; de Carvalho Neto, D.P.; Enriquez, H.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analyzed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef]
- Silva, C.F.; Schwan, R.F.; Dias, E.S.; Wheals, A.E. Microbial diversity during maturation and natural processing of coffee cherries of Coffea arabica in Brazil. Int. J. Food Microbiol. 2000, 60, 251–260. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Fang, M.; Suppavorasatit, I.; Cadwallader, K.R. Effect of post-harvest drying process on chlorogenic acids, antioxidant activities and CIE-Lab color of Thai Arabica green coffee beans. Food Chem. 2022, 366, 130504. [Google Scholar] [CrossRef]
- Somporn, C.; Kamtuo, A.; Theerakulpisut, P.; Siriamornpun, S. Effect of shading on yield, sugar content, phenolic acids and antioxidant property of coffee beans (Coffea Arabica L. cv. Catimor) harvested from north-eastern Thailand. J. Sci. Food Agric. 2012, 92, 1956–1963. [Google Scholar]
- Barbosa, M.D.S.G.; dos Santos Scholz, M.B.; Kitzberger, C.S.G.; de Toledo Benassi, M. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Jitjaroen, W.; Kongngoen, R.; Panjai, L. The equations of coffee Brixter index: The boosting of sugar concentration in post-harvest by using low temperature, low relative humidity. Eur. Food Res. Technol. 2024, 250, 311–323. [Google Scholar] [CrossRef]
- Specialty Coffee Association of America Cupping Protocol (SCAA). SCAA Protocols. Cupping Specialty Coffee. America. 2015. Available online: https://www.scaa.org/PDF/resources/cupping-protocols.pdf (accessed on 3 February 2024).
- Chandrasekar, V.; Viswanathan, R. Physical and thermal properties of coffee. J. Agric. Eng. Res. 1999, 73, 227–234. [Google Scholar] [CrossRef]
- Wongsa, P.; Khampa, N.; Horadee, S.; Chaiwarith, J.; Rattanapanone, N. Quality and bioactive compounds of blends of Arabica and Robusta spray-dried coffee. Food Chem. 2019, 283, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Official Methods of Analysis of AOAC International, 17th ed.; Official Method 979.12; AOAC International: Gaithersburg, MD, USA, 2000.
- Akbar, A.; Medina, A.; Magan, N. Resilience of Aspergillus westerdijkiae strains to interacting climate-related abiotic factors: Effects on growth and ochratoxin A production on coffee-based medium and in stored coffee. Microorganisms 2020, 8, 1268. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Moreno, F.L.; Osorio, C.; Velasquez, S.; Ruiz, Y. Chemical and sensory evaluation of cold brew coffees using different roasting profiles and brewing methods. Food Res. Int. 2021, 141, 110141. [Google Scholar] [CrossRef] [PubMed]
- Constantino, L.M.; Gil, Z.N.; Montoya, E.C.; Benavides, P. Coffee Berry Borer (Hypothenemus hampei) Emergence from Ground Fruits Across Varying Altitudes and Climate Cycles, and the Effect on Coffee Tree Infestation. Neotrop. Entomol. 2021, 50, 374–387. [Google Scholar] [CrossRef]
- Silvia, E.; Sidebang, B. Engineering properties of coffee beans from various colors of coffee cherries. Agric. Agric. Sci. Procedia 2015, 3, 274–277. [Google Scholar]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Figueroa Campos, G.A.; Sagu, S.T.; Saravia Celis, P.; Rawel, H.M. Comparison of batch and continuous wet-processing of coffee: Changes in the main compounds in beans, by-products and wastewater. Foods 2020, 9, 1135. [Google Scholar] [CrossRef]
- Putri, D.P.; Andriansyah, R.C.E.; Setiyoningrum, F.; Yulianti, L.E.; Hidayat, D.D. Physicochemical properties of Robusta coffee at various roasting levels using different roaster types. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2023; Volume 69, p. 03016. [Google Scholar]
- Baggenstoss, J.; Perren, R.; Escher, F. Water content of roasted coffee: Impact on grinding behaviour, extraction, and aroma retention. Eur. Food Res. Technol. 2008, 227, 1357–1365. [Google Scholar] [CrossRef][Green Version]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-Estrada, M.T. Coffee silver skin: Characterization, possible uses, and safety aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef] [PubMed]
- Sinaga, R.R.; Maryana, N.; Hidayat, P. Diversity and foraging activity of coffee insect pollinators in land near and far from the forest of North Sumatra, Indonesia. Biodiversitas J. Biol. Divers. 2024, 25, 240–248. [Google Scholar] [CrossRef]
- Mintesnot, A.; Dechassa, N. Effect of altitude, shade, and processing methods on the quality and biochemical composition of green coffee beans in Ethiopia. East Afr. J. Sci. 2018, 12, 87–100. [Google Scholar]
- Konstantinidis, N.; Franke, H.; Schwarz, S.; Lachenmeier, D.W. Risk assessment of trigonelline in coffee and coffee by-products. Molecules 2023, 28, 3460. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bicho, N.C.; Leitao, A.E.; Ramalho, J.C.; de Alvarenga, N.B.; Lidon, F.C. Identification of chemical clusters discriminators of Arabica and Robusta green coffee. Int. J. Food Prop. 2013, 16, 895–904. [Google Scholar] [CrossRef]
- Moon, J.K.; Yoo, H.S.; Shibamoto, T. Role of roasting conditions in the level of chlorogenic acid content in coffee beans: Correlation with coffee acidity. J. Agric. Food Chem. 2009, 57, 5365–5369. [Google Scholar] [CrossRef]
- Kulapichitr, F.; Borompichaichartkul, C.; Suppavorasatit, I.; Cadwallader, K.R. Impact of drying process on chemical composition and key aroma components of Arabica coffee. Food Chem. 2019, 291, 49–58. [Google Scholar] [CrossRef]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Influence of drying techniques on sensory profile and chlorogenic acid content of instant coffee powders. Meas. Food 2022, 6, 100030. [Google Scholar] [CrossRef]
- Li, X.; Yang, L.; Hao, M.; Song, T.; He, Y.; Yang, M.; Zhang, J. Chlorogenic acid as an indispensible partner of caffeic acid in coffee via selective regulation of prooxidative actions of caffeic acid. Food Res. Int. 2023, 173, 113482. [Google Scholar] [CrossRef]
- Moon, J.K.; Shibamoto, T. Formation of volatile chemicals from thermal degradation of less volatile coffee components: Quinic acid, caffeic acid, and chlorogenic acid. J. Agric. Food Chem. 2010, 58, 5465–5470. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ayala, L.F.; Perez-Gonzalez, A.; Reina, M.; Guzman-Lopez, E.G.; Galano, A. Morning Antioxidants: Molecular Insights on the Chemistry of Coffee Components. Preprints 2023, 2023062138. [Google Scholar] [CrossRef]
- Knopp, S.; Bytof, G.; Selmar, D. Influence of processing on the content of sugars in green Arabica coffee beans. Eur. Food Res. Technol. 2006, 223, 195–201. [Google Scholar] [CrossRef]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the Impacts of Postharvest Processing on the Microbiota and Metabolite Profiles during Green Coffee Bean Production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef]
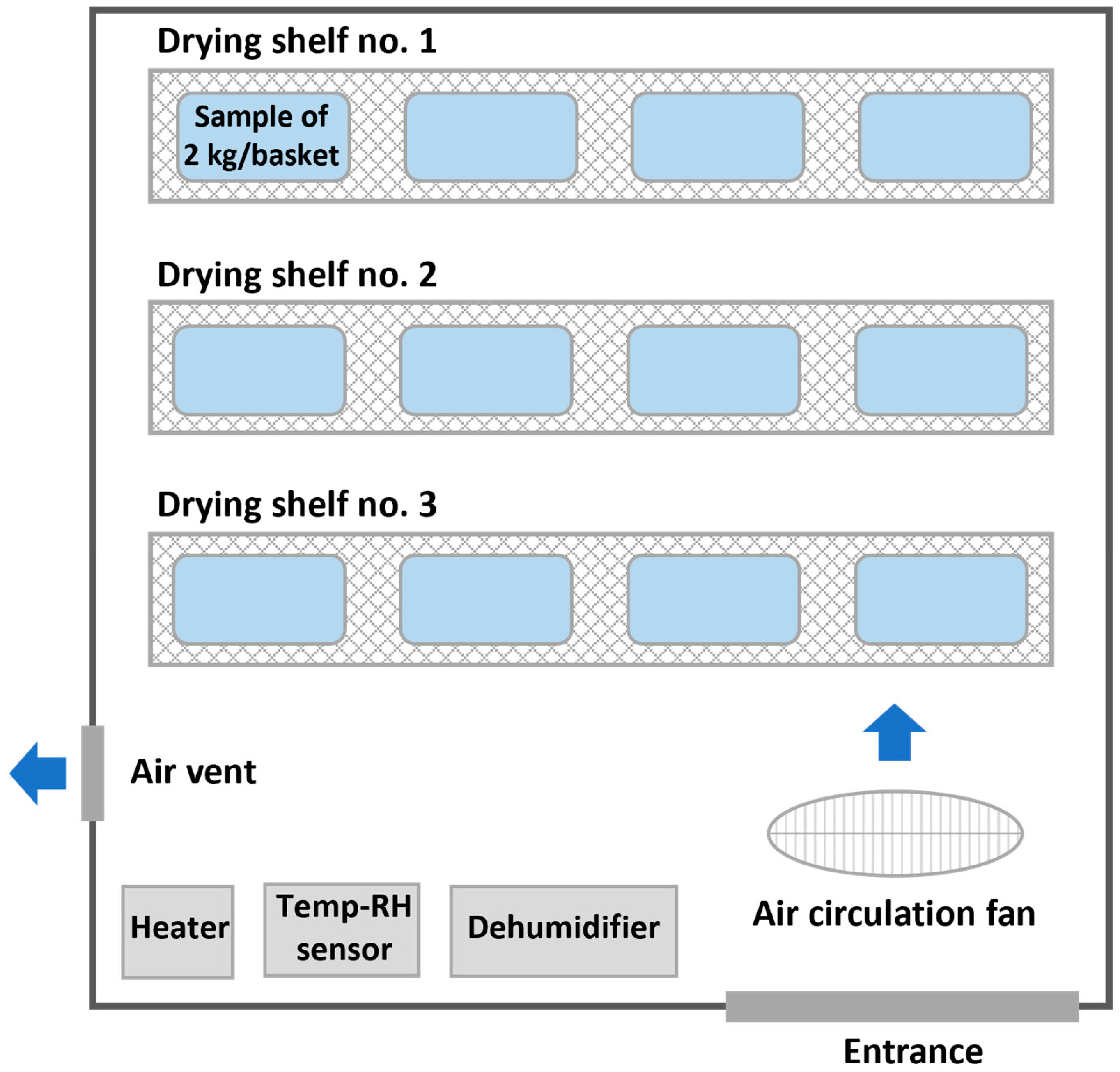
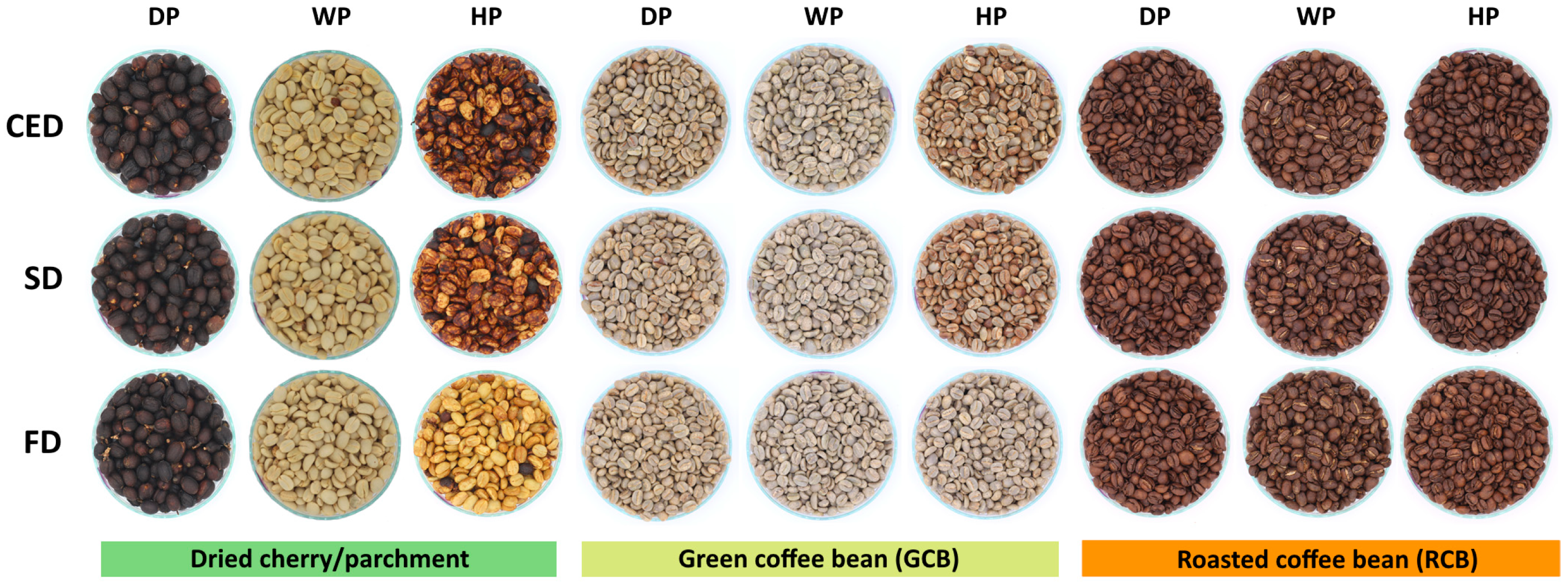


| Drying Technique | Processing | T (°C) | RH (%) | Drying Time (Day) | Final MC (% w.b.) |
|---|---|---|---|---|---|
| CED | DP | 20–30 | 50–55 | 28 ± 0.05 a | 11.00 ± 0.06 a |
| WP | 20–30 | 50–55 | 10 ± 0.01 d | 11.20 ± 0.10 a | |
| HP | 20–30 | 50–55 | 14 ± 0.01 c | 9.69 ± 0.09 bc | |
| SD | DP | - | - | 21 ± 0.03 b | 10.96 ± 0.30 ab |
| WP | - | - | 9 ± 0.07 d | 9.44 ± 0.07 b | |
| HP | - | - | 13 ± 0.13 c | 9.52 ± 1.26 c | |
| FD | DP | 30–40 | 50–55 | 15 ± 0.07 c | 10.99 ± 0.14 ab |
| WP | 30–40 | 50–55 | 8 ± 0.25 e | 9.40 ± 1.00 c | |
| HP | 30–40 | 50–55 | 9 ± 0.06 e | 9.95 ± 0.54 bc |
| Sample | TD (g/mL) | L* | a* | b* | MC (% w.b.) | aw | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCB | RCB | GCB | RCB ns | GCB | RCB | GCB | RCB ns | GCB | RCB | GCB | RCB | |
| CED-DP | 1.21± 0.01 ab | 0.74± 0.02 ab | 51.91± 0.51 bc | 41.60± 0.61 | 1.85± 0.18 ab | 2.82± 0.12 abc | 8.53± 0.31 ab | 1.54± 0.30 | 8.48± 0.22 b | 1.99± 0.05 c | 0.52± 0.02 c | 0.40± 0.04 ab |
| CED-WP | 1.18± 0.02 bcd | 0.71± 0.02 bc | 50.57± 0.21 d | 41.69± 0.12 | 0.71± 0.17 d | 2.76± 0.11 bc | 7.32± 0.31 b | 1.65± 0.47 | 10.28± 0.41 ab | 1.74± 0.01 d | 0.61± 0.00 a | 0.39± 0.06 b |
| CED-HP | 1.23± 0.01 a | 0.75± 0.01 a | 52.70± 0.15 b | 41.35± 0.07 | 2.48± 0.47 a | 2.67± 0.31 c | 10.02± 1.30 a | 1.83± 0.56 | 10.37± 0.97 ab | 1.92± 0.07 c | 0.62± 0.00 a | 0.44± 0.02 a |
| SD-DP | 1.18± 0.02 bcd | 0.73± 0.01 a | 52.14± 0.56 bc | 41.60± 0.23 | 1.92± 0.01 ab | 2.87± 0.44 abc | 10.00± 0.51 a | 1.84± 0.19 | 8.57± 0.20 b | 2.18± 0.03 b | 0.53± 0.01 c | 0.45± 0.01 a |
| SD-WP | 1.20± 0.01 bc | 0.70± 0.01 bc | 52.49± 0.11 bc | 41.85± 0.36 | 1.10± 0.07 cd | 2.50± 0.16 c | 8.25± 0.29 ab | 1.65± 0.27 | 9.73± 1.06 ab | 1.95± 0.02 c | 0.58± 0.00 b | 0.38± 0.02 b |
| SD-HP | 1.16± 0.01 d | 0.70± 0.01 bc | 52.13± 0.04 bc | 41.75± 0.45 | 2.52± 0.25 a | 2.57± 0.17 c | 10.27± 0.84 a | 1.36± 0.23 | 11.23± 0.56 a | 1.96± 0.02 c | 0.58± 0.01 b | 0.41± 0.02 ab |
| FD-DP | 1.19± 0.03 bc | 0.69± 0.02 c | 51.55± 0.35 cd | 42.35± 0.67 | 2.57± 0.34 a | 3.55± 0.28 a | 9.5± 0.26 ab | 2.44± 0.46 | 9.25± 0.59 ab | 1.86± 0.09 cd | 0.57± 0.01 b | 0.42± 0.04 ab |
| FD-WP | 1.17± 0.02 cd | 0.69± 0.02 c | 52.34± 0.23 bc | 41.81± 0.33 | 0.93± 0.16 cd | 2.64± 0.27 c | 8.19± 0.97 ab | 1.53± 0.23 | 8.78± 0.06 b | 2.26± 0.02 ab | 0.53± 0.01 c | 0.39± 0.03 b |
| FD-HP | 1.15± 0.01 d | 0.72± 0.01 abc | 54.07± 0.42 a | 41.24± 0.88 | 1.82± 0.54 ab | 3.43± 0.35 ab | 9.66± 0.52 ab | 2.46± 0.43 | 9.41± 0.86 ab | 2.40± 0.07 a | 0.53± 0.00 c | 0.42± 0.04 ab |
| Sample | Caffeine (mg/mL) | Trigonelline (mg/mL) | Chlorogenic Acid (mg/mL) | Caffeic Acid (mg/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| GCB | RCB | GCB | RCB | GCB | RCB | GCB | RCB ns | |
| CED-DP | 1.01± 0.02 a | 0.76± 0.48 a | 0.68± 0.11 a | 0.23± 0.01 b | 205.38± 2.42 a | 24.44± 3.17 b | 0.45± 1.12 a | 0.19± 0.17 |
| CED-WP | 0.91± 0.03 cd | 0.71± 0.01 ab | 0.54± 0.01 bc | 0.22± 0.02 c | 79.61± 2.34 c | 23.37± 0.94 cd | 0.41± 0.39 ab | 0.18± 0.03 |
| CED-HP | 0.92± 0.02 ab | 0.64± 0.01 cd | 0.51± 0.04 bc | 0.21± 0.01 d | 109.08± 0.94 bc | 24.05± 3.38 b | 0.39± 0.99 ab | 0.19± 0.01 |
| SD-DP | 0.95± 0.02 ab | 0.68± 0.01 bc | 0.61± 0.09 abc | 0.24± 0.01 a | 189.99± 3.13 a | 25.99± 0.56 a | 0.41± 0.30 ab | 0.20± 0.01 |
| SD-WP | 0.88± 0.03 cd | 0.72± 0.02 ab | 0.57± 0.03 abc | 0.22± 0.01 c | 76.81± 1.65 c | 24.53± 0.68 bc | 0.38± 0.41 ab | 0.20± 0.01 |
| SD-HP | 0.87± 0.04 cd | 0.68± 0.00 bc | 0.48± 0.03 d | 0.21± 0.02 d | 123.82± 0.76 b | 25.65± 0.32 a | 0.30± 0.25 b | 0.19± 0.01 |
| FD-DP | 0.90± 0.01 cd | 0.65± 0.00 cd | 0.64± 0.04 ab | 0.23± 0.00 b | 122.42± 0.56 b | 22.77± 0.52 c | 0.40± 0.11 ab | 0.18± 0.02 |
| FD-WP | 0.82± 0.01 d | 0.68± 0.01 bc | 0.55± 0.07 bc | 0.22± 0.01 c | 68.35± 5.68 c | 21.20± 1.51 d | 0.45± 0.21 a | 0.19± 0.01 |
| FD-HP | 0.86± 0.02 cd | 0.60± 0.02 d | 0.50± 0.02 bc | 0.22± 0.00 c | 103.98± 3.49 bc | 21.53± 0.93 d | 0.37± 0.66 b | 0.18± 0.02 |
| Sample | Sucrose (%) | Fructose (%) | Glucose (%) | |||
|---|---|---|---|---|---|---|
| GCB | RCB | GCB | RCB | GCB | RCB | |
| CED-DP | 6.15 ± 0.04 bc | ND | 3.75 ± 0.40 a | 2.07 ± 0.41 a | ND | ND |
| CED-WP | 5.15 ± 0.04 bc | ND | 1.30 ± 0.57 bc | 0.95 ± 0.29 bc | ND | ND |
| CED-HP | 9.55 ± 1.76 a | ND | 1.20 ± 0.65 c | 1.15 ± 0.20 bc | ND | ND |
| SD-DP | 5.60 ± 0.33 bc | ND | 2.20 ± 0.33 b | 1.33 ± 0.25 b | ND | ND |
| SD-WP | 6.60 ± 0.41 bc | ND | 1.77 ± 0.41 bc | 1.07 ± 0.50 bc | ND | ND |
| SD-HP | 7.45 ± 0.61 ab | ND | 1.70 ± 0.16 bc | 0.95 ± 0.18 bc | ND | ND |
| FD-DP | 5.23 ± 0.73 bc | ND | 1.53 ± 0.04 bc | 0.96 ± 0.33 bc | ND | ND |
| FD-WP | 4.35 ± 0.37 c | ND | 1.10 ± 0.08 c | 0.60 ± 0.08 c | ND | ND |
| FD-HP | 5.55 ± 0.04 bc | ND | 1.60 ± 0.04 bc | 0.68 ± 0.15 bc | ND | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aung Moon, S.; Wongsakul, S.; Kitazawa, H.; Saengrayap, R. Influence of Post-Harvest Processing and Drying Techniques on Physicochemical Properties of Thai Arabica Coffee. AgriEngineering 2024, 6, 2198-2213. https://doi.org/10.3390/agriengineering6030129
Aung Moon S, Wongsakul S, Kitazawa H, Saengrayap R. Influence of Post-Harvest Processing and Drying Techniques on Physicochemical Properties of Thai Arabica Coffee. AgriEngineering. 2024; 6(3):2198-2213. https://doi.org/10.3390/agriengineering6030129
Chicago/Turabian StyleAung Moon, Sai, Sirirung Wongsakul, Hiroaki Kitazawa, and Rattapon Saengrayap. 2024. "Influence of Post-Harvest Processing and Drying Techniques on Physicochemical Properties of Thai Arabica Coffee" AgriEngineering 6, no. 3: 2198-2213. https://doi.org/10.3390/agriengineering6030129
APA StyleAung Moon, S., Wongsakul, S., Kitazawa, H., & Saengrayap, R. (2024). Influence of Post-Harvest Processing and Drying Techniques on Physicochemical Properties of Thai Arabica Coffee. AgriEngineering, 6(3), 2198-2213. https://doi.org/10.3390/agriengineering6030129








